Introduction
Methylglyoxal (MG) is a highly reactive dicarbonyl compound, being a major precursor in the formation of molecular adducts. Reference Chen, Simar, Lambert, Mercier and Morris1,Reference Melo, Benatti and Ignacio-Souza2 MG originates non-enzymatically as a by-product of glycolysis Reference Phillips and Thornalley3 by degradation of glyceraldehyde-3-phosphate and dihydroxyketone phosphate, intermediates of the glycolytic pathway, as well as a consequence of lipid metabolism. Reference Miyata, Inagi and Asahi4,Reference Miyata, Van Ypersele de Strihou, Kurokawa and Baynes5 MG levels have been shown to be elevated in diabetes and to be associated to the development of diabetic complications. Reference Matafome, Rodrigues, Sena and Seiça6 Nonetheless, westernised diets are composed of highly processed foods that are rich not only in fat, sugar and salt but also contain potentially harmful compounds known as advanced glycation end products (AGEs). Reference Uribarri, del Castillo and de la Maza7 In addition to food preparation methods, which use high temperatures and potentiate the production of AGEs, maternal diabetes or metabolic syndrome can also increase the bioavailability of AGEs to the newborn through the milk, given that both are related to increased plasma AGEs, which are able to pass through the blood milk barrier. Reference Li and Yang8,Reference Francisco, Barella and da Silveira9 In physiological conditions, endogenously formed MG is metabolised, detoxified and converted into D-lactate by the glyoxalase system. Reference McLellan, Phillips and Thornalley10 This system was first described by Dakin and Dudley in 1913, being identified in tissues such as pancreas, liver, muscle tissue, heart, kidney, blood, spleen and brain. Reference Dakin and Dudley11 In this sense, glyoxalase system is an evolution-conserved critical defence mechanism against the glycation of proteins, lipids and nucleic acids. Reference Rabbani, Xue and Thornalley12,Reference Monnier and Taniguchi13
Postnatal (PN) early environmental and nutritional disturbances are critical for the developmental origins of health and disease. Clinical and experimental studies have demonstrated acute and long-term effects of such early disturbances on growth and metabolism later in life. Reference Francisco, Barella and da Silveira9-Reference Hales and Barker16 There is a growing interest in the possible adverse human health outcomes associated with the early exposure to chemical products commonly present in diet. Several studies demonstrated that this exposure during critical stages of development, such as pregnancy and lactation, may ‘program’ individuals to the development of chronic non-communicable diseases, such as obesity, diabetes and hypertension. Reference Francisco, Barella and da Silveira9-Reference Valério Prates, Ribeiro and Pavanello19 In this sense, maternal diet and metabolic status have also been implicated in the higher probability of obesity and metabolic syndrome development in offspring throughout life, despite the contribution of breastmilk MG is unknown. Reference Francisco, Barella and da Silveira9 Moreover, infant formulas, which are used worldwide as a substitute for breastmilk, also exhibit high levels of MG and AGEs. The content of glycotoxins in infant formula exceeds that of breastmilk by hundred folds. Reference Federico, Gori, Randazzo and Vierucci20-Reference Prosser, Carpenter and Hodgkinson22 Thus, exposing infants to these nutritional contaminants early in life may contribute to the development of cardiometabolic disorders at adulthood and disclosing the mechanisms is urgent.
Recently, we demonstrated that maternal oral treatment with MG during lactation leads to dyslipidaemia and disrupted glucose homeostasis in the offspring, programming the adult offspring to develop the type 2 diabetic phenotype. Reference Francisco, Barella and da Silveira9 Nevertheless, in that study, we cannot attribute such events to the direct effect of MG that may have passed through the milk. Thus, this study was designed to evaluate the direct effect of MG exposure during the suckling period on the offspring. Thereby, we hypothesised that PN offspring exposure to MG in the first 2 weeks of lactation could lead to long-term impairment of metabolic homeostasis in the adult life.
Materials and methods
Ethical approval
The handling of animals and experimental procedures were done according to ARRIVE guidelines, the rules of National Council of Animal Experiments Control (CONCEA) and the Brazilian Society of Science in Laboratory Animals (SBCAL) and approved by the Ethics Committee on Animal Use of Universidade Estadual de Maringá – CEUA/UEM (protocol number 3830171215).
Experimental design and treatment
Wistar rats (70-day-old) were housed in the Animal Facility of the Laboratory of Cell Secretion Biology, Department of Biotechnology, Genetic and Cell Biology of State University of Maringá, in polypropylene cages (45 × 30 × 15 cm) maintained on a 12:12 h light-dark cycle (07:00 lights on) and controlled temperature (22.0°C ± 2°C). After 1 week of adaptation, the animals were mated in a ratio of three females (n = 24) to each male (n = 8). Pregnant rats were accommodated in individual cages throughout the pregnancy and nursing period. At delivery (PN1), animals were divided into two groups: CO (n = 48) offspring treated with saline (0.9% NaCl, i.p.) and MG group (MG; n = 48) offspring treated with MG (20 mg/kg of BW i.p. Sigma-Aldrich, São Paulo, São Paulo, Brazil). Litter size was standardised for 8 pups per mother (preferentially male) to minimise the competition in the breastfeeding and provide similar conditions between the offsprings. The treatment of the offspring was initiated at delivery and occurred between 4 and 5 pm, throughout the first 2 weeks of the suckling period, from PN1 to PN14. From PN14 until weaning the offspring remained with their mothers who received standard chow (Nuvital, Curitiba, Paraná, Brazil) and had unlimited access to food and water throughout lactation period. Throughout the experimental period food intake and BW were evaluated daily.
Experimental procedures
At weaning (PN21), male offspring were housed in polypropylene cages (three to four rats per cage), under same conditions of their mothers. The offspring from both groups received standard chow (Nuvital, Curitiba, Paraná, Brazil) and had unlimited access to food and water until PN90. Body weight was evaluated throughout experimental period. At PN90, a batch of offspring (n = 12–15/group) were 12-h fasted, anesthetised with sodium thiopental (45 mg/kg, i.p., Thiopentax, Cristália, Itapira, São Paulo, Brazil) and euthanised for blood, white adipose tissue, liver, pancreas and kidney sample collection. Blood was collected by inferior vena cava puncture, with sterile needle and syringe. For each experimental procedure, offspring from at least three litters per group were used to avoid litter effects.
Intravenous glucose tolerance test
At PN90, other batch of adult offspring (n = 10–12/group), from both experimental groups, were anesthetised (Ketamine 75 mg/kg; Xylazine 15 mg/kg, i.m. Cristália, Itapira, São Paulo, Brazil) and then submitted to the implantation of a silicone cannula (Silastic, Dow Corning, Midland, MI, USA) in the right jugular vein for intravenous glucose tolerance test (ivGTT). The test was performed in overnight fasted conscious rats, as previously described. Reference Gomes, Tófolo and Rinaldi23 Briefly, blood samples (100 µL) were collected before and after 5, 15, 30 and 45 min of an intravenous injection of glucose (1 g/kg). Blood samples were centrifuged at (10,000 rpm for 5 min) for plasma collection, and the plasma stored at −20°C for subsequent quantification of glucose and insulin. Animals used for the ivGTT were not used in any other experimental procedures.
Blood glucose, lipid profile and fructosamine measurements
Blood samples were centrifuged (10,000 rpm for 5 min) and plasma was used for the measurements of glucose, total cholesterol, high density lipoprotein (HDL), triglycerides and fructosamine by enzymatic colorimetric method with commercial kits (Gold Analisa, Belo Horizonte, Minas Gerais, Brazil). Low density lipoprotein (LDL) was calculated according to the Friedewald equation: LDL = Total Cholesterol − (HDL + Triglycerides/5). Reference Berlanga, Cibrian and Guillén24 Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated using the formula: serum insulin (mmol/L) × (blood glucose (mmol/L)/22.5. Reference Antunes, Elkfury, Jornada, Foletto and Bertoluci25
Plasma insulin measurement
Plasma insulin was measured by radioimmunoassay in a gamma counter (Wizard2 Automatic Gamma Counter, TM-2470, PerkinElmer, Shelton, CT, USA). It was used as standard human insulin and anti-rat insulin antibody (Sigma-Aldrich, St. Louis, MO, USA) and recombinant human insulin labelled I125 (PerkinElmer, Shelton, CT, USA). The intra-assay coefficients of variation were in the range 8%–10%. The limit of detection was 0.006 ng/ml. The measurements were taken in a single assay.
Morphological analyses of pancreas, liver and kidney
Liver, kidney and pancreas samples were fixed in 10% formalin and embedded in histological paraffin. Nonserial sections (5 µm thick) were performed using a Leica RM2145 microtome (Leica Biosystems, Richmond, VA, USA). Liver and pancreas sections were stained with haematoxylin and eosin. Liver and kidney sections were stained with Picrosirius Red and counter-stained with haematoxylin. Photomicrographs were made in a light microscope coupled to a digital camera (DM500 plus 199 ICC50 HD, Leica Microsystems, Wetzlar, Germany).
Analysis of the pancreatic islet area was performed using 20 digital images (×400 magnification) from each animal (n = 5 animals/group). The results were expressed as µm Reference Melo, Benatti and Ignacio-Souza2 .
To evaluate liver lipid inclusion, stereological analysis was performed in HE stained sections (1000× magnification), with a mesh of 594 points. Similarly, liver fibrosis assessment was done by stereology in fields where portal triads were present, with a same mesh of 594 points.
To assess the glomerulus area and capsular space, coronal sections (400× magnification) where glomeruli with well-defined renal capillaries can be seen were used. For analysis of glomerulus count by area, three micrographs per field of each animal were used and then the count was performed, the result was expressed in number/field.
Stereological analysis was performed using Image-Pro Plus software (version 6.0, Media Cybernetics, Rockville, MD, USA). Glomerulus area and number, capsular space and pancreatic islet area assessment were performed using the ICY software (Institut Pasteur, Paris, France. http://icy.bioimageanalysis.org/).
Biochemical analyses of hepatic and renal GSH, SOD, CAT, LOOH and MPO
Liver and kidney samples were collected, fractionated and processed to evaluate biochemical markers of oxidative stress and inflammatory parameters. After being weighed, tissue portions were homogenised separately in 200 mm potassium phosphate buffer (pH 6.5). Part of the homogenate was used for quantification of reduced glutathione (GSH) levels. The other part was centrifuged (20 min at 9000×g) and the supernatant was used for Superoxide Dismutase (SOD), Catalase (CAT) and Lipid Hydroperoxide (LOOH) measurements as previously described. Reference Borges, Ferreira and da Silva26 The precipitate was used for the analysis of Myeloperoxidase enzyme activity (MPO), according to Borges et al. Reference Borges, Ferreira and da Silva26
Statistical analyses
Statistical analysis of the data and the construction of the graphics were performed using GraphPad Prism version 6.01 (GraphPad software, Inc., La Jolla, CA, USA). All data were submitted to DʼAgostino-Pearson omnibus K2 normality test and analysed using unpaired Student’s t-test. Results were expressed as the mean ± standard error of means and p < 0.05 was considered significantly different.
Results
Postnatal early MG exposure reduces fat mass and weight gain without reducing food intake
MG-exposed offspring presented reduced body weight since PN10 until PN90, compared with the CO offspring (p < 0.05; Fig. 1a, 1b). Accordingly, PN early exposure to MG decreased naso-anal length (p < 0.01; Fig. 1c). Despite there is no difference on absolute food intake after weaning (data not show), when corrected by the body weight, MG animals presented increased food intake under the same period (p < 0.001; Fig. 1d, 1e). In addition, MG group had twofold (p < 0.0001; Fig. 1f), 37% (p < 0.01; Fig. 1g) and fivefold (p < 0.0001; Fig. 1h) less periepididymal, retroperitoneal and mesenteric fat mass, respectively, as compared with CO.
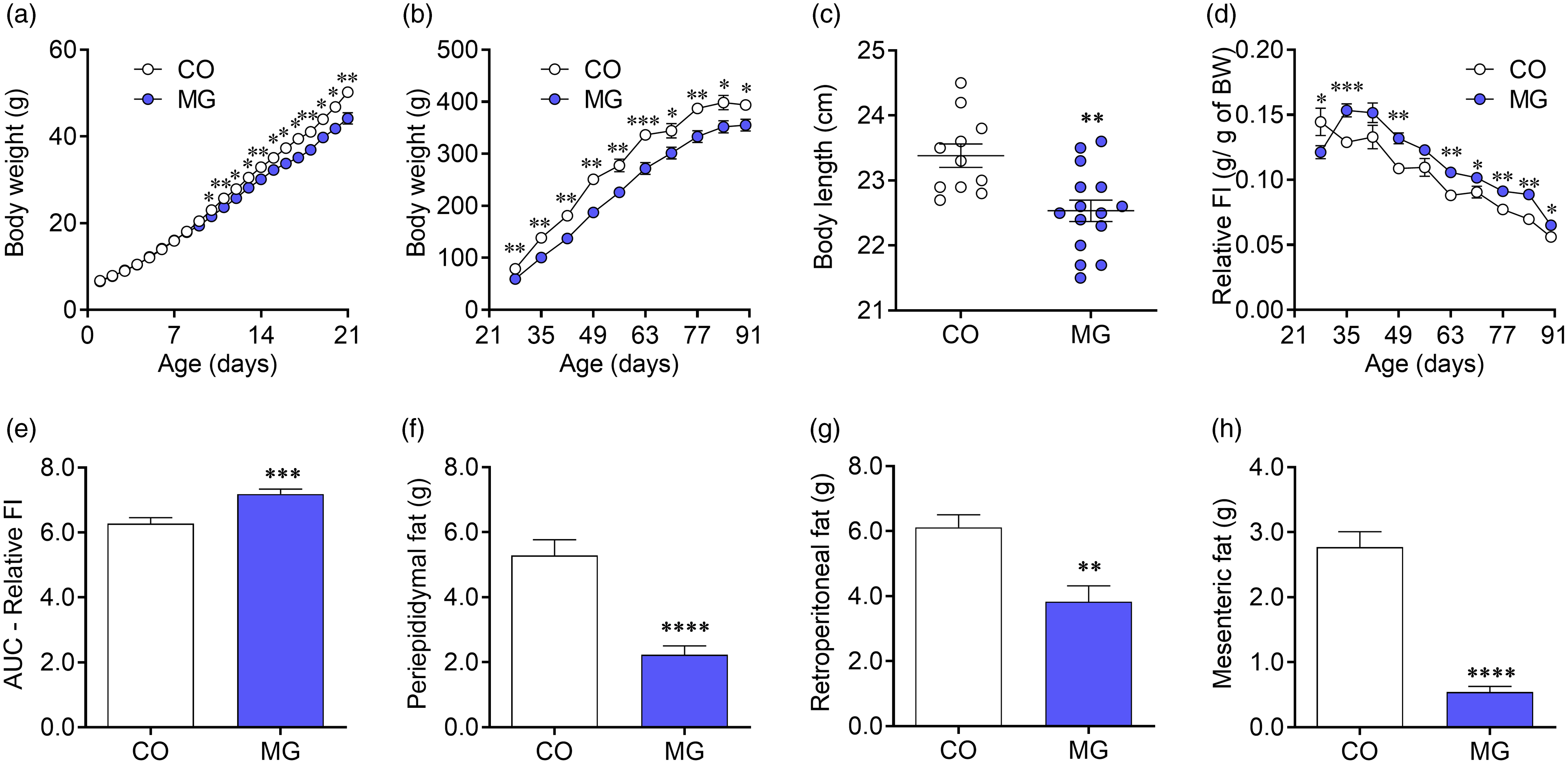
Fig. 1. Effects of postnatal early exposure to MG on body composition and food intake. Body weight before (a) and after (b) weaning, body length (c) at adulthood, relative food intake (d), AUC of relative food intake (e), periepididymal (f), retroperitoneal (g) and mesenteric (h) fat pads. Data are presented as mean ± SEM (n = 12–15). To compare the experimental groups Student’s t-test was used, where *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. SEM, standard error of mean.
Postnatal early MG exposure induces insulin resistance, pancreatic islet hypertrophy and dyslipidaemia
Plasma glucose levels did not differ from CO group neither during the ivGTT nor at fasting (Fig. 2a, 2b, 2e). However, MG-treated animals showed increased plasma insulin levels throughout the ivGTT (p < 0.05; Fig. 2c) leading to twofold greater AUC of blood insulin levels compared with CO group (p < 0.01; Fig. 2d). We also observed higher fasting insulin levels (p < 0.05; Fig. 2f) and increased HOMA index (p < 0.05; Fig. 2g) in MG-treated offspring. Accordingly, blood fructosamine was higher in the MG group (p < 0.01; Fig. 2h). Although MG-treated animals present no difference in pancreas mass compared to CO animals (Fig. 2j), pancreatic islets hypertrophy was observed (p < 0.01; Fig. 2k, 2l).
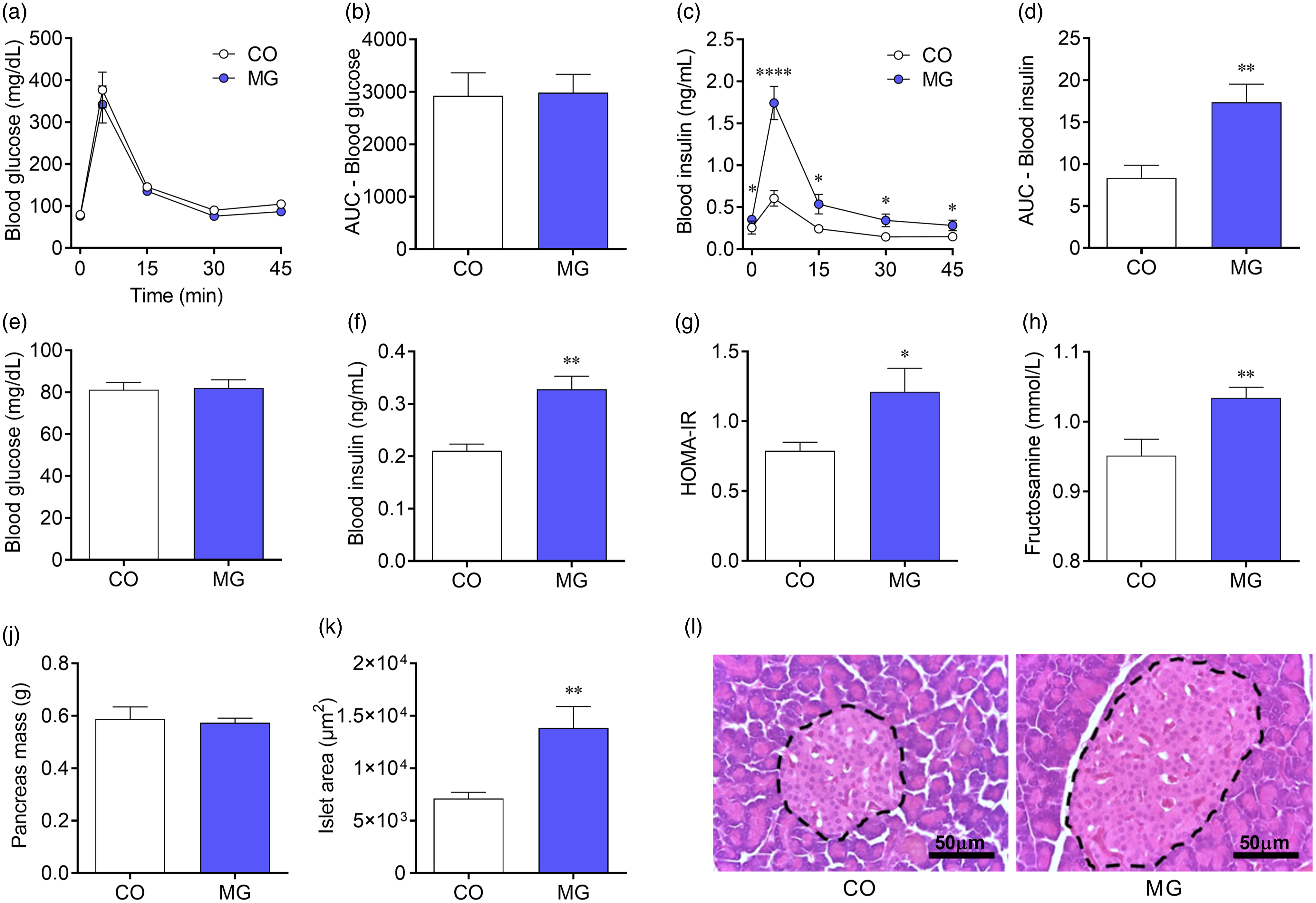
Fig. 2. Postnatal early exposure to MG induces insulin resistance and pancreatic islet hypertrophy. Blood glucose (a) and AUC of blood glucose (b) during the ivGTT. Blood insulin (c) and AUC of insulin (d) during the ivGTT. Fasting blood glucose (e), fasting blood insulin (f), HOMA-IR (g), blood fructosamine (h), pancreas mass (j), pancreatic islets area (k) and representative photomicrographs (×400 magnification, scale bars = 50 µm) showing sections of pancreatic islets stained whit haematoxylin and eosin (l). Data are presented as mean ± SEM (n = 10–15). To compare the experimental groups Student’s t-test was used, where *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. SEM, standard error of mean.
Despite no changes in total cholesterol (Fig. 3a), MG-treated offspring were dyslipidemic, showing reduced HDL-cholesterol (p < 0.01; Fig. 3b) and higher LDL cholesterol levels (p < 0.001; Fig. 3c). Lower triglycerides levels were also observed in MG-treated offspring (p < 0.001; Fig. 3d).
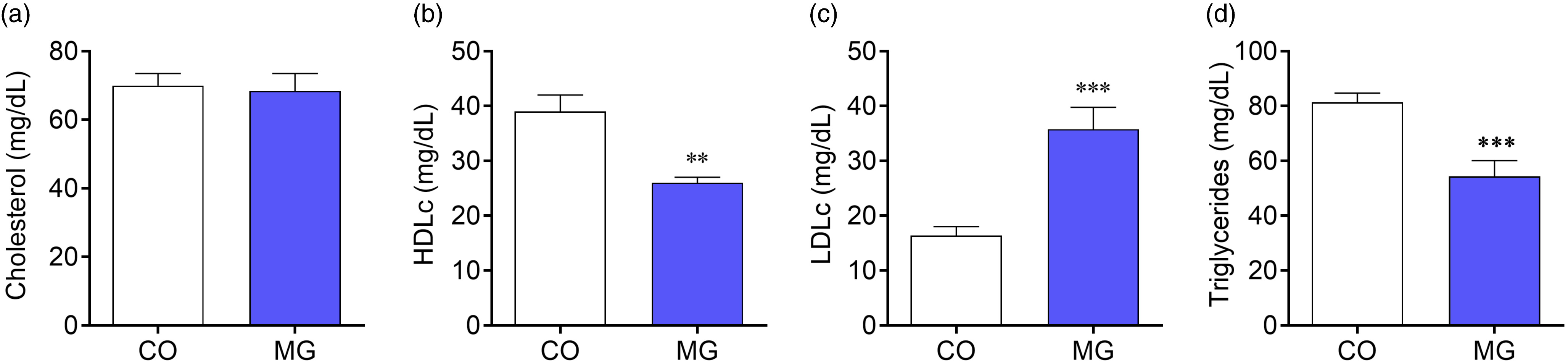
Fig. 3. Effect of postnatal early exposure to MG on the blood lipid profile. Cholesterol (a), HDL cholesterol (b), LDL cholesterol (c) and triglycerides levels (d). Data are presented as mean ± SEM (n = 8–10). To compare the experimental groups Student’s t-test was used, where *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. SEM, standard error of mean.
Postnatal early exposure to MG causes hepatic steatosis and aggravates markers of liver and kidney oxidative stress, inflammation and fibrosis
Adult MG offspring presented lower liver mass (p < 0.01; Fig. 4a) than CO offspring. In addition, they also showed increased lipid inclusion and perivascular fibrosis (p < 0.05; Fig. 4g, 4j). Increased enzymatic activity of SOD (p < 0.01; Fig. 4b) and CAT (p < 0.05; Fig. 4c) were observed in the liver of MG group. In turn, lower levels of GSH (p < 0.05; Fig. 4d) were found in the liver of MG offspring, together with higher LOOH levels (p < 0.01; Fig. 4e) and MPO activity (p < 0.01; Fig. 4f).
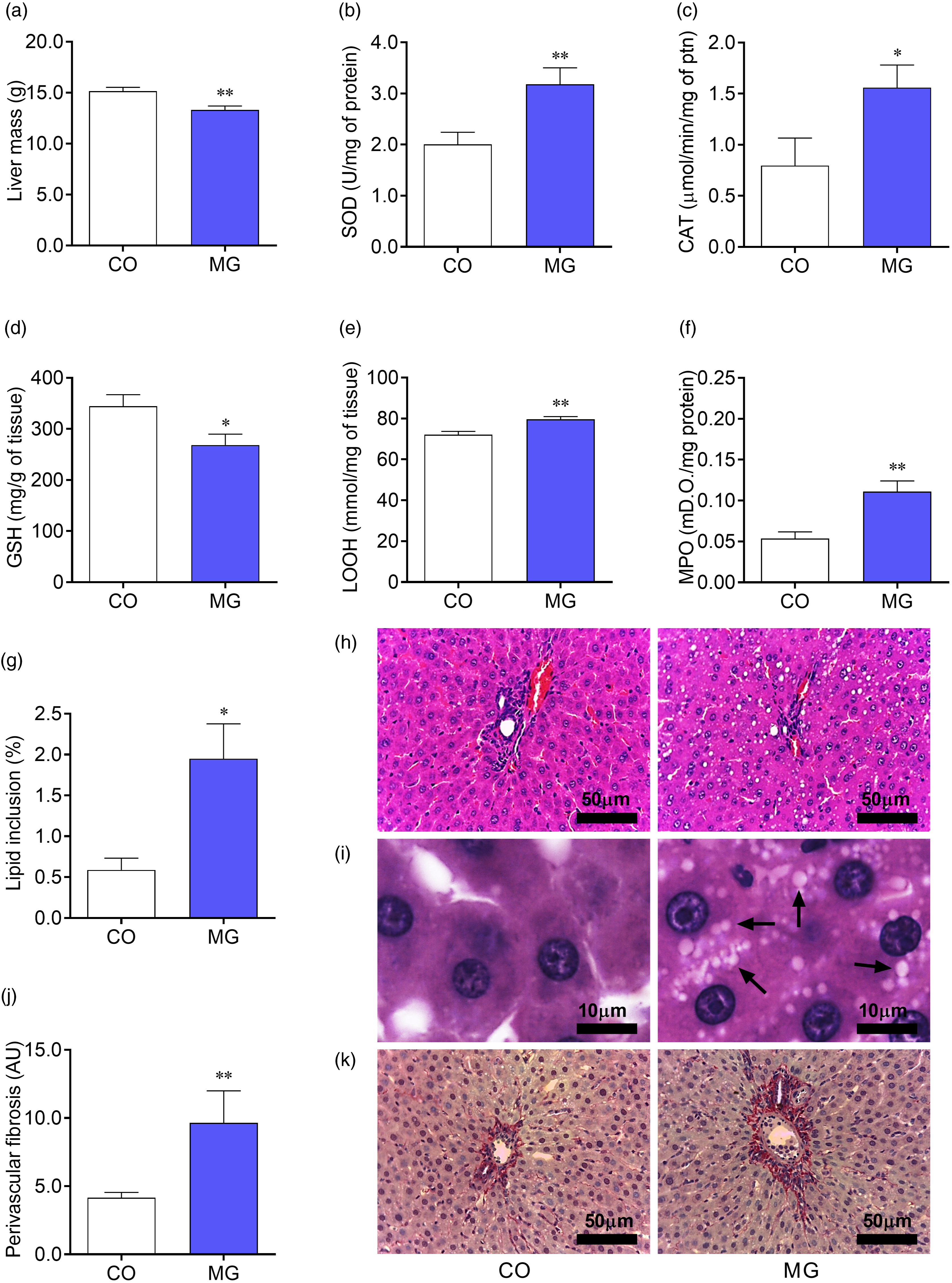
Fig. 4. Effects of postnatal early exposure to MG on the liver. Liver mass (a), enzymatic activity of SOD (b) and Catalase (c), hepatic GSH content (d), LOOH content (e) and enzymatic activity of MPO (f). Quantitative analysis of lipid inclusion in the liver (g) and quantitative analysis of hepatic perivascular collagen deposition (j). Representative photomicrographs (h: ×400 magnification, scale bars = 50 µm and i: ×1000 magnification, scale bars = 10 µm) showing sections of liver stained whit haematoxylin and eosin, black arrows indicate lipid inclusions (i). Representative photomicrographs (×400 magnification, scale bars = 50 µm) showing sections of liver stained whit picrosirius red, collagen appears red (k). Data are presented as mean ± SEM (n = 8–10). To compare the experimental groups Student’s t-test was used, where *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. SEM, standard error of mean.
Similarly, MG offspring showed lower kidney mass (p < 0.05; Fig. 5a). Although no difference in glomeruli number (Fig. 5g), MG group had increased glomerular area (p < 0.05; Fig. 5h) and reduced capsular space (p < 0.01; Fig. 5i), in addition to increased pericapsular fibrosis (p < 0.01; Fig. 5j, 5k). Increased enzymatic activity of SOD (p < 0.001; Fig. 5b) and CAT (p < 0.01; Fig. 5c) were also observed in the kidney of MG group. Renal GSH levels were lower (p < 0.05; Fig. 5d), while LOOH levels (p < 0.05; Fig. 5e) and MPO activity (p < 0.01; Fig. 5f) were higher in the kidney from MG, compared with CO group.

Fig. 5. Effects of postnatal early exposure to MG on the kidney. Kidney mass (a), enzymatic activity of SOD (b) and Catalase (c), renal GSH content (d), LOOH content (e) and enzymatic activity of MPO (f). Quantitative analysis of glomeruli number (g), quantitative analysis of glomerular area (h), quantitative analysis of capsular space (i) and quantitative analysis of pericapsular fibrosis (j). Representative photomicrographs (×400 magnification, scale bars = 50 µm) showing sections of kidney stained whit picrosirius red, collagen appears red (k). Data are presented as mean ± SEM (n = 8–10). To compare the experimental groups Student’s t-test was used, where *p < 0.05, **p < 0.01 and ***p < 0.001. SEM, standard error of mean.
Discussion
In this study, we demonstrated that early PN exposure to MG, during the first 2 weeks of lactation, leads to development of metabolic disturbances, namely glycaemic and lipid dysmetabolism, at adulthood (Supplementary Fig. S1). It is important to note that the dosage of MG used in this work was superior than the achieved by the infant formulas in some studies but, as aforementioned, lower than that used in our previous work. Reference Francisco, Barella and da Silveira9-Reference Dittrich, Hoffmann, Stahl, Müller, Beckmann and Pischetsrieder29 In order to evaluate the possibility of metabolic programming, both CO and MG groups were investigated at adult life, being MG-treated animals insulin resistant and dyslipidemic. Interestingly, MG offspring were hyperphagic, although we have observed reduced body weight combined with decreased fat stores and increased hepatic lipid deposition. Impairment of oxidative stress markers and morphological changes in liver and kidney were also observed at adulthood. Thus, we show for the first time, that the early PN exposure to MG leads to the development of metabolic syndrome features and markers of tissue oxidative, inflammatory and fibrotic damage at adulthood, hallmarks of late-diabetic complications.
At PN90, MG group presented less body weight and body length than CO group. MG was suggested to cause a deleterious effect on growth hormone release and action in type 1 diabetic patients. Reference Berlanga, Cibrian and Guillén24 The impaired body weight gain in MG-treated animals can also be attributed to the impairment of adipose tissue development. Rodrigues et al. Reference Rodrigues, Matafome, Santos-Silva, Sena and Seiça30,Reference Rodrigues, Matafome and Sereno31 have consistently shown that MG impairs adipose tissue capillarisation, further limiting adipose tissue expandability in obesity due to decrease of blood supply and conducting to insulin resistance. In this sense, several studies have shown the role of hypoxia on adipose tissue dysfunction and consequent decrease of adipokines secretion. Reference Rodrigues, Matafome and Sereno31,Reference Hosogai, Fukuhara and Oshima32 Normal expansion of the adipose tissue is based on a regulated interaction between adipogenesis and angiogenesis, leading to lower adipocyte volume and preventing hypoxia and inflammation. On the other hand, limited capillarisation makes the distance between central and peripheral adipocytes greater than the maximum oxygen diffusion distance, leading to hypoxia. Reference Rutkowski, Davis and Scherer33 Thus, disturbance in tissue oxygenation, inefficient angiogenesis and vascular network impairment may probably be the basis of questions related to adipose tissue dysfunctions with regard to adequate adipocyte growth and accumulation of fat stores. MG has a direct inhibitory effect on adipose tissue angiogenesis, compromising its healthy growth, which is important not only at adulthood but also during critical phases of development. These previous findings are aligned with the effects on adipose tissue impairment caused by the exposure to MG during the first 14 days of life in our study, a stage of development where deficits in angiogenesis and adipogenesis may have a crucial impact on future adipose tissue function. Adipose tissue dysfunction due to early exposure to MG is further suggested by the alteration of the lipid profile, showing lower levels of HDL cholesterol, a marker of impaired adipocyte function. Moreover, we also showed that precocious exposure to MG leads to lower body weight, despite a higher relative food intake throughout life. Decreased adipose tissue may in fact lead to lower production of leptin, which can in turn be the associated with a poorer regulation of food intake. However, other nutrient-sensing mechanisms may also be involved, which should be addressed in future studies.
In this study, we clearly demonstrate by ivGTT and HOMA-IR that MG animals were insulin resistant. Previous studies have already demonstrated the effects of MG in inhibiting the insulin pathway and the intimate relationship between high MG levels and insulin resistance in humans, Reference Biswas and Kumar34 rodents Reference Sheader, Benson and Best35 and cell cultures. Reference Riboulet-Chavey, Pierron, Durand, Murdaca, Giudicelli and Van Obberghen36 The mechanisms of MG-induced insulin resistance are still under debate, being oxidative stress and inflammation crucial. Nevertheless, under such circumstances, beta-cells are under stress and their exhaustion tend to be accelerated. It is known that dicarbonyl stress has an important role in the development of pancreatic beta cell toxicity. Reference Rabbani, Xue and Thornalley12 This toxic effect can lead to pancreatic islets hypertrophy, caused by the decrease in beta cell function and increased reactive oxygen species production, Reference Franco, Dias-Rocha and Fernandes37 which is in accordance with our results in this animal model of metabolic programming.
Several studies have shown that the quantification of fructosamine levels is relevant to evaluate the level of total glycated proteins. Reference Meerwaldt, Links, Zeebregts, Tio, Hillebrands and Smit38,Reference da Silva Lima, de Moura and Passos39 Accordingly, in our study, MG animals have increased blood fructosamine levels. In a previous study, we demonstrated that maternal MG treatment, during lactation, increased blood and milk fructosamine levels; further, their pups have increased blood fructosamine levels at adult life. Reference Francisco, Barella and da Silveira9 In the current study, we show that direct exogenous MG intraperitoneal administration to offspring also leads to increased blood fructosamine levels at adulthood. It is important to emphasise that this work is the first to use intraperitoneal injections of MG in newborn rats. Thus, we established a dose three times lower than that used in the previous work, 60 mg/kg/day orally administered in dams during suckling period. Reference Francisco, Barella and da Silveira9 Thus, given that an oral route was too difficult to perform in newborn pups, we have used the intraperitoneal approach with a lower dose than used in previous studies to avoid possible toxic doses do the newborns.
Elevated MG levels are found parallel to oxidative stress as well as AGEs; however, not always these data are accompanied by high blood glucose levels. Reference Chen, Hosokawa, Bumbalo and Leahy40-Reference Chang, Wang and Wu43 In this study, MG animals showed higher activity of SOD and CAT in the liver and kidney, together with lower GSH levels, which can indicate an increase of reactive oxygen species and their detoxification mechanisms. Moreover, in our study, MG group showed a depletion of GSH, which may reflect the compensatory activation of GSH-dependent antioxidant and detoxification pathways and predispose to oxidative stress in these animals. Such results are in accordance with the higher levels of LOOH in the liver and kidney of MG-treated rats, an indirect measure of oxidative stress-induced damage. Reference Borges, Ferreira and da Silva26 In this sense, reduced GSH content have been reported to be related to decreased activity of glyoxalase system and impaired detoxification of MG, leading to increasing levels of MG and circulating AGEs. Reference Thornalley44
In the liver, such observations were closely related to the development of hepatic steatosis at an early stage of the development of non-alcoholic fatty liver disease, Reference Cha, Hwang, Heo and Jun45 as well as inflammatory infiltrates and the development of fibrosis. Reference Neves, Rodrigues and Sereno46 Similar observations were also made in adult rats fed a high-fat diet, as well as by other authors, and may be closely related with adipose tissue dysfunction, which causes an increased fatty acids flux to the liver. Reference Neves, Rodrigues and Sereno46,Reference Gaens, Niessen and Rensen47 Despite all the available studies related to MG exposure at adult life, our study shows for the first time the deleterious effects on the metabolism after MG exposure in the early life, at adulthood.
Berlanga et al. Reference Berlanga, Cibrian and Guillén24 demonstrated that there is an intimate relationship between high levels of MG and AGEs and renal diseases. More, physiological doses of MG were shown to resemble in Wistar rats the renal lesions observed in diabetic rats. Reference Rodrigues, Matafome and Crisóstomo48 However, more studies are needed to confirm whether these diseases are directly related to kidney mass loss. We found in this study that the early exposure to MG leads to the modification of kidney morphology. Development of chronic kidney disease, renal fibrosis and the onset of kidney failure is directly related to the increased levels of MG, Reference Markova, Hüttl and Oliyarnyk49 leading to whole organ injury, which includes lower glomerular capsule and filtration rate, in addition to the well stablished role in the reactive oxygen species generation. Reference Rodrigues, Matafome and Crisóstomo48
Our results demonstrate that the MG group also has higher levels of MPO in the liver and kidney, indicating a proinflammatory state that can cause organ damage. The relationship of oxidative stress, inflammation, AGEs and their precursors, and their relevant role in the development of renal diseases, is already known. Reference Uribarri, del Castillo and de la Maza7 In addition, studies have shown that a diet with low levels of AGEs had a significant reducing effect on inflammatory markers, oxidative stress and improved insulin sensitivity in resistant patients. Reference Vlassara50-Reference Mark, Poulsen and Andersen52
The present study shows that early PN exposure to MG induces oxidative stress, inflammation and fibrosis markers in pancreas, liver and kidney, which are related to metabolic dysfunction features, such as dyslipidaemia, hyperinsulinemia and insulin resistance, increasing the risk for diabetes and cardiometabolic diseases. Overall, these results confirm lactation as an important period for health or disease programming and suggest the careful use of infant formulas in the newborn diets, as well as the need for better maternal diet during the lactation period.
Supplementary materials
For supplementary material for this article, please visit https://doi.org/10.1017/S204017442100074X
Acknowledgements
All authors are grateful to Maroly Valentim Alves Pinto for technical assistance.
Financial support
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES; Finance Code 001); Paraná Science Foundation (Fundação Araucária) and the Portuguese Foundation of Science and Technology (Strategic Projects UID/NEU/04539/2013 and UIDB/NEU/04539/2020). Funding agencies had no involvement in any phase of this study.
Conflicts of interest
The authors declare no conflict of interest.
Ethical standards
None.








