Outpatient parenteral antibiotic therapy (OPAT) is a safe, effective, and cost-saving alternative to prolonged inpatient hospitalization for patients who require long durations of intravenous antimicrobial therapy.Reference Ruh, Parameswaran, Wojciechowski and Mergenhagen 1 – Reference Vargas-Palacios, Meads and Twiddy 6 OPAT enables earlier transitions out of the acute-care setting, reduces the duration of hospitalization, and is associated with high levels of patient satisfaction.Reference Durojaiye, Bell, Andrews, Ntziora and Cartwright 2 – Reference Saillen, Arensdorff and Moulin 4
Although the benefits of OPAT are well recognized, long-term intravenous antimicrobial treatment carries substantial risk of antimicrobial toxicity and complications of intravenous catheters. Multiple studies report rates of adverse events during a typical OPAT course ranging from 6% to 44%; the most common undesirable outcomes include adverse drug events (ADEs) and vascular access complications.Reference Ruh, Parameswaran, Wojciechowski and Mergenhagen 1 , Reference Suleyman, Kenney, Zervos and Weinmann 7 – Reference Shrestha, Shrestha and Everett 13 These complications cause harm to patients, increase healthcare utilization, and diminish the benefits that OPAT programs offer patients and health systems. Minimizing patient risk requires substantial clinical and administrative infrastructure to ensure that treatment is safe and effective.Reference Schmidt, Hearn, Gabriel, Spencer and McCurdy 14 – Reference Madaline, Nori and Mowrey 20
The adverse effects caused by antimicrobial therapy are well established. Among hospitalized patients, antibiotic-associated ADEs are common during treatment and when used for prophylaxis around surgery, with a linear relationship between the duration of therapy and the risk of ADEs.Reference Tamma, Avdic, Li, Dzintars and Cosgrove 21 , Reference Branch-Elliman, Ripollone and O’Brien 22 Vancomycin is associated with increased rates of ADEs, including nephrotoxicity, when compared to other gram-positive agents.Reference Keller, Williams, Gavgani, Hirsch, Adamovich, Hohl, Gurses and Cosgrove 8 , Reference Tamma, Avdic, Li, Dzintars and Cosgrove 21 Nephrotoxicity risk is compounded when vancomycin is administered in combination with β-lactam antibiotics, which commonly occurs in the OPAT setting if a patient is diagnosed with a polymicrobial infection or if no specific organism is isolated.Reference Branch-Elliman, Ripollone and O’Brien 22 – Reference Moenster, Linneman, Finnegan, Hand, Thomas and McDonald 25 Recent studies suggest that daptomycin may offer a safe and effective alternative with reduced need for OPAT staff intervention compared to vancomycin.Reference Shrestha, Mason and Gordon 12
Given the well-established concerns regarding antimicrobial toxicity and the challenges of balancing convenience and comfort with safety and effectiveness, we sought to compare the rates and timing of ADEs and healthcare utilization between patients receiving OPAT antibiotic treatment with vancomycin and daptomycin to inform clinical decision making.
Methods
Setting
We performed a single-center, retrospective observational cohort study of patients receiving treatment with either daptomycin or vancomycin in the OPAT program of a large tertiary-care academic medical center. Patients enrolled in the OPAT program require consultation with an infectious disease physician, including the determination of the need for >14 days of parenteral antibiotics following hospital discharge. All enrolled patients have documentation in an integrated inpatient/outpatient electronic health record (EHR) outlining diagnosis, antimicrobial type, dose, anticipated treatment duration, and recommendations for weekly laboratory safety monitoring, based on Infectious Diseases Society of America (IDSA) OPAT Guidelines.Reference Tice, Rehm and Dalovisio 16 Laboratory data, clinic visit notes, and telephone notes are entered into the EHR by OPAT staff. Notes from home visits by external infusion companies and visiting nurse agencies were not available.
Cohort identification
Adult patients receiving their initial OPAT treatment course for management of skin and soft-tissue infections, bone and joint infections (including hardware-associated infections and diabetic ulcer infections), bacterial endocarditis, and bacteremia or endovascular infections who were treated with a regimen containing vancomycin or daptomycin were eligible for inclusion. The study period was July 1, 2013, through September 30, 2016. Patients were excluded for the following reasons: the initial hospital discharge was to hospice care, OPAT enrollment occurred as an outpatient, post-discharge management and follow-up were with a provider outside of the study site’s Infectious Disease OPAT clinic, the patient was receiving chronic renal replacement therapy, or if death occurred prior to completion of OPAT treatment (Fig. 1).

Fig. 1 Patient selection. aSkin and soft-tissue infections, bone and joint infections, endocarditis, and bacteremia/endovascular infections. Abbreviations: OPAT, outpatient parenteral antibiotic therapy; VAN, vancomycin; DAP, daptomycin.
Data collection and definitions
Dates of enrollment, hospital discharge, and infection diagnosis were extracted from the OPAT database. The cohort entry date was defined as the date of discharge from the initial hospitalization. Data abstracted from the EHR included demographics, insurance status, baseline clinical and laboratory characteristics (Table 1), microbiology results (site of culture and bacterial organism), other antimicrobials administered during OPAT treatment, location of disposition and receipt of OPAT treatment (home, long-term acute care, or skilled nursing facility), type of vascular access used for infusion, recommended and actual duration of OPAT, frequency of clinic visits and telephone calls with OPAT clinic staff, availability of safety lab testing results, and the occurrence and type of ADEs related to antibiotic therapy. Laboratory testing was considered discordant with IDSA OPAT guidelines if recommended labs were unavailable for review by the treating OPAT physician in the EHR for >1 week of the patient’s treatment course.Reference Tice, Rehm and Dalovisio 16 Charlson comorbidity index (CCI) was calculated using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) or International Classification of Disease, Tenth Revision (ICD-10) codes obtained from hospital fiscal databases.Reference Quan, Sundararajan and Halfon 26 , Reference Li, Evans, Faris, Dean and Quan 27
Table 1 Patient Characteristics
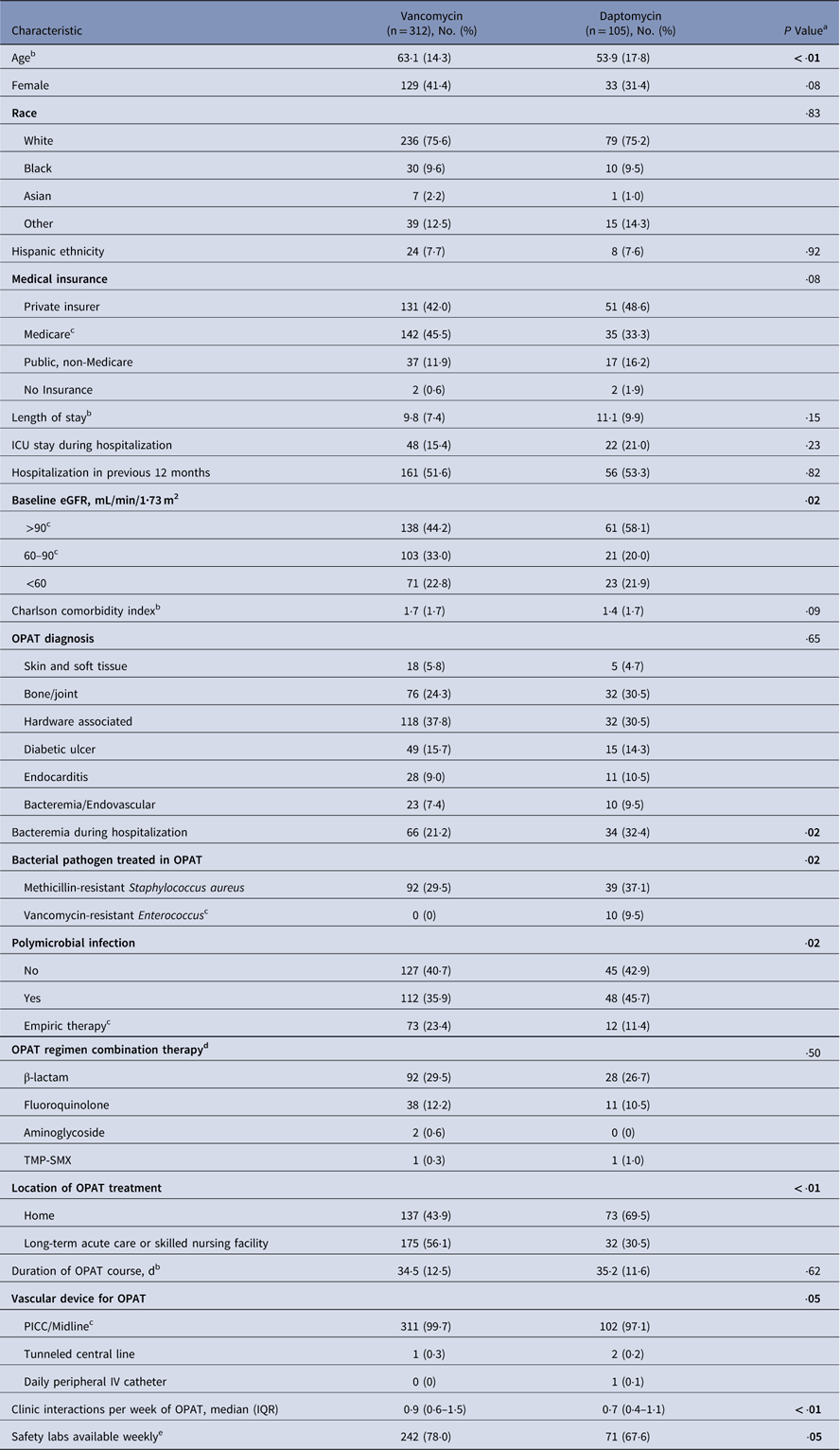
Note. eGFR, estimated glomerular filtration rate; ICU, intensive care unit; IQR, interquartile range; IV, intravenous; OPAT, outpatient parenteral antibiotic therapy; PICC, peripherally inserted central catheter; TMP-SMX, trimethoprim-sulfamethoxazole; VAN, vancomycin.
a Bold values indicate statistical significance.
b Data presented as mean (standard deviation).
c P≤·05 for univariate comparison of the category.
d Only receipt of combination therapy with β-lactam or fluoroquinolone used in multivariate regression model.
e Missing data (VAN=1).
Outcomes
The primary outcome was defined as a change or early discontinuation of the antibiotic of interest due to an ADE occurring >7 days prior to the anticipated end date of treatment. An ADE was defined as harm or injury to the individual attributed to the antimicrobial agent according to the treating OPAT physician, as documented in clinic or telephone notes. Secondary outcomes were time from OPAT enrollment to occurrence of ADE, unplanned hospital readmissions and emergency room visits during the 30-day window after completion of OPAT, and change or early discontinuation of the antibiotic of interest due to reason other than ADE occurring >7 days prior to the anticipated end date of treatment. Hospital readmissions and emergency room visits were not independently counted as ADEs, though they may have been related to an ADE.
Data analysis
Baseline characteristics of patients receiving vancomycin and daptomycin were compared using the Student t test, the Fisher exact test, the Mann-Whitney U test, and the χ2 test as appropriate. The primary analysis was performed using logistic regression, adjusting for 6 variables chosen a priori based on prior studies: age, CCI, location of OPAT treatment, receipt of combination therapy with either β-lactam or fluoroquinolone, baseline renal function, and availability of weekly safety labs.Reference Ruh, Parameswaran, Wojciechowski and Mergenhagen 1 , Reference Bhavan, Brown and Haley 5 , Reference Schmidt, Hearn, Gabriel, Spencer and McCurdy 14 , Reference Karino, Kaye and Navalkele 23 – Reference Moenster, Linneman, Finnegan, Hand, Thomas and McDonald 25 , Reference Huck, Ginsberg, Gordon, Nowacki, Rehm and Shrestha 28 To measure the association between antimicrobial choice and hospital readmission and emergency room visits, 3 variables were chosen a priori for inclusion in a logistic regression model: age, CCI, and location of OPAT treatment.Reference Bhavan, Brown and Haley 5 , Reference Schmidt, Hearn, Gabriel, Spencer and McCurdy 14 , Reference Huck, Ginsberg, Gordon, Nowacki, Rehm and Shrestha 28 A sensitivity analysis including patients who died during the OPAT treatment course was completed to ensure that our results were robust to inclusion and exclusion criteria.
In addition, a time-to-event analysis was completed. Patients were censored at completion of OPAT treatment, discontinuation of vancomycin or daptomycin, or after loss to follow up and cumulative incidence curves were generated.
Data were collected and analyzed using Microsoft Access 2010 software (Microsoft, Redmond, WA) and SAS version 9·3 software (SAS Institute, Cary, NC).
Ethical considerations
The Institutional Review Board of Beth Israel Deaconess Medical Center approved this study prior to data collection and analysis.
Results
Study population and baseline characteristics
In total, 417 patients met inclusion criteria, including 312 (74·8%) who received vancomycin and 105 (25·2%) who received daptomycin (Fig. 1). We excluded 2 patients from our analysis of the primary outcome due to loss to follow-up.
Baseline characteristics are summarized in Table 1. The most common OPAT diagnoses were bone and joint infections and hardware-associated infections. The mean patient age was 60·8 years, and 38·9% of the patient cohort were female. The distribution of combination regimens was similar among patients receiving daptomycin and vancomycin; the most common additional agents were β-lactams and fluoroquinolones. Patients who received vancomycin were more likely to have mild renal impairment with an estimated glomerular filtration rate (eGFR) of 60–90 mL/min/1·73 m2 (33·0% vs 20·0%). Patients who received vancomycin were also more likely to have recommended safety laboratory results available to the treating OPAT provider each week (78·0% vs 67·6%). Of 105 patients patients receiving daptomycin, 10 (9·5%) had an infection with vancomycin-resistant Enterococcus.
After stratifying by the location of OPAT, (Supplementary Table 1), patients receiving daptomycin therapy at home had the lowest severity of illness (CCI>1; 26·0%) compared to those receiving daptomycin therapy at a long-term acute-care or skilled nursing facility and those receiving vancomycin at either location. Most patients receiving treatment in a long-term acute-care or skilled nursing facility had Medicare health insurance , both for daptomycin (56·3%) and vancomycin (52·6%). Patients receiving home infusions of vancomycin had the highest frequency of clinic interactions, with a median of 1·1 per week.
Outcomes
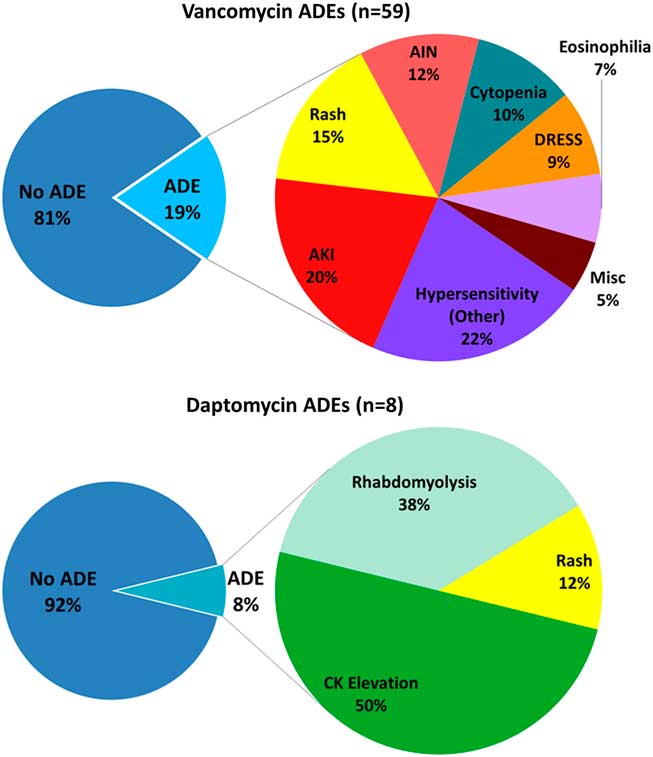
Patients receiving vancomycin had higher rates of ADEs resulting in change or early discontinuation of therapy (19·0% vs 7·6%; P<·01) (Table 2). In the 59 vancomycin-treated patients with ADEs, the most common were hypersensitivity reactions (22%), acute kidney injury (20%), rash (15%), and acute interstitial nephritis (12%). Among the 8 daptomycin-treated patients with ADEs, the most common were asymptomatic creatine kinase (CK) elevations (50%), rhabdomyolysis (38%), and rash (12%) (Fig. 2). In multivariate logistic regression analysis, vancomycin remained an independent predictor of ADE (adjusted odds ratio [aOR], 3·71; 95% confidence interval [CI], 1·64–8·40) (Table 3). Inclusion of patients who died (N=5) did not change the association.

Fig.2 Rates and categories of ADEs among recipients of vancomycin and daptomycin as part of their OPAT regimen. Abbreviations: ADEs, adverse drug events; AIN, acute interstitial nephritis; AKI, acute kidney injury; CK, creatine kinase; DRESS, drug reaction with eosinophilia and systemic symptoms; OPAT, outpatient parenteral antibiotic therapy.
Table 2 Unadjusted Rates of Primary and Secondary Outcomes With Vancomycin and Daptomycin

Note. ADE, adverse drug event; DAP, daptomycin; VAN, vancomycin.
a Bold values indicate statistical significance.
b Missing data (VAN=2).
c Missing data (VAN=2, DAP=2).
d Missing data (VAN=3, DAP=2).
Table 3 Multivariate Logistic Regression Model of Adverse Drug Events Leading to a Change or Early Discontinuation of Antibiotic Therapy
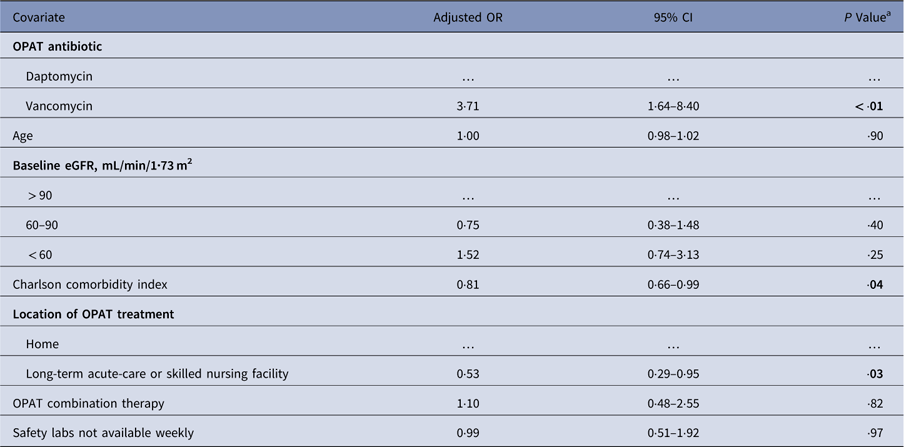
NOTE. OR, odds ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; OPAT, outpatient parenteral antibiotic therapy; OR, odds ratio.
a Bold values indicate statistical significance.
Daptomycin-treated patients received longer durations of therapy prior to onset of ADEs (P<·01). After 7 and 28 days of therapy, 4·5% and 16.7% of vancomycin patients experienced the primary outcome versus 0% and 6·7% of daptomycin patients (Fig. 3, cumulative incidence curve).

Fig. 3 Cumulative incidence (with 95% confidence intervals) for adverse drug events among recipients of vancomycin and daptomycin as part of their OPAT regimen. Patients were censored at completion of OPAT treatment, discontinuation of vancomycin or daptomycin, or after loss to follow-up.
Therapy changes for non-ADE events were higher in vancomycin-treated patients (10·0% vs 2·9%; P=·03). Among the 31 vancomycin-treated patients with therapy changes, the reasons included microbiologic results (26%), physician choice (16%), dosing challenges (13%), peripherally inserted central catheter-related issues (13%), and patient preference to discontinue parenteral therapy (13%). For the 3 daptomycin-treated patients with therapy changes, the reasons included OPAT physician concern for clinical failure, readmission for pneumonia, and patient preference to discontinue parenteral therapy. Rates of unplanned hospital readmission (vancomycin 30·3% vs daptomycin 32·0%) and emergency room visitation (vancomycin 34·0% vs daptomycin 35·0%) were similar.
Discussion
This study is one of the largest published cohorts to compare therapy-related outcomes among OPAT patients and provides additional data to inform selection of antimicrobial agents. Patients receiving vancomycin had higher rates of ADEs resulting in change or early discontinuation of treatment and higher rates of healthcare utilization when compared to daptomycin-treated patients (aOR, 3·71; 95% CI, 1·64–8·40). This association persisted throughout the duration of the OPAT treatment course, from week 1 to beyond week 8 of therapy. The incidence of ADEs leading to change or discontinuation of therapy in our cohort (16%) is similar to those reported in other published studies.Reference Ruh, Parameswaran, Wojciechowski and Mergenhagen 1 , Reference Suleyman, Kenney, Zervos and Weinmann 7 – Reference Lee, Tam and Weigel 9 , Reference Shrestha, Mason and Gordon 12
The types and severity of ADEs in vancomycin- versus daptomycin-treated patients were substantively different (Fig. 2). The most common reason for therapy change in the daptomycin-treated patients was asymptomatic CK elevation, while hypersensitivity syndromes, organ dysfunction, and cytopenias were observed in the vancomycin group. Notably, 3 of 7 daptomycin patients who developed a skeletal muscle ADE were also taking a statin at the time of discharge. Some cases of skeletal muscle toxicity may have been reduced through a robust discharge medication reconciliation process. However, in some high-risk patients, discontinuing the statin may have outweighed any potential benefits. This high-risk population is also at increased risk of kidney injury, further complicating the clinical decision-making process.
The toxicity of intravenous antibiotics, particularly vancomycin, necessitates a careful review of the clinical indication for the expanded-spectrum gram-positive coverage. A significant number of patients in our cohort received vancomycin empirically without a confirmed microbiologic diagnosis of a resistant organism (23·4%). This treatment decision was driven in part by cases in which patients did not have a microbiologic culture obtained in the setting of an infectious process often caused by methicillin-resistant Staphylococcus aureus (MRSA) or coagulase-negative Staphylococci, such as skin and soft-tissue infections (5·8%), bone and joint infections (24·3%), and hardware-associated infections (37·8%). Careful consideration of MRSA risk is important before committing patients to a prolonged course of vancomycin. If microbiology results are not conclusive, alternative means of MRSA risk stratification, such as MRSA nasal screening, may be useful to tailor decision making. Negative nasal screening has a high negative predictive value and is useful in many instances for narrowing antimicrobial coverage.Reference Acuna-Villaorduna, Branch-Elliman, Strymish and Gupta 29
The IDSA OPAT guidelines recommend weekly safety laboratory monitoring for patients receiving home infusions of vancomycin and daptomycin. These guidelines were last updated in 2004, however, and logistical challenges result in significant variation in real-world clinical practice.Reference Keller, Williams, Gavgani, Hirsch, Adamovich, Hohl, Gurses and Cosgrove 8 , Reference Keller, Ciuffetelli and Bilker 30 , Reference Lane, Marschall and Beekmann 31 Availability of OPAT laboratory testing is associated with a lower risk of hospital readmission and higher OPAT success.Reference Ruh, Parameswaran, Wojciechowski and Mergenhagen 1 , Reference Huck, Ginsberg, Gordon, Nowacki, Rehm and Shrestha 28 We did not find a significant association between frequency of safety laboratory monitoring and reduced risk of adverse events; however, our study had limited power to detect a difference in this outcome between the 2 exposures groups.
These findings are limited in several ways. First, there is potential for residual confounding present in all observational designs. This may explain our finding that receipt of therapy at a long-term acute-care or skilled nursing facility was associated with a lower risk of medication change due to ADE. A higher burden of comorbidities was noted among patients at facilities (Supplementary Table 1). In a sensitivity analysis including patients who died, the association between receipt of therapy at a long-term acute-care or skilled nursing facility and the primary outcome did not persist (P=·12), suggesting that unmeasured confounding may be driving this finding. Second, as a single-center analysis, this cohort and the clinical practices in our center may not be reflective of those in other OPAT clinics. In addition, anticipated OPAT duration of at least 14 days was a qualification for enrollment; shorter courses that are at lower risk of ADEs were not included. To ensure that only clinically significant ADEs were reflected in our results, we limited our primary outcome to ADEs that resulted in a therapy change or discontinuation. We also did not consider common adverse events, such as red man syndrome, that may negatively impact a patient’s quality of life without resulting in a change in therapy. There was a high rate of hospital readmission in this cohort as compared to other studies, potentially reflecting a sicker patient population, although a long follow up period—extended 30 days beyond the completion of OPAT therapy—may also have impacted this result.Reference Suleyman, Kenney, Zervos and Weinmann 7 , Reference Keller, Williams, Gavgani, Hirsch, Adamovich, Hohl, Gurses and Cosgrove 8 , Reference Means, Bleasdale, Sikka and Gross 10 , Reference Shrestha, Mason and Gordon 12 , Reference Schmidt, Hearn, Gabriel, Spencer and McCurdy 14 , Reference Madaline, Nori and Mowrey 20
Our study is one of the few published analyses comparing adverse events and healthcare utilization associated with 2 of the most commonly prescribed gram-positive agents in the OPAT setting, daptomycin and vancomycin. Other strengths include the size of the cohort included, the breadth of infectious diagnoses considered, and the completeness of the data with minimal missing covariates or outcomes. With an organized OPAT program involving physicians, nurses, and administrative staff, pertinent clinical and demographic data for each patient was documented at the time of enrollment and clinical follow up, optimizing data capture.
Pre-enrollment evaluation of patients with an infectious disease consultation and sufficient clinical support to ensure a complete care transition reduces adverse events and overall OPAT costs.Reference Conant, Erdman and Osterholzer 17 , Reference Muldoon, Snydman, Penland and Allison 19 , Reference Madaline, Nori and Mowrey 20 Careful consideration is necessary when selecting an antibiotic agent for long-term therapy, including the risk of selection for antibiotic resistance, costs, ease of administration, need for monitoring, and the known risk of complications, including ADEs. By defining the primary outcome as ADEs that required a change or discontinuation of therapy, the results of this study are more directly related to the choice of antimicrobial agent used for treatment, a programmatic decision for an OPAT clinic, rather than outcomes related to healthcare delivery, nursing care, or patient preferences. Our study informs inpatient clinicians and infectious disease OPAT providers regarding the risks of ADEs among recipients of vancomycin versus daptomycin.
The perceived drawbacks of daptomycin—primarily cost and broader spectrum of activity—must be weighed against the apparent challenges of long-term vancomycin: high rates of clinically significant ADEs and utilization of OPAT clinic resources. While our study did not include an economic analysis, our results suggest that vancomycin, although less expensive on a per-dose basis, is associated with complications that may render it more expensive when used for prolonged therapy in the outpatient setting. Notably, Medicare recipients (42·4% of this study’s cohort) without supplemental insurance are often restricted in their long-term antibiotic options with regard to type of antimicrobial agent and site of therapy. As our analysis demonstrates, daptomycin is a safer alternative to vancomycin for gram-positive therapy in OPAT. Therefore, a Medicare recipient with a low burden of comorbidities and the ability to self-administer a once-daily medication may be better served receiving daptomycin home infusions. In addition to the direct medical costs associated with complications, vancomycin is also associated with other societal costs attributable to lost time and work productivity. For example, vancomycin infusions last for 1–2 hours, typically multiple times per day, compared to <30 minutes once daily for daptomycin. Prospective analysis to compare the rates of clinical success and ADEs would alleviate some of the confounding challenges present in this study, and further cost-effectiveness analyses of these 2 medications would greatly inform the decisions of health systems beyond the wholesale price of the medication.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.107
Acknowledgments
The authors acknowledge Linda Baldini, RN, the BIDMC InSIGHT Core, and the Center for Healthcare Delivery Science for their assistance with data retrieval.
Financial support
This study did not receive any funding and was conducted as part of our routine work. WBE is supported by a Veterans Integrated Service Network (VISN)-1 Career Development Award.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.