Introduction
The spined soldier bug (SSB), Podisus maculiventris (Say) (Hemiptera: Pentatomidae), is a generalist predator used as a biological control agent in North America and Europe primarily for the control of lepidopteran and coleopteran pests (De Clercq, Reference De Clercq, Schaefer and Panizzi2000). As with most beneficial insects, current methods for mass rearing of P. maculiventris involve rearing on live insect prey. Hence, the nutritional value or food quality of prey is critical for the fitness of this predator (De Clercq et al., Reference De Clercq, Merlevede and Tirry1998; Strohmeyer et al., Reference Strohmeyer, Stamp, Jarzomski and Bowers1998; Mahdian et al., Reference Mahdian, Kerckhove, Tirry and De Clercq2006). Development of immatures, consumption capacity or production of eggs, in response to feeding, has been used to define the food quality of prey (Legaspi & Legaspi, Reference Legaspi and Legaspi2004). In addition, specific biochemical parameters in P. maculiventris nymphs and adults have been considered to analytically determine food quality, such as lipid and protein profiles (Legaspi et al., Reference Legaspi, Shapiro and Legaspi2004; Shapiro & Legaspi, Reference Shapiro and Legaspi2006) and vitellogenin content in females (Shapiro et al., Reference Shapiro, Wasserman, Greany and Nation2000). However, the effect of prey food quality on the digestive capacity of the predator is largely unknown.
Nymphs and adults of P. maculiventris mechanically and enzymatically process their prey extra-orally, by inserting the stylet and injecting digestive enzymes through the salivary canal into the prey, then suck the partially digested material into their own gut where digestion is completed (Cohen, Reference Cohen1990, Reference Cohen1995). Protease activity in P. maculiventris salivary secretions is mainly based on serine proteases, whereas cysteine proteases and exopeptidases are predominant in the gut (Stamopoulos et al., Reference Stamopoulos, Diamantidis and Chloridis1993; Bell et al., Reference Bell, Down, Edwards, Gatehouse and Gatehouse2005; Álvarez-Alfageme et al., Reference Álvarez-Alfageme, Martínez, Pascual-Ruiz, Castañera, Diaz and Ortego2007). A number of studies have revealed that the quality and quantity of digestive enzymes in phytophagous insects can be altered in response to changes in dietary protein (Broadway & Duffey, Reference Broadway and Duffey1988; Felton, Reference Felton1996). However, Habibi et al. (Reference Habibi, Backus, Coudron and Brandt2001) showed that, in contrast to phytophagous hemipterans, P. maculiventris manifested minimal differences in their salivary protein profile after feeding on different prey. Thus, the capacity to distinguish and respond to dietary stimuli appears to differ between phytophagous and entomophagous hemipterans.
The aim of this study was to examine the effects of different prey regimes on the performance and digestive physiology of P. maculiventris nymphs. Specifically, P. maculiventris nymphs were fed on Colorado potato beetle (CPB), Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae), larvae; Egyptian cotton leafworm (ECW), Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae), larvae; Calliphora spp. (CAL) (Diptera: Calliphoridae) pupae or a mixture of the three prey.
Materials and methods
Insects
A laboratory colony of CPB from Ávila (Spain) was reared on potato plants, Solanum tuberosum cv. Kennebec. A laboratory colony of ECW was reared on a semi-artificial diet, modified from Poitout & Bues (Reference Poitout and Bues1970) by the addition of 0.63% (w/w) Wesson's salt mixture. Larvae and pupae of CAL were obtained from a local fishing tackle retailer. Nymphs of P. maculiventris were purchased from Koppert Sistemas Biológicos (Almería, Spain) and reared for one generation on larvae of CPB or ECW or pupae of CAL before use in trials. All laboratory colonies were reared in environmental chambers at 25±2°C, 70±10% RH and a 16:8 h (L:D) photoperiod.
Insect bioassays
Feeding assays were performed with third instar P. maculiventris nymphs placed singly in ventilated plastic dishes (30 mm height×90 mm Ø) that contained filter paper and cotton soaked with water and were maintained in an environmental chamber as described above. Two to three larvae of CPB, ECW, pupae of CAL or a mixture of the three prey were provided daily until P. maculiventris nymphs reached the fifth instar. Twenty replicas were made for each prey regime, and the weight of each P. maculiventris nymph were recorded before release (newly molted, less than 12 hours, third instar nymphs) and at the end of the bioassay (fifth instar nymphs allowed to feed for 24 hours). The weight gain of P. maculiventris nymphs was calculated as the difference between the initial and the final weight.
At the end of the assay, P. maculiventris nymphs were placed at −20°C for about 5 min and then dissected in ice-cold dH2O and salivary glands and midguts extracted for enzymatic determinations. Midguts were homogenised in 500 μl of 0.15 M NaCl, centrifuged at 10,000 g for 5 min and the supernatants individually frozen to provide 18–20 samples of each feeding regime. Salivary glands were pooled in groups of two to provide 9–10 samples, homogenised in 150 μl of 0.15 M NaCl, centrifuged, and the supernatants stored frozen until needed.
Digestive protease assays
Unless otherwise stated, insect protease activities were determined at 30°C, at their optimum pH of activity in 1 ml of reaction mixture. Total protein in midgut extracts was determined according to the method of Bradford (Reference Bradford1976) with BSA as the standard. All substrates were purchased from Sigma Chemical Co. (St Louis, Missouri, USA).
The protease activities present in the salivary glands and midgut of P. maculiventris nymphs were determined following the conditions described by Álvarez-Alfageme et al. (Reference Álvarez-Alfageme, Martínez, Pascual-Ruiz, Castañera, Diaz and Ortego2007). All assays were performed using 30 μl of salivary gland extract or 20 μl of midgut extract. Trypsin-like activity was assayed using 1 mM BApNa (Nα–benzoyl-DL-arginine-p-nitroanilide) as substrate and incubating for 24 h at pH 9.0 (salivary glands) or pH 10.0 (midgut), chymotrypsin-like activity using 0.25 mM SA2PPpNa (N-succinyl-(alanine)2-proline-phenylalanin-p-nitroanilide) as substrate and incubating for 24 h at pH 9.5 (salivary glands) or pH 10.0 (midgut), cathepsin B-like activity using 50 μM ZAA2MNA (N-carbobenzoxy-alanine-arginine-arginine 4-methoxy-β-naphthyl amide) as substrate and incubating for 24 h (salivary glands) or 4 h (midgut) at pH 7.0 with buffer that contain 1 mM L-cysteine, leucine aminopeptidase-like activity using 1 mM LpNa (L-leucine p-nitroanilide) as substrate and incubating for 24 h at pH 7.0 (salivary glands) or 2 h at pH 7.5 (midgut), and carboxypeptidase B-like activity using 1 mM HA (hippuryl-L-arginine) as substrate and incubating for 24 h at pH 7.0 (salivary glands) or 7.5 (midgut). Spectrophotometric measurements were made using a Hitachi U-2000 spectrophotometer (Tokyo, Japan), and readings taken at 410 nm for pNa substrates, 520 nm for ZAA2MNA, and 570 nm for HPA and HA by the ninhydrin procedure.
Zymograms
Electrophoretic detection of proteolytic forms was performed by 0.1% (w/v) gelatine-containing, 0.1% (w/v) SDS, 12% (w/v) polyacrylamide gel electrophoresis under non-denaturing conditions (Lantz & Ciborowski, Reference Lantz and Ciborowski1994) using a Bio-Rad Mini-Protean II Electrophoresis Cell system. The ratio of acrylamide to bis-acrylamide was 37.5:1. Samples of salivary glands and midguts of P. maculiventris nymphs contained 3 μg of total protein, whereas CPB and ECW midgut extracts and CAL pupae full body extracts contained 2, 0.5 and 3 μg of total protein, respectively. After migration at 4°C, gels were transferred to a 2.5% (v/v) aqueous solution of Triton X-100 for 30 min at room temperature, to allow renaturation of the proteases. Gels were then placed in the following activation buffers for 24 h at 30°C: 0.1M Tris-HCl and 10 mM L-cysteine, pH 7.0; or 0.1 M glycine-NaOH, pH 9.0. Proteolysis was stopped by transferring the gels into a staining solution [0.3% (w/v) Coomassie Blue R-250 in 40% (v/v) methanol and 10% (v/v) acetic acid]. The gels were destained in 25% (v/v) methanol and 10% (v/v) acetic acid. Bands of proteolytic activity were visualised against the blue background of the gel.
Statistical analysis
Weight gain, duration of stadia and protease activities of P. maculiventris nymphs fed with different prey species were analyzed by means of the Student-Newman-Keuls-test. Differences between treatments were considered significant at the P⩽0.05 level.
Results
No differences in development and weight gain were observed when P. maculiventris nymphs were fed different prey species (CPB, ECW or CAL) (table 1). However, an increase in weight gain and a reduction in the duration of the stadia were observed for nymphs fed with a mixture of the three species. To investigate the physiological background, biochemical analysis were carried out on insects dissected at the end of the feeding assay. Biochemical analysis showed that the activities of the proteases (trypsin-, chymotrypsin-, cathepsin B-, leucine aminopeptidase- and carboxypeptidase B-like) present in the salivary glands of P. maculiventris nymphs were not affected by the different prey regimes, whereas the activities in the midgut were determined by the prey (table 1). Thus, trypsin- and chymotrypsin-like activities were high in P. maculiventris midguts when the nymphs were fed on ECW, low when fed on CPB or CAL and intermediate when fed with a mixture of the three species. Likewise, P. maculiventris nymphs fed on CPB, ECW or the MIX diet presented higher midgut cathepsin B-, leucine aminopeptidase- and carboxypeptidase B-like activities than when fed with CAL.
Table 1. Performance and digestive protease activity of P. maculiventris nymphs feeding on L. decemlineata (CPB) larvae, S. littoralis (ECW) larvae, Calliphora spp. (CAL) pupae or a mixture (MIX) of the three prey.

a Assays were performed with third instar nymphs of P. maculiventris feeding on different prey until they reached the fifth instar. Data are the mean±SE (n=18 for CPB, 19 for BAW, 20 for CAL and 18 for MIX).
b Protease activity is expressed as nmoles of substrate hydrolyzed min−1 mg−1 protein. Data are the mean±SE (n=9–10 for salivary glands and 18–20 for midguts).
Row means followed by the same letter are not significantly different from each other (Student-Newman-Keuls test, P⩽0.05).
Gel assays were performed to look for differences in protease forms in salivary glands and midgut of P. maculiventris nymphs fed with different prey. The proteolytic profile in salivary glands was not affected by the different prey regimes, and the protease forms were different to those present in the prey (fig. 1a). On the contrary, the midguts from P. maculiventris nymphs feeding on CPB, ECW, CAL or a mixture of the three prey presented different proteolytic profiles, and the banding patterns closely resembled those of their prey (fig. 1b). Minimal differences in banding patterns were obtained when the zymograms with P. maculiventris salivary glands and midguts were performed at two different pHs, 7.0 and 9.0 (data not shown). With respect to the effect of pH in the protease forms of the prey, different banding patterns were obtained for CPB at pH 7.0 and 9.0, whereas similar results were observed with ECW (figs 1a, b). No protease forms were detected with CAL at any of the pH tested (figs 1a, b).
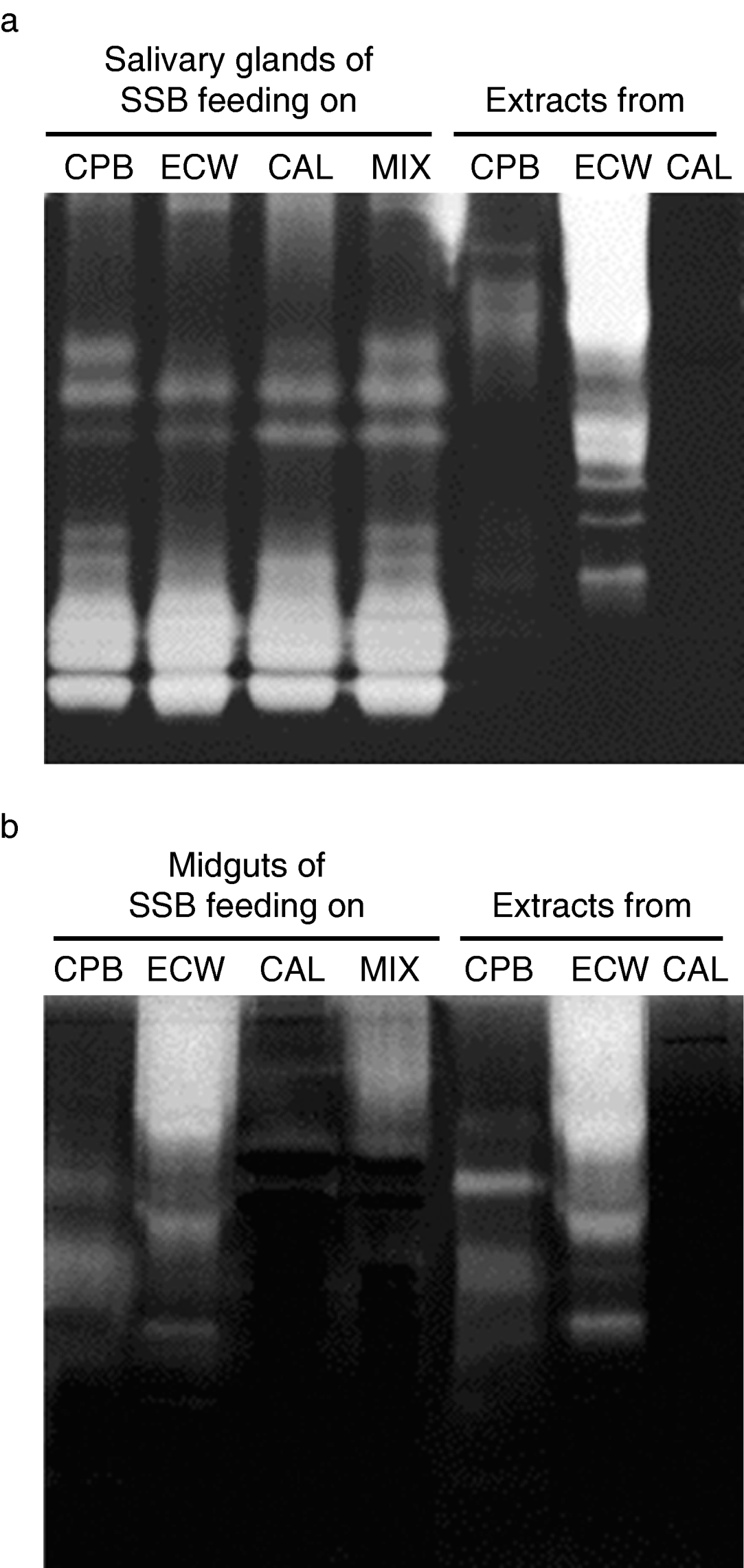
Fig. 1. Gelatin-containing SDS-PAGE gels of salivary glands and midgut of P. maculiventris (SSB) nymphs feeding on L. decemlineata (CPB) larvae, S. littoralis (ECW) larvae, Calliphora spp. (CAL) pupae or a mixture (MIX) of the three prey. Extracts from CPB, ECW and CAL larval midgut and CAL full body were also analyzed. Gels were incubated for 24 h at 30°C with (a) 0.1 M glycine-NaOH, pH 9.0 or (b) 0.1M Tris-HCl and 10 mM L-cysteine, pH 7.0.
Discussion
The involvement of serine proteases, especially trypsin and chymotrypsin, in extra-oral digestion of heteropteran predators is well documented (Boyd, Reference Boyd2003; Bell et al., Reference Bell, Down, Edwards, Gatehouse and Gatehouse2005; Oliveira et al., Reference Oliveira, Oliveira, Guedes and Soares2006). We have found that, in addition to these two activities, cathepsin B-, leucine aminopeptidase- and carboxypeptidase B-like activities were also detected in the salivary glands of P. maculiventris nymphs. Bell et al. (Reference Bell, Down, Edwards, Gatehouse and Gatehouse2005) also provided evidences for the presence of cysteine proteases and carboxypeptidases in the salivary glands of this species. It has been reported that the salivary composition of phytophagous hemipterans may vary in response to diet composition of the host plant (Miles, Reference Miles, Minks and Harrewijn1987; Habibi et al., Reference Habibi, Backus, Coudron and Brandt2001). However, information regarding the capacity of entomophagous hemipterans to respond to dietary stimuli is very scarce. Habibi et al. (Reference Habibi, Backus, Coudron and Brandt2001) showed that P. maculiventris manifested minimal differences in banding patterns of the saliva proteins after feeding on different prey. Our results go further, indicating that the proteolytic activity in the salivary glands of P. maculiventris nymphs was not affected by prey species. It has been suggested that variable salivary composition in phytophagous hemipterans might indicate induction of new proteins in preparation for continued feeding on a new host plant (Habibi et al., Reference Habibi, Backus, Coudron and Brandt2001). However, generalist predators may consume a large variety of prey species in a short period of time, and changes in salivary composition in respond to dietary stimuli of one specific prey may not represent a utility for the consumption of the next prey.
Gut proteolysis in P. maculiventris is mainly performed by cysteine proteases, aminopeptidases and carboxypeptidases (Stamopoulos et al., Reference Stamopoulos, Diamantidis and Chloridis1993; Bell et al., Reference Bell, Down, Edwards, Gatehouse and Gatehouse2005; Álvarez-Alfageme et al., Reference Álvarez-Alfageme, Martínez, Pascual-Ruiz, Castañera, Diaz and Ortego2007). Biochemical analysis showed that, in contrast to what happen with salivary glands, the relative activity of these proteases in the midgut depends on the prey. Thus, nymphs fed on CPB, ECW or the MIX diet presented higher midgut cathepsin B-, leucine aminopeptidase- and carboxypeptidase B-like activities than when fed with CAL. Likewise, trypsin- and chymotrypsin-like activities were detected in the P. maculiventris midgut when the nymphs were fed on ECW or the MIX diet, but very low levels were found when fed on CPB or CAL. Interestingly, trypsin- and chymotrypsin-like activities are the major digestive proteases in ECW (Lee & Anstee, Reference Lee and Anstee1995), whereas CPB mainly rely on cysteine proteases (Thie & Houseman, Reference Thie and Houseman1990) and only traces of proteolytic activity were found in CAL pupae (data not shown). Moreover, the zymograms proved that the proteolytic profiles of midguts from P. maculiventris nymphs feeding on CPB, ECW and CPB closely resembled those of their prey. All together, these results suggest that P. maculiventris may utilize enzymes from the prey they consume. However, none of the main proteases present in the salivary glands, and presumably injected in the prey, were recovered in the gut.
Nymphs of P. maculiventris fed with a mixture of the three prey attained higher weights and shorter developmental time than those reared on a single prey. In predation studies where a choice of prey is presented simultaneously, polyphagous predators can improve their fitness or fecundity by feeding on mixed diets. For example, Podisus nigrispinus (Dallas) displayed higher egg and nymphal production on a diet of Tenebrio molitor L. (Coleoptera: Tenebrionidae) and Musca domestica L. (Diptera: Muscidae), compared with diets consisting of either species exclusively (Zanuncio et al., Reference Zanuncio, Molina-Rugama, Serrao and Pratissoli2001). Under this scenario, polyphagous predators are expected to select prey that would be of the best nutritional quality (Strohmeyer et al., Reference Strohmeyer, Stamp, Jarzomski and Bowers1998; Legaspi & Legaspi, Reference Legaspi and Legaspi2004). However, prey selection may be determined by other factors, such as prey size and mobility (De Clercq et al., Reference De Clercq, Wyckhuys, De Oliveira and Klapwijk2002) and the avoidance of toxic unpalatable prey (Traugott & Stamp, Reference Traugott and Stamp1996). The results of the present study indicate that, as a consequence of the extra-oral digestion, the digestive proteases of the prey are sucked by P. maculiventris nymphs into its own gut. Thus, when consuming a variety of prey, a broader range of proteases will be available that may facilitate the process of digestion. Likewise, the overall performance of P. maculiventris reared on insect-free artificial diet is inferior to those reared on live prey (De Clercq et al., Reference De Clercq, Merlevede and Tirry1998; Wittmeyer & Coudron, Reference Wittmeyer and Coudron2001). Differences in nutritional quality, lack of feeding stimulants and diet presentation may explain these results (De Clercq et al., Reference De Clercq, Merlevede and Tirry1998). Still, the use by the predator of prey-derived enzymes may also help to explain the higher nutritional value of prey over artificial diets.
In summary, the effects of different prey regimes on the performance and digestive physiology of P. maculiventris nymphs have been assessed. It is noteworthy that P. maculiventris apparently utilizes enzymes from the prey they consume that may facilitate the process of digestion. Physiological analysis would be particularly informative for insight into which combinations of prey enhance the efficiency of digestion and what physiological processes are involved.
Acknowledgements
We thank Dr Viñuela (ETSIA-UPM, Spain) for providing the laboratory colony of S. littoralis and Dr González-Nuñez for collecting adults of L. decemlineata.




