Introduction
The oceans are the largest ecosystems on Earth (Klimpel et al., Reference Klimpel, Palm, Busch, Kellermanns and Rückert2006). More than two-thirds of the world's surface is covered by seawater, with an average depth of 3800 m. Although deep-sea ecosystems represent the largest biome of the global biosphere, knowledge regarding their biodiversity is still scant (Danovaro et al., Reference Danovaro, Company, Corinaldesi, Onghia, Galil, Gambi, Gooday, Lampadariou, Luna, Morigi, Olu, Polymenakou, Ramirez-llodra, Sabbatini and Sarda2010), and this is particularly true for parasites being hosted by deep-sea fish. According to Bray et al. (Reference Bray, Littlewood, Herniou and Williams1999), the pioneer study regarding the parasites of deep-sea fish was that of Moseley in 1880. Later, Manter (Reference Manter1934) described some digeneans from deep-sea fish from the Tortugas Islands. Rohde (Reference Rohde1988) presented a comparative analysis of the monogeneans from deep-sea teleost and surface-water fish from south-eastern Australia, suggesting that the diversity of monogeneans is higher in surface-water marine fish. Later on, Klimpel et al. (Reference Klimpel, Busch, Kellermanns, Kleinertz and Palm2009) presented a comprehensive list of metazoan parasites of deep-sea fish (>200 m depth); of the listed species (more than 900), only 10% were monogeneans, making them uncommon parasites in contrast with trophically transmitted parasites, such as Digenea and Nematoda (Campbell et al., Reference Campbell, Haedrich and Munroe1980). However, Ñacari & Oliva (Reference Ñacari and Oliva2016) recently analysed the parasite fauna of deep-sea fish from northern Chile (Atacama Trench) and found 44 parasite species in a sample of five teleost species; 9 of them (20.4%) were monogeneans. Clearly, conclusions about the biodiversity of parasites of deep-sea fish must be treated with caution because only a small proportion of deep-sea fish have been studied for parasites (see Klimpel et al., Reference Klimpel, Busch, Kellermanns, Kleinertz and Palm2009; Ñacari & Oliva, Reference Ñacari and Oliva2016). Among the monogeneans, Acanthocotyle Monticelli, 1888, a monogenean parasite of the skin of skates and stingrays, includes nine recognized species (Kearn et al., Reference Kearn, Whittington, Chisholm and Evans-Gowing2016), and just one (A. pacifica) has been found in a deep-sea skate from the northern Pacific (Puget Sound). The valid species of the genus include Acanthocotyle lobianchi Monticelli, 1888; A. elegans Monticelli, 1890; A. greeni Macdonald & Llewellyn, 1980; A. pacifica Bonham & Guberlet, 1938; A. patagonica Kuznetsova, 1971; A. pugetensis Bonham & Guberlet, 1938; A. urolophi Kearn et al., 2016; A. verrilli Goto, 1899; and A. williamsi Price, 1938, which occur in the North Atlantic and North Pacific as well as the South Pacific (Tasmania).
Here, we describe a new species of Acanthocotyle from the skin of Gurgesiella furvescens deBuen, 1959 (Rajidae) caught off Valparaiso, Chile. A molecular comparison with A. urolophi, the only member of the genus with genetic data (large subunit (LSU) rDNA), was also performed. This is the first described monogenean in deep-sea skates from South America.
Materials and methods
Collection and examination of samples
Two specimens of the dusky finless skate, G. furvescens (Rajidae), were obtained as a by-catch of the deep-sea shrimp (Heterocarpus reedi) and the yellow squat lobster (Cervimunida johni) fisheries in the vicinity of Valparaiso (33°S, 72°W), central Chile, using a midwater trawl, at 350–450 m depth. The fish were immediately frozen (−18°C) on-board and transported to the laboratory for parasitological analyses. After thawing, the skates were dissected and examined for metazoan ectoparasites. A total of 12 specimens of the new species were obtained from the skin of the host, ten of which were used for morphological studies and were fixed in AFA (alcohol:formalin:acetic acid), preserved in 70% alcohol and subsequently stained (Gomori's trichrome). Figures were made with a drawing tube. Measurements are in millimetres (mean plus range in parentheses) unless otherwise indicated. The remaining two specimens were preserved in 95% ethanol for molecular studies.
Phylogenetic analysis
Preserved parasites were placed individually into 1.5-ml Eppendorf tubes for DNA extraction. The DNA of each individual was isolated following a modified protocol based on Miller et al. (Reference Miller, Dykes and Polesky1988) involving treatment with sodium dodecyl sulphate, digestion with proteinase K, NaCl protein precipitation and subsequent ethanol precipitation. For molecular analyses, the nuclear LSU rDNA and the mitochondrial (mt) gene cytochrome c oxidase 1 (COI) were used. LSU rDNA was amplified by polymerase chain reaction (PCR) with the forward primer C1 (5′-ACCCGCTGAATTTAAGCAT-3′) and the reverse primer D2 (5′-TGGTCCGTGTTTCAAGAC-3′) (Chisholm et al., Reference Chisholm, Morgan, Adlard and Whittington2001); COI mtDNA was amplified using the forward primer L-CO1 (5′-TTTTTTGGGCATCCTGAGGTTTAT-3′) and the reverse primer H-COX1 (5′-TAAAGAAAGAACATAATGAAAATG-3′) (Littlewood et al., Reference Littlewood, Rohde and Clough1997).
Each PCR reaction had a final volume of 35 μl, including 5 standard units of GoTaq DNA polymerase (Promega, Madison, Wisconsin, USA), 7 μl 5× PCR buffer, 5.6 μl MgCl2 (25 mm), 2.1 μl bovine serum albumin (BSA; 10 mg/ml), 0.7 μl of deoxynucleotide triphosphate (dNTP) (10 mm), 10 pm of each primer and 7 μl template DNA. A BoecoEcogermany M-240R Thermal Cycler (Boeckel, Hamburg, Germany) was used with a cycling profile as follows: 30 temperature cycles programmed at a slow temperature ramp rate. Cycle 1 was 95°C for 3 min, 45°C for 2 min and 72°C for 90 s. This was followed by four cycles of 95°C for 45 s, 50°C for 45 s and 72°C for 90 s, then a further 25 cycles at 95°C for 20 s, 52°C for 20 s and 72°C for 90 s. The mix was held at 72°C for 5 min to complete extension and then the temperature was dropped to 4°C. For the COI PCR, there was an initial denaturation step at 95°C (5 min), followed by 35 cycles of 95°C (1 min), 48°C (2 min) and 72°C (21 min), with a final extension step at 72°C (10 min). The PCR products were directly sequenced (Macrogen, Seoul, Korea; http://www.macrogen.com).
Sequences were edited and assembled using ProSeq v2.9 (Filatov, Reference Filatov2002). The fragments obtained from the LSU rDNA gene were aligned using the Clustal 2 software package (Larkin et al., Reference Larkin, Blackshields, Brown, Chenna, Mcgettigan, Mcwilliam, Valentin, Wallace, Wilm, Lopez, Thompson, Gibson and Higgins2007) with sequences of related monogeneans obtained from GenBank (table 1). All new DNA sequences were deposited in GenBank, and accession numbers are given in table 1.
Table 1. GenBank accession numbers for the sequences (LSU rDNA) of eight monogenean species, including Acanthocotyle gurgesiella n. sp.
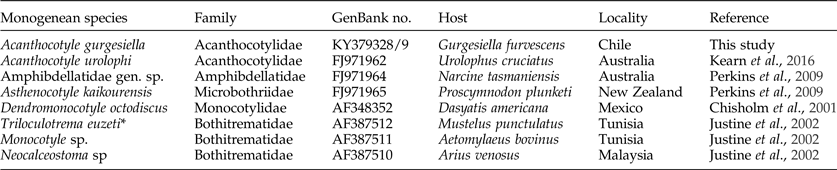
*The sequence AF387512 was uploaded as Triloculotrema sp., but not included in the article by Justine et al. (Reference Justine, Jovelin, Neifar, Mollaret, Lim, Hendrix and Euzet2002). Boudaya & Neifar (Reference Boudaya and Neifar2016) indicated that this sequence corresponds to T. euzeti.
The sequences were compared using maximum likelihood (ML) in the Mega v6 software package (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013). The analyses were performed using the GTR + I evolution model chosen according to the Akaike information criterion (AIC) as implemented in Mega v6. To determine the nodal support, a 1000-bootstrap analysis was implemented. In addition, to compare the genetic distances among specimens, we calculated the pairwise p-distances in the same software. The sequence of the monogenean Neocalceostoma sp. (AF387516) was used as the outgroup.
Results
Acanthocotyle gurgesiella n. sp.
Acanthocotylidae Monticelli, 1903; Acanthocotyle Monticelli, 1888.
Description
Based on ten adult whole mounts (ten stained). Total body length, including pseudohaptor 4.19 (3.07–5.72) and nearly parallel-sided, maximum body width 0.94 (0.63–1.18). Three anterior adhesive lobes on each side of head with a single aperture (fig. 1A). At the level of the posterior end of the pharynx, there is a more or less pronounced constriction or neck marking off the head region from the body proper. Pseudohaptor nearly circular 0.89 (0.60–1.10) × 0.91 (0.64–1.25), with 36–40 rows of hooks with sharp recurved tips (average 38, mode 40) (fig. 1C). The rows are easily counted peripherally, but towards the centre of the pseudohaptor it is difficult to determine to which row a spine belongs. The total number of hooks ranged from 201 to 331. True haptor with 16 marginal hooks (14 peripheral and 2 central) located near the anterior border of the pseudohaptor (fig. 1D). Pharynx globular 0.24 (0.11–0.33) × 0.25 (0.15–0.36). Intestinal caeca without diverticula. Two excretory bladders anterior to vitellaria field on each side of the body. Sense organs conspicuous at or near anterior margin of head. Eyes absent. Male genital aperture slightly to right of mid-ventral line just behind level of intestinal bifurcation and armed with one spear-like spine (fig. 1B). Cirrus pouch relatively large, curved, containing an internal seminal vesicle and short cirrus; vas deferens enlarged and constricted, two external seminal vesicles; paired prostatic vesicles present, one on each side of cirrus pouch, extending from level of middle of anterior seminal vesicle to genital aperture (fig. 1B). Testes 35 (28–43), posterior to ovary, arranged in 2–3 rows. Ovary globular, 0. 241 (0.190–0. 284) in length by 0.237 (0.206–0.283) in width. Vitellaria extracaecal, consisting of numerous elongate and discrete follicles, extending from level of ovary to near posterior end of body proper. Seminal receptacle 0.059 (0.054–0.062) in diameter, postero-dorsal to ovary, vagina absent. Ootype not observed. Uterine pore dextral and dorsal, at level of anterior part of pharynx. Eggs not observed.

Fig. 1. The morphology of Acanthocotyle gurgesiella to show: (A) the holotype, ventral view, (B) genitalia, (C) pseudohaptor and (D) the larval haptor. aa, Anterior adhesive lobes; ph, pharynx; up, uterine pore; ut, uterus; bl, excretory bladder; sr, seminal receptacle; vd, vas deferens; vi, vitelline follicles; lh, larval haptor; ps, pseudohaptor; te, testes; in, intestine; ov, ovary; id, vitelline duct; sv, bipartite seminal vesicle; ed, evaculatory duct; ag, accessory glands; od, oviduct; ss, spear-like spine; rg, male accessory gland reservoir.
Taxonomic summary
Type host
Gurgesiella furvescens (Rajidae) De Buen, 1959.
Type locality
Waters off Valparaiso, Chile (33°S, 72°W).
Specimens deposited
Holotype USNM 1422088 (United States National Museum). Paratypes USNM 1422089–1422090 (two stained and mounted specimens), MZUC-UCCC 45095–45096 (Museo de Zoología, Universidad de Concepción) (two stained and mounted specimens), MHN-UNMSM 3348 (Museo de Historia Natural, Universidad Nacional Mayor de San Marcos) (two stained and mounted specimens).
Etymology
The specific name refers to the generic name of the host.
Remarks
The presence of a large and discoidal pseudohaptor bearing numerous radial rows of sclerites with sharp recurved tips, a true but small haptor and the lateral opening of the uterine pore allows us to include the new species in the genus Acanthocotyle. Among the known species of the genus, A. gurgesiella can be identified by a combination of morphological characteristics, including the number of testes, number of radial rows of sclerites in the pseudohaptor, aperture of the genital pore and shape of the vitelline follicles.
The new species shows a genetic divergence of 3.2–3.7% (LSU rDNA gene) with A. urolophi, the only species for which molecular data are available.
Phylogenetic analyses
For the LSU rDNA region, two sequences of 890 bp were obtained. Intraspecific genetic variability was 0.3% (three polymorphic sites of 890 bp). Sequences were aligned and cut down to 410 bp, the size of the smaller sequence available at GenBank. Genetic distance between the new species and A. urolophi was 3.2–3.7%. Two sequences (424 bp) (GenBank accession numbers KY379330, KY379331) of the COI gene were obtained. Intraspecific variability was 0.5% (two polymorphic sites).
Discussion
To date, nine species of Acanthocotyle are recognized. Kearn et al. (Reference Kearn, Whittington, Chisholm and Evans-Gowing2016) recently revised the genus, including a key to identify the known species. As stated by Kearn et al. (Reference Kearn, Whittington, Chisholm and Evans-Gowing2016), this genus was described by Monticelli in 1888 to accommodate the new species A. lobianchi, a parasite of the ray Raja clavata from Naples (Florida, USA). Since then, 14 nominal species have been described, and three of them have been considered nomen nudum (A. brachyuropsi Kuznetsova, 1971 and A. scobini Kuznetsova, 1971) or taxon inquirenda (A. monticellii Scott, 1902). Acanthocotyle borealis Brinkmann, 1940 was synonymized with A. verrilli Goto,1899 and A. merluccii (Van Beneden & Hesse, 1863) was transferred to the genus Anthocotyle. All the described species in the genus are parasites of skates of the Rajiformes families Rajidae (genus Raja) and Arhynchobatidae (genus Bathyraja), except A. urolophi, a parasite of Urolophus cruciatus (Myliobatiformes). Seven of the nine valid species of Acanthocotyle are parasites of the skin of skates of the genus Raja. The host of the new species, the skate G. furvescens, is a member of the Rajidae, suggesting host specificity for Acanthocotylidae.
As noted by Kearn et al. (Reference Kearn, Whittington, Chisholm and Evans-Gowing2016), the composition and classification of Acanthocotylidae Price, 1936 is not clear, mainly due to the lack of clear and unequivocal taxonomic characters. Furthermore, there are some reported (named) species that have never been described formally or are incompletely described, as noted by Dawes (Reference Dawes1946). Despite the early comment by Dawes (Reference Dawes1946), three species were described by Kuznetsova (Reference Kuznetsova1971) in short sentences and with no illustrations. Accordingly, 10 of the 14 nominal species have been recognized as valid according to Kearn et al. (Reference Kearn, Whittington, Chisholm and Evans-Gowing2016), who suggest that the best morphological and/or meristic characters to discriminate species in Acanthocotyle include testes number, the number of radial rows of pseudohaptor sclerites, the location of the opening of the uterine pore (left or right side of the body) and the shape of vitelline follicles (discrete or not). All the recognized species except A. pugetensis have more than ten testes. With regards to the number of radial rows of pseudohaptor sclerites, only three species (A. patagonica, A. urolophi and A. verrilli) show 40 or fewer rows as in the species described here. The uterine pore opening is on the right side in A. urolophi, A. verrilli and the new species. Vitelline follicles are discrete in A. urolophi but diffuse in A. verrilli and the new species. The male genital aperture is not armed with one spear-like spine in A. verrilli but is armed in the new species.
Molecular analysis supported our morphological studies in differentiating A. gurgesiella from A. urolophi, the only representative of the genus for which genetic information (LSU rDNA) is available.
Acknowledgement
Thanks to Dr J.T. Timi (Universidad Nacional de Mar del Plata, Argentina) for providing translations of Kuznesova (Reference Kuznetsova1971).
Financial support
Partially financed by FONDECYT 1140173 (to M.E.O.). The Millennium Institute of Oceanography (IMO), IC120019 also provided support.
Conflict of interest
None.




