Introduction
Bipolar disorder is an unstable condition. Relapse rates between 73% and 90% are frequently reported at follow-up, with nearly 50% of the relapses occurring within 2 years from the start of treatment with mood stabilizers (Gitlin et al. Reference Gitlin, Swendsen, Heller and Hammen1995; Bowden & Krishnan, Reference Bowden and Krishnan2004). This suggests the probable presence of an underlying pathology that we are not yet targeting pharmacologically. One approach to increase our understanding of the underlying pathology and to help make diagnostic and treatment decisions is with biomarkers (Phillips et al. Reference Phillips, Glasier and Brodsky2008; Kapczinski et al. Reference Kapczinski, Dias, Kauer-Sant'anna, Brietzke, Vazquez, Vieta and Berk2009; Frey et al. Reference Frey, Andreazza, Houenou, Jamain, Goldstein, Frye, Leboyer, Berk, Malhi, Lopez-Jaramillo, Taylor, Dodd, Frangou, Hall, Fernandes, Kauer-Sant'anna, Yatham, Kapczinski and Young2013). For bipolar disorder, an ideal biomarker would be quick to obtain, inexpensive, minimally invasive, reflect current mood and predict future changes and direction. Biomarkers obtained from neuroimaging, gene and protein analysis are still technically demanding and costly (Lakhan et al. Reference Lakhan, Vieira and Hamlat2010). Alternatively, biomarkers from peripheral blood may offer some advantages. One example is brain-derived neurotrophic factor (BDNF) in plasma or serum, which is reduced in depressed, manic and hypomanic patients but returns to healthy levels in euthymic patients (Lin, Reference Lin2009).
To identify additional biomarkers, oxidative stress offers an arguably rational approach since it may play a potentially crucial role in the pathophysiology of bipolar disorder (Andreazza et al. Reference Andreazza, Cassini, Rosa, Leite, De Almeida, Nardin, Cunha, Cereser, Santin, Gottfried, Salvador, Kapczinski and Goncalves2007; Machado-Vieira et al. Reference Machado-Vieira, Andreazza, Viale, Zanatto, Cereser, Da Silva Vargas, Kapczinski, Portela, Souza, Salvador and Gentil2007a ; Kunz et al. Reference Kunz, Gama, Andreazza, Salvador, Cereser, Gomes, Belmonte-De-Abreu, Berk and Kapczinski2008; Ng et al. Reference Ng, Berk, Dean and Bush2008; Manji et al. Reference Manji, Kato, Di Prospero, Ness, Beal, Krams and Chen2012). Oxidative stress is an imbalance between reactive oxygen species and the antioxidant defence system or a dysregulation of its control (Jones, Reference Jones2006b ). Possible markers of oxidative stress include oxidants, oxidation products, antioxidants and antioxidant enzymes (Jones, Reference Jones2006b ). In bipolar disorder several measures suggestive of oxidative stress have already been reported, mostly based on the activity of enzymes superoxide dismutase, catalase and glutathione peroxidase, glutathione reductase and glutathione S-transferase (Kuloglu et al. Reference Kuloglu, Ustundag, Atmaca, Canatan, Tezcan and Cinkilinc2002; Andreazza et al. Reference Andreazza, Cassini, Rosa, Leite, De Almeida, Nardin, Cunha, Cereser, Santin, Gottfried, Salvador, Kapczinski and Goncalves2007, Reference Andreazza, Kapczinski, Kauer-Sant'anna, Walz, Bond, Goncalves, Young and Yatham2009; Kunz et al. Reference Kunz, Gama, Andreazza, Salvador, Cereser, Gomes, Belmonte-De-Abreu, Berk and Kapczinski2008).
Rather than measuring enzyme activities in blood cells, it is possible to gain insight into whole body oxidative stress directly by measuring blood levels of glutathione. Glutathione is a tripeptide with a cysteine, which can be present in either a reduced state (as a free sulfhydryl) or an oxidized state (as part of a disulfide). Total plasma glutathione and the ratio of reduced:oxidized change with ageing and disease states and can be a useful indicator of disease risk (Jones, Reference Jones2006a ; Essex, Reference Essex2009). Plasma glutathione has several advantages as a measure of whole organism oxidative status (Schafer & Buettner, Reference Schafer and Buettner2001; Jones, Reference Jones2006a ) because it integrates the redox status of all organs. Thus, cells release reduced and oxidized glutathione in proportion to their intracellular status and plasma levels reflect the inter-organ balance of glutathione (Deneke &d Fanburg, Reference Deneke and Fanburg1989). Glutathione levels are decreased in post-mortem prefrontal cortex from patients with bipolar disorder, major depression and schizophrenia (Gawryluk et al. Reference Gawryluk, Wang, Andreazza, Shao and Young2011b ) but blood glutathione levels have not been reported. Our hypothesis was that we would detect reductions in plasma glutathione levels in bipolar patients compared with healthy controls, and we should explore correlations with clinical variable in well-characterized patients.
We have investigated whether blood levels of glutathione and its redox state were altered in bipolar patients and correlated with clinical variables or mood symptoms. We will show that glutathione levels and redox state can detect a level of oxidative stress even in subsyndromal patients with normal BDNF that appears to relate to the age of onset of the disorder.
Method
Ethics
The study was approved by the local research ethics committee and was in accordance with the Helsinki Declaration of 1975. All participants provided written informed consent before entering the study.
Subjects and participant assessments
A total of 50 patients fulfilling Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR) criteria (APA, 2000) for bipolar I disorder were recruited from the OXTEXT Database, at the Warneford Hospital, Oxford, UK. All patients were assessed using the Structured Clinical Interview for DSM disorders (SCID) and a qualified psychiatrist confirmed the diagnosis. All patients were subsyndromal at the time of sample collection. Inclusion criteria for OXTEXT were: participant is willing and able to give informed consent for participation in the study, aged 16 years or above, diagnosed with bipolar disorder I by DSM-IV criteria, currently using, or suitable for, mood monitoring and a patient of the local National Health Service (NHS) trust. There were no exclusion criteria except failure to meet the above.
Assessment of self-reported mood scores
All patients were completing self-rating scales for depression (Quick Inventory of Depressive Symptomology; QIDS) and mood elevation (Altman Self-Rating Mania Scale) weekly, using the True Colours SMS texting or online system. These data was analysed for the 4 weeks before and after the collection of the blood sample.
The control group comprised 50 healthy volunteers, matched for age and sex, recruited from the local community in the same geographical area as the patients. The Mini-International Neuropsychiatric Interview (MINI; Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998) was administered to the controls in order to rule out any psychiatric disorder.
Sociodemographic and clinical data had been collected through an extensive interview with the patient group; further information – pharmacological data, smoking habits, alcohol or substance abuse – was obtained from patient and control groups at the time of blood letting.
Blood collection and processing
Each subject had a 5 ml blood sample collected by venepuncture into a BD vacutainer containing ethylenediaminetetra-acetic acid (EDTA) (no. 367525; BD, USA). Plasma was processed according to the protocol described in Rahman et al. (Reference Rahman, Kode and Biswas2006). Briefly, plasma was separated by centrifugation immediately following venepuncture. In order to maintain the reduced:oxidized glutathione ratio, 100 μl of 0.6% (w/v) sulfosalicylic acid were added to 200 μl of separated plasma for the measurement of glutathione at a later time. Sulfhydryl oxidation was minimized by the presence of the metal chelator EDTA and the rapid addition of 5-sulfosalicylic acid, which not only acidifies the sample and precipitates protein but also inhibits γ-glutamyl transferase. Biological samples must be acidified quickly to minimize the oxidation of reduced glutathione to glutathione and to mixed disulfides, and also to inactivate γ-glutamyl transpeptidase. The samples were then frozen and stored for a period up to 3 months at –80°C.
Measurement of BDNF
The plasma BDNF level was measured using the Human BDNF DuoSet ELISA kit (DY248; R&D Systems, USA). A quantity of 100 μl of the plasma sample frozen for BDNF analysis was pipetted into the enzyme-linked immunoassay (ELISA) plate, and the assay was carried out according to the instructions provided. The sample BDNF concentration was interpolated from a BDNF standard curve. BDNF concentrations are expressed in ng/ml plasma.
Measurement of glutathione
Both total reduced glutathione (GSH) and its oxidized disulfide metabolites, where it is conjugated to either glutathione (GSSG) or cysteine to form a mixed disulfide, were measured using Tietze's recycling assay as described by Rahman et al. (Reference Rahman, Kode and Biswas2006). In this assay, reduced glutathione reacts with 5,5′- dithiobis-2-nitrobenzoic acid (DTNB) to form a coloured product and the enzyme glutathione reductase is used to reduce oxidized difulfides of glutathione with each cycle of reduction producing 5-thio-2-nitrobenzoic acid (TNB) as a coloured product. The recycling assay is rapid, reproducible and sensitive (detection limit of 100 nm-GSH). Reduced glutathione was used as the standard. Oxidized glutathione (all forms of glutathione disulfide) was measured by first reacting reduced glutathione present in the sample with of 2-vinylpyridine. Glutathione disulfide reductase uses both glutathione disulfide and mixed disulfides including glutathione–cysteine as substrates (Eriksson & Mannervi, Reference Eriksson and Mannervi1970; Jones, Reference Jones2006b ), which will overestimate glutathione disulfide. Therefore, to correct for this, we used literature values for the relative contribution of each oxidized form to calculate the amount of GSSG as reported previously (Samiec et al. Reference Samiec, Drews-Botsch, Flagg, Kurtz, Sternberg, Reed and Jones1998; Jones et al. Reference Jones, Carlson, Mody, Cai, Lynn and Sternberg2000). Reduced and oxidized glutathione levels were measured and other parameters were calculated from these values and based on published values for the proportion of oxidized glutathione present as the disulfide and mixed disulfides of which glutathione–cysteine is the main species (Jones et al. Reference Jones, Carlson, Samiec, Sternberg, Mody, Reed and Brown1998, Reference Jones, Carlson, Mody, Cai, Lynn and Sternberg2000, Reference Jones, Mody, Carlson, Lynn and Sternberg2002; Jones & Liang, Reference Jones and Liang2009).
Redox state calculations
The redox state is a function of the reducing potential of the redox pair (its ratio) and its reducing capacity (absolute concentrations). The redox state can be estimated from the Nernst equation where ΔE = ΔE° – RT/nF ln Q, where ° indicates standard conditions, R is the gas constant, T is the absolute temperature, n is the number of electrons transferred, F is the Faraday constant and Q is the mass action equilibrium constant. The Nernst equation for the redox state of glutathione is: E = –240 – (61.5/2) log ([GSH]2/[GSSG]) mV at pH 7 and 37°C (Schafer & Buettner, Reference Schafer and Buettner2001; Jones, Reference Jones2006a ).
Statistical analysis
Statistical analyses were performed with Prism 5 (GraphPad, USA). Data were tested for normality with the D'Agostino and Pearson omnibus normality test to determine the subsequent appropriate statistical tests. To determine differences between two means, we used an unpaired, two-tailed t test for data that were normally distributed and a Mann–Whitney test for data that were not normally distributed. To determine correlations between variables, we used Pearson's product-moment correlation for data that were normally distributed and Spearman's rank correlations for data that were not normally distributed. As there is no consensus regarding statistical thresholds and corrections for multiple correlations (Curtin & Schulz, Reference Curtin and Schulz1998; Moran, Reference Moran2003; Nakagawa, Reference Nakagawa2004), we present exact p values for all the correlations explored. However, we only assume significance for those correlations that have appropriate p values after applying the Bonferroni correction (Rice, Reference Rice1989). Demographic and clinical characteristics were analysed using χ 2 and the t test.
Results
Glutathione levels
The participants' demographic and clinical characteristics are summarized in Table 1. There were no differences in glutathione levels between patients with or without cigarette smoking or those with or without substance abuse (alcohol or other drugs). Nor did glutathione levels differ with regard to pharmacological treatment (i.e. dividing the group by mood stabilizers, antipsychotics or antidepressants).
Table 1. Demographic and clinical characteristics of the sample population

s.d., Standard deviation.
a Statistically significant differences (p ⩽ 0.05) between bipolar patients and healthy controls were determined by χ 2 or Student's t test.
In bipolar patients both total glutathione (Fig. 1 a) and reduced glutathione (Fig. 1 c) were lower compared with healthy controls. The oxidation status of glutathione can be expressed in several ways and regardless of whether it was expressed as absolute concentrations of oxidized (Fig. 1 b), percentage oxidized (Fig. 1 d), a ratio of reduced:oxidized (Fig. 1 e) or as a reducing potential (Fig. 1 f), glutathione was more oxidized in bipolar patients. These data suggest that glutathione levels are lower and more oxidized in bipolar patients.

Fig. 1. Plasma glutathione levels and oxidation state differ between healthy controls and subsyndromal bipolar patients. Total and oxidized glutathione were measured and the other parameters were calculated. Means were compared with either a t test (a, c, d) for normally distributed data or the Mann–Whitney test (b, e, f) for non-normally distributed data. Each data point represents an average of assay triplicates, with n = 50 for each group.
BDNF levels
Plasma BDNF levels in the bipolar patients were the same as those in healthy controls (Fig. 2).

Fig. 2. Plasma brain-derived neurotrophic factor concentrations ([BDNF]) do not differ between healthy controls and subsyndromal bipolar patients. Means were compared with the Mann–Whitney test. Data points represent averages of assay duplicates, with n = 50 for healthy controls and n = 49 for bipolar patients.
Relationship between glutathione levels and mood
The patients were subsyndromal, so only 12% had scores equal to or higher than 6 on the Altman Self-Rating Mania Scale and 28% had a mean score of 10 or higher on the QIDS. Subsequent analyses were based on the average mood score for the entire 8 weeks (there was no difference in the average scores for either depression or mania in the 4 weeks before or after the blood sampling). Neither the QIDS scores (online Supplementary Fig. S1) nor the Altman scores (online Supplementary Fig. S2) correlated with any of the glutathione parameters. We performed additional exploratory data analysis by plotting the data as ‘small multiples’ (Tufte, 2001) (online Supplementary Fig. S3), but no trends between mood scores and biochemical parameters were evident.
Relationship between glutathione levels and clinical variables
The data relating demographics to total and oxidized glutathione are presented as pairwise scatterplots (Figs 3 and 4). The exact p values are given in the figures. Using the conventional threshold p of 0.05, there are three significant correlations out of a possible 14 (Figs 3 and 4). Only the total and the oxidized glutathione measures were correlated with the clinical variables since the others, i.e. reduced, percentage oxidized, reduced:oxidized ratio and redox state are all derived from the measures of total and oxidized glutathione. Applying the Bonferroni correction (Rice, Reference Rice1989) there is one significant correlation: total glutathione level is related to the age of onset of bipolar disorder (Fig. 3a).
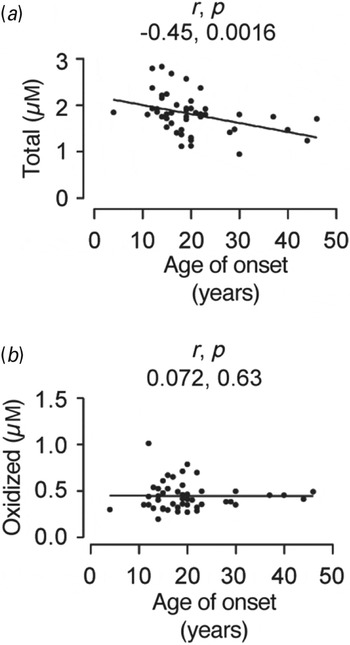
Fig. 3. Scatterplots showing the correlation between age of onset in years (x-axis) and plasma levels of total (a) and oxidized (b) glutathione (y-axis) in bipolar patients. As the parameters were not normally distributed, we used Spearman's rank correlation (r). Both r and exact p values are presented on the plots. Only (a) reached statistical significance after applying the Bonferroni correction for multiple comparisons (where p = 0.0016; 0.05 divided by 14 comparisons).

Fig. 4. Scatterplots showing demographic data of bipolar patients plotted against plasma total and oxidized glutathione levels (x-axis). As most of the parameters were not normally distributed, we used Spearman's rank correlation (r). Both r and exact p values are presented on the plots, values reaching statistical significance at the conventional threshold of p = 0.05. None of these reached statistical significance after applying the Bonferroni correction for multiple comparisons.
Discussion
Oxidative stress is emerging as a factor correlated with neurological dysfunction in neurodegenerative diseases such as amyotrophic lateral sclerosis, Parkinson's disease (Schulz et al. Reference Schulz, Lindenau, Seyfried and Dichgans2000), and, more recently, in affective disorders such as depression and bipolar disorder (Ng et al. Reference Ng, Berk, Dean and Bush2008). Endogenously, oxidative damage is reversed by glutathione, the tripeptide γ-l-glutamyl-l-cysteinylglycine. Although glutathione is central to the oxidative stress hypothesis of central nervous system disorders, the role it plays in these processes is poorly defined. Biochemically, glutathione directly reduces proteins with oxidized sulfhydryl groups and indirectly reduces superoxide and hydrogen peroxide through the cooperative action of enzymes such as glutathione peroxidase, glutathione reductase and catalase. The mechanism as to how the cellular redox state and redox metabolism link to brain disorders is not clear, but given that damage to cultured neurons induced by exogenous oxidants (free radical oxygen species) is prevented by BDNF (Gong et al. Reference Gong, Wyatt, Baker and Masserano1999), redox is playing a role, possibly through glutathione. Glutathione may also act through non-redox mechanisms, either by acting as a reserve pool of the neurotransmitter glutamate (Koga et al. Reference Koga, Serritella, Messmer, Hayashi-Takagi, Hester, Snyder, Sawa and Sedlak2011) or through allosteric control of the N-methyl-d-aspartate (NMDA) receptor (Varga et al. Reference Varga, Jenei, Janaky, Saransaari and Oja1997).
In this study, both total glutathione and reduced glutathione were reduced in the plasma of the subsyndromal bipolar patient group, while the oxidized form was increased. There was consequently a fall in the ratio between the reduced and oxidized forms and redox state compared with a matched group of healthy controls. None of the plasma glutathione parameters was correlated with current mood as self-assessed by the QIDS for depression (Rush et al. Reference Rush, Trivedi, Ibrahim, Carmody, Arnow, Klein, Markowitz, Ninan, Kornstein, Manber, Thase, Kocsis and Keller2003) or the Altman Self-Rating Mania Scale for elation (Altman et al. Reference Altman, Hedeker, Peterson and Davis1997) within the bipolar group, but the patients were basically subsyndromal. The magnitude of reductions in glutathione parameters was greatest in those with the latest age of onset of mood disorder. Thus, our study supports the notion that oxidative imbalance may be involved in the pathophysiology of bipolar disorder. It is consistent with a number of other studies, including those that measured glutathione in post-mortem brain samples of bipolar patients (Wang et al. Reference Wang, Shao, Sun and Young2009; Gawryluk et al. Reference Gawryluk, Wang, Andreazza, Shao, Yatham and Young2011a , Reference Gawryluk, Wang, Andreazza, Shao and Young b ), as well as a magnetic resonance spectroscopy (MRS) study (Das et al. Reference Das, Tanious, Fritz, Dodd, Dean, Berk and Malhi2013) carried out in major depressive disorder, that have suggested that oxidative stress plays an important role in the depressive symptomology of bipolar disorder.
Glutathione levels and redox state
Plasma glutathione measures are interrelated but do provide somewhat different information. For example, absolute concentrations are the relevant measure for enzymes that use reduced or oxidized glutathione. Reduced glutathione concentration is directly related to the extent of protein S-glutathionylation, which can affect regulation (Gilbert, Reference Gilbert1990). Conversely, the glutathione redox state is a good measure of the plasma reduction–oxidation state, which in turn, reflects the oxidative state of the whole body (Schafer & Buettner, Reference Schafer and Buettner2001; Jones, Reference Jones2006b ). The redox potential is preferred as it takes into account the two-electron stoichiometry, its dependence on absolute concentrations of reduced and oxidized glutathione, and its expression in units of volts, so enabling more direct comparison with other systems and studies (Schafer & Buettner, Reference Schafer and Buettner2001). For this study, we measured total and oxidized glutathione and then calculated several parameters to enable comparison with other studies in the literature on plasma glutathione levels. Our healthy control population had a mean of –125 mV, which is close to the range reported for healthy individuals with a mean of –137 ± 9 mV (Jones, Reference Jones2006a). The bipolar group had a mean of –91 mV, which is approximately equivalent to the oxidative stress load associated with the heavy drinking of alcohol, a cysteine-poor diet, type 2 diabetes or cigarette smoking (Jones et al. Reference Jones, Mody, Carlson, Lynn and Sternberg2002; Jones, Reference Jones2006a ).
Glutathione and mood
We did not find any significant correlation between the mood scores of the patients and their plasma glutathione level. Mood was averaged over a relevant interval of 8 weeks, and, while subsyndromal, many patients were reporting significant depressive symptoms. Thus, the glutathione system is not a simple state marker driven by mood (perhaps in contrast with BDNF – see below). Open-label and double-blind clinical studies have suggested that the antioxidant N-acetylcysteine is beneficial in treating symptoms associated with bipolar depression (Magalhaes et al. Reference Magalhaes, Dean, Bush, Copolov, Malhi, Kohlmann, Jeavons, Schapkaitz, Anderson-Hunt and Berk2011; Berk et al. Reference Berk, Copolov, Dean, Lu, Jeavons, Schapkaitz, Anderson-Hunt and Bush2008, Reference Berk, Dean, Cotton, Gama, Kapczinski, Fernandes, Kohlmann, Jeavons, Hewitt, Moss, Allwang, Schapkaitz, Cobb, Bush, Dodd and Malhi2012; Dean et al. Reference Dean, Bush, Copolov, Kohlmann, Jeavons, Schapkaitz, Anderson-Hunt and Berk2012). Our findings instead suggest that the residual depressive symptoms and oxidative stress would not necessarily be relieved in parallel. It is possible that other clinical targets related to the age of onset like cognitive impairment would be more appropriate (Bourne et al. Reference Bourne, Aydemir, Balanza-Martinez, Bora, Brissos, Cavanagh, Clark, Cubukcuoglu, Dias, Dittmann, Ferrier, Fleck, Frangou, Gallagher, Jones, Kieseppa, Martinez-Aran, Melle, Moore, Mur, Pfennig, Raust, Senturk, Simonsen, Smith, Bio, Soeiro-De-Souza, Stoddart, Sundet, Szoke, Thompson, Torrent, Zalla, Craddock, Andreassen, Leboyer, Vieta, Bauer, Worhunsky, Tzagarakis, Rogers, Geddes and Goodwin2013).
Glutathione and age of onset
What is particularly intriguing from our study is the correlation between plasma glutathione levels and the age of onset of bipolar disorder (Fig. 3). This survives appropriate correction for multiple comparisons but is nevertheless unexpected. One might have predicted that oxidative stress would be cumulative over time and age of disease onset and duration of symptoms would correlate with increased oxidative state. However, the correlation is the opposite of this expectation, with later onset correlated with decreased total glutathione, although no correlation with oxidized glutathione was seen (Fig. 3). Length of illness was not correlated with the glutathione measures. At present we cannot separate cause and effect for the correlation with age of onset. It seems possible that the increased oxidative stress may contribute as a risk factor to later onset of bipolar disorder. This implies that correcting oxidative stress might be a means of preventing disease onset, rather than a treatment for established illness.
BDNF and bipolar disorder
BDNF plays a crucial role in neurogenesis and neuroplasticity in the developing and adult brain and is implicated in bipolar disorder, depression and cognitive dysfunction (Post, Reference Post2007). BDNF is able to cross the blood–brain barrier (Pan et al. Reference Pan, Banks, Fasold, Bluth and Kastin1998), so blood BDNF levels are reflective of brain BDNF levels across species (Klein et al. Reference Klein, Williamson, Santini, Clemmensen, Ettrup, Rios, Knudsen and Aznar2011). Several studies have investigated BDNF levels in serum samples and fewer using plasma samples from bipolar patients (Palomino et al. Reference Palomino, Vallejo-Illarramendi, Gonzalez-Pinto, Aldama, Gonzalez-Gomez, Mosquera, Gonzalez-Garcia and Matute2006; Machado-Vieira et al. Reference Machado-Vieira, Dietrich, Leke, Cereser, Zanatto, Kapczinski, Souza, Portela and Gentil2007b ; Monteleone et al. Reference Monteleone, Serritella, Martiadis and Maj2008; Dias et al. Reference Dias, Brissos, Frey, Andreazza, Cardoso and Kapczinski2009; Tramontina et al. Reference Tramontina, Andreazza, Kauer-Sant'anna, Stertz, Goi, Chiarani and Kapczinski2009; Suwalska et al. Reference Suwalska, Sobieska and Rybakowski2010; Grande et al. Reference Grande, Kapczinski, Stertz, Colpo, Kunz, Cereser, Kauer-Sant'anna, Frey, Vieta and Magalhaes2012) with varying results. Lithium responders (both partial and excellent) did not have significantly different BDNF levels, whereas the lithium non-responders had significantly lower BDNF levels (Suwalska et al. Reference Suwalska, Sobieska and Rybakowski2010). This finding was then further confirmed measuring plasma BDNF levels in excellent lithium responders (Rybakowski & Suwalska, Reference Rybakowski and Suwalska2010). A consolidated meta-analysis has confirmed that BDNF levels are decreased in manic/hypomanic or depressive periods but are near normal in euthymia (Lin, Reference Lin2009). Ours is the largest study of its kind to date and supports the latter summary. BDNF levels may be a useful biomarker to confirm patient responsiveness to pharmacological intervention.
Limitations of the study
This is the first study to investigate glutathione in the plasma of bipolar patients. However, it would be very useful to correlate plasma glutathione levels with brain glutathione levels, which is something no study has yet done. Any future comparison of reduced glutathione measured in vivo using MRS requires accompanying blood glutathione levels to determine whether peripheral levels are reflective of the values observed in the brain.
Mature patients have long illness histories and may have a range of co-morbidities. We cannot rule out the effect of medications or other factors such as diet and exercise which were not controlled for and could have some influence on glutathione levels. Although smoking does have an impact on redox states (by about 20–30 mV), in this case, no significant correlation was seen between redox levels in smokers versus non-smokers in the bipolar patient cohort. This suggests that smoking did not add any further to the oxidative insult already seen in bipolar patients. We sought a validating correlation with a range of clinical features within the patient group. Years of illness or number of episodes might be expected to reflect illness intensity but did not strongly correlate with redox state. Unfortunately, these clinical measures are themselves uncertain proxy measures of long-term stressors. It is interesting that age of onset, which is largely unambiguous, did show the predicted relationship and so offers some validation of the redox findings.
Conclusion
We speculate that it is whole body oxidative stress that should be measured as the target biomarker for antioxidant treatments trialled for bipolar disorder (Magalhaes et al. Reference Magalhaes, Dean, Bush, Copolov, Malhi, Kohlmann, Jeavons, Schapkaitz, Anderson-Hunt and Berk2011; Berk et al. Reference Berk, Dean, Cotton, Gama, Kapczinski, Fernandes, Kohlmann, Jeavons, Hewitt, Moss, Allwang, Schapkaitz, Cobb, Bush, Dodd and Malhi2012; Dean et al. Reference Dean, Bush, Copolov, Kohlmann, Jeavons, Schapkaitz, Anderson-Hunt and Berk2012). Moreover, it would be informative to measure plasma glutathione redox to determine if it responds in parallel with clinical measures in intervention studies with antioxidants such as N-acetylcysteine (Magalhaes et al. Reference Magalhaes, Dean, Bush, Copolov, Malhi, Kohlmann, Jeavons, Schapkaitz, Anderson-Hunt and Berk2011; Berk et al. Reference Berk, Dean, Cotton, Gama, Kapczinski, Fernandes, Kohlmann, Jeavons, Hewitt, Moss, Allwang, Schapkaitz, Cobb, Bush, Dodd and Malhi2012, Dean et al. Reference Dean, Bush, Copolov, Kohlmann, Jeavons, Schapkaitz, Anderson-Hunt and Berk2012) or ebselen (Singh et al. Reference Singh, Halliday, Thomas, Kuznetsova, Baldwin, Woon, Aley, Antoniadou, Sharp, Vasudevan and Churchill2013). Our results are consistent with an important role for oxidative stress in bipolar disorder. Hitherto this has often been assumed to be a simple consequence of the accumulated burden of the illness experience. Glutathione levels are decreased and redox state compromised. This might account for cognitive impairment on the one hand and physical co-morbidity on the other. The correlations of glutathione parameters with age of onset are thought provoking because they imply an alternative or at least additional hypothesis: could impaired handling of oxidative stress be a risk factor for late-onset bipolar disorder? Clearly we need a careful comparison of early- and late-onset cases and better prospective longitudinal data to decide on the relative contribution of oxidative stress before and after illness onset. Clarification of this relationship will strengthen the potential for redox state to be a focus for treatment. Measurement of peripheral glutathione would then provide an important mediating biomarker of any drug or behavioural intervention.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291714000014.
Acknowledgements
This article presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research (grant reference no. RP-PG-0108-10087), the Instituto Carlos III (PI080180), the Centro de Investigación en Red de Salud Mental, CIBERSAM and the Generalitat de Catalunya to the Bipolar Disorders Group (2009 SGR 1022). A.R.R. is funded by the Spanish Ministry of Education through a Juan de la Cierva contract (JCI-2009-04329). N.S. is funded by the Department of Pharmacology, University of Oxford. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Declaration of Interest
G.M.G. received grants/research support, consulting fees and honoraria within the last 3 years from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Cephalon/Teva, Eisai, Eli Lilly, Lundbeck, Otsuka, P1Vital, Roche, Sanofi-Aventis, Servier, Sunovion and Takeda. J.R.G. acted as expert witness for Dr Reddys Laboratories and is chief investigator on the CEQUEL (Comparative Evaluation of QUEtiapine-Lamotrigine) trial to which GlaxoSmithKline has contributed and supplied investigational drugs and placebo. E.V. has served as consultant, advisor or speaker for the following companies: Almirall, AstraZeneca, Bial, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Johnson and Johnson Janssen, Jazz, Lundbeck, Merck-Sharp and Dohme, Novartis, Organon, Otsuka, Pfizer Inc., Sanofi-Aventis, Servier, Takeda and UBC.







