Introduction
The first recorded incidence of buffalopox dates back to 1934 and occurred in Lahore in undivided India (Sharma, Reference Sharma1934). This was followed by regular reports of both mild and severe forms of the disease from various states of India (Sehgal et al., Reference Sehgal, Ray, Ghosh and Arora1977). In the mild form, lesions are localized on the udder, teats and inguinal region (Sharma, Reference Sharma1934; Singh and Singh, Reference Singh and Singh1967), over the parotid, and the base and inner surface of the ear and eyes (Bhatia, Reference Bhatia1936; Wariyar, Reference Wariyar1937; Mallick and Dwivedi, Reference Mallick and Dwivedi1982; Mallick, Reference Mallick1988). In the severe form, the lesions are generalized (Ramakrishna and Ananthapadmanabham, Reference Ramakrishna and Ananthapadmanabham1957; Chandra et al., Reference Chandra, Garg, Rana and Rao1987). However, generalized forms of the disease are infrequent these days as the lesions are mostly confined to the udder, teats, and sometimes on the thighs and hindquarters of the affected animals. Infection in milk animals leads to mastitis, which contributes to reduction in milk yield and the working capacity of draft animals. In severe cases of mastitis, a permanent reduction in milk yield is also recorded (Singh et al., Reference Singh, Hosamani, Balamurugan, Satheesh, Shingal, Tatwarti, Bambal, Ramteke and Yadav2006c). Although buffaloes are primarily affected, cows and humans are occasionally involved. Outbreaks of buffalopox are reported not only from India but also from many other countries including Pakistan, Egypt, Nepal and Bangladesh. Recently, similar outbreaks of pox-like-infections caused by Vaccinia virus (VACV)-like agents viz. Cantagalo (Damaso et al., Reference Damaso, Espostio, Condit and Moussatche2000) and Aracatuba viruses (de Souza Trindade et al., Reference de Souza Trindade, da Fonseca, Marques, Nogueira, Mendes, Borges, Peiro, Pituco, Bonjardim, Ferreira and Kroon2003) have been reported from Brazil.
Aetiology
The causative agent of buffalopox, buffalopox virus (BPXV), is classified in the Orthopoxvirus (OPV) genus of the subfamily Chordopoxvirinae and the family Poxviridae. The BP4 strain (Department of Bacteriology and Hygiene, Haryana Agricultural University, Hisar, India) is regarded as the reference strain of the virus (Singh and Singh, Reference Singh and Singh1967; Baxby and Hill, Reference Baxby and Hill1969).
Virus morphology
Earlier electron microscope studies of BPXV have shown structural similarity with other OPVs. The size of BPXV isolates BP Giza 72 (Egyptian) and BP4 (Hisar, India) was reported to be 270×700×100 Å (Skalinskii and Tantawi, Reference Skalinskii and Tantawi1974). There were no differences in appearance between the Indian and Egyptian isolates of BPXV nor between BPXV and VACV. Various developmental stages of the virus in chorioallantoic membrane (CAM), including the modes of release of C- and M-forms of mature virions, are probably analogous to those of VACV (Skalinskii and Tantawi, Reference Skalinskii and Tantawi1974; Bloch and Lal, Reference Bloch and Lal1975). Later studies have indicated that the mature virus particle is brick-shaped measuring 280–330 nm×200–250 nm in size (Bloch and Lal, Reference Bloch and Lal1975; Lal and Singh, Reference Lal and Singh1977; Mohanty et al., Reference Mohanty, Rai, Mallick and Kumar1989). However, the developmental forms in the cytoplasm appear oval or spherical with or without a central core (Bloch and Lal, Reference Bloch and Lal1975; Sehgal et al., Reference Sehgal, Ray, Ghosh and Arora1977). The intracytoplasmic B-type inclusions of BPXV resemble those of cowpox virus (CPXV), but appear different from Guarnieri bodies of VACV, which are abundant, small, irregular, eosinophilic and granulated (Singh and Singh, Reference Singh and Singh1967; Chandra et al., Reference Chandra, Garg, Rana and Rao1987).
Physicochemical properties
BPXV is resistant to ether but sensitive to chloroform, bile salts, pH and heat (Lal and Singh, Reference Lal and Singh1977). Complete loss of infectivity of strain BP4 (Buffalo pox virus reference strain) has been reported at 56°C in 90 min (Chandra et al. Reference Chandra, Garg, Rana and Rao1987), while strain BPH-80 (BPXV isolated from an outbreak in 1980 in Hissar, Haryana, India) showed a drop in infectivity titer of log10 2.74 at 56°C in 30 min and complete loss at 56°C in 60 min (Kumar et al., Reference Kumar, Yadav, Chandra and Garg1987). The virus becomes inactivated according to the first-order exponential kinetics in one-component fashion with a velocity constant (K) of 0.31. The cationic stabilization to thermal inactivation test revealed an increased inactivation rate in the presence of bivalent cations such as Mg++ ions. The BPH-80 strain of BPXV and VACV, though sensitive to heat, were able to produce pock lesions on CAM at 40.5±0.5°C, whereas the BP4 strain of BPXV failed to produce lesions. However, all three viruses (BP4, VACV and BPH-80) produced pock lesions at 38±0.5°C (Kumar et al., Reference Kumar, Yadav, Chandra and Garg1987).
Serological relationships
The BPXV cross-reacts with fowlpox virus (FPV), sheeppox virus (SPPV) and goatpox virus (GTPV) in gel diffusion tests (Mathew, Reference Mathew1975). BPXV antiserum also cross-reacts with FPV in the hemagglutination inhibition (HI) test, but rabbit anti-VACV serum failed to react with FPV in this test (Mathew, Reference Mathew1975). The existence of a close relationship between BPXV and VACV has been affirmed by employing cross-neutralization and cross-precipitation tests (Singh and Singh, Reference Singh and Singh1967; Kataria and Singh, Reference Kataria and Singh1970; Baxby and Hill, Reference Baxby and Hill1971; Lal and Singh, Reference Lal and Singh1973). However, no cross-precipitation of BPXV was seen with swinepox virus (SWPV) in the gel precipitation test, although they share a common nucleoprotein (NP) antigen in the Agar gel precipitation test (AGPT) and immunoelectrophoresis (IEP) tests (Kataria and Singh, Reference Kataria and Singh1970; Baxby and Hill, Reference Baxby and Hill1971; Lal and Singh, Reference Lal and Singh1973). The serum neutralizing and precipitating antibodies in rabbits inoculated with BPXV appeared 10 days post inoculation (dpi), while delayed type hypersensitivity (DTH) reaction was detected from day 12 onwards. Both the humoral and cellular immune responses are essential in recovery from BPXV infection (Chandra et al., Reference Chandra, Singh, Garg and Rao1990).
The serological relationship of BPXV with VACV and CPXV has been studied using agar gel immunodiffusion (AGID), IEP, serum neutralization test (SNT) and complement fixation test (CFT). The pattern of cross-reaction in AGID and IEP was indicative of a closer relationship of BPXV with VACV than CPXV (Kataria and Singh, Reference Kataria and Singh1970). The precipitin pattern observed in AGID and IEP showed that BPXV shares four and three antigenic components with VACV, respectively. The SNT titers of rabbit anti-BPXV serum were 256, 16 and eight against BPXV, VACV and CPXV, respectively. VACV and CPXV antisera neutralized BPXV at a 1:4 titer. The homologous and cross complement-fixing (CF) activities of BPXV, VACV and CPXV antisera further established a close antigenic relationship amongst these viruses. The rabbit anti-BPXV serum fixed complement at a 1:640 titer with BPXV antigen and up to 1:80 and 1:40 with VACV and CPXV antigens, respectively (Kataria and Singh, Reference Kataria and Singh1970). The above studies are confirmatory of the close antigenic relationships among these viruses, but in SNT, the BPXV behaves differently from VACV and CPXV despite possessing common antigenic components and these differences are further manifested in higher neutralization titers with homologous antisera compared with heterologous ones. In toto, these observations suggest that BPXV is sufficiently different from VACV and CPXVs (Kataria and Singh, Reference Kataria and Singh1970; Singh and Singh, Reference Singh and Singh1967; Baxby and Hill, Reference Baxby and Hill1969) to be classified as a separate entity within the Variola-Vaccinia (VV) group of poxviruses.
Molecular biology
Virion polypeptide analysis
Virion polypeptides of purified BPXV have been analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The polypeptide profiles of purified BPXV strain BPBB (a buffalo isolate) and BPXV strain BPBH (a human isolate) from an outbreak that occurred in the Bhilai district of Madhya Pradesh, India and VACV strain WR yielded 26, 19 and 29 polypeptides, respectively (Maan and Kalra, Reference Maan and Kalra1995). Of these, six, three and seven polypeptides of BPBB, BPBH and VACV, respectively, were glycoprotein in nature. The number of major polypeptides for these isolates were 5, 4 and 4, respectively, in that order, with molecular weights ranging from 347 to 14.2 kDa. The BPXV-BPBB and -BPBH isolates shared four polypeptides (42, 26, 18.2 and 14.6 kDa), whereas VACV shared only one polypeptide of 18.1 kDa with BPXV isolates. On the other hand, two glycoproteins of 26 and 14.8 kDa were shared by all three viruses. In addition, a 42 kDa glycoprotein was a common major protein amongst BPBB and BPBH. Thus, the polypeptide profile did not provide conclusive data on the antigenic relationships among these viruses. Recently, Singh et al. (Reference Singh, Balamurugan, Hosamani, Satheesh, Rasool and Yadav2006a) identified and characterized the viral polypeptides of three Indian BPXV isolates and the reference virus (BP4) in 12% SDS-PAGE, which showed that all four BPXV isolates were similar and contained more than 25 polypeptides with molecular weights ranging from 14.2 kDa to >180 kDa. Synthesis of early and late polypeptides of these viruses could be tracked in vivo using 35S-methionine labeling.
Restriction enzyme (RE) analysis of viral genomic DNA
RE profiles of 13 isolates of BPXV and VACV digested with HindIII have been shown to be nearly identical. However, 12 Maharashtra BPXV isolates could be differentiated from a Hisar strain and the three strains of VACV that have been used in India (King Institute, Madras; Patwadangar, Nainital and U.S.S.R.). The 12 BPXV isolates appear to be repeated isolations. One of these isolates of BPXV was indistinguishable from VACV in its biological properties, but differed in RE profile from three VACV strains (Dumbell and Richardson, Reference Dumbell and Richardson1993).
Bhat et al. (Reference Bhat, Bhat, Mishra, Kumar and Singh1993) compared the GTPV, SPPV and BPXV by RE using HindIII, EcoRI, BamHI and PstI REs and found that these viruses are clearly different from each other. Further, the RE profiles generated by HindIII, BamHI and PstI of BPXV were identical to that of VACV. The PstI profile of BPXV (BP4 strain) was different from that of the ‘A strain’ of BPXV studied by Baxby and Hill (Reference Baxby and Hill1971) and three smallpox vaccine virus strains previously used in India. These results confirmed the conservation of genome regions among OPVs such as VACV, CPXV and BPXV (Esposito and Knight, Reference Esposito and Knight1985; Pilaski et al., Reference Pilaski, Rosen and Darai1986). The thymidine kinase (TK) gene in strain BP4 was localized in the BPXV genome in BamHI; HindIII and two EcoRI fragments of the viral genome, when hybridized to a cloned VACV TK probe. Bhat et al. (Reference Bhat, Mishra and Bhat1994) also demonstrated weak hybridization signals between capripoxvirus TK and BPXV (BP4 strain) TK, indicating TK homology between the orthopox and capripox groups of viruses.
Sequence and phylogenetic studies on BPXV
Sequence analysis based on the nucleotide (nt) and amino acid (aa) sequences of the viruses provides a better understanding of the evolutionary relationships among the closely related virus species. Sequences of a number of OPVs are now available in the database. To determine the relationship of BPXV with other member species of OPV, we sequenced a number of BPXV genes that play vital roles either in pathogenesis or morphogenesis as established in the VACV model. These include major envelope genes of BPXV homologues of VACV genes, viz. envelope proteins that bind cellular heparan sulfate (H3L and A27L) and chondroitin sulfate (D8L). These genes have been shown to play a prime role in VACV attachment and cell entry (Chung et al., Reference Chung, Hsaio, Chang and Chang1998; Hsiao et al., Reference Hsiao, Chung and Chang1999; Lin et al., Reference Lin, Chung, Heine and Chang2000). Nucleic acid sequences of selected genes of BPXV isolates showed >99% sequence identity not only among themselves but also with VACV. Many substitutions in the genes that were unique to both BPXV and VACV among all OPVs were identified. Phylogenetic analysis based on structural protein genes including H3L, A27L, D8L (Singh et al., Reference Singh, Hosamani, Balamurugan, Satheesh, Rasool and Yadav2006b) and B5R (Singh et al., Reference Singh, Balamurugan, Hosamani, De, Chandra Naik, Krishnappa and Yadav2007) and non-structural protein (H4L) homologue genes (Table 1), that are highly conserved across the genus, showed that BPXV is phylogenetically closely related to VACV vaccine strains (Dumbell and Richardson, Reference Dumbell and Richardson1993; Singh et al., Reference Singh, Hosamani, Balamurugan, Satheesh, Rasool and Yadav2006b) (Fig. 1).

Fig. 1. Phylogenetic analysis of OPVs. Unrooted tree is constructed based on the aa sequence of the highly conserved gene of RNA polymerase (H4L homologue of VACV Co strain) using MEGA 3.1. The scale beneath the tree represents the number of nucleotides per substitution in BPXV per site. The sequences representing BPXV AUR03 and BPXV VIJ96 have been submitted to GenBank under the accession numbers DQ916289 and DQ916290, respectively.
Table 1. Oligonucleotide primers used for amplification of the BPXV homologue of H4L gene in VACV

Recently, we analyzed samples derived from outbreaks where only cows or concurrently both cows and buffaloes were affected. A few of these isolates were adapted in Vero cells and analyzed by electron microscopy (EM) (Fig. 2), polymerase chain reaction (PCR) (Table 2), PCR–restriction fragment length polymorphism (RFLP) and sequencing. The sequence of the hemagglutinin (HA) gene from a total of eight virus isolates was determined for phylogenetic analysis. The HA gene is useful in the differentiation of OPV species (Ropp et al., Reference Ropp, Jin, Knight, Massung and Espositu1995; Damaso et al., Reference Damaso, Espostio, Condit and Moussatche2000). Sequence analysis of the HA genes revealed that the majority of the pox-like outbreaks in cows were caused by a single virus entity, BPXV, implying a wider host spectrum of the virus apart from buffaloes and humans. Cow and buffalo isolates shared high sequence identity (more than 98%) among themselves (Table 3). These isolates also showed high sequence identity with other homologous gene sequences of various species of OPV available in the GenBank database. Among OPVs, they shared 97.2–98.9% and 94.6–98.7% sequence similarity with VACV at the nt and aa levels, respectively. Similar outbreaks of disease in cows and humans in Brazil are caused by Aracatuba virus (de Souza Trindade et al., Reference de Souza Trindade, da Fonseca, Marques, Nogueira, Mendes, Borges, Peiro, Pituco, Bonjardim, Ferreira and Kroon2003) and Cantagalo virus (Damaso et al., Reference Damaso, Espostio, Condit and Moussatche2000; Nagasse-Sugahara et al., Reference Nagasse-Sugahara, Kisielius, Ueda-Ito, Curti, Figueiredo, Cruz, Silva, Ramos, Silva, Sakurai and Salles-Gomes2004) that have been reported to be closely related to VACV based on phylogenetic studies. Aracatuba virus showed 95.7–96.4% identity with VACV, in comparison to 97.2–98.9% sequence identity of the BPXV isolates with VACV strains. This was also consistent with the phylogenetic analysis carried out on the aa sequence of the HA genes. However, BPXV isolates appeared to be more closely related to VACV than Aracatuba virus to VACV. Further, Aracatuba virus also showed deletions of six codons between nt 249 and 254 in comparison to BPXV or VACV sequences. However, full-genome-based phylogeny would provide more conclusive evidence on the phylogeny of BPXV isolates in light of the previous observations that the BPXV is a clade of VACV.

Fig. 2. Mature virions (A–E) in intercellular space of cells infected with poxvirus isolated from buffaloes. Viruses are typically brick-shaped with outer envelope and biconcave core.
Table 2. Comparison of various tests in the detection and isolation of BPXV from clinical samples in India (1999–2006)

Source: unpublished data.
+=Positive; −=negative; ND=not done; CIE=counter-immunoelectrophoresis; CPE=cytopathic effects.
Table 3. Sequence identity (percentage) of BPXV with representative species of OPVs based on HA gene nt and deduced aa sequences

Further, analysis of the sequence of the BPXV homologue of the C18L gene of VACV revealed marked changes in relation to the corresponding VACV sequence (unpublished data). PCR amplicons corresponding to the VACV complete open reading frame (ORF) generated from four BPXV isolates revealed that the gene is disrupted in the latter, implying that BPXV lacks a functional gene homologue. Work is in progress in our laboratory to develop a diagnostic PCR based on this gene to differentiate BPXV from other members of OPV and particularly VACV, with which it is very closely related.
Epidemiology
After the first report of buffalopox in 1934, disease has been regularly reported from buffalo-rearing areas involving different states of India. The disease has mainly been recorded in young and old buffaloes during the epidemics. However, cattle have also been occasionally affected (Ghosh et al., Reference Ghosh, Arora, Sehgal, Ray and Wattal1977). Transmissibility of BPXV was experimentally studied (Singh et al., Reference Singh, Bhat, Mishra and Singh1996) in a number of animal species, which revealed a wide host range, viz. buffaloes, cows at high dose of virus, guinea pigs and suckling mice (BALB/c and Swiss white strains). Strain BPH-80 produced pox lesions in rabbits, 6-week-old mice, cow and buffalo calves, and chicken. Sheep, goat, fowl and adult mice (BALB/c and Swiss white) were refractory to experimental BPXV infection (Kumar et al., Reference Kumar, Yadav, Chandra and Garg1987).
An overall disease prevalence rate of 10.13% was reported in one study, in which there was no significant difference between prevalence in adult males (13.63%) and females (13%) and young males (6.32%) and females (5.24%) (Kumar et al., Reference Kumar, Yadav, Chandra and Garg1987). Mastitis due to secondary bacterial infections has been recorded in 50% of affected lactating buffaloes. A higher prevalence rate (23.4–79.4%) was reported in another buffalopox outbreak from Mandya and Mysore (Karnataka; Muraleedharan et al., Reference Muraleedharan, Raghavan, Murthy, Murthy, Swamy and Prasanna1989). A recent outbreak of buffalopox in Aurangabad (Maharashtra) caused a morbidity rate of up to 45% (Singh et al., Reference Singh, Hosamani, Balamurugan, Satheesh, Shingal, Tatwarti, Bambal, Ramteke and Yadav2006c). Although 5.6% seropositivity in buffaloes has been reported using CFT from various parts of Egypt (Iwad et al., Reference Iwad, Saber, Amin and Yousef1981), the spread of disease was rapid in buffaloes but cows, sheeps and goats in the same areas remained unaffected. Biting flies such as Lyperosia exigua, Musca crossirostris and Musca vicina aggravated the sores. Therefore, it was contended that flies may be involved in mechanical transmission (Muraleedharan et al., Reference Muraleedharan, Raghavan, Murthy, Murthy, Swamy and Prasanna1989).
Zoonosis
Buffalopox is an important contagious viral malady of buffaloes of all ages, occurring in epidemic proportions in countries including India, where buffaloes are reared. The Joint Expert Committee on Zoonosis declared buffalopox as one of the important zoonotic diseases. The committee also emphasized that the mode of transmission of buffalopox to human subjects and other epidemiological features appear similar to cowpox (Chandra et al., Reference Chandra, Garg, Rana and Rao1987).
Human beings including smallpox-vaccinees contract infection upon close contact with buffalopox virus-infected animals, but the extent of disease is greater in non-immunized individuals. The appearance of gross lesions in human beings maintaining close proximity with pox-affected buffaloes, attendants and milkers has been reported from time to time (Wariyar, Reference Wariyar1937; Ghosh et al., Reference Ghosh, Arora, Sehgal, Ray and Wattal1977; Mehrotra et al., Reference Mehrotra, Verma and Somvanshi1981; Mitra and Chatterji, Reference Mitra and Chatterji1986; Kumar et al., Reference Kumar, Yadav, Chandra and Garg1987; Kolhapure et al., Reference Kolhapure, Deolankar, Tupe, Raut, Basu, Dama, Pawar, Joshi, Padbidri, Goverdhan and Banerjee1997; Raut et al., Reference Raut, Tatwarti, Deolankar, Kolhapure and Tupe1997; Jain, Reference Jain1998; Singh et al., Reference Singh, Gupta, Randhawa and Sharma1999, Reference Singh, Hosamani, Balamurugan, Satheesh, Shingal, Tatwarti, Bambal, Ramteke and Yadav2006c). The lesions are mainly confined to the hands (Fig. 3), forehead, face, buttocks and legs. Occasionally, lymphadenopathy has also been recorded. Human-to-human transmission has not been reported. However, Kolhapure et al. (Reference Kolhapure, Deolankar, Tupe, Raut, Basu, Dama, Pawar, Joshi, Padbidri, Goverdhan and Banerjee1997), after detailed analysis of a 1996 buffalopox outbreak in Maharashtra, opined that (i) the detection of antibodies in 70% of in-contact persons and 17% of individuals who had no history of contact either with infected buffaloes or humans is a public health concern, (ii) the detection of antibodies in these humans could be an indication of sub-clinical infection which might lead to exposure of the general population to BPXV, and (iii) the virus, after repeated passage in humans, may acquire virulence as is evident from the comparison of BPXV strains of the Maharashtra outbreak and the reference strain (BP4) isolated 40 years ago. Milking of affected animals is one of the major modes of spread. Accordingly, 28% of milkers were affected, including children aged 8 and 11 years, in a Dhulia district outbreak in 1976. This led to a detailed survey of 30 families (n=240; milkmen: 56; morbid: 36) having buffalopox cases in six villages, in which buffalopox attacks had affected up to 69.7% of the milkmen. It was further noted that out of 39 persons who developed lesions, 23% had primary vaccination while 64.1% had both primary and booster vaccination (Ghosh et al., Reference Ghosh, Arora, Sehgal, Ray and Wattal1977). Until recently, BPXV was considered host-specific infecting only buffaloes but our experiments on biological transmission of BPXV (BP4 strain) indicated that BPXV could be transmitted to cow calves if given in a high dose (Singh et al., Reference Singh, Bhat, Mishra and Singh1996). Recently, we characterized virus isolates recovered from cows affected with pox-like disease by sequencing of HA and inclusion A-type inclusion (ATI) genes and found that the causative agent was BPXV and not CPXV (Yadav, Reference Yadav2006).

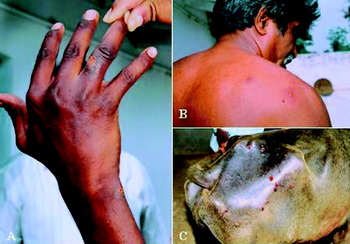
Fig. 3. Clinical cases of buffalopox in humans and buffaloes in an outbreak that occurred in the Nellore district of Andhra Pradesh, India during August 2006. Pox-like lesions are found on the fingers, fore arm (A) and scapular region (B) of the milker associated with an affected herd. In buffalo, lesions are mostly found on the teats and udder and some times on the sacral region (C).
Experimental pathogenesis
Rabbits
Rabbits inoculated with 0.3 ml of a 10% (w/v) BPXV-infected CAM suspension by the intravenous route developed cutaneous pock lesions in the skin on day 4 without any gross lesions in the internal organs such as lungs, spleen, mesenteric lymph nodes, heart, kidney or brain except for liver which showed numerous small necrotic spots. However, the presence of virus in most of these organs was demonstrated by inoculating the tissue suspensions in rabbit skin (Srinivasappa and Garg, Reference Srinivasappa and Garg1976). Intra-dermal (i.d.) inoculation of rabbits with BPXV indicated an eclipse period of 12 h. The virus was detectable in the skin at 15 h post-infection (hpi) and in the regional lymph nodes by 36 hpi followed by primary viremia at 48 hpi. Typical pock lesions were found on the skin, at the site of primary inoculation, after an incubation period of 48–72 hpi. The virus was first detected in lungs on day 4, and in liver and spleen on day 5 and release of virus from these organs led to secondary viremia, followed by spread of virus in kidneys, stomach, intestines and gonads between 7 and 14 dpi (Chandra et al., Reference Chandra, Singh and Garg1985).
Gross lesions on the internal organs included focal or diffuse necrotic areas on lungs, liver and spleen after day 5 pi. Individual lesions measuring approx. 2 mm in diameter appeared on the skin and in the stomach, intestine and uterus day 7 onwards. Histopathological changes included intra-alveolar and intra-bronchial hemorrhages, and degenerative fatty changes in the liver. During the recovery phase there were multinuclear syncytial cells. Virus particles could be visualized by EM in the skin, lungs, liver and spleen lesions (Chandra et al., Reference Chandra, Singh, Garg and Varshney1986).
Buffalo calves
Buffalo calves inoculated with BPXV by the i.d. route showed increased temperature and localized skin lesions at 12 dpi (Tantawi et al., Reference Tantawi, Khamis, Saber, Shalaby and Omar1976). However, buffaloes inoculated with CPXV and BPXV revealed that (i) BPXV produced larger lesions than CPXV and (ii) duration for buffalopox was shorter (Tantawi et al., Reference Tantawi, Fayed and El Sherif1979a, Reference Tantawi, Fayed, Shalaby and Skalinskyb). Both of these viruses as well as VACV inoculated in lactating buffaloes produced only localized lesions on the teats and udder without generalization. Complete protection between CPXV and BPXV was demonstrated upon challenge. The in-contact control buffalo did not show pock lesions although it had a rise in body temperature (Iwad et al., Reference Iwad, Saber, Amin and Yousef1981). Likewise, BP4 strain produced typical skin lesions, nasal discharge and diarrhea in buffalo calves upon i.d. inoculation (Rana et al., Reference Rana, Garg, Chandra, Singh and Chandra1985). Virus could be recovered from internal organs between 5 and 9 dpi and from the skin after 10 dpi and the disease lasted 13–15 days.
Clinical signs and lesions
The incubation period in animals is comparatively shorter (2–4 days) than in humans (3–19 days) (Ghosh et al., Reference Ghosh, Arora, Sehgal, Ray and Wattal1977). The disease may be localized or generalized with mild to severe disease associated invariably with mastitis in almost 50% of the affected animals. Experimental inoculation (i.d.) of female buffaloes with BPXV (Egyptian) led to local erythema and cutaneous eruptions throughout the body between 7 and 25 dpi with complete recuperation from the disease within a month. The skin lesions are more common on the udder, the base of the ear and other less hairy areas like the inguinal region. Otitis has also been reported due to secondary bacterial complications.
Molecular diagnosis
The conventional isolation methods of BPXV include either passaging in CAM or inoculation of specific hosts – buffalo or cattle. Although various serological assays have been developed for diagnosis of buffalopox including AGID, counter-immunoelectrophoresis (CIE), SNT, ELISA and immunoperoxidase test (IPT), these tests fail in accurate diagnosis of the disease because of antigenic cross-reactivity. Conventional biological and serological methods have limitations in identification and differentiation of OPVs. Analysis of virion polypeptides by SDS-PAGE has enabled genus and species differentiation but not between closely related members of the same genus (Arita and Tagaya, Reference Arita and Tagaya1977; Esposito et al., Reference Esposito, Obijeski and Nakano1977). However, RE–DNA profiling (Esposito and Knight, Reference Esposito and Knight1985) has been a proven method for poxvirus identification but requires virus propagation and viral genomic DNA isolation, which is time-consuming.
PCR-based assays have been described for diagnosis and differentiation of OPVs like VACV, Variola virus (VARV), monkeypox virus (MPXV) and CPXV, based on inclusion and HA genes (Knight et al., Reference Knight, Massung, Esposito, Becker and Darai1995; Ropp et al., Reference Ropp, Jin, Knight, Massung and Espositu1995; Meyer et al., Reference Meyer, Ropp and Esposito1997, Damaso et al., Reference Damaso, Espostio, Condit and Moussatche2000). PCR using the primers CoPV-3 and CoPV-4 has been used for diagnosis of OPVs including CPXV, camelpox virus (CMLV), MPXV, raccoonpox virus (RCNV), VACV (Funahashi et al., Reference Funahashi, Sato and Shida1988; Meyer and Rziha, Reference Meyer and Rziha1993) and BPXV (Singh et al., Reference Singh, Hosamani, Balamurugan, Satheesh, Rasool and Yadav2006b). Amplification of full-length A type inclusion gene using CoPV-1 and CoPV-2 (Funahashi et al., Reference Funahashi, Sato and Shida1988) primer set is useful in the differentiation of OPVs (Table 4). The primers amplify 2.8 and 3.7 kb fragments in CMLV and CPXV, respectively, and 3.2 kb (approx.) in BPXV. The inclusion gene is localized in two fragments of HindIII digest of BPXV genomic DNA (Kolte, Reference Kolte1998). Primer pair ATI-up and ATI-low, which amplify a fragment of the inclusion gene, is also used for differentiation of OPVs (Meyer et al., Reference Meyer, Ropp and Esposito1997). Size of the amplicons in BPXV is 1587 bp (unpublished data) unlike 1603 bp in VACV-WR strain, 1673 bp in CPXV-Brighton strain and 880 bp in CMLV CP-1 strain (Meyer et al., Reference Meyer, Ropp and Esposito1997).
Table 4. Oligonucleotide primers for diagnosis of BPXV

In our laboratory, we also characterized genomic DNA of BPXV and CMLV employing DNA amplification fingerprinting by RAPD-PCR for differentiation of BPXV from CMLV, using whole viral genomic DNA isolated from purified virus preparations. The DNA amplification fingerprint (DAF) profiles of BPXV and CMLV were clearly different. Differentiation of BPXV and CMLV as well as between various isolates of BPXV was also possible by DNA amplification fingerprinting employing PCR amplified full-length inclusion gene sequences (3.2 kb) of these viruses as template. The DAF profiles for BPXV as well as CMLV were quite different. Similarly, DAF profiles of closely related isolates of BPXV were also different. These studies were carried out using four random primers, one of which could amplify a fragment of >2.2 kb specifically in CMLV indicating that DAF can potentially lead to the development of virus-specific PCR. Further, sequencing of the above CMLV-specific fragment may be useful in designing primers and probes for specific diagnosis of the virus.
Control measures
In countries where the disease is endemic and animal movement is difficult to restrict, disease control becomes complex. The problem is compounded by lack of precise diagnostics and prophylactics. Further, the contamination of buffalo meat for export is another problem. The social belief, cultural issues and economic considerations prevent India from having a slaughtering policy for cattle. In the absence of suitable immunopropylaxis and restriction on animal movement, employing sanitary measures such as segregation of the infected animals is the only means of containing the spread of infection. In our laboratory, recently a live attenuated vaccine was prepared using Vero cell-adapted BPXV (VIJ96) and compared (unpublished data) with the already available reference strain (BP4) vaccine. We found that the BP4 vaccine was still superior in terms of potency compared with the Vero cell-adapted vaccine. Work is in progress towards the optimization of attenuation level and dosage of this newly developed vaccine.
Perspectives
BPXV infects primarily buffaloes but can also cross species barriers and infect humans (milkmen and researchers) handling either the infected animals or the virus itself as is evident from many outbreaks that have been recorded (Kolhapure et al., Reference Kolhapure, Deolankar, Tupe, Raut, Basu, Dama, Pawar, Joshi, Padbidri, Goverdhan and Banerjee1997; Raut et al., Reference Raut, Tatwarti, Deolankar, Kolhapure and Tupe1997; Singh et al., Reference Singh, Hosamani, Balamurugan, Satheesh, Shingal, Tatwarti, Bambal, Ramteke and Yadav2006c). Similarly, buffalopox zoonosis was reported from villages in Jalgaon, Dhule and Beed districts of Maharashtra state during 1992–1996 outbreaks. Neutralizing antibodies were detected not only in the sera of affected humans but also in in-contact individuals. It was opined that seropositivity in young individuals from the endemic area, who were neither vaccinated for smallpox nor had any contact with buffaloes or history of any poxvirus disease, was suggestive of occurrence of sub-clinical infection. A few children who had no contact with infected animals also showed clinical manifestations with disseminated lesions on the face, arm and buttocks, and thus were suspected to have acquired infection through their infected parents or other family members indicating a possibility of person-to-person transmission. Therefore, in the light of discontinuation of smallpox vaccination, control of buffalopox outbreaks would be crucial as this may emerge as a serious zoonotic disease in India. The situation is worsened by the fact that pox outbreaks in buffaloes have become very frequent in recent years, with outbreaks in Raigarh (Madhya Pradesh) in 1990, Namakkal (Tamil Nadu) in 1995, Beed, Nasik and Aurangabad (Maharashtra) during 1996–2003, and more recently in Bangalore (Karnataka) in 2004, and Nellore (Andhra Pradesh) during 2005–2006. It is worthwhile to consider that since humans and animals live in close association, these viruses may become pathogenic to man under certain conditions and establish disease in humans. Persisting human infection may sometimes be the origin of an outbreak in herds.
Further, the use of antibiotics in treatment of secondary infections in buffalopox-infected animals poses a public health hazard because of the possible presence of antibiotic residues in milk and meat. Contamination of buffalo meat with BPXV also has implications in international trade. In the absence of suitable diagnostic assays and prophylactic measures, effective strategies cannot be devised for the control of buffalopox virus infection in the countries where BPXV or VACV-like agents have been reported to be prevalent. This warrants a detailed systematic study on virus epidemiology, existence of reservoir host, biological transmission, and molecular organization of BPXV in comparison with other closely related viruses, which may pave the way for development of a suitable vaccine for control of the disease. Frequent epidemics of buffalopox in the Indian subcontinent and transmissibility of BPXV across buffaloes, humans and cows are important public health concerns in the wake of cessation of small pox vaccination around the world.
Acknowledgements
We thank the Director, Indian Veterinary Research Institute, for providing the facility to carry out BPXV research. The BPXV project work was supported by grants from the Department of Biotechnology, Government of India, New Delhi, India (BT/PR 3200/AAQ/01/131/2002).