Significant outcomes
∙ We found a significant negative correlation between emotional blunting and the neural activation of the left amygdala during processing of positive affect in patients.
∙ We found a significant negative correlation between stereotyped thinking and the volume of the right amygdala in patients.
Limitations
∙ Block design is not optimal to detect trial-by-trial differences.
Introduction
Negative symptoms in schizophrenia (NSS) are characterised by a decrement in various behaviours such as facial expression, speech, pleasurable activities, and goal directed activity (Reference Earnst and Kring1). NSS have been shown to be persistent (Reference Keefe, Harvey and Lenzenweger2), predict a poorer prognosis with respect to functional outcome (Reference Milev, Ho, Arndt and Andreasen3), and being associated with diminished quality of life (Reference Bow-Thomas, Velligan, Miller and Olsen4), and currently available treatments for schizophrenia are only effective to some degree in alleviating these symptoms (Reference Murphy, Chung, Park and McGorry5).
The neural correlates of NSS remain elusive. Large-scale neuronal fronto-limbic networks have been implicated in NSS (Reference Hovington and Lepage6). Neuroimaging studies have associated NSS with structural brain changes in the prefrontal cortex, medial temporal lobes and their interconnecting structures (Reference Bora, Fornito and Radua7–Reference Yoshida, McCarley and Nakamura13).
The amygdala is a limbic structure consisting of several nuclei located bilaterally in the anterior region of the medial temporal lobe. The amygdala plays a key role in emotional and cognitive processing (Reference Ousdal, Reckless, Server, Andreassen and Jensen14). Studies on amygdala volume in schizophrenia remain inconclusive. A previous meta-analysis of 15 voxel-based morphometry studies (n=390, assessing regional changes in grey matter volume) showed significant volume reductions of the amygdala in schizophrenia (Reference Honea, Crow, Passingham and Mackay15,Reference Levitt, Bobrow, Lucia and Srinivasan16). However, this finding was not replicated in an original study of ultra high-risk individuals (n=135), first-episode psychosis patients (n=162) and chronic schizophrenia patients (n=89) (Reference Velakoulis, Wood and Wong17). A region-of-interest volumetric study (assessing global amygdala volume) found no differences between schizophrenia subjects and healthy controls (n=40) with regard to amygdala volume, but a negative correlation between the volume of the hippocampus-amygdala complex and clinical ratings of negative symtoms and thought disturbances (Reference Rajarethinam, DeQuardo and Miedler18).
A review of 12 functional magnetic resonance imaging (fMRI) studies suggests diminished neural activation of the amygdala in response to emotional stimuli (emotional facial expressions, pictures with emotional content) among patients with schizophrenia (Reference Aleman and Kahn19). Altered activation of the amygdala during emotion processing have also been associated with negative symptoms such as blunted affect (Reference Rauch, Reker and Ohrmann20–Reference Lepage, Sergerie, Benoit, Czechowska, Dickie and Armony23).
Thus previous neuroimaging studies have identified structural and functional alterations that suggest an involvement of the amygdala in the emergence of NSS. However, previous studies that investigate emotion processing and the amygdala in schizophrenia have neither combined structural and functional neuroimaging within the same subjects and experimental session, nor done more extensive subphenotypisation of the clinical ratings of NSS.
Aims of the study
The general aim of this study was to investigate the neural correlates of NSS using two neuroimaging metrics: structural and functional MRI. First, we hypothesised that there is a decreased amygdala volume and neural activation during emotion processing in patients compared with controls. Second, we hypothesised that there is a correlation between amygdala volume, neural activation, and clinical ratings of NSS. Within the same subjects and experimental session, we used automated amygdala volumetry, fMRI together with an experimental paradigm for eliciting amygdala activity (Hariri’s Faces Matching Task (Reference Hariri, Mattay and Tessitore24)), and clinical ratings of negative symptoms to investigate the neural correlates of NSS in 28 patients with schizophrenia and 28 healthy controls.
Materials and methods
Subjects
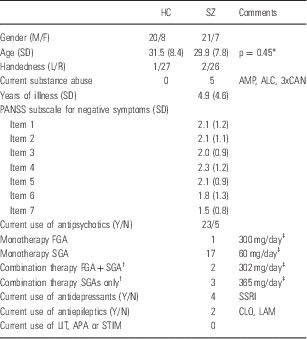
The study sample consisted of patients with a DSM-IV (Diagnostic and Statistical Manual, 4th edition) diagnosis of schizophrenia (n=28) and healthy controls (n=28). Subjects participated in an on-going multi-center study called Thematically Organized Psychosis (TOP) Study at the University of Oslo, Norway. All subjects were between 18 and 42 years old and born in Norway. Demographic and clinical variables are listed in Table 1. We analysed the first consecutively collected subjects in both groups that were recruited in the TOP study.
Table 1 Sample characteristics
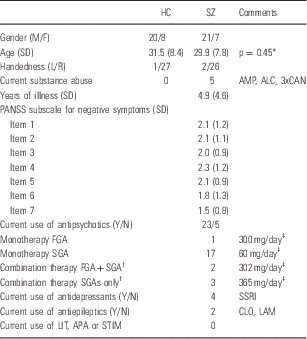
ALC, overconsumption of alcohol; AMP, amphetamine; APA, antiparkinson agents; CAN, cannabis; CLO, clonazepam; FGA, first generation antipsychotics; LAM, lamictal; LIT, lithium; SD, standard deviation; SGA, second generation antipsychotics; SSRI, selective serotonin reuptake inhibitor agents (two with escitalopram, one with citalopram, and one with sertraline); STIM, stimulant medication.
The subject groups consisted of 28 schizophrenia subjects (SZ) and 28 healthy controls (HC). Age and sex were normally distributed in both groups (Shapiro Wilks W tests: prob<W<0,0001).
* T-test, two-tailed, unequal variance.
† Two different antipsychotics.
‡ Chlorpromazin equivalents, mean value.
The patient group was recruited from four major psychiatric hospitals and outpatient clinics that together cover most of the population in Oslo. All patients underwent a thorough clinical assessment by specialist trained psychologists and physicians. Clinical diagnoses were determined using the Structured Clinical Interview for DSM-IV axis 1 disorders (SCID-I) module A–E (Reference Spitzer, Williams, Gibbon and First25), with an overall interrater agreement for diagnostic categories of 82%, κ=0.77 (95% CI 0.60–0.94). Psychosocial function was assessed with the Global Assessment of Function scale, split version. Current psychotic symptoms were rated with the Positive and Negative Syndrome Scale (PANSS), with intraclass coefficients of 0.73 and 0.86 for the positive and negative sub-scales respectively (Reference Engh, Friis and Birkenaes26). We excluded three patients with psychosis that had not been diagnosed with schizophrenia, and three other patients due to faulty anatomical scans. Healthy controls were randomly selected from the Norwegian national population register. Healthy controls were residents in the same catchment area as patients, and were screened for mental illness by trained psychologists using the Primary Care Evaluation of Mental Disorders (Prime-MD) (Reference Spitzer, Williams and Kroenke27). Control subjects with current or previous somatic illness, or substance misuse disorder (including alcohol overuse) were excluded. Patients and controls were matched at group level with regard to age, sex, and handedness (see Table 1).
The study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate, and was conducted in accordance with the Helsinki declaration. After complete description of the study to participants, written informed consent was obtained from all subjects.
Clinical ratings
We used PANSS to assess the severity of current schizophrenia symptoms (Reference Kay, Fiszbein and Opler28). Data was collected from clinical interviews and family reports. The following seven items were rated using a scale ranging from 1 (absent) to 7 (severe): blunted affect (N1), emotional withdrawal (N2), poor rapport (N3), passive/apathetic social withdrawal (N4), difficulty in abstract thinking (N5), lack of spontaneity and flow of conversation (N6), and stereotyped thinking (N7).
Depressive symptoms in patients were rated with the Inventory of Depressive Symptomatology (Reference Rush, Giles, Schlesser, Fulton, Weissenburger and Burns29) (IDS; n=22) and the Calgary Depression Scale for Schizophrenia (Reference Addington, Addington and Schissel30) (CDSS; n=22). Thirteen patients were rated with both scales. The mean number of days between clinical ratings of depressive symptoms and the MRI scan were 292. The mean total score on the IDS was 17.7 (SD±10.3), and no patient reached the threshold for ‘severe’ (39–48 points) or ‘very severe’ (49–84 points). The mean total score on the CDSS was 6.5 (SD±4.6), thus indicating a specificity between 77% and 82%, and a sensitivity of 85% and 92%, for prediction of a major depressive episode in schizophrenia. Given the distance in time between clinical depression ratings and the MRI scan, and the absence of severe depressive symptoms, we chose not to adjust for depressive symptoms in our statistical analyses.
Experimental task
We used Hariri’s Faces Matching Task (Reference Hariri, Mattay and Tessitore24), previously described in (Reference Ousdal, Anand Brown and Jensen31). The Faces Matching Task has been widely used and validated in several studies, and is known to elicit amygdala activity during emotion processing (Reference Carre, Murphy and Hariri32).
In the Faces Matching Task, participants select the stimulus (one of two, displayed at the bottom of the screen) that matches a target stimulus (displayed at the top of the screen). The images were either human faces expressing affect (faces matching task) or geometrical shapes (control task). Participants completed five blocks of the face matching task, where each block consisted of six emotion-specific face trios derived from a standard set of facial affect pictures (Reference Tottenham, Tanaka and Leon33). Subjects were scanned during two sessions on the same day. In one of the sessions we presented faces expressing positive affect, and in the other faces expressing negative affect (anger and fear). To avoid bias, the presentation order was chosen randomly for each subject. Interleaved between the blocks, participants completed five blocks of the control task. Each trial (faces or shapes) was presented for 5.4 s with no inter-stimulus interval, for a total block length of 32.64 s. Total scanning time was 10 min, both sessions included. Stimuli were visually presented to participants using the E-prime software (version 1.0 Psychology Software Tools Inc., Pittsburgh, PA, USA) and VisualSystem (NordicNeuroLab, Bergen, Norway). Responses were recorded through MR-compatible ResponseGrips (NordicNeuroLab). After each session the target stimuli were shown again to the participants and this time the task was to assess valence and arousal for each face (on two different nine-point scales).
Image acquisition
All participants underwent MRI scanning on a 1.5 T Siemens Magnetom Sonata scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a standard head coil. T2*-weighted echoplanar images (EPI) were acquired for blood-oxygen-level-dependent (BOLD) data. Each image consisted of 24 axial slices. Each slice was 4 mm thick with a resolution of 3.5×3.5×5 mm and inter-slice interval of 1 mm. Each session comprised 144 volumes (16 volumes/block). We used a BOLD EPI sequence (TR=2040 ms, TE=50 ms, flip angle=90°, matrix 64×64, FOV 192×192 mm). We discarded dummy volumes. For co-registration of EPI scans and volumetric assessment of the amygdala, we acquired two sagittal T1-weighted magnetisation prepared rapid gradient echo volumes were acquired with the Siemens tfl3d1_ns pulse sequence (TE=3.93 ms, TR=2730 ms, TI=1000 ms, flip angle=7°; FOV=24 cm, voxel size=1.33×0.94×1 mm3, number of partitions=160). These high-resolution anatomical scans were subsequently averaged together, after rigid-body registration, to increase the signal to noise ratio. There was no scanner upgrade during the study period, and patients and controls were scanned consecutively. A neuroradiologist evaluated all scans in order to rule out brain pathology.
Functional neuroimaging analysis
Functional neuroimaging data was analysed using FEAT (FMRI Expert Analysis Tool) version 6.00, part of FSL (Reference Jenkinson, Beckmann, Behrens, Woolrich and Smith34). In our first-level analysis we used the following pre-statistics processing; orientation to standard space (MNI152) using FLIRT (Reference Jenkinson and Smith35), motion correction using MCFLIRT (Reference Jenkinson, Bannister, Brady and Smith36); non-brain removal using BET (Reference Smith37); spatial smoothing using a Gaussian kernel of FWHM 8.0 mm; grand-mean intensity normalisation of the entire 4D data set by a single multiplicative factor; high pass temporal filtering with a cutoff of 90 s (Gaussian-weighted least-squares straight line fitting, with σ=45.0 s). Time-series statistical analysis was carried out using FILM with local autocorrelation correction (Reference Woolrich, Ripley, Brady and Smith38). Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z>2.3 and a (corrected) cluster significance threshold of p=0.05 (Reference Worsley39). Parameter estimates (PE) and a contrast PE (COPE) (affective faces>geometric shapes) was calculated for each voxel using a general linear model. In our second level analyses we used FLAME (FMRIB’s Local Analysis of Mixed Effects) stage 1 and stage 2 with automatic outlier detection (Reference Beckmann, Jenkinson and Smith40–Reference Woolrich, Jbabdi and Patenaude43). Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z>2.3 and a (corrected) cluster significance threshold of p=0.05 (Reference Worsley39). We did a region-of-interest (ROI) analysis of neural activation in the left and right amygdala respectively. We derived probabilistic ROI masks of the amygdala from the Harvard-Oxford Cortical Atlas supplied with FSL. The Z-statistic images in our ROI analyses were thresholded using a corrected significance threshold of p=0.05 (at the voxel level). We included age, gender, and current use of antipsychotics (yes/no) as co-variates in our regression model. Antipsychotics were coded as binary variables since normalisation of neuroleptic refers to dopamine blockade and other subcortical structures but the amygdala (for which the effect of antipsychotics is not firmly established) (Reference Scherk and Falkai44).
Structural neuroimaging analysis
We used FIRST 1.2 for automatic segmentation of the left and right amygdala (Reference Patenaude, Smith, Kennedy and Jenkinson45). In FIRST the outline of the amygdala has a default setting from 336 manual tracings that provide the mean variations and a statistical model for the amygdala. We used FIRST to compute meshes representing the volumetric outputs. We applied boundary correction that further refined the delineation of the amygdala. We calculated intracranial volumes and derived a scaling factor for each participant using SIENAX. The scaling factor was derived from the difference in size of each individual brain in relation to the MNI 152 standard brain. We used this scaling factor to normalise all volumetric measures in each subject.
Clinical correlations
Statistical analyses were performed in JMP 9 (SAS Institute, Boston, MA, USA). The alpha level was set to 0.05 for all tests. First, the association between each of the negative symptom items (n=6) of the PANSS and amygdala activation (left and right, respectively) was determined by calculating Spearman’s ρ. Second, we used multiple regression analysis to determine the effect of amygdala volume on the association between clinical ratings and neural activation. Third, we used multiple regression analysis to investigate the association between amygdala volume and clinical ratings while adjusting for age, sex, and antipsychotic treatment. Antipsychotic treatment was coded as a binary variable.
Results
There was no significant between-group difference in the accuracy rate during the Faces Matching Task. The mean response time (Mean RT) was significantly longer (p<0.001) for patients (Mean RT=1231 ms) than for healthy controls (Mean RT=1065 ms). Both groups activated the amygdala bilaterally during the Faces Matching Task (Fig. 1). However, we did not find any statistically significant between-group differences in whole brain activation or regional amygdala activation during task performance (Table 2). In the patient group we found a statistically significant and negative correlation between the clinical ratings of blunted affect and left amygdala activation during presentation of the positive affective stimuli (Spearman’s ρ −0.5177, p=0.0046; Fig. 2). The results from the non-parametric correlation analysis survived Bonferroni correction for multiple tests (n=7). Using a multiple regression analysis, we found no significant effect of amygdala volume on this association (p=0.42). We did not find a significant effect of group on amygdala volumes while adjusting for age, sex, and antipsychotic treatment. We also found that right amygdala volume predicted stereotyped thinking (p=0.043; adjusted for age, gender and treatment with antipsychotics; Fig. 3). However this did not survive Bonferroni correction for multiple comparisons.

Fig. 1 Neural activation of the amygdala in schizophrenia during processing of emotional stimuli (p<0.05, corrected; left: positive affect; right: negative affect).

Fig. 2 Correlation between emotional blunting (PANSS, item N1) and COPE values in left amygdala of schizophrenia subjects. Dotted lines demark 95% confidence interval.

Fig. 3 Correlation between stereotyped thinking (PANSS, item N7) and volume of right amygdala (dotted red line demarks 95% confidence interval, blue line demarks mean value of right amygdala size).
Table 2 fMRI results (affective faces>geometrical shapes) for whole brain analyses, cluster based corrected p<0.05

fMRI, functional magnetic resonance imaging; HC, healthy controls; Neg, affective faces with negative affects in the experiment; Pos, affective faces with positive affects in the experiment; SZ, schizophrenia patients.
The table shows local maxima in the largest cluster for each of the experimental situations. There were no significant differences when schizophrenia patients were compared with healthy controls.
* Harvard Oxford Cortical and Subcortical Atlases are population based probability maps for 48 cortical and 21 subcortical structures.
Discussion
We used magnetic resonance imaging together with an emotional task, and clinical measures of symptom severity, to test the hypothesis that NSS are associated with structural and functional alterations of the amygdala.
We did not find any between-group differences in amygdala activation. Thus our results contradict previous studies of amygdala activation in schizophrenia (Reference Rauch, Reker and Ohrmann20–Reference Lepage, Sergerie, Benoit, Czechowska, Dickie and Armony23). One putative reason for the lack of between-group differences in our study is the characteristics of the baseline condition of our task. It has previously been shown that when an emotional stimulus (i.e. emotional facial expressions) in contrasted with a non-emotional stimulus of another category (i.e a fixation cross), the task effect may be diminished (Reference Aleman and Kahn19,Reference Whalen46)
We did not find any between-group differences in amygdala volume. Our negative finding is not corroborated by a previous meta-analysis (Reference Honea, Crow, Passingham and Mackay15) that estimates amygdala volume reductions to 6–8% in schizophrenia (compared with healthy controls). The lack of volume reduction may be attributed to the short duration of illness in our cohort (4.9 years, SD 4.6), and that medial temporal structural changes are not apparent in the early phases of schizophrenia (Reference Velakoulis, Wood and Wong17,Reference Kubicki, Shenton and Salisbury47,Reference Salgado-Pineda, Baeza and Perez-Gomez48). However, this is contradicted by Malchow et al. (Reference Malchow, Hasan and Schneider-Axmann49), that finds a decrease of amygdala volume also in recent onset schizophrenia.
We found a statistically significant negative correlation between blunted affect and left amygdala activation during processing of positive affect. Considering the function of the amygdala in emotion regulation (Reference Phelps and LeDoux50), cognitive processing (Reference Schaefer and Gray51) and relevance detection (Reference Ousdal, Jensen, Server, Hariri, Nakstad and Andreassen52), it is reasonable that there is an association between amygdala and negative symptoms of reduced expression in schizophrenia, as indicated by the present results. Indeed, this is in accordance with a model of flattened affect, proposed by Aleman and Kahn (Reference Aleman and Kahn19). Based on neuroimaging data they suggested that flattened affect is due to amygdala lesions in combination with reduced connectivity with the prefrontal cortex. We also found that right amygdala volume predicts stereotyped thinking. Although the correlation between volume and stereotyped thinking is only nominally significant, the results are in line with earlier findings indicating an association between negative symptoms and amygdala features in schizophrenia (Reference Rajarethinam, DeQuardo and Miedler18).
Factor analyses of schizophrenia symptoms implicate a specific domain of negative symptoms that can be further subdivided into factors of reduced expression, and asociality/avolition (Reference Stevenson, Mikels and James53). The negative symptoms for which we found correlations with amygdala features (blunted affect and stereotyped thinking) belong to the reduced expression-factor. This supports the notion that subdivision of negative symptoms has a neural substrate that is associated with the amygdala.
Strengths of our study are the acquisition of both structural and functional data from within the same subjects and session, the homogeneous patient sample in terms of diagnosis (only schizophrenia), clinical characteristics (i.e. duration of illness) and geographical distribution. We were also able to control for potential confounders that affect amygdala neuroimaging (i.e. substance misuse, and current treatment with antidepressants and antiepileptics).
Our study has limitations. First, we used a block design in the experiment, which is superior in detecting the localisation of neural activity, but not as good as event-related designs with regard to specificity in the degree of activation. In this study we infer that there is an association between the degree of amygdala activation and clinical ratings. We acknowledge that an event-related design may have been more appropriate in this respect (Reference Birn, Cox and Bandettini54). Block designs may also contribute to the continuous cognitive control and top-down modulation of affective expression and arousal in response to affective stimuli, and this could potentially bias brain activation patterns in that affective processing may be kept attenuated (Reference Schafer, Schienle and Vaitl55).
In conclusion, this study reaffirms that NSS are associated with alterations of both structure and function of amygdala, in particular with regard to negative symptoms related to reduced expression.
Acknowledgement
The authors thank Dr. Kjetil Nordbø Jørgensen for contributing to acquisition and quality checking of data.
Authors’ contributions: All authors made a substantial intellectual contribution to the study. In particular, Christoffer Rahm performed the analyses, interpreted the results, and wrote the first draft of the manuscript. Benny Liberg assisted in the analyses, and in the writing of the manuscript. Greg Reckless made substantial contributions to acquisition and quality checking of data. Olga Ousdal and Ingrid Melle made a substantial contribution to the conception and design of the study, and acquisition of data. Ole A. Andreassen and Ingrid Agartz conceived the study, participated in its design and coordination and helped finalise the manuscript. All authors read and approved the final manuscript.
Financial Support
Christoffer Rahm received funding from Schizofreniförbundet, and the Strategic Research Committee, Karolinska Institutet and Stockholm County Council, Sweden. Benny Liberg received funding from Svenska Läkaresällskapet (The Swedish Society of Medicine, SLS-403101), and the Strategic Research Committee, Karolinska Institutet and Stockholm County Council, Sweden.
Conflicts of Interest
Ole A. Andreassen received speakers honorarium from GSK, Lundbeck, Otsuka.
Ethical Standards
The authours assert that all precedures contributing to this work comply with the ethical standards of the relevant ational and institutional committees on human experimentation and with the Declaration of Helsinki of 1975, as revised in 2008.