Partial anomalous pulmonary venous connection (PAPVC) occurs when one to three of the pulmonary veins fail to open directly into the left atrium and instead drain into the right atrium or one of its venous tributaries. Its incidence has been reported in some autopsy series to be about 0.7%.Reference Alsoufi, Cai and Van Arsdell1 The most common form of PAPVC is the superior caval type (87–97% of cases), where the PAPVCs drain into the superior caval vein and in most cases (92%), a sinus venosus atrial septal defect coexists.Reference Sojak, Sagat and Balazova2 Different patch techniques (single or double) have been described to repair such defect with the concept of closing the atrial septal defect and redirecting the anomalous pulmonary venous drainage into the left atrium. However, in case of high drainage of the PAPVC (above the cavoatrial junction), these techniques may be complicated by stenosis of the superior caval vein or the redirected pulmonary veins as well as sinus node dysfunction.Reference Said, Burkhart and Schaff3,Reference Kottayil, Dharan and Menon4 Therefore, we prefer to perform the Warden procedure at our institute in such cases. In this retrospective study, we share our experience over a period of 10 years with the Warden procedure in 65 cases showing drainage of the PAPVC above the cavoatrial junction.
Patients and methods
In this study, we obtained the approval of our ethical committee to retrospectively collect and analyse the data of patients receiving the Warden procedure for repair of PAPVC (above the cavoatrial junction) over a period of 10 years starting from January, 2010 to January, 2020. During this period, a total of 173 cases of PAPVCs were repaired of which 65 patients received the Warden procedure. The remaining 108 cases had the PAPVCs below the cavoatrial junction and were repaired with the single-patch technique. Due to its retrospective nature, the need for patient consent for inclusion in the study was waived. The diagnosis of PAPVC was based on history taking (e.g., recurrent respiratory tract infections and exertional dyspnea), clinical examination (e.g., wide fixed splitting of second heart sound and ejection systolic murmur over the pulmonary area), investigations in the form of electrocardiogram and chest X-ray (e.g., showing evidence of right-sided cardiac enlargement and features of increased pulmonary blood flow), and transthoracic echocardiography. Multi-slice computed tomography angiography on the heart and major vessels was done if any suspicion regarding the anatomy exists. Demographic, clinical, and surgical data were collected from medical charts. Anatomical details of the defects were retrieved from echocardiographic reports as well as multi-slice computed tomography images and included the following: the number of the anomalous pulmonary veins, distance between the proximal pulmonary venous opening in the superior caval vein and the cavoatrial junction, site and size of the sinus venosus atrial septal defect, degree of tricuspid regurgitation, biventricular function, presence of left superior caval vein, and other associated congenital anomalies. Post-operative data were retrieved from hospital records including the need for cardiac pacing; duration of mechanical ventilation, intensive care unit, and hospital stays; adverse events; and in-hospital mortality. Intra-operative transesophageal echocardiography was done for patients to rule out superior caval vein or pulmonary venous obstruction and residual shunts. Pulmonary venous obstruction was defined if the pulsed wave Doppler gradient across any pulmonary vein was >2 mmHg. The presence of stenosis at the Warden anastomosis was defined by Doppler gradients across the cavoatrial junction of >5 mmHg or loss of biphasic pattern in the superior caval vein.Reference Aggarwal, Gadhinglajkar and Sreedhar5,Reference Tacy, Wong and MillerHance6 Patients were followed up in the outpatient clinic through clinical examination, transthoracic echocardiography, and Holter monitoring (when needed) at 3 and 6 months post-operatively and then at regular intervals of 1 year. Post-operative sinus node dysfunction was defined as persistent sinus bradycardia (<50 beats/minutes), pauses of more than 3 seconds, or persistent nodal rhythm > 1 week, especially with the need for permanent pacemaker. Early mortality was defined as the death occurring within the primary hospitalization (in-hospital mortality) and up to 30 days after discharge, while late mortality was defined as any death occurring 30 days after discharge from the hospital following the original repair.
Surgical technique
After full median sternotomy, the pericardium was opened longitudinally with in situ preparation of a pericardial patch. The superior caval vein was adequately mobilised along its whole length with division of the azygos vein. Heparin was then administered and cardiopulmonary bypass was established via aorto-bicaval cannulation inserting a right-angled metal-tip cannula very high at the insertion of the innominate vein into the superior caval vein. A persistent left superior caval vein was snared when found to be small with a large bridging vein and was cannulated with a separate right-angled metal-tip cannula when found to be large in size with a small or absent bridging vein. Myocardial protection was achieved through systemic cooling to 30 C and antegrade cold blood cardioplegia through the aortic root. The superior caval vein was then divided above the uppermost insertion of the anomalous pulmonary veins and its caudal end was oversewn or patched with untreated autologous pericardium. Early in this study, we used to augment the superior caval stump with an untreated autologous pericardial patch to avoid any pulmonary vein obstruction, but later on, we skipped this step because we found no significant benefit on the post-operative gradients across the pulmonary veins. After high right atriotomy, the native untreated pericardial patch was now used to close the sinus venosus atrial septal defect rerouting the pulmonary veins into the left atrium. If the inter-atrial septum was found intact, an atrial septal defect was created by excising the fossa ovalis and incising the limbus superiorly until an atrial septal defect of an adequate size was achieved. If the sinus venosus atrial septal defect was found small, it was enlarged to an adequate size to avoid any baffle obstruction. Finally, the right atrial appendage was amputated and anastomosed to the cranial end of the superior caval vein via continuous polypropylene 0/5 or 0/6 suture with intermittent locking, after cutting off any trabeculae to avoid superior caval obstruction. In five cases (7.7%), a pericardial patch was used to augment the anterior surface of the superior caval vein-to-right atrium anastomosis to avoid excessive tension at the cavoatrial junction. In cases associated with the bilateral PAPVCs, the left PAPVC was simply divided at its distal end and re-implanted into the left atrial appendage.
Statistical analysis
Collected data were coded to facilitate their manipulation and entered into Microsoft Access, where data analysis was performed using Statistical Package of Social Science software version 22 in windows 7. The descriptive analysis for qualitative data was carried out in the form of numbers and percentages and for quantitative data in the form of arithmetic means as a measure of central tendency and standard deviations as a measure of dispersion. Survival was shown as Kaplan–Maier curve.
Results
Pre-operative data
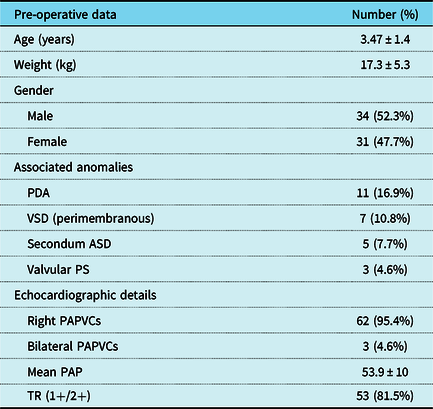
In total, 65 cases receiving the Warden procedure for the repair of PAPVCs were included in this study of which 34 were males (52.3%) and 31 were females (47.7%). The mean age of the study group was 3.47 ± 1.4 years (range 6 months to 7.5 years), while the mean weight was 17.3 ± 5.3 kg (range 4–30 kg). Pre-operative echocardiographic assessment revealed right-sided PAPVCs in 95.4%, while 4.6% of cases showed bilateral PAPVCs. In total, 28 cases had 1 PAPVC (43%), 32 cases had 2 connections (49%), and 5 cases had 3 connections (8%). A persistent left superior caval vein was detected in 10 cases (15.4%). In total, 60 cases had sinus venosus atrial septal defect, while the remaining five cases (7.7%) had intact inter-atrial septum and an atrial septal defect was created. Multi-slice computed tomography angiography was done in 38 cases (58%). Associated anomalies were found in 26 cases (40%). As regards pre-operative intervention, only one case had the history of previous repair of sinus venosus atrial septal defect 1 year earlier using single-patch technique with residual symptomatic (face puffiness) stenosis of the superior caval vein as confirmed by echocardiography and computed tomography angiography. Table 1 summarises pre-operative patient characteristics.
Table 1. Pre-operative patient characteristics
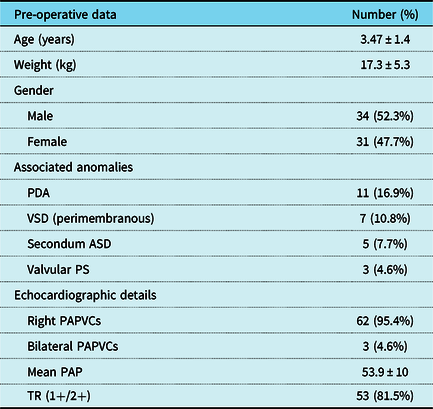
*ASD = atrial septal defect; PAP = pulmonary artery pressure; PAPVCs = partial anomalous pulmonary venous connections; PDA = patent ductus arteriosus; Ps = pulmonary stenosis; SD = standard deviation; TR = tricuspid regurgitation; VSD = ventricular septal defect
Operative data
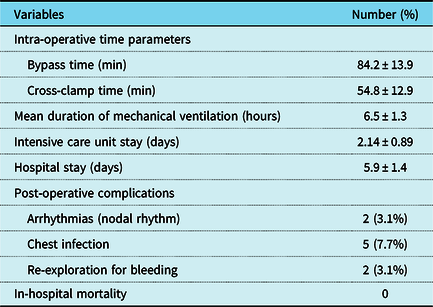
Intra-operative transesophageal echocardiography confirmed the presence of the PAPVC in all patients. The mean bypass time was 84.2 ± 13.9 minutes (range 55–120 minutes), while the mean cross clamp time was 54.8 ± 12.9 minutes (range 20–90 minutes). In the 11 cases associated with patent ductus arteriosus, the ductus was doubly ligated through the sternotomy incision before going onto cardiopulmonary bypass. In the seven cases associated with perimembranous ventricular septal defect, the defect was closed through the right atrium by a polytetrafluoroethylene patch, while in the five cases associated with secundom atrial septal defect, the defect was closed with a patch of autologous untreated pericardium. The cases associated with valvular pulmonary stenosis were managed with valvotomy until a suitable Hegar dilator could be passed through the pulmonary valve.
In total, 18 cases (27.5%) received patch augmentation of the superior caval stump to avoid any pulmonary vein stenosis early in the study. However, the remaining 47 cases (72.5%) were repaired without this step, since post-operative transthoracic echocardiography showed no significant difference in the gradients across the PAPVCs in both groups (1.50 ± 0.31 mmHg and 1.62 ± 0.20 mmHg, respectively, with a p-value of 0.07).
Post-operative data
The mean intensive care unit stay was 2.14 ± 0.89 days (range 1–5 days), while the mean hospital stay was 5.9 ± 1.4 days (range 4–11 days). Regarding post-operative complications, two cases (3.1%) developed nodal rhythm immediately after surgery which returned spontaneously to sinus rhythm on the 1st and 2nd post-operative days, respectively. In addition, five cases (7.7%) developed chest infection post-operatively and were managed with antibiotics and non-invasive ventilatory support. Also, two cases were re-explored at the night of surgery due to excessive bleeding from the thymic bed and sternal wires, respectively. Fortunately, with careful dissection, we did not have any case of phrenic nerve injury in our series. There was no in-hospital mortality and no case showed evidence of superior caval vein or pulmonary vein obstructions and residual shunts in the transthoracic echocardiography done before discharge. Table 2 summarises operative and early post-operative results.
Table 2. Operative and post-operative results

Follow up
Patients could be followed up for a mean period of 7.8 ± 1.2 years after discharge. Follow up was complete in 62 patients (95%). In total, three patients skipped the follow up due to the loss of updated contact data. Only one case of late mortality (1.6%) in all patients followed up was detected 3 years after the Warden procedure and was related to accidental poisoning (95% confidence interval, 8.071–9.096) (Fig 1).

Figure 1. Kaplan–Meier curve showing survival among group.
No stenosis was detected in the Warden anastomosis with a Doppler gradient across the cavoatrial junction <5 mmHg in all patients. In addition, no patient developed pulmonary venous obstruction during the follow-up period.
Discussion
In general, PAPVC shows a spectrum of variants according to the site of drainage to the superior caval vein. The higher the opening of the PAPVCs above the cavoatrial junction, the longer the patch required to baffle the pulmonary veins across the sinus venosus atrial septal defect into the left atrium. This may result in superior caval obstruction, especially if small, in single-patch technique. On the other hand, augmenting the cavoatrial junction with a second patch in double-patch technique can injure the sinoatrial node or its artery resulting in sinus node dysfunction.Reference Stewart, Bailliard and Kelle7 The Warden technique avoids any cavoatrial incision and therefore carries the lowest risk of sinus node dysfunction. In addition, the Warden procedure limits the incidence of superior caval obstruction by the baffle created to shunt blood from the PAPVCs to the left atrium with a good growth potential.Reference Okontaa and Agarwal8
In our series, we report no persistent sinus node dysfunction or the need for permanent pacemaker. This is consistent with some studies reporting significantly lower incidence of sinus node dysfunction or sick sinus syndrome after the Warden procedure than after the double-patch technique.Reference Tao, Pan and Lin9 On the other hand, we had no cases of early or late superior caval obstruction in our study. This result has been also reported by Shahriari et al in 54 patients.Reference Shahriari, Rodefeld and Turrentine10 However, the key point in the avoidance of superior caval obstruction in the Warden procedure is the establishment of an adequate tension-free anastomosis between the divided superior caval vein and the amputated right atrial appendage.Reference Chandra, Gupta, Nath, Kazmi, Grover and Gupta11 This can be achieved by extensive mobilisation of the superior caval vein both caudally and cranially, wide opening of the right atrial appendage, excision of any trabeculae at the appendage, and enlargement of the anterior surface of the superior caval vein-to-right atrium anastomosis in some cases by a patch when any doubt about anastomotic tension still exists.
Post-operative stenosis of the baffled pulmonary veins is also an issue that must be taken care of. The sinus venosus atrial septal defect should be enlarged if found to be small and restrictive at the time of repair in order to avoid such complication.Reference Zhu, Kotani and Chetan12 Some surgeons recommend, in addition, routine patch closure of the superior caval stump.Reference Said, Burkhart and Dearani13 Nevertheless, we found, by time, that there is no statistically significant difference in the post-operative gradients across the pulmonary veins with or without patch augmentation of the superior caval stump and therefore omitted this step to save more time for other steps. Finally, only one case of late mortality occurred in our series due to a non-cardiac cause which represents an excellent outcome of the Warden procedure. Similarly, other studies reported mortality rates after the Warden procedure of 0–3%.Reference DiBardino, McKenzie and Heinle14,Reference Park, Kwak and Lee15
Few studies represented long-term outcomes of the Warden procedure with an adequate sample size specifically in the paediatric age group.Reference Alsoufi, Cai and Van Arsdell1,Reference Yong, Griffiths and Robertson16 Therefore, we tried, in this study, to represent a reasonable number of children with the same pathology receiving the same procedure over a long follow-up period in order to emphasise, beside other studies, the reproducibility of the Warden procedure. However, our study still carries the limitation of the retrospective observational nature and single-center experience.
Conclusion
The Warden procedure is a safe approach for the repair of PAPVCs draining above the cavoatrial junction with good long-term results and low incidence of complications like sinus node dysfunction and pulmonary vein and superior caval vein obstruction.
Acknowledgements
None.
Financial Support
None.
Conflicts of Interest
None.
Ethical Standards
Not applicable.