Introduction
Roads cause pollution from maintenance and traffic (Angold Reference Angold1997) in terms of gases, fine and coarse particulate matter, airborne particulate-bound trace metals and metals (Klos et al. Reference Klos, Rajfur, Waclawek and Waclawek2009; Amato et al. Reference Amato, Viana, Richard, Furger, Prévôt, Nava, Lucarelli, Bukowiecki, Alastuey and Reche2011). Earlier studies have focused on deposition of road pollutants with increasing distance from the road (e.g. Pagotto et al. Reference Pagotto, Remy, Legret and Le Cloirec2001; Klos et al. Reference Klos, Rajfur, Waclawek and Waclawek2009; McAdam et al. Reference McAdam, Steer and Perrotta2011; Škrbic et al. Reference Škrbić, Milovac and Matavulj2012). Factors such as wind (Bari et al. Reference Bari, Rosso, Minciardi, Troiani and Piervittori2001; Venkatram et al. Reference Venkatram, Snyder, Isakov and Kimbrough2013), traffic speed (Coelho et al. Reference Coelho, Farias and Rouphail2005), tree cover and topography (González et al. Reference González, Pignata and Orellana2003) shape dispersal patterns of road pollutants. Angold (Reference Angold1997) found that traffic pollutants affected natural vegetation up to 80 m from the road with a maximum edge effect of 200 m, whereas Viskari et al. (Reference Viskari, Rekilä, Roy, Lehto, Ruuskanen and Kärenlampi1997) reported declining inorganic and organic pollutant deposits in snow samples and moss bags from 30 m to 60 m from the road. Also de-icing salts disperse widely from roads to the environment in winter (Blomqvist & Johansson Reference Blomqvist and Johansson1999; Thunqvist Reference Thunqvist2004).
Lichens, as symbiotic associations between a fungus (mycobiont) and algae and/or cyanobacteria (photobiont), are sensitive to air pollutants (e.g. Nash Reference Nash and Nash2008). They take up elements through their entire surface, and are useful monitoring organisms for metals (Garty Reference Garty, Kranner, Beckett and Varma2002; Carreras et al. Reference Carreras, Wannaz, Perez and Pignata2005; Giordani Reference Giordani2007; Purvis & Pawlik-Skowroska Reference Purvis, Pawlik-Skowroska, Avery, Stratford and van West2008), sea salt deposition (Nash & Lange Reference Nash and Lange1988; Figueira et al. Reference Figueira, Pacheco, Sousa and Catarino2002) and trace elements (van Dobben et al. Reference van Dobben, Wolterbeek, Wamelink and ter Braak2001). Elements are taken up in various lichen compartments: being deposited on surfaces and intercellular spaces, bound to exchange sites on the cell wall, and entering the cytoplasm (Brown & Brown Reference Brown and Brown1991). Accumulation of metals in lichens may result from a mechanism involving organic acid production (Sarret et al. Reference Sarret, Manceau, Cuny, Van Haluwyn, Deruelle, Hazemann, Soldo, Eybert-Berard and Menthonnex1998). Epiphytic lichens taking up most nutrients from wet and dry depositions can promptly detect changes in heavy metal deposition (Loppi & Pirintsos Reference Loppi and Pirintsos2003), although accumulation of heavy metals by lichens is complex and affected by factors such as exposure time, age, size of thallus, species-specific features, environmental and physiochemical factors (Garty Reference Garty2001; Nimis et al. Reference Nimis, Andreussi and Pittao2001; Pawlik-Skowroska et al. Reference Pawlik-Skowroska, di Toppi, Favali, Fossati, Pirszel and Skowronski2002; Hauck Reference Hauck2008).
Effects of pollution on lichen viability are often investigated as photosynthetic apparatus functioning and membrane permeability status (Beltman et al. Reference Beltman, de Kok, Kuiper and van Hasselt1980). Decreasing Chl a/b-ratio concurred with increasing Cu content in lichens (Chettri et al. Reference Chettri, Cook, Vardaka, Sawidis and Lanaras1998). Excess Mn reduced Chl concentrations and Chl fluorescence parameters, and degraded chloroplasts in Hypogymnia physodes photobionts (Hauck & Paul Reference Hauck and Paul2005). Pollutants may also upset the physiological balance between lichen bionts, resulting in degradation of the organism (Brown & Beckett Reference Brown and Beckett1984).
With increasing road traffic, more comprehensive studies quantifying pollution elements and their dispersal distances in the local roadside environment are necessary. Methods using lichens as monitoring organisms should be refined to facilitate biomonitoring of pollutants. In particular, there is a need for studies during cold seasons with application of de-icing salts, during which visible damage, even of trees, often occurs (e.g. Viskari & Kärenlampi Reference Viskari and Kärenlampi2000). Furthermore, more knowledge is needed to select good indicator species: are lichen species with a higher surface area/biomass-ratio better than more compact foliose growth forms? Do species from leached, oligotrophic bark accumulate elements more efficiently than those from more cation-rich broad-leaved deciduous stands? Can viability measures such as Chl fluorescence parameters or growth rate be used as simple proxies for road pollutants in standardized lichen transplantation experiments? By transplanting four epiphytic lichen species with contrasting growth forms and nutrient requirements, we aim to study species-specific distance-dependent accumulation of pollutants including de-icing salts along a highway in southern Norway. By quantifying elemental accumulation and functional lichen responses across gradients in distance from a road, we aim to gather knowledge that can improve biomonitoring techniques.
Methods
Study area
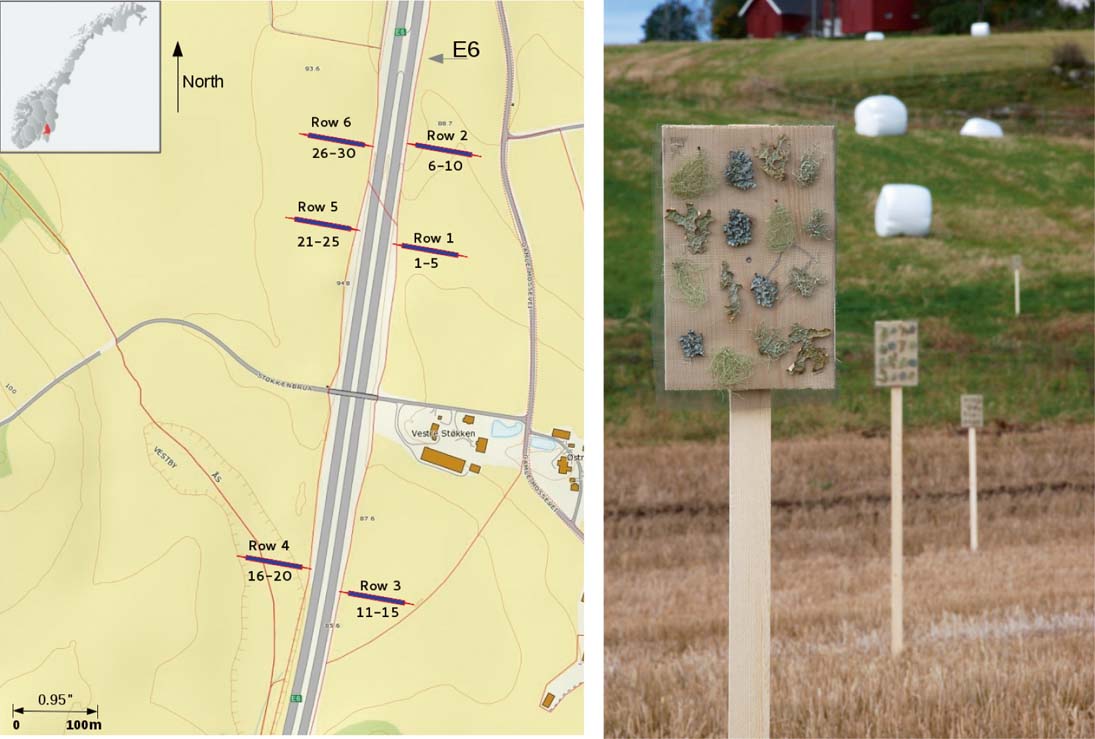
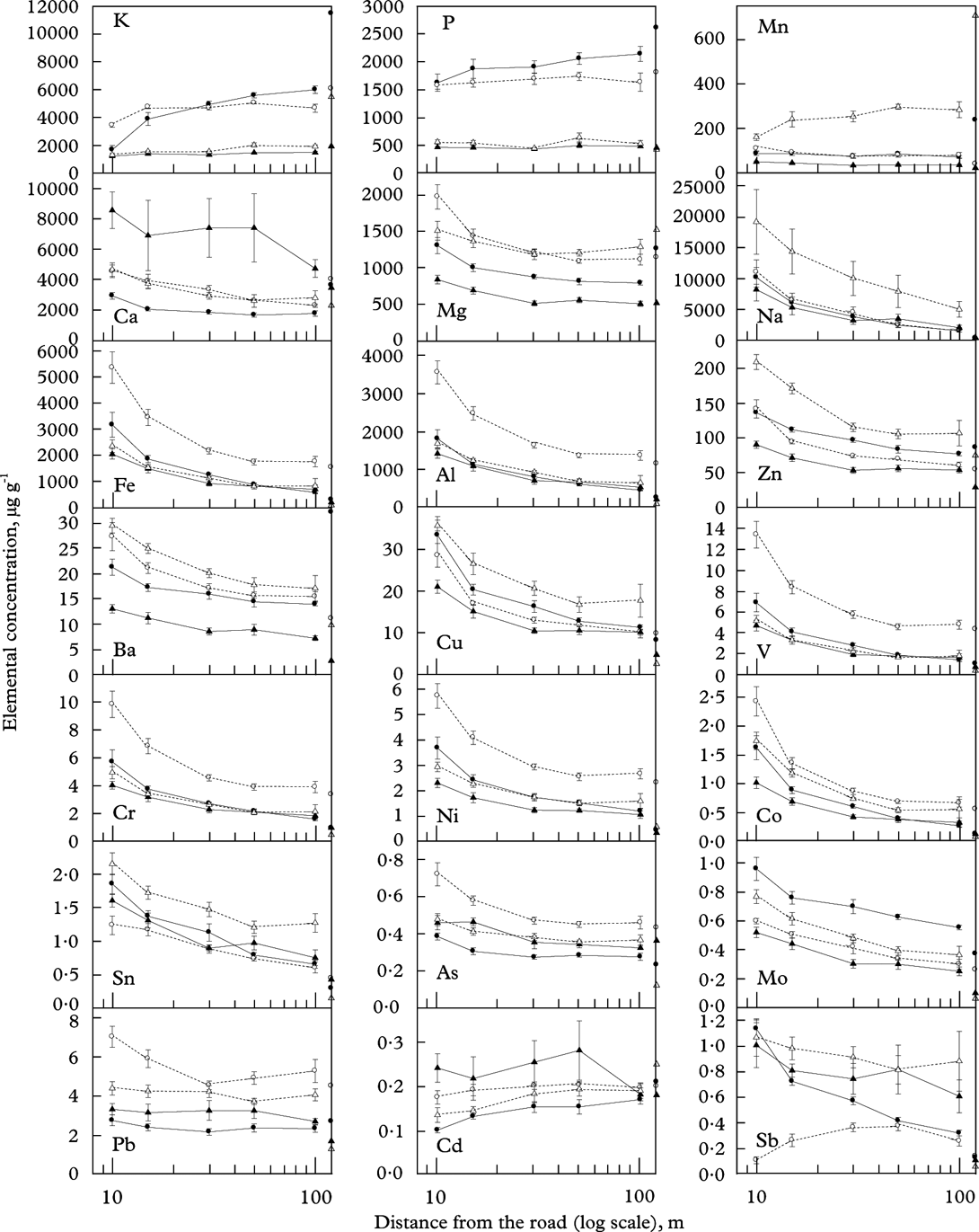
The study was carried out along the E6, the main north-south highway in Norway, 30 km south of Oslo, SE Norway (59°64′N and 10°74′E; 100–150 m a.s.l). Three lines perpendicular to the E6 were selected. Along each line, three transects were established on both sides of the road (Fig. 1A). The land tilted (≈2%) towards E6 on its east side. On the west side, the terrain was more flat except for one row with a 2–3 m deep depression. Lichens were transplanted onto five vertical boards 20×30 cm supported on posts 2 m above the ground and positioned at 10, 15, 30, 50 and 100 m from the road in each of the six transects (Fig. 1B). Four replicates of each lichen species were attached in a randomized design on each board. Lichens were fastened by flax thread in four rows on a nylon net attached to each board (Fig. 1B). Thus, lichens had a vertical position mimicking their natural location on tree trunks. In total, 30 boards, all facing the road, were set up on 25 September 2011 and harvested on 26 March 2012 during a season with episodic high de-icing salt application, road dust produced by spiked tyres, and high traffic-related pollution. We used agricultural landscapes, in which distance from the road was not confounded by factors such as forest density, tree composition and vegetation.
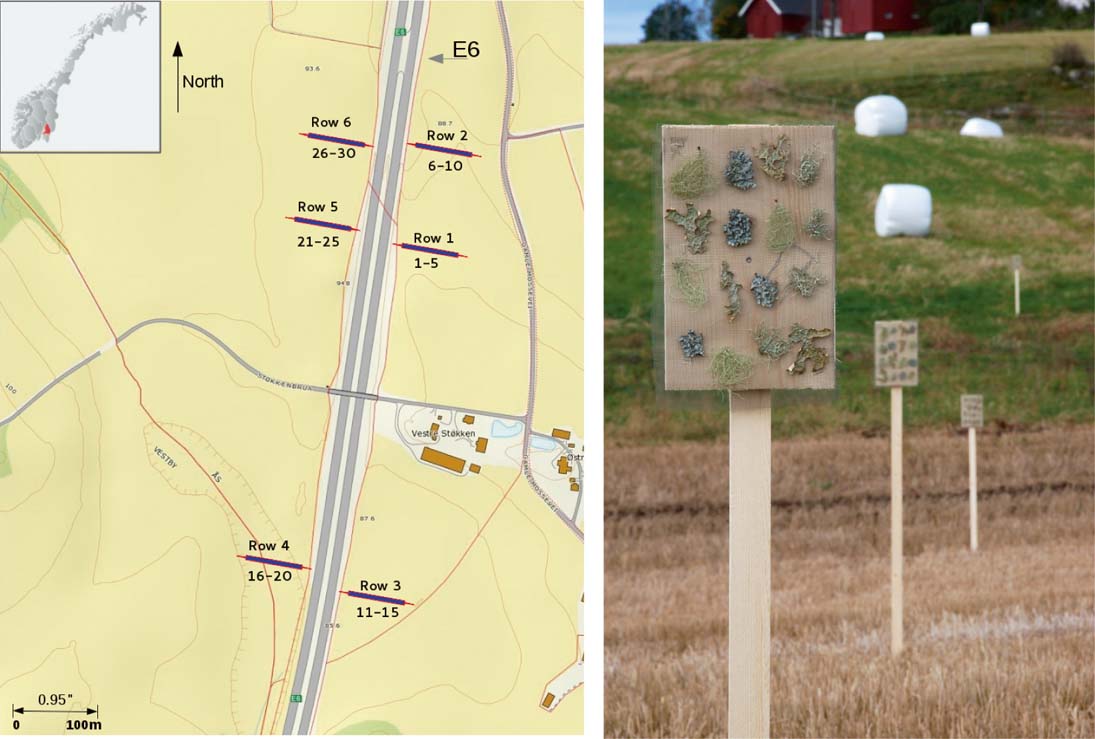
Fig. 1. Study area, the inset shows the position within Norway with location of transects (left); stands with lichen transplants near the E6 highway (right). Photograph: Olena A. Yemets. In colour online.
The climatic data for the field experiment obtained from the eKlima database (the nearby Ås meteorological station) showed that maximum precipitation occurred in December 2011 (112·3 mm) and minimum in March 2012 (13·1 mm). The average monthly air temperature varied from 12·2°C (September) to −3·0°C (February); both months were warmer than normal by 1·3 and 1·8°C, respectively. During transplantation, prevailing winds came from the south. The study area had 15 759 passing cars per day in a single direction at Korsegården. The percentage of heavy vehicles varied from 11·9 to 14·0%. The road was periodically treated with de-icing salts (particularly by NaCl): 14·5 kg m–2 was applied during the study period.
Study species
We used two fruticose [Ramalina farinacea (L.) Ach., Usnea dasopoga (Ach.) Nyl.], and two foliose [Lobaria pulmonaria (L.) Hoffm., Parmelia sulcata Taylor] lichens. The first mentioned species of each growth form grows on bark with relatively high pH, the other in oligotrophic habitats (Gauslaa Reference Gauslaa1985, Reference Gauslaa1995). Lobaria pulmonaria has a primary green-algal photobiont (Dictyochloropsis reticulata) and N2-fixing cyanobacterium Nostoc in small internal cephalodia. It was collected from Fagus sylvatica in Larvik, Norway (59°05′31″N, 9°56′52″E; 200 m a.s.l.) in old open-shaded forests. It is highly susceptible to air pollution (Hallingbäck Reference Hallingbäck1986; Sigal & Johnston Reference Sigal and Johnston1986). All other lichens had the green alga Trebouxia as their only photobiont. The pollution-resistant P. sulcata (von Arb et al. Reference von Arb, Mueller, Ammann and Brunold1990; Bennett Reference Bennett2002) was collected on isolated Tilia cordata Mill. along a small farm road in Ås, Norway (59°40′N, 10°45′E; 100–150 m a.s.l). Ramalina farinacea, susceptible to air pollution (van Dobben & ter Braak Reference van Dobben and ter Braak1999), was collected in open Populus tremula stands in Hvaler, SE Norway (59°03′13–14″N, 10°56′27–30″E; 5–10 m a.s.l). Usnea dasopoga belongs to a genus recognized as pollution-susceptible, especially to SO2 (Conti & Cecchetti Reference Conti and Cecchetti2001; Carreras et al. Reference Carreras, Wannaz, Perez and Pignata2005). Thalli were taken from Betula spp. and Pinus sylvestris in open, W-facing rocky forest in Töckfors, W Sweden (59°29′16–19″N, 11°52′13–16″E; 120–135 m a.s.l).
Laboratory analyses
After transplantation, lichens were collected air dry from the stands, and labelled. Additional control thalli had been kept air dry at −20°C since the transplantation started. Freezing is the recommended method for long-term storage of lichen thalli for later experiments (Honegger Reference Honegger2003). Before analyses, we removed debris from the air dry lichens.
Prior to transplantation, 30 thalli per species were randomly selected, placed on wet filter paper and sprayed with de-ionized water, to recover at low light (10 µmol m–2 s–1 PPFD) for 24 h. Then maximal photosystem II activity (F v/F m) after 15 min dark adaptation was measured with a modulated fluorometer (PAM-2000, Walz, Effeltrich, Germany). After transplantation, all thalli were placed on plastic nets in contact with paper hydrated with de-ionized water in plastic containers (26×56×6 cm). The containers were covered with cling film to enhance humidity, and exposed for 24 h at 10 µmol m–2s–1 PPFD before F v/F m was again recorded. This hydration protocol ensured minimal leaching before elemental analyses. Dry mass (DM) of each thallus was weighed (±0·1 mg) before and after the field experiment. DM was not corrected to oven-dry DM, but both start and end measurements were made in seasons with low temperature outside and heating inside, meaning that air humidity was fairly low and similar. Unpublished data on the dry mass/wet mass correction factor for four foliose and fruticose lichens showed low interspecific contrasts (total range: 0·890–0·921) in our laboratory during initial measurements. Thus, the value of RGR may not be exact, but because correction factors of different lichens follow each other in the laboratory, the relative differences between species should be real. Growth was calculated as relative growth rate (RGR; mg g–1 day–1)=[ln(DMend/DMstart)] * 1000/Δt where Δt is the number of days between DM measurements at the start and end of transplantation (Evans Reference Evans1972).
Each thallus was powdered in a mill with Teflon balls (Precellys 24-Dual tissue homogenizer, Bertin Technologies, France). Chl a and b were quantified immediately after grinding in four randomly selected thalli of each species at the start, and in all thalli after harvest. Ground material (4–10 mg) was placed in Eppendorf tubes and extracted in 1·5 ml MgCO3-saturated dimethyl sulfoxide (DMSO). The Eppendorf tubes were placed in a water bath (60°C) for 40 min for Chl extraction (Palmqvist & Sundberg Reference Palmqvist, Sundberg, Kranner, Beckett and Varma2002). After an incubation period, the Eppendorf tubes were centrifuged by Hettich Universal 16 centrifuge (Germany) at 12 900 rpm for 2 min. The absorbance of the supernatant was measured with a Shimadzu UV-2101PC spectrophotometer at 649, 665 and 750 nm. Absorbance values at 649 and 665 nm were corrected against the absorbance at 750 nm and Chl a and b concentrations were calculated (Wellburn Reference Wellburn1994).
The powder left after chlorophyll analyses from all four specimens of each species from one stand was pooled and treated as one sample for elemental analyses, carried out at the Department of Plant and Environmental Sciences, NMBU, Ås. Powdered and homogenized lichen DM (0·2 g) was put into Teflon tubes. Samples were then mineralized with concentrated HNO3 (5 ml), with an added 1 ml Milli-Q water (MQ H2O) and 250 µl internal standard (IS). Tubes with samples were transferred to an ultraCLAVE III Microwave Digestor (MLS GmbH Mikrowellen-Labor-Systeme, Milestone S.r.l.) under 50 bar pressure and 260°C for 2·5–3 h. The clear extract from each sample was passed to plastic tubes, and MQ H2O was added up to 50 ml. Calcium (Ca), potassium (K), magnesium (Mg), sodium (Na), iron (Fe), manganese (Mn), aluminium (Al), chromium (Cr), copper (Cu), nickel (Ni) and zinc (Zn) were analyzed by an inductively coupled plasma optical emission spectrometer (ICP-OES – Perkin-Elmer Optima 5300 DV, Perkin Elmer Co., Waltham, MA, USA) whereas phosphorous (P), barium (Ba), arsenic (As), cadmium (Cd), cobalt (Co), molybdenum (Mo), lead (Pb), tin (Sn), antimony (Sb) and vanadium (V) were determined by using an inductively coupled plasma mass spectrometer (ICP-MS – Agilent 7500ce, Agilent Technologies, Palo Alto, CA, USA). Elements were reported as concentrations. Analytical quality was checked against Standard Reference Materials (SRM) No. NCS DC 73348 “Bush Branches and Leaves” and No. NCS ZC 73013 “Spinach”. The “Bush Branches and Leaves” certified reference material had an average recovery of 93·5%, while the “Spinach” certified reference material had 106·3%.
Statistical analyses
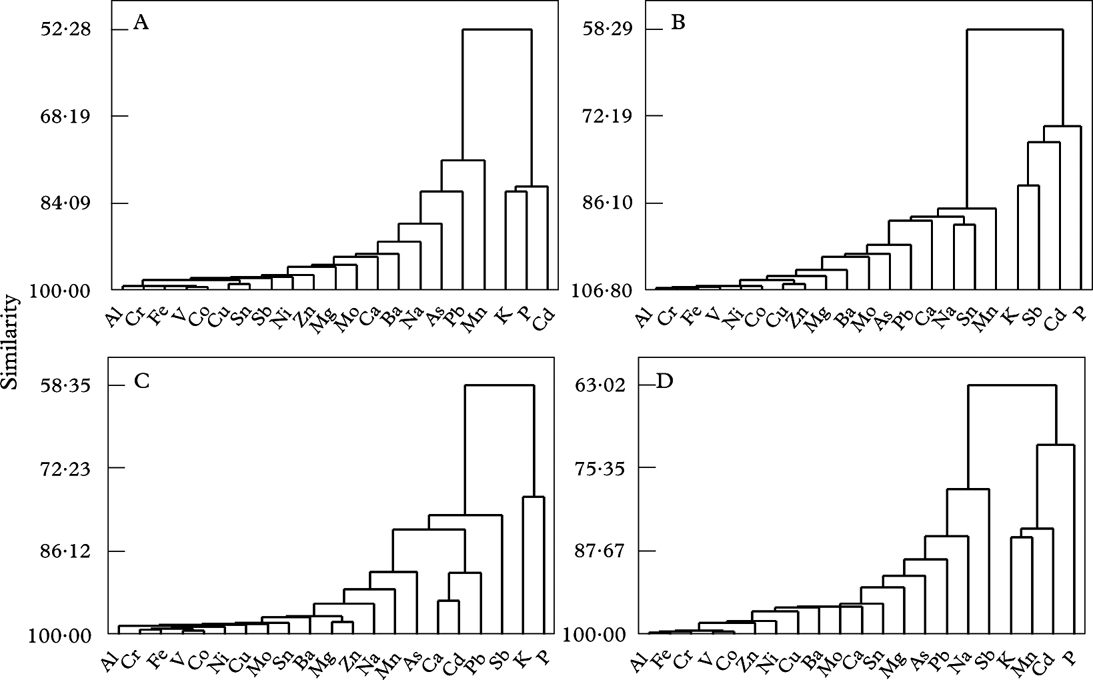
Before statistical analyses, data distributions were examined. Most elemental data were log-transformed to improve distributions for ANOVA. All statistical analyses were run by MINITAB 16 (Minitab Inc., State College, PA, USA). Species-wise dendrograms (single linkage) used correlation coefficient distance on log-transformed values of elemental concentration.
Results
Distance-dependent elemental accumulation patterns
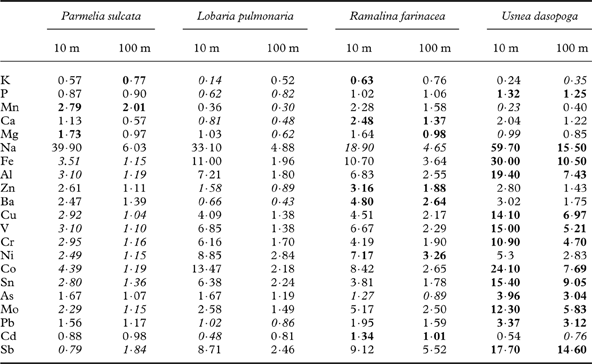
Effects of the side of the road (i.e. east or west) were few and weak (data not shown). Thus, transects on both roadsides were combined in all analyses. Most elements (Ca, Mg, Na, Fe, Al, Zn, Ba, Cu, V, Cr, Ni, Co, Sn, As, Mo) strongly decreased (P<0·001) with increasing distance from the road (Fig. 2; Table 1). The decline was strongest close to the road. There was no significant distance×species interaction for any of these elements (Table 1), meaning that they declined in similar ways in all species. The species-specific concentration of each of these elements was higher, at least closer to the road, than the background (control) levels (Table 2). For Na, Fe, Al, Cu, V, Cr, Ni, Co, Sn, Mo and Sb, the concentration even at 100 m distance was higher than control levels in all species (Fig. 2; Table 2). Pb also tended to decrease with distance, but the decrease was weak (P=0·009).
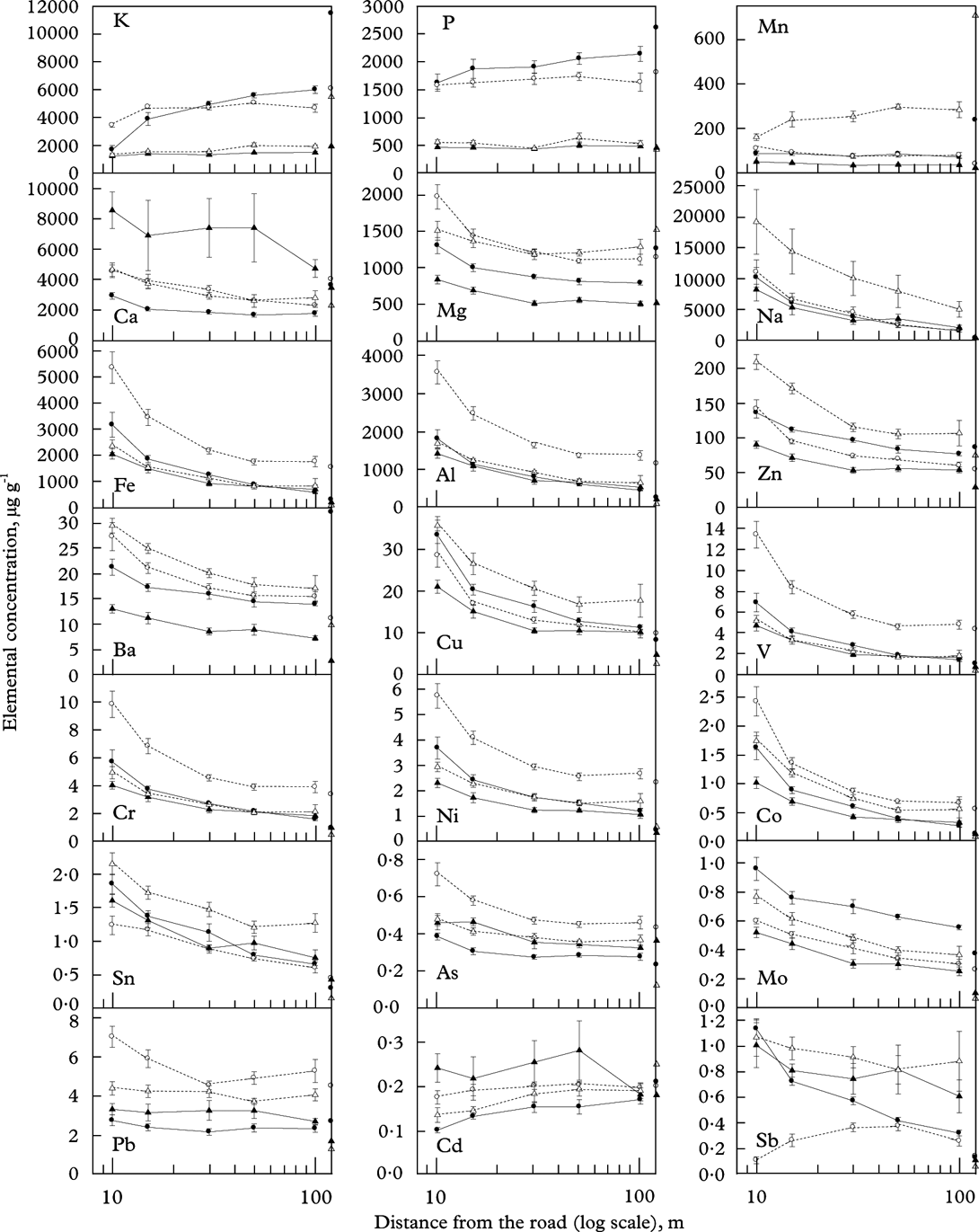
Fig. 2. The concentration of elements measured in Lobaria pulmonaria (•), Parmelia sulcata (○), Ramalina farinacea (▴) and Usnea dasopoga (▵) subsequent to transplantation from 25 September to 26 March at five distances (x-axis; log-scale), averaged across six transects along a highway (means ± 1SE; n=6 are given). Concentrations at start for one pooled sample of several thalli are given for each species at the right y-axes.
Table 1. Results of two-way ANOVAs for elemental concentrations with factors species and distance from the road (mean values across species and distances in Fig. 2).
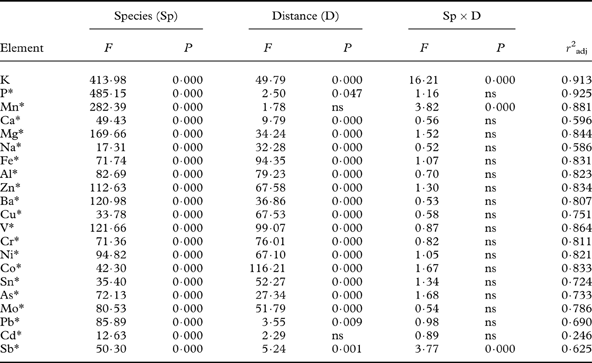
* The ANOVA was run on log-transformed values. Degrees of freedom: Species (3), distance (4), species×distance (12), error (100), total (119).
Table 2. Elemental accumulation factors in elements studied at 10 and 100 m distance from the road relative to background values.
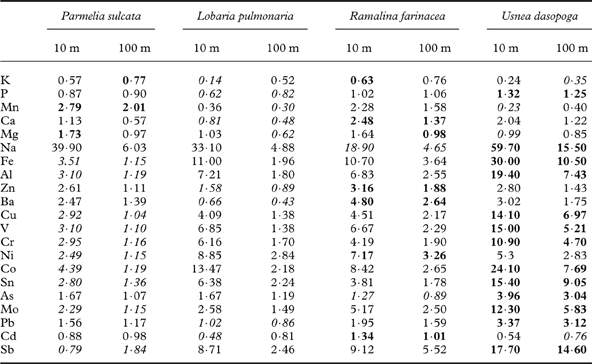
Factors are computed by dividing the mean concentration of each element at the end of transplantation at the nearest and most distant location by the control values before the start. Elements are ranked in the sequence given in Fig. 2 giving the concentrations. The highest and lowest factors for each element are given in bold and italics, respectively.
Some elements showed different patterns. Macronutrients (K and P) as well as Mn increased with distance from the road in some species but not in others (Fig. 2), evidenced by a highly significant distance×species interaction for K and Mn (Table 3). The increase with distance was particularly strong for K in L. pulmonaria, with a 3-fold increase from 10 m to 100 m. Both L. pulmonaria and U. dasopoga experienced substantial losses in K and Mn during transplantation, even at 100 m, relative to controls (Fig. 2; Table 2).
Table 3. Two-way ANOVAs for relative growth rate and viability parameters [maximal quantum yield of PSII (Fv/Fm), total chlorophyll content, and Chl a/b-ratio] with factors species and distance from the road (data given in Fig. 4).
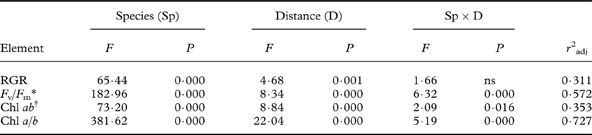
Transformations: *(F v/F m)2-transformed values. †log-transformed values. Degrees of freedom: species (3), distance (4), species×distance (12), error (460), total (479).
Finally, some elements with low concentrations (Pb, Cd and Sb) exhibited weak or no trends with distance from the road (Fig. 2; Table 1). Among them, Cd and partly Pb hardly changed in concentration during transplantation, whereas Sb increased strongly from control levels at 100 m (Table 2). Highly contrasting species-specific trends occurred for Sb; it strongly declined in L. pulmonaria, but increased with distance up to 50 m in P. sulcata (Fig. 2).
The cluster analyses (Fig. 3) visualize structures in the set of elemental concentrations across distance. For all species, macronutrients (K and P) belonged to the cluster most distant from the main heavy metal cluster. Heavy metals, except Cd, Mn, Sb and Pb, were closely linked. In all species, the response of Na was more similar to most heavy metals than to K, P or Cd (Fig. 3).

Fig. 3. Species-wise dendrograms (single linkage) based on correlation coefficient distance for log-transformed values of elemental concentration subsequent to transplantation from 25 September to 26 March at five distances in each of six transects along a highway. A, Lobaria pulmonaria; B, Parmelia sulcata; C, Ramalina farinacea; D, Usnea dasopoga.
Species-specific elemental concentrations
The controls representing background levels represented a number of thalli pooled into one sample. Therefore, no statistical tests could be done with control values. Yet it seems that many elements already occurred in species-specific levels from the start (Fig. 2). For example, the Mn concentration was 25 times higher in U. dasopoga than in R. farinacea, whereas Fe was nearly 20 times higher in P. sulcata than in U. dasopoga. The total pool of elements measured prior to transplantation varied from 0·72% (R. farinacea) to 2·01% (L. pulmonaria) of DM, mainly because K (1·14%) and P (0·26%) concentrations were six times higher in L. pulmonaria.
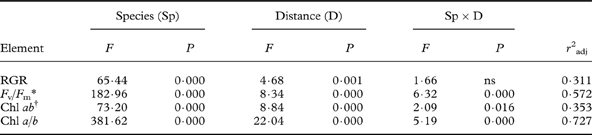
After transplantation, the average total pool of elements measured differed much less between the species, from 1·60% (R. farinacea) to 2·08% (U. dasopoga). There was close to a doubling in U. dasopoga (1·09% at the start), whereas L. pulmonaria slightly declined (from 2·01 to 1·76%). Inspecting the species-specific accumulation factors for individual elements (Table 2), the heavy metal accumulation increased from foliose to fruticose lichens in the order: P. sulcata<L. pulmonaria<R. farinacea≪U. dasopoga.
In all species, concentrations of most elements showed similar responses to distance from the road, particularly Al, Fe, Cr, V, Co, Ni, Cu, Ba, Zn (Figs 2 & 3). Nevertheless, the concentration of all elements differed strongly between species. Species affected concentrations of K, P, Ca, Mg, Mn, Cd and Sb more than did the distance (Table 3). The two-way ANOVAs with distance and species as factors accounted for a high proportion of the variation in most elements (59–93%; Table 1). Only for Cd did factors studied account for a small part of the variation (25%; Table 1). Many elements had substantially higher concentrations in either P. sulcata or U. dasopoga (inhabiting acidic and oligotrophic bark) than in the other two species from richer bark (Fig. 2). Parmelia sulcata had the highest levels of most elements (Fe, Pb, V, Cr, Ni, As, Co; Fig. 2), followed by U. dasopoga (Na, Mn, Zn, Ba, Cu, Sn, and maybe Sb). The highest Na mean in U. dasopoga (10 m) exceeded the control level by almost 60 times (Table 2). Lobaria pulmonaria had the highest concentrations of macronutrients (K and P), at least in less polluted sites, and also of Mo, whereas R. farinacea had the highest just in Ca and Cd. In the species richest in Ca and Cd (R. farinacea) as well as Na (U. dasopoga), the standard errors for these elemental means were exceptionally high (Fig. 2).
Lichen growth and viability along the road
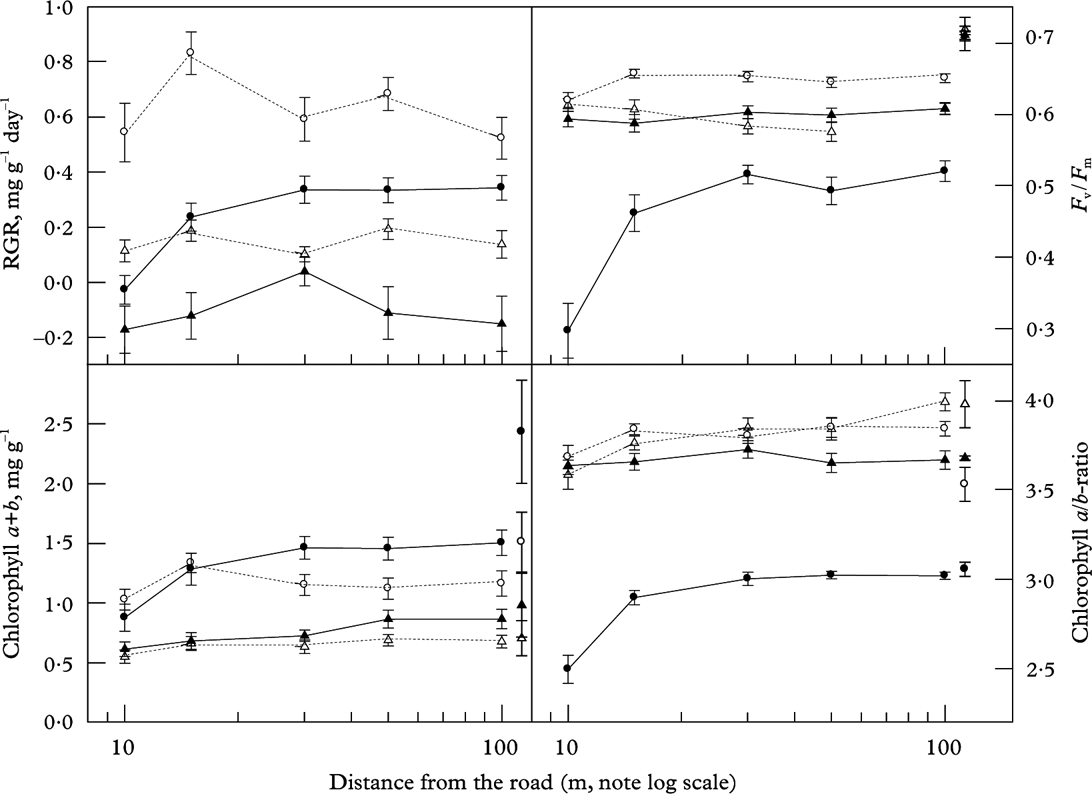
Mean RGR recorded from 25 September 2011 to 26 March 2012 differed highly significantly between the species (Fig. 4; Table 3). Parmelia sulcata grew fastest (0·614 ± 0·048 mg g–1 d–1; mean ± 1SE; n=120), followed by L. pulmonaria (0·232 ± 0·034 mg g–1 d–1) and U. dasopoga (0·148 ± 0·020 mg g–1 d–1), whereas R. farinacea (−0·123 ± 0·046 mg g–1 d–1) experienced a net loss in DM. RGR clearly increased only for L. pulmonaria (from −0·026 ± 0·052 near the road to 0·344 ± 0·045 mg g–1 d–1 at 100 m); RGR values for other species did not show clear distance responses (Fig. 4). In the two-way ANOVA, species and distance accounted for 32% of the variation in RGR (Table 3).

Fig. 4. Relative growth rate (RGR), maximal quantum yield of PSII (F v/F m), total chlorophyll content, and Chl a/b-ratio in L. pulmonaria (•), P. sulcata (○), R. farinacea (▴) and U. dasopoga (▵) after a transplantation period from 25 September to 26 March at five distances (x-axis; log-scale), averaged across six transects along a highway. Means ± 1SE; n=24 are given. Start levels for viability measures are given as symbols with standard error bars for each species near the right y-axes (n=4 for chlorophyll parameters, and n=30 for F v/F m).
All species had high and similar maximal quantum yields of PSII (F v/F m) before transplantation (Fig. 4). Afterwards, F v/F m became depressed in all species, particularly in L. pulmonaria (Fig. 4). F v/F m differed highly significantly between species and distances, which accounted for 57% of its variation (Table 3). Additionally, L. pulmonaria became much more photoinhibited at 10–15 m compared to more distant locations (Fig. 4). Although P. sulcata had the highest F v/F m at all distances, thalli closest to the road were significantly more photoinhibited than the two fruticose species which were not influenced by distance (Fig. 4).
Concentrations of Chl a + b were greater in the two foliose compared to the two fruticose species by a factor of two (Fig. 4; Table 3). Within each growth form type, the species from the most oligotrophic bark had slightly less Chl ab than those from more cation-rich bark. All species were lower in Chl ab near the road; the depression was strongest for L. pulmonaria, having nearly twice as much Chl ab at 100 m compared to 10 m. Even at 100 m, L. pulmonaria, unlike other species, experienced substantial Chl ab loss relative to the start (Fig. 4). The Chl a/b-ratio was relatively similar in all species apart from L. pulmonaria, which had a substantially lower ratio (Fig. 4), particularly close to the road. The two-way ANOVA accounted for as much as almost 73% of the variation in Chl a/b-ratio. However, at 30–100 m, the Chl a/b-ratio was little changed in any species (Fig. 4).
In general, growth and viability were depressed at smaller spatial scales (≤15 m; Fig. 4) than the scales affecting the concentration of most elements (≥50–100 m; Fig. 2; Table 2). Best subset multiple regression analyses modelling RGR from thallus-based viability parameters resulted in a good model only for L. pulmonaria (r 2 adj = 0·438). Chl ab, Chl a/b-ratio and particularly F v/F m contributed significantly. In U. dasypoga, total Chl ab and Chl a/b-ratio contributed significantly, but accounted for just 11·5% of the variation in RGR. For the two other species, viability parameters explained only 4% of the variation in RGR (multiple regressions not shown).
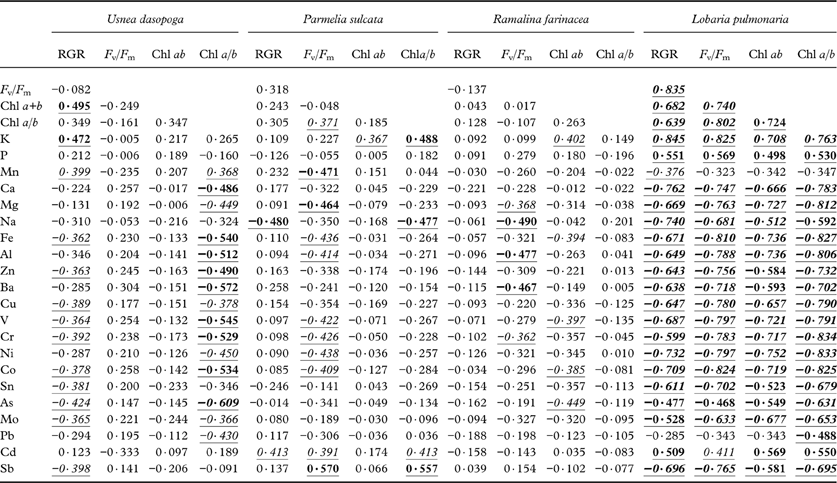
In L. pulmonaria, growth and viability strongly correlated with the concentration of all elements apart from Mn and Pb (Table 4). High K, P and Cd concentrations were associated with high viability and growth; all the remaining elements adversely impacted growth and viability of L. pulmonaria (P-values mostly <0·001; Table 4). In a best subset regression analysis using elemental concentrations for explaining RGR in L. pulmonaria (data not shown), the best one-variable model included the positive effects of K (r 2 adj=0·703). Adding the adverse effects of Cr and Fe resulted in the best multiple model (r 2 adj=0·760). This is in contrast with other species, particularly with P. sulcata and R. farinacea. RGR in P. sulcata only had a significant negative correlation with Na (P=0·007); otherwise no other heavy metals negatively correlated with growth rate, or had weak positive relationships (Table 4). For R. farinacea, there were no significant relationships between any of the elements and RGR. RGR in U. dasopoga correlated negatively, but this was only weakly significant (P<0·05) with most heavy metals, whereas high RGR was associated with high K concentration (P=0·008; Table 4). In this species, a fairly strong decline in the Chl a/b-ratio was associated with high heavy metal concentration. Interpreting best subset regression analyses for these three species was not easy. The best single-variable model merely accounted for 14·8% (R. farinacea) to 20·3% (P. sulcata) of the variation in RGR. Only in U. dasopoga (19·5%) was K concentration positively related to RGR as in L. pulmonaria. In all three species, r 2 adj continuously increased by adding more and more elements, but we refrain from giving multiple models because of high intercorrelation.
Table 4. Pearson correlation coefficients between species-specific relative growth rate (RGR), maximal quantum yield of PSII (Fv/Fm), total chlorophyll concentration (Chl ab) and chlorophyll a/b-ratio (Chla/b) versus log-transformed concentrations of analyzed elements across six transects and five distances.
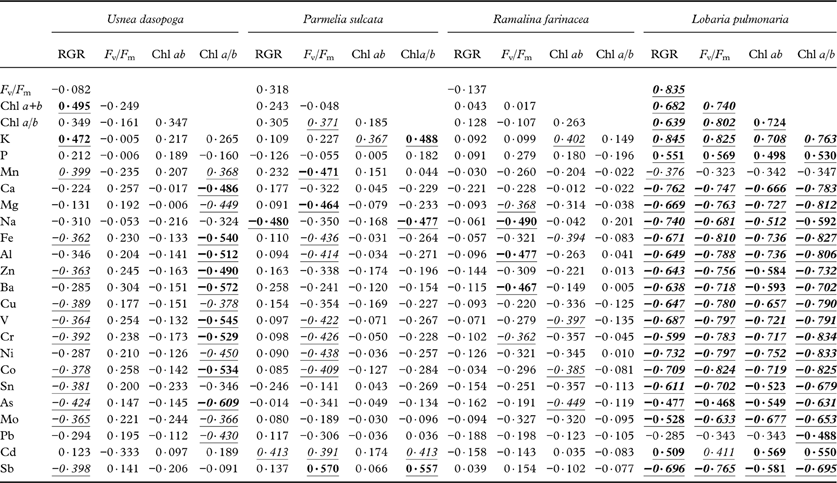
Significant values are underlined. P- level: <0·05 (italic); <0·01 (bold); <0·001 ( bold italic ). n=30.
Discussion
Our study was carried out in winter when growth rates are low due to low temperatures (Larsson et al. Reference Larsson, Solhaug and Gauslaa2012). The ranking of species with respect to RGR corresponds to their Norwegian altitudinal and latitudinal distributions (http://nhm2.uio.no/lav/web/index.html). The fastest growing species, P. sulcata, extends to northernmost Norway and occurs frequently up to 1100 m elevation. Usnea dasopoga and L. pulmonaria, with intermediate RGR values, mainly grow below 800 m and are less frequent in N Norway whereas R. farinacea, with mean negative RGR, is absent from the northernmost county and is rare above 300 m. We thus believe that the ranking of species-specific overall RGRs measured in winter reflects the species thermal niche rather than species-specific susceptibility to road pollutants. In addition, fruticose lichens are more susceptible to fragmentation and this may have contributed to their low RGR. In general, viability parameters are hardly affected by traffic-related pollutants at distances ≥30 m. The long-term photoinhibitory damage at distances ≥30 m during transplantation, evidenced by species-specific reductions in F v/F m relative to the start (Fig. 4), reflects the higher light exposure in the sunny and dry period at low temperatures (Bidussi et al. Reference Bidussi, Gauslaa and Solhaug2013) before harvesting in March than the light experienced by control thalli on trees with ± shading canopies in source habitats. At distances ≥30 m, the strongest photoinhibition occurred in the high-light susceptible L. pulmonaria, and the lowest in the high-light resistant species P. sulcata (consistent with Gauslaa & Solhaug Reference Gauslaa and Solhaug1996). Also the strong chlorophyll degradation in L. pulmonaria (Fig. 4) is consistent with its earlier reported susceptibility to high light (Gauslaa & Solhaug Reference Gauslaa and Solhaug1999).
In addition to species-specific responses to changed light, growth and viability responded to road proximity (Fig. 4; Table 3). The high heavy metal- (Branquinho et al. Reference Branquinho, Brown, Máguas and Catarino1997; Cabral Reference Cabral2002) and air pollutant-susceptibility (e.g. Coxson et al. Reference Coxson, Björk and Bourassa2014) of L. pulmonaria probably caused its low RGR and depressed viability near the road. The other species were remarkably resistant to traffic pollutants, although chlorophyll degradation slightly increased with decreasing distance from the road. The chlorophyll pool was substantially higher in the foliose than in the fruticose lichens (Fig. 4). Because high nutrient availability stimulates the photobiont rather than the mycobiont (Palmqvist Reference Palmqvist2000), species-specific contrasts in chlorophylls are probably connected to the higher control levels of macronutrients in the foliose species (Fig. 2).
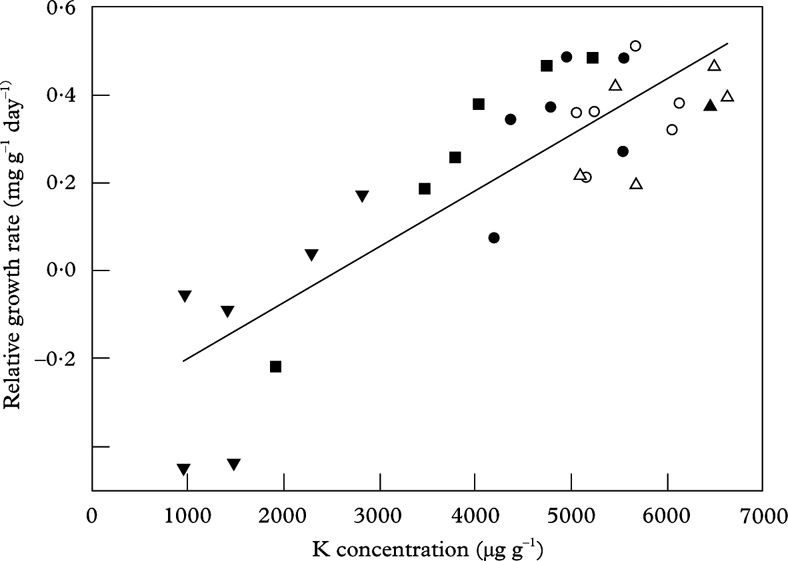
Macronutrients (P and K) increased with distance from the road in susceptible species (Fig. 2). Potassium was the most important element for growth in L. pulmonaria (Fig. 5) as in U. dasopoga (Table 4). Both experienced K loss during transplantation (Fig. 2). Garty et al. (Reference Garty, Kloog and Cohen1998), Haffner et al. (Reference Haffner, Lomsky, Hynek, Hallgren, Batic and Pfanz2001) and Marques et al. (Reference Marques, Freitas, Wolterbeek, Steinebach, Verburg and De Goeij2005) found that lichen K concentration correlates inversely with the electric conductivity of lichens and suggested leakage of this element as a result of air pollutants. We consider the reduction of K in L. pulmonaria (Fig. 5) and U. dasopoga near the road to be pollution-induced leaching from damaged membranes.
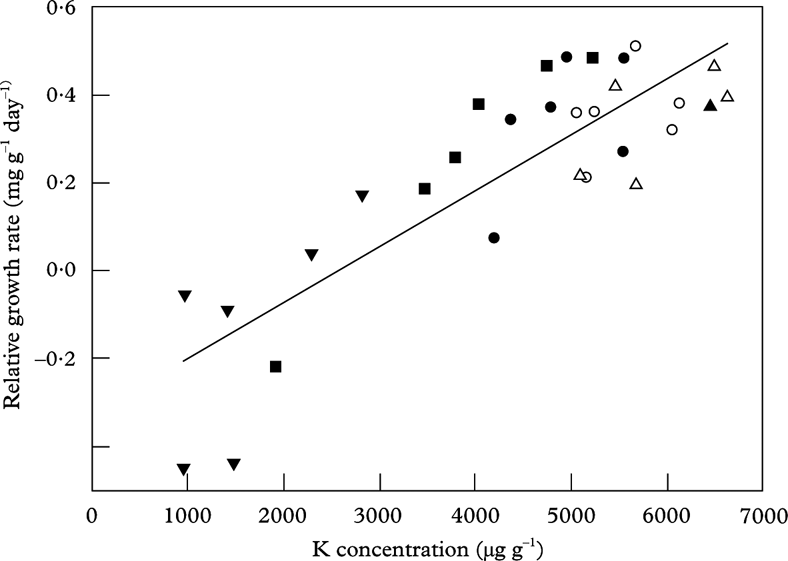
Fig. 5. The relationship between relative growth rate (RGR) and potassium concentration in Lobaria pulmonaria measured after a transplantation period of 25 September to 26 March at five distances (▼: 10 m; ▪: 15 m; •: 30 m; ○: 50 m; ▵: 100 m) replicated in six transects along a highway. Regression line: RGR=0·000127*K – 0·327; n=30; r 2 adj=0·703; P<0·001.
The highly significant increase in most heavy metals (Fe, Al, Zn, Ba, Cu, V, Cr, Ni, Co, Sn, As, Mo; Table 1) in all species (Fig. 2; Table 2) along the road is consistent with major influences of road pollutants over large distances. Most are traffic-related pollutants (Monaci et al. Reference Monaci, Moni, Lanciotti, Grechi and Bargagli2000; Ozaki et al. Reference Ozaki, Watanabe and Kuno2004; Amato et al. Reference Amato, Viana, Richard, Furger, Prévôt, Nava, Lucarelli, Bukowiecki, Alastuey and Reche2011). Corrosion of vehicles is a main source of heavy metal pollution (Zn, Co, Cu, Cr, Fe and Ni; Norrström Reference Norrström2005). Ni, V and As are related to fuel combustion (Minganti et al. Reference Minganti, Capelli, Drava, De Pellegrini, Brunialti, Giordani and Modenesi2003: Tasić et al. Reference Tasić, Rajšić, Tomašević, Mijić, Aničić, Novaković, Marković, Marković, Lazić, Radenković and Gungor2008), Cr, Cu, Zn and Pb are associated with diesel engines and wearing of brakes (Garty Reference Garty2001; Pacyna & Pacyna Reference Pacyna and Pacyna2001; Jozic et al. Reference Jozic, Peer and Türk2009), whereas Ca, Al and Fe are road and soil dust elements (van Dobben et al. Reference van Dobben, Wolterbeek, Wamelink and ter Braak2001; Tasić et al. Reference Tasić, Rajšić, Tomašević, Mijić, Aničić, Novaković, Marković, Marković, Lazić, Radenković and Gungor2008). Elements accumulated in lichens probably originate from road dust and exhaust emissions. The increase in Mn and Cd with distance (Fig. 2) may result from accumulation of other heavy metals with a stronger affinity to available ion exchange sites (Rinne & Barclayestrup Reference Rinne and Barclayestrup1980).
Sodium, the quantitatively most important road pollutant in winter, strongly accumulated particularly in U. dasopoga (Fig. 2, Table 2). It is probably caused by de-icing salt (Viskari et al. Reference Viskari, Rekilä, Roy, Lehto, Ruuskanen and Kärenlampi1997; Blomqvist & Johansson Reference Blomqvist and Johansson1999). The high retention of this monovalent cation in lichens exposed to rain, even weeks after the last application of de-icing salts, was unexpected. Because Na and heavy metals are closely correlated in the roadside environment during winter (Fig. 3), it is difficult to separate their influence on lichen viability. In species-specific best subset regression analyses of RGR based on elemental concentration (data not shown), Na is the single best predictor for RGR in P. sulcata (r 2 adj= 0·203), and is maintained in all best multiple models. This is not the case for the three other species in which Na is first included at a stage when its participation hardly improves the r 2 adj. Because sea water improves functioning of cultivated lichen photobionts (Wieners et al. Reference Wieners, Mudimu and Bilger2012), salt is hardly highly toxic.
The fruticose lichens, U. dasopoga in particular, were most efficient in accumulating road pollutants (Table 2), probably because of their large, freely exposed surface area. Their generally lower start concentration of most elements is probably caused by stronger coupling to airborne elements than the two foliose lichens attached to the bark surface. The higher concentration in P. sulcata of most heavy metals after transplantation is probably caused mainly by its higher content of these elements at the start (Fig. 2), presumably caused by local dust in the small rural farm road along which this species was collected. Many heavy metals disperse further than 100 m from the road (Table 2), as reported elsewhere (Klos et al. Reference Klos, Rajfur, Waclawek and Waclawek2009). Using the interpretative scale of Frati et al. (Reference Frati, Brunialti and Loppi2005), the substantial increase compared to control values for Na, Fe, Al, Ni, Cr, V, Co, Mo, As, Sn and Sb can be classified for the EC class as “severe accumulation” in all species studied.
In conclusion, lichens are useful for biomonitoring pollutants in roadside ecosystems. Six months transplantation along a highway during winter caused significant accumulation of heavy metals and Na, often beyond 100 m. The heavy metal accumulation increased from foliose to fruticose lichens in the order: P. sulcata<L. pulmonaria<R. farinacea≪U. dasopoga. By comparing the long spatial gradient in heavy metal accumulation with the shorter gradients in reduced viability, it is clear that substantial heavy metal accumulation can take place without a measurable adverse effect on lichen performance, even in the most susceptible species, L. pulmonaria. Measurement of metal accumulation is thus a more sensitive way of monitoring road pollutants than recording growth and lichen viability.
Thanks to Astrid Skrindo in Statens Vegvesen, Vegdirektoratet (Norwegian Public Roads Administration) for providing funding for this project, and to Kjell A. Gai for help during fieldwork and analyses.