INTRODUCTION
Rhagodia preissii (Family Chenopodiaceae) is a shrub species currently being assessed for its ability to contribute to grazing systems in Western Australia. A range of species of Rhagodia are distributed throughout Western Australia but R. preissii is the first to be commercially grown and adopted by growers, who are seeking forage plants that are more tolerant to drought than the pasture species they currently use. R. preissii performs well in nutrient-poor soils, is drought tolerant and produces substantial dry matter of reasonable nutritive value with up to 23% protein and an estimated digestibility of 60–65% (H. Norman, personal communication). Of particular interest, however, is its potential activity against sheep gastrointestinal parasites. A recent study found that extracts from this species contained significant anthelmintic activity in in vitro larval development assays with Haemonchus contortus (Kotze et al. Reference Kotze, O'Grady, Emms, Toovey, Hughes, Jessop, Bennell, Vercoe and Revell2009). Extracts from this species were also lethal (as indicated by a complete cessation of movement) towards adult H. contortus worms in in vitro assays. With the increasing impact of drench resistance worldwide (Kaplan, Reference Kaplan2004), including Australia (Besier and Love, Reference Besier and Love2003), forage plants with anti-parasitic properties may offer a practical option in an integrated management plan to provide a degree of control of gastrointestinal parasites (Athanasiadou and Kyriazakis, Reference Athanasiadou and Kyriazakis2004; Jackson and Miller, Reference Jackson and Miller2006; Athanasiadou et al. Reference Athanasiadou, Githiori and Kyriazakis2007).
The aim of this study was to determine whether the anthelmintic effects observed in vitro could be replicated in vivo by grazing sheep infected with Trichostrongylus colubriformis on R. preissii in a field situation. We compared faecal egg counts in sheep that grazed R. preissii with sheep that grazed conventional annual pasture species. In addition we examined the grazing behaviour of sheep offered R. preissii, and took samples from plants readily eaten by the animals as well as those which remained uneaten. This allowed for examination of the impact of some plant secondary compounds (oxalates, saponins, tannins) on both grazing behaviour and anthelmintic properties. These compounds were examined due to their known anthelmintic effects (saponins, tannins) and known anti-nutritional effects (all 3 groups) (Hungerford, Reference Hungerford1990; Athanasiadou and Kyriazakis, Reference Athanasiadou and Kyriazakis2004), as well as reports of anthelmintic tannins in other Rhagodia species (Kotze et al. Reference Kotze, O'Grady, Emms, Toovey, Hughes, Jessop, Bennell, Vercoe and Revell2009), and a previous observation of frothiness in R. preissii extracts (Kotze, unpublished data) suggesting the presence of saponins.
MATERIALS AND METHODS
Experimental design
The experiment was conducted at the Badgingarra Research Station, Department of Agriculture and Food Western Australia (approximately 200 km north of Perth; 30º19′S, 115º32′E) from July to September 2008. Stands of R. preissii shrubs were planted in September 2006. The plants were derived from seed collections from wild populations over a wide geographical range (I. Pullbrook, personal communication). The sheep grazing experiment was approved by the Animal Ethics Committees of CSIRO Floreat (project 0712) and the Department of Agriculture and Food WA (approval number 5-07-44). Forty 2-year-old merino wethers (mean live weight 52·35±0·04 kg) were selected from a larger flock of sheep that had previously had access to R. preissii at the same site. All sheep were drenched orally with 13 ml of Q-drench (Abamectin 1 g/L, Albendazole 25 g/L, Closantel 37·5 g/L. Levamisole 40 g/L; Jurox Pty Ltd, AUS; 1 ml/5 kg live weight) and placed in a clean paddock (pasture species described below) for 2 weeks prior to the experiment to remove any existing parasite burdens.
On day 1 of the experiment, sheep were stratified by live weight and randomly allocated into 4 groups of 10 sheep. Two of the groups, referred to subsequently as ‘shrub’ groups, were allocated to plots of R. preissii shrubs with inter-row pasture consisting of annual species. The other 2 groups, referred to subsequently as ‘pasture’ groups, had access to only annual pasture. One of the shrub groups received a single oral dose of 30 000 infective T. colubriformis larvae on day 4. Similarly, 1 group of 10 sheep that grazed only annual pasture also received this dose of larvae on day 4. The 4 groups are thus defined as: ‘shrub-control’, ‘shrub+worms’, ‘pasture-control’, and ‘pasture+worms’.
Sheep were rotationally grazed for 7 weeks, with a new plot every week. Weekly live weights and body condition scores were used to monitor animal performance throughout the experiment. This was done at the same time of day each week to minimize variation in live weight due to gut fill. Individual faecal samples were collected per rectum for faecal egg counts (FEC) on day 0 to ensure all sheep were parasite free, and then weekly from week 3 to week 7 to assess the parasite burden of the 4 groups. Sheep were removed from their plots at the end of week 7 and were drenched with 12 ml of Cydectin SE (Moxidectin 1 g/L + sodium selenate; Fort Dodge Pty Ltd, AUS; 1 ml/5 kg live weight). From week 2 onwards the ‘pasture’ groups were fed 100 g of lupins/head/day, and the ‘shrub’ groups 200 g/head/d, fed twice weekly, to correct for a decline in live weight caused by a limited supply of pasture in the plots.
Dry matter intake was estimated by subtracting the biomass (pasture or R. preissii) remaining at the end of each grazing period (weekly) from the biomass on offer at the start, corrected for the growth rate of the pasture or R. preissii shrubs.
Parasites and faecal egg counts
T. colubriformis infective-stage larvae were sourced from Dr Malcolm Knox, CSIRO Livestock Industries, Armidale, NSW. The solution was diluted with water to make a concentration of approximately 1000 larvae/ml. This was administrated on day 4 of the experiment via 3×10 ml doses using a 15 ml syringe with a T attachment. The dosage of larvae (30 000) was selected based on previous work in which this dosage resulted in moderate worm burdens of 400–1200 eggs per gramme (epg) faeces (Miller et al. Reference Miller, Blaire, Birtles, Reynolds, Gill and Revell2000). Faecal samples were refrigerated and faecal worm egg counts (FEC) conducted the next day. The number of eggs per gramme (epg) of fresh faeces was determined using a flotation technique (Whitlock, Reference Whitlock1948) using a saturated salt solution (density = 1·2).
Pasture and shrubs
The annual pasture consisted mostly of capeweed (Arctotheca calendula), with smaller quantities of radish (Raphanus raphanistrum), annual ryegrass (Lolium rigidum), perennial grass species (temperate grasses: e.g., perennial ryegrass – Lolium perenne, cocksfoot – Dactylis glomerata, phalaris – Phalaris aquatica; subtropical, warm season grasses: e.g., kikuyu – Pennisetum clandestinum, Rhodes grass – Chloris gayana; native perennial grasses: eg, kangaroo grass – Themeda triandra, red grass – Bothriochloa macra, tall windmill grass – Chloris ventricosa), serradella (French serradella – Ornithopus sativus, yellow serradella – Ornithopus compressus), and blue lupins (Lupinus angustifolius). The plot sizes for the ‘pasture’ groups varied from 525 to 880 m2 and for the ‘shrub’ groups from 645 to 1400 m2. The sizes were greater for the ‘shrub’ group in order to provide enough feed-on-offer for live weight maintenance.
The biomass of annual pasture in the ‘pasture’ plots and the inter-row of the ‘shrub’ plots was estimated from 5 quadrats (0·33×0·33 m) collected randomly along a ‘W’ transect in each plot before and after grazing. Hand shears were used to harvest all above-ground vegetable matter. The growth rate of the annual pasture was estimated from quadrat cuts taken within 5 pasture exclusion cages, placed in a ‘W’ transect, in the ‘pasture’ and ‘shrub’ plots. Each ‘shrub’ plot contained an average of 114 R. preissii shrubs. Shrub biomass was estimated using a modified ‘Adelaide technique’ (Andrew et al. Reference Andrew, Noble and Lange1979). The growth rate of R. preissii from the beginning (week 1) and end (week 7) of the experimental period was estimated using this technique on 40 bushes on an adjacent block of ungrazed R. preissii.
After the sheep had grazed each R. preissii plot, individual shrubs were classified as eaten or uneaten based on whether there was any evidence that the sheep had fed on them. Eaten shrubs were not necessarily completely eaten, but were classified as eaten if it was clear that foliage had been removed by the animals. Leaf samples of R. preissii from 5 eaten and 5 uneaten bushes in each plot (i.e., each week) were collected and assayed for plant secondary metabolites and anthelmintic activity, as described below. All pasture and R. preissii samples were dried at 65°C for 3 days, sieved to remove sand, and weighed. Eaten R. preissii and weekly bulked pasture samples from the ‘pasture’ and ‘shrub’ plots were ground through a 1 mm sieve for later nitrogen analysis (using a combustion technique with an Elementor instrument, Waite Analytical Services, Adelaide, Australia).
The parasite load on the annual pasture in the ‘pasture’ and ‘shrub’ plots and on the R. preissii bushes was assessed (Albany Animal Health Laboratories, Department of Agriculture and Food Western Australia) before grazing commenced. Plant material was sampled following the technique of Taylor (Reference Taylor1939) along an ‘N’ transect. When sampling a R. preissii shrub, 4 samples were plucked from different places around the bush.
Nematode larval development assays
Dried, ground subsamples of individual eaten (n=60) and uneaten (n=60) R. preissii (100 mg) were weighed into 2 ml microcentrifuge tubes, and 1 ml of distilled water added, and the tubes were placed onto a roller wheel for 48 h at room temperature. The mixture was then centrifuged at 9000 g for 30 min and the supernatant recovered, and stored at −20°C for use in bioassays. In vitro anthelmintic activity was tested as described previously (Kotze et al. Reference Kotze, O'Grady, Emms, Toovey, Hughes, Jessop, Bennell, Vercoe and Revell2009) using larval development assays with Haemonchus contortus from the anthelmintic susceptible Kirby1982 strain, isolated from the field at the University of New England Kirby Research Farm (as described by Albers and Burgess (Reference Albers and Burgess1988)). Infected animals were housed at the McMaster Laboratories of CSIRO Livestock Industries, Armidale, New South Wales. Sheep faeces were sent in vacuum-sealed bags by courier to the Queensland Biosciences Precinct Laboratory at St Lucia, Brisbane. Nematode eggs were recovered from the faeces by sieving and sucrose density-gradient centrifugation as described previously (Kotze et al. Reference Kotze, O'Grady, Emms, Toovey, Hughes, Jessop, Bennell, Vercoe and Revell2009), and used immediately for larval development assays. Eggs were dispensed into wells of 96-well plates containing solid agar (2%) overlaid with aliquots of plant extract (10 μl) (equivalent to the water extractable material in 1 mg of the original dried plant sample) (control wells received water only). The next day, the newly hatched larvae were fed with a live culture of E. coli. After 5 days, the assays were terminated by addition of iodine to each well and the number of fully developed infective stage larvae (L3) present in each well was counted. The percentage larval development was calculated by expressing the numbers of larvae in treated wells (mean of 3 samples/plant extract) as a percentage of the mean number in control wells.
The role of tannins in the observed anthelmintic activity of some plant extracts was examined using polyvinylpolypyrrolidine (PVPP) as described previously (Kotze et al. Reference Kotze, O'Grady, Emms, Toovey, Hughes, Jessop, Bennell, Vercoe and Revell2009). Aliquots (400 μl) of extract from groups of 4 plants which had shown almost complete inhibition of larval development in the initial screening assays (development < 5%) were pooled and divided into 2, with one half remaining untreated while the other half was incubated on a roller wheel in the presence of 10% (w/v) PVPP overnight. The PVPP was removed by centrifugation at 2200 g for 5 min. Both untreated and PVPP-treated extracts were used to prepare a series of 2-fold diluted solutions which were added (10 μl) to larval development assays.
Plant secondary compounds
Samples from eaten and uneaten R. preissii bushes were analysed for saponin and oxalate concentrations. Saponin levels were quantified using a simple assay described in principle by Harborne (Reference Harborne1973) in which the formation of a persistent foam following shaking is used to indicate saponin content. A standard curve was constructed using a commercially available powdered saponin extract from quillaja bark (Sigma-Aldrich). A stock solution (40 mg/ml in H2O) was used to make a series of dilutions (2–0·1 mg/ml) which were shaken in a Savant shaker (FastPrep FP120) for 1·5 min, allowed to sit for 15 min, and the foam levels measured using a digital micrometer. The froth measurements (in mm) for each dilution were imported into GraphPad Prism, and fitted with a 5th order polynomial trend line, for use as a standard curve. Plant extracts prepared as described above (100 mg extract in 1 ml of water) were diluted 25-fold and shaken, and the amount of foam was converted into a saponin concentration using the standard curve.
The levels of oxalates in extracts from eaten and uneaten plants were determined using a Trinity Biotech Diagnostics kit (oxalate procedure No. 591) based on the method of Ilarslan et al. Reference Ilarslan, Palmer, Imsande and Horner(1997). Briefly, plant samples were dried and ground through a 1 mm screen, and 100 mg samples were then added to 2 ml of EDTA buffer solution, homogenized and rinsed, sonicated for 1 min at 4–5 Watts, and shaken gently in a water bath at 55°C overnight. Water extracts were centrifuged (8 min at 10 000 g) and the supernatant was used in the enzymatic and colorimetric assay. The amount of oxalate in the plant tissue was calculated using a standard curve constructed with an oxalate standard (591-11Trinity Biotech), with confirmation of linearity of the standard curve.
Statistical analyses
All statistical analyses for larval development were conducted using Genstat 10.2 (Lawes Agricultural Trust, 2007). Live weight and body condition scores (BCS) are reported as arithmetic means with s.e. Faecal egg counts (FEC) were log-transformed (log10(x+1)) before statistical analysis. Live weight, BCS and FEC data were analysed with a mixed model (REML) with repeated measures, with the treatment (R. preissii or annual pasture), parasite dose, individual sheep and time as factors. The FEC data were analysed using the transformed FEC from all animals, and also after correction of the FEC data from worm-dosed animals to account for infections derived from pasture within the plots over the course of the experiment. This correction was done by subtracting the mean of the control animals (infections acquired from pasture alone) from the FEC of each animal in the worm-dosed groups.
One-way ANOVA was used to compare the estimated daily intake of R. preissii by animals in the ‘shrub-control’ group with those in the ‘shrub+worms’ group, and the percentage of R. preissii shrubs on-offer that were actually grazed, using plots as replicates because the 2 groups with access to R. preissii were each moved onto new plots each week. The toxicity of eaten and uneaten R. preissii extracts to parasite larvae (LDA) was analysed by t-test. Transformed data are reported in the results as back-transformed means and s.e., while all other data are reported as arithmetic means with s.e.
RESULTS
Pasture load and sheep FEC data
No parasite larvae were found on the control pasture or the R. preissii bushes. However, 12 612 larvae/kg dry matter were present on the R. preissii inter-row pasture prior to the commencement of grazing. Of the larvae found, 50% were Trichostrongylus, 40% were Teladorsagia and 10% were Nematodirus.
Faecal egg counts over the course of the 7 weeks of the experiment are shown in Fig. 1. Worm eggs were first observed in high numbers in the faeces of ‘+worms’ sheep in week 4, and peaked in week 5, at an average of 1537 epg for those in the ‘control’ group and 800 epg for those in the ‘shrub’ group. There was a large variation in FEC within the ‘+worms’ groups, with a co-efficient of variation for weeks 4 to 7 of 130–205%. The mean FEC in the ‘shrub + worms’ group was lower than the ‘pasture + worm’ group in weeks 4–7 (mean FEC in ‘shrub + worms’ as a percentage of the ‘pasture + worms’ group was 26%, 52%, 80%, and 31% at weeks 4, 5, 6 and 7, respectively). However, there was no significant effect of treatment (shrub versus pasture) (P > 0·05), even after correction of the data in the ‘+ worms’ groups to account for the FEC measured in the ‘no worms’ groups.
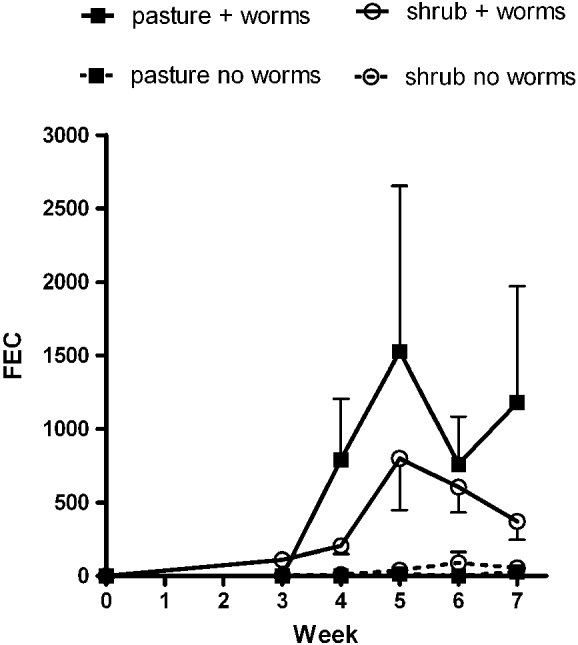
Fig. 1. Back-transformed means of faecal egg counts (eggs per g faeces±s.e.) of sheep (n = 10) grazing annual pasture (squares) or Rhagodia preissii (circles), with or without receiving a single oral dose of 30 000 Trichostrongylus colubriformis larvae.
Intake of R. preissii and pasture, and animal live weights
A striking observation made early in the trial was the selectivity shown by the sheep towards individual R. preissii plants. The sheep grazed some plants heavily while leaving adjacent plants untouched (Fig. 2). If a shrub was eaten at all, it was in most cases quite heavily eaten. We therefore monitored the response to individual plants and scored each plant as either eaten or uneaten. The percentage of R. preissii shrubs on offer that were eaten (partially or completely) ranged from 18% to 47% across all of the shrub plots grazed, apart from 1 plot used in week 7 in which 80% of shrubs were eaten (Fig. 3A). No difference was found between the non-parasitized and parasitized groups (P = 0·56) or time (plot) (P = 0·45).

Fig. 2. Photographs of adjacent Rhagodia preissii plants, one eaten the other uneaten, in plots that had been grazed for 1 week.
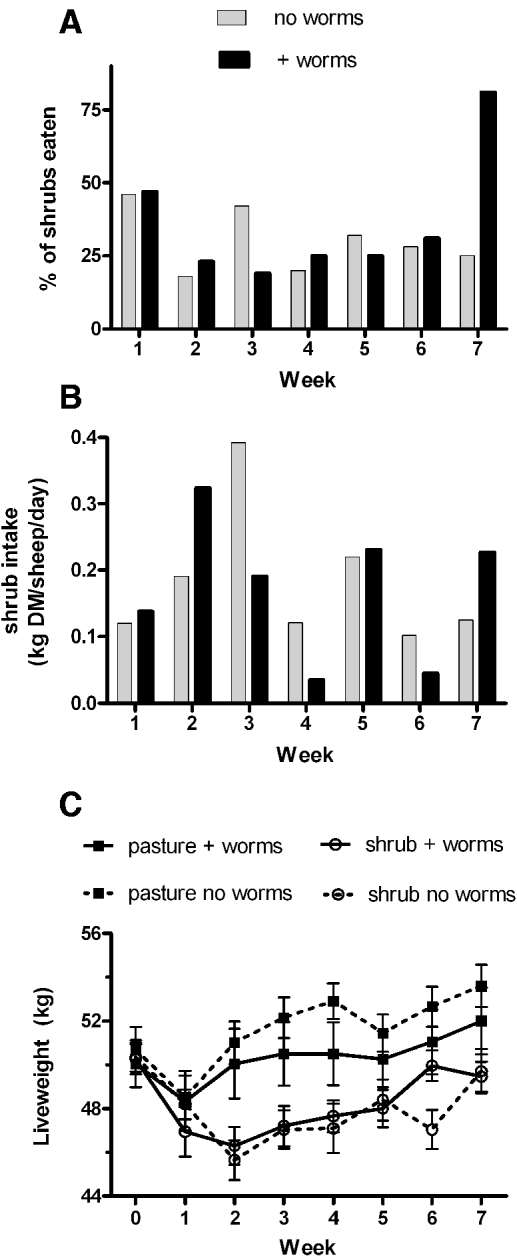
Fig. 3. Intake of Rhagodia preissii and animal live weights. (A) The percentage of R. preissii shrubs eaten in each plot for sheep (n=10) that were non-parasitized (grey bars) or parasitized (black bars). (B) Estimate of daily intake of R. preissii (kg DM/sheep/day) in each plot for shrub group sheep that were non-parasitized (grey bars) or parasitized (black bars). (C) Average live weight (kg±s.e.) of sheep (n = 10) that were non-parasitized or parasitized grazing annual pasture (squares) or R. preissii (circles) for a period of 7 weeks.
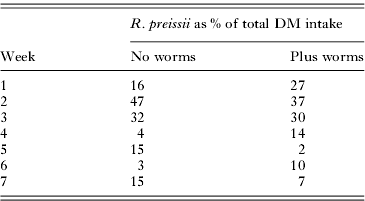
Feed intake for the 2 shrub groups varied considerably over the course of the trial (Fig. 3B). Over the whole trial period though there was no difference in the amount of shrub material consumed by the parasitized and non-parasitized groups (kg DM/sheep/day) (P = 0·835). The minimum quantity consumed was 0·04 kg/sheep/day and the maximum was 0·40 kg/sheep/day. The percentage of total DM intake made up by R. preissii for each week of the trial is shown in Table 1. Shrub intake as a percentage of the diet varied considerably across the trial, but was generally quite low. Total feed intake for the 4 groups were: pasture-control 1·2±0·11; pasture+worms 1·6±0·09; shrubs-control 1·2±0·11 kg; shrubs+worms 1·0±0·10 kg DM/head/day. The average protein content of R. preissii, inter-row annual pasture in the R. preissii plots, and annual pasture in the ‘pasture’ plots was 13·8%±0·21, 8·8%±0·15 and 8·5%±0·48, respectively. Daily protein intake of the control and treatment groups was 10·3% and 13·7% of the daily feed intake.
Table 1. Rhagodia preissii intake as a percentage of total DM intake (R. preissii, pasture, lupins) for each week of the trial in the worm-dosed and no worm groups
Live weight initially declined in the first week of grazing in all groups from an initial weight of 50·3 kg±0·04, but increased in the groups of animals grazing pasture (parasitized and non-parasitized) from week 1, and the ‘shrub’ groups from week 2 (Fig. 3C) after supplementation with lupin grain commenced. There was a significant difference (P < 0·005) between ‘pasture’ (51 kg±0·01) and ‘shrub’ groups (48·1 kg±0·01) over the 7-week period. No difference in live weight was found between sheep that were parasitized compared to non-parasitized sheep (P=0·614).
Larval development assays
Water extracts from 60 selected and 60 unselected individual R. preissii shrubs (collected over the course of the trial) were tested for their toxicity against parasite larvae (% larval development) as shown in Fig. 4. For both eaten and uneaten plants there was a large spread in the effects on larval development, from a complete inhibition of development in some cases to no effect at all in others (Fig. 4A). There was no difference between the mean larval development for extracts from eaten plants (58%±5) compared to uneaten plants (53%±5) (P > 0·05), indicating that there was no relationship between the grazing response of the sheep to the R. preissii shrubs and the level of in vitro anthelmintic activity (Fig. 4A). In addition, when the data were examined separately for worm-treated and control groups, there were no differences in mean anthelmintic activity of eaten and uneaten plants within each group (Fig. 4B), indicating that worm-infected sheep did not consume more anthelmintic material from R. preissii than control sheep (with a much lower worm burden, from Fig. 1).
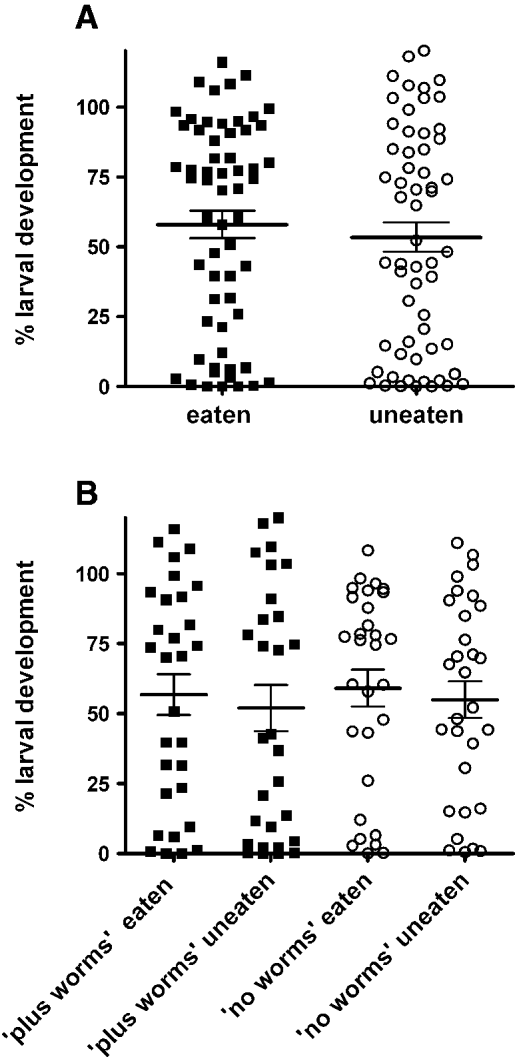
Fig. 4. Effects of plant extracts on Haemonchus contortus larval development in vitro. (A) extracts from eaten and uneaten plants, pooled data from ‘plus worms’ and ‘no worms’ groups; (B) extracts from eaten and uneaten plants within either the ‘plus worms’ or ‘no worms’ groups. Each data point represents mean, n = 8 (pooled data from 2 experiments, each with quadruplicate assays). Horizontal lines show mean±s.e. (n = 60 in A, 30 in B).
Plant secondary compounds
PVPP treatment did not affect the anthelmintic activity of the extracts (Fig. 5), indicating that the observed activity was most likely not due to tannins.
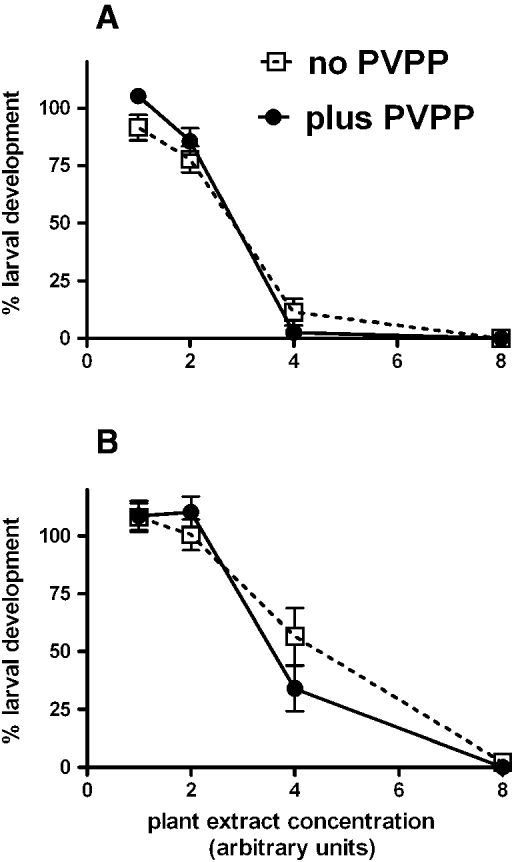
Fig. 5. Effect of PVPP on toxicity of pooled samples of extracts from 4 eaten plants (A) or 4 uneaten plants (B) in larval development assays with Haemonchus contortus. Extracts not treated with PVPP are shown as dashed lines; extracts treated with PVPP are shown as solid lines. Each data point represents mean±s.e., n = 8 (pooled data from 2 experiments, each with quadruplicate assays). Concentration units represent addition of 10 μl neat (or 2-, 4- or 8- fold diluted) plant extract to assays.
An assay for saponin levels in the plant extracts was first examined in terms of linearity. Fig. 6A shows that the a standard curve using a commercial saponin extract showed a standard curve with 2 phases; an initial sharp rise in frothiness followed by a region of almost linear increase in frothiness as the saponin level increased. Saponin levels were subsequently estimated with reference to the frothiness observed with the commercial saponin extract in 60 eaten plant samples and 59 uneaten samples (insufficient material was available for one of the uneaten plant samples) (Fig. 6B). The various extracts showed a range of saponin levels from zero up to almost 80% (w/w). There was no difference in the mean concentration between eaten and uneaten plants (eaten = 14±2%, uneaten = 19±2%, P = 0·11) indicating that there was no relationship between saponin level and sheep grazing response. In addition, there was no correlation between saponin level and in vitro anthelmintic activity across the 119 plant samples (r 2 = 0·005).

Fig. 6. Saponin and oxalate levels in extracts from eaten and uneaten plants. (A) Standard curve of foam level versus content of saponin (extract from quillaja bark). (B) Saponin levels in eaten or uneaten plants. Each data point represents a single foam measurement. Horizontal lines show mean±s.e. (n = 60 eaten, 59 uneaten plants). (C) Oxalate levels in eaten and uneaten plant samples. Each data point represents mean, n = 2 (duplicate assays). Horizontal lines show mean±s.e. (n = 10 eaten and uneaten).
Oxalate levels were measured in 10 samples of eaten and uneaten plants (Fig. 6C). The levels were slightly higher in the eaten plants (eaten = 4·3±0·3%, uneaten = 3·0±0·3%, P = 0·004). There was no relationship between oxalate levels and in vitro anthelmintic activity (r 2 = 0·003).
DISCUSSION
Including Rhagodia preissii as part of the forage on offer to parasitized sheep did not reduce their parasite burdens significantly as assessed by FEC relative to sheep grazing on annual pasture species. While the FEC for the parasitized group was lower than the pasture group for weeks 4–7 of the trial, with reductions of up to 74% in mean FEC, the differences were not significant (P > 0·05). Several factors may be important in this lack of a significant in vivo effect despite promising in vitro data from an earlier study (Kotze et al. Reference Kotze, O'Grady, Emms, Toovey, Hughes, Jessop, Bennell, Vercoe and Revell2009). These include a low level of intake of the shrubs, and lower in vitro anthelmintic activity of the shrubs at the time of this trial than in our previous study (discussed below). In addition, the many host pharmacokinetic factors which can contribute to an inability to translate in vitro anthelmintic activity into in vivo success could have played a role (Athanasiadou et al. Reference Athanasiadou, Githiori and Kyriazakis2007).
A striking feature of the trial was the selective nature of the grazing behaviour of sheep in the R. preissii plots. Both parasitized and non-parasitized animals were highly selective in which R. preissii plants they consumed. On average about one third of R. preissii shrubs in each plot showed evidence of being eaten. The remainder were left completely untouched. The same selectivity for individual plants has been observed with sheep grazing Atriplex nummularia (Norman et al. Reference Norman, Friend, Masters, Rintoull, Dynes and Williams2004), which is another chenopod shrub native to Australia. The overall intake of the R. preissii plants was quite low; the average daily intake of R. preisii was approximately 175 g/day for both parasitized and non-parasitized sheep. The sheep on the R. preissii plots showed lower live weights than those on pasture, and required supplementary feeding with lupins at twice the rate of the pasture-fed sheep. It is clear that the high degree of plant selectivity and the low intake of R. preissii limited the amount of ‘anthelmintic’ leaf material that was consumed. This raises a need to examine means to increase its uptake (discussed below) if in vivo anthelmintic effects are to be observed.
The grazing selectivity did not appear to be associated with the in vitro anthelmintic activity as eaten and uneaten plants showed an equivalent range of toxicities in larval development assays, and a similar mean percentage inhibition of larval development. However, it should be noted that the relationship between anthelmintic activity and grazing selectivity has been tested to only a limited degree by the present study. For example, we did not look at the grazing selectivity of individual sheep to determine whether high FEC individuals within a group showed a different pattern of plant choice, nor the relationship between the grazing pattern of high FEC sheep and individual plant anthelmintic activities. In addition, our in vitro larval development assays may not accurately reflect the in vivo anthelmintic activity of different plants towards adult worm stages. However, despite these limitations, the present study would suggest that under our experimental conditions, and with the type of data we have collected, there was no relationship between grazing selectivity and anthelmintic activity. Hence, at least 2 groups of plant secondary compounds would seem to be active in this species. One group has a strong influence on whether the plant is grazed by sheep, while another group confers anthelmintic activity to some plants. Our study indicates that saponins and oxalates are not responsible for either the grazing-deterrent or anthelmintic effects, while tannins are most likely not responsible for the anthelmintic effects. The role of tannins cannot be excluded completely, however, due to the possibility that our plant sample drying methods (65°C for 3 days) may have reduced the tannin levels in the extracts. One group of compounds that may be associated with the anthelmintic activity is the phytoecdysteroids, which have been found in high concentrations in Rhagodia baccata (Dinan et al. Reference Dinan, Sarker, Bourne, Whiting, Sik and Rees1999). In particular, R. baccata contained high levels of 20-hydroxyecdysone (20E), which is known to induce abnormal moulting and death in many arthropods, and is toxic towards free-living larval stages of H. contortus (Rogers, 1963; Graham et al. Reference Graham, Kotze, Fernley and Hill2010). However, the anthelmintic agents in the R. preissii sampled in the present study remain unknown, and should be the subject of further research.
The in vitro anthelmintic activity in the shrub samples was lower than expected given the activity seen with this species in our earlier study (Kotze et al. Reference Kotze, O'Grady, Emms, Toovey, Hughes, Jessop, Bennell, Vercoe and Revell2009); mean percentage development of 29% and 25% for plants sampled in September 2007 and February 2008, respectively, compared to 58% and 53% from Fig. 4. The plants sampled previously were from an adjacent plot to that used for the present trial, and hence there were no major differences in soil type or topography apparent. The samples collected for the present experiment were from more mature shrubs than the previous collection and were not flowering or fruiting, unlike some of the previous samples (February 2008). This lack of activity at the time of the present field trial was further demonstrated by some subsequent sampling at the same site in February 2009 in which extracts from 62 individual plants (out of the original 120 from the present study) showed a mean percentage inhibition of development of approximately 30% (Kotze, unpublished data). The effect of the plant's physiological stage (growing or reproductive) on the uptake or anthelmintic properties of R. preissii is unknown, but should be a key area for future research. It will be important for future field trials of this sort to ensure that the plants are showing an optimal level of anthelmintic activity at the time of the trial. However, this will require more knowledge on the factors that influence the anthelmintic activity shown by different R. preissii plants. An ability to monitor the levels of the anthelmintic agents would enable optimal use of the shrubs.
The saponin concentrations were very high in some cases (up to 80% (w/w)). However, this may be due to the use of a commercially available saponin reference compound (extract from quillaja bark) which may be quite different in its physical properties (specifically, degree of foaminess) than the saponins found in R. preissii. However, the advantage of reference to this standard over the reporting of actual foaminess levels (in units of height) of the different samples is that it will allow for standardization of the assay. That is, reporting of sample frothiness with reference to the commercial saponin will allow such measurements in different laboratories to be compared directly even if their physical shaking procedures to generate the foam differ. Hence, for the present study, the saponin data should be viewed as an approximation of the saponin levels for the purpose of comparing plant samples, rather than an accurate measure of absolute amounts.
Previous studies using bioactive forages to reduce parasite burdens in vivo have been inconsistent (Hoste et al. Reference Hoste, Jackson, Athanasiadou, Thamsborg and Hoskin2006). There are currently no set guidelines outlining the best way to conduct in vivo experiments. Several suggestions have been made by Athanasiadou et al. (Reference Athanasiadou, Githiori and Kyriazakis2007) in a recent review of this problem to simplify the comparison of studies of different bioactive forages. They recommend that the plant compounds responsible for the anthelmintic activity should be isolated and their concentrations measured and documented, as well as animal performance measures, indicators of immunity and behavioural observations. In the present study we have measured animal performance; however, at present we have no ability to measure bioactive concentrations. The value of behavioural observations has been illustrated by the grazing selectivity issue that it has identified with R. preissii in the present study.
The R. preissii used for the present trial were from different genetic backgrounds, being derived from seed collections from wild populations over a wide geographical range. To date there has been no systematic selection within this species so there remains considerable variability between plants, unlike the consistency expected with highly selected cultivars. The grazing selectivity, in vitro anthelmintic activity, and plant secondary compound assays illustrate this variability across the plants. Given this genetic variability, and the data from the present study showing that grazing selectivity and anthelmintic activity were not directly related under our experimental conditions, there appears to be potential to select within the R. preissii plant population for both increased animal preference and increased anthelmintic activity. Such increases in preference would appear to be essential if the shrub is to be of practical use, while the lack of significant anthelmintic effects observed in the trial, alongside the great variation seen in in vitro anthelmintic activity among separate plant samples, demonstrates an additional need to select for this property. Hence, future efforts to increase the utility of this shrub species as both a forage and anthelmintic plant should address both these traits in plant-breeding programmes.
In the short term though, if R. preissii is to have an anthelmintic effect in a commercial situation, we may need to increase the amount or the frequency that is eaten. Animal preference is a function of a food's odour, taste, and texture, with post-ingestive consequences of nutrients and toxins from the food (Provenza et al. Reference Provenza, Villalba, Haskell, MacAdam, Griggs and Wiedmeier2007). Intake of R. preissii would have been influenced by the selectivity for individual shrubs and the complementary phytochemicals of the inter-row pasture and lupin supplement. Intake of shrub may also be influenced by the level of nematode infections, and the presence of nematode larvae on pasture (Hutchings et al. Reference Hutchings, Kyriazakis, Anderson, Gordon and Coop1998, Reference Hutchings, Kyriazakis, Gordon and Jackson1999). For example, in the present study, the intake of R. preissii may have been affected by a grazing deterrent associated with the parasite load on the inter-row pasture, compared to an absence of such contamination on the control pasture plots. However, we consider this unlikely given the relatively low level of contamination in the shrub inter-row pasture. Animals may also choose to eat more R. preissii if it is offered in different combinations of plants or supplements. Sheep may be encouraged to consume greater amounts through behavioural techniques and grazing management strategies; for example, by grazing it with a mixture of complementary forage species, or by providing restricted access to a familiar food that helps offset any toxins or negative feedback responses (Shaw et al. Reference Shaw, Villalba and Provenza2006). These grazing strategies will require more study. From the anthelmintic perspective, benefit may be gained in the short term through optimizing the use of the shrub (plant maturity, reproductive phase, soil type etc) based on an ability to coordinate grazing with periods when the plant is expressing its anthelmintic compounds at their highest levels.
ACKNOWLEDGMENTS
Assistance from farm staff from Badgingarra Research Station is gratefully acknowledged. Malcolm Knox (CSIRO Livestock Industries, Armidale NSW) is thanked for supply of parasite larvae, Geoff Mitchell (DAFWA) for assistance with faecal egg counts, and Allan Rintoul (CSIRO Livestock Industries, Floreat WA) for oxalate analysis. Financial support was provided to N. Zadow by the Growers Group Alliance. This work was partly supported by the Future Farm Industries Co-operative Research Centre and Meat & Livestock Australia.









