INTRODUCTION
Bovine besnoitiosis is a cattle disease caused by the cyst-forming apicomplexan parasite Besnoitia besnoiti. Although low mortality rates are reported (up to 10%) (Bigalke, Reference Bigalke1968), it is a chronic and a debilitating disease that causes local and systemic clinical signs. Thus, severe economic losses are incurred, mainly due to poor body condition, decreased milk production, and transient or definitive sterility in bulls. Additionally, the hides of affected animals are of low value for leather production (reviewed by Jacquiet et al. Reference Jacquiet, Liénard and Franc2010 and Álvarez-García et al. Reference Gutiérrez-Expósito, Ortega-Mora, Marco, Boadella, Gortázar, San Miguel-Ayanz, García-Lunar, Lavín and Álvarez-García2013).
In Europe, during the last century, the disease was restricted to the Pyrenees and Alentejo region in Portugal. However, recent outbreaks of bovine besnoitiosis have been reported in other European countries, and it is considered to be re-emergent due to the increased number of cases and the geographic dissemination over the last two decades (EFSA Journal, 2010; reviewed by Álvarez-García et al. Reference Gutiérrez-Expósito, Ortega-Mora, Marco, Boadella, Gortázar, San Miguel-Ayanz, García-Lunar, Lavín and Álvarez-García2013). The disease has been described in France, Portugal, Spain, Germany, Switzerland, Italy and Hungary (Besnoit and Robin, Reference Besnoit and Robin1912; Cortes et al. Reference Cortes, Reis, Waap, Vidal, Soares, Marques, Pereira da Fonseca, Fazendeiro, Ferreira, Caeiro, Shkap, Hemphill and Leitão2006b ; Alzieu, Reference Alzieu2007; Fernández-García et al. Reference Fernández-García, Risco-Castillo, Pedraza-Díaz, Aguado-Martínez, Álvarez-García, Gómez-Bautista, Collantes-Fernández and Ortega-Mora2009; Rostaher et al. Reference Rostaher, Mueller, Majzoub, Schares and Gollnick2010; Gentile et al. Reference Gentile, Militerno, Schares, Nanni, Testoni, Bassi and Gollnick2012; Basso et al. Reference Basso, Lesser, Grimm, Hilbe, Sydler, Trösch, Ochs, Braun and Deplazes2013; Hornok et al. Reference Hornok, Fedák, Baska, Hofmann-Lehmann and Basso2014).
Bovine besnoitiosis is also present in Africa, where it is widely distributed in sub-Saharan countries. In particular, the disease seems to be spreading in smallholder farming areas, limiting livestock production (as reported by farmers in Zimbabwe) (Chatikobo et al. Reference Chatikobo, Choga, Ncube and Mutambara2013), and it has been reported on several occasions in South Africa from the 1950s until the present day (Bigalke, Reference Bigalke, Ristic and McIntyre1981; Dubey et al. Reference Dubey, van Wilpe, Blignaut, Schares and Williams2013). The disease is also present in Israel (Neuman, Reference Neuman1972; Frank et al. Reference Frank, Pipano and Rosenberg1977; Goldman and Pipano, Reference Goldman and Pipano1983), and there have also been reports of it in Russia, China, Kazakhstan, Korea, Uzbekistan and Venezuela (Olias et al. Reference Olias, Schade and Mehlhorn2011), although these have not been confirmed. The existence of B. besnoiti infection in dairy, beef and mixed cattle from South Australia (Nasir et al. Reference Nasir, Lanyon, Schares, Anderson and Reichel2012) and Brazil (Uzêda et al. Reference Uzêda, Andrade, Corbellini, Antonello, Vogel and Gondim2014) could not be confirmed using complementary serological tools (enzyme-linked immunosorbent assay (ELISA), immunofluorescence antibody test (IFAT) and western blot). Confirmation of 17 and 9% seroprevalence rates reported in Egypt for cattle and water buffalo, respectively, is also necessary (Ashmawy and Abu-Akkada, Reference Ashmawy and Abu-Akkada2014).
The current spread of besnoitiosis in Europe has emphasized the need for prevalence studies to identify areas where the disease may be endemic and to determine the extent of the outbreaks (EFSA Journal, 2010). Recent seroprevalence studies carried out in Spain have confirmed that (i) the disease is found in beef cattle kept under extensive husbandry conditions and (ii) it is endemic and extremely widespread in northern mountainous regions (87·3% herd prevalence and 50% animal prevalence) (Urbasa-Andía Mountains and the Pyrenees) (Álvarez-García et al. Reference Álvarez-García, Fernández-García, Gutiérrez-Expósito, Ruiz-Santa Quinteria, Aguado-Martínez and Ortega-Mora2014; Gutiérrez-Expósito et al. in press). Rinaldi et al. (Reference Rinaldi, Maurelli, Musella, Bosco, Cortes and Cringoli2013) applied an ELISA to test sera from 88 cattle farms in southern Italy, where the disease had not been previously reported. Serological findings of 83 and 44·1% herd and animal prevalence, respectively, were reported. However further confirmation is required as no additional independent assays were used. In contrast, in non-endemic areas in central Spain, the number of seropositive animals increased up to 90·5%, and 43·2% exhibited clinical signs (Fernández-García et al. Reference Fernández-García, Álvarez-García, Risco-Castillo, Aguado-Martínez, Marcén, Rojo-Montejo, Castillo and Ortega-Mora2010). The incidence of clinical cases reported also varied depending on whether the disease was emerging (15–40% per year) or endemic (1–10% per year) (Cortes et al. Reference Cortes, Reis, Waap, Marcelino, Vaz, Nunes, Fanzendeiro, Caeiro and Leitão2006a ; Fernández-García et al. Reference Fernández-García, Álvarez-García, Risco-Castillo, Aguado-Martínez, Marcén, Rojo-Montejo, Castillo and Ortega-Mora2010; reviewed by Jacquiet et al. Reference Jacquiet, Liénard and Franc2010; Schares et al. Reference Schares, Basso, Majzoub, Rostaher, Scharr, Langenmayer, Selmair, Dubey, Cortes, Conraths and Gollnick2010; Liénard et al. Reference Liénard, Salem, Grisez, Prévot, Bergeaud, Franc, Gottstein, Alzieu, Lagalisse and Jacquiet2011). These studies support the hypothesis that infection spreads slowly within affected herds and could last at least several months or years.
Given the importance of the disease, the dynamics of B. besnoiti infection in cattle is reviewed in depth, and available knowledge on relevant parasite/host-dependent factors in the pathogenesis of the infection is summarized. Moreover, important questions requiring further clarification are also raised, taking into account the limitations of the few experimental infections carried out in cattle in the past.
BESNOITIA BESNOITI LIFE CYCLE AND TRANSMISSION
Besnoitia besnoiti is taxonomically closely related to Toxoplasma gondii and Neospora caninum (Ellis et al. Reference Ellis, Holmdahl, Ryce, Njenga, Harper and Morrison2000). Parasites of the genus Besnoitia are classified in the phylum Apicomplexa, family Sarcocystidae, sub-family Toxoplasmatinae, and this genus comprises 10 species (B. besnoiti, B. bennetti, B. jellisoni, B. wallacei, B. tarandi, B. darlingi, B. caprae, B. akadoni, B. neotomofelis and B. oryctofelisi) (Nganga et al. Reference Nganga, Kanyari and Munyua1994; Dubey and Lindsay, Reference Dubey and Lindsay2003; Dubey et al. Reference Dubey, Shkap, Pipano, Fish and Fritz2003a , Reference Dubey, Sreekumar, Rosenthal, Lindsay, Grisard and Vitor b , Reference Dubey, Sreekumar, Rosenthal, Vianna, Nylund, Nikander and Oksanen2004, Reference Dubey, Sreekumar, Donovan, Rozmanec, Rosenthal, Vianna, Davis and Belden2005; Oryan and Azizi, Reference Oryan and Azizi2008; Dubey and Yabsley, Reference Dubey and Yabsley2010). Among these species, B. besnoiti, B. tarandi, B. caprae and B. bennetti cause a similar disease in different species of ungulates (bovids, wild ruminants, goats and equids, respectively).
The definitive host of B. besnoiti is unknown; thus, its life cycle remains to be elucidated. The role of a carnivorous species was suspected, although such a species has not been identified yet (Diesing et al. Reference Diesing, Heydorn, Matuschka, Bauer, Pipano, de Waal and Potgieter1988; Basso et al. Reference Basso, Schares, Gollnick, Rütten and Desplazes2011). Cattle and wild bovids act as intermediate hosts of this parasite (Pols, Reference Pols1960; Basson et al. Reference Basson, Van Niekerk, McCully and Bigalke1965). Both the fast multiplying tachyzoites and slowly multiplying bradyzoites in tissue cysts (Fig. 1) develop in these animals, leading to the acute and the chronic stage, respectively (reviewed by Jacquiet et al. Reference Jacquiet, Liénard and Franc2010; Olias et al. Reference Olias, Schade and Mehlhorn2011; reviewed by Álvarez-García et al. Reference Gutiérrez-Expósito, Ortega-Mora, Marco, Boadella, Gortázar, San Miguel-Ayanz, García-Lunar, Lavín and Álvarez-García2013).

Fig. 1. Asexual stages of Besnoitia besnoiti. (A) Tachyzoites proliferating in a monolayer cell culture; (B) Tissue cysts in a haematoxylin-eosin-stained skin section.
Both tachyzoites and bradyzoites are crescent-shaped and contain common and typical apicomplexan organelles that have been previously detailed in bradyzoites. Bradyzoites are located inside tissue cysts composed of three layers: the outer layer, with connective-like tissue, the middle layer, which includes host nuclei, and the parasitophorous vacuole membrane (PVM) containing the bradyzoites. The host cell nucleus is enclosed in the cyst layer above the PVM, which is a feature that is distinct from other tissue cyst-forming coccidian parasites (Dubey et al. Reference Dubey, Shkap, Pipano, Fish and Fritz2003a , Reference Dubey, van Wilpe, Blignaut, Schares and Williams2013; Fernández-García et al. Reference Fernández-García, Risco-Castillo, Pedraza-Díaz, Aguado-Martínez, Álvarez-García, Gómez-Bautista, Collantes-Fernández and Ortega-Mora2009).
Mechanical transmission of B. besnoiti was achieved experimentally either with a syringe or with blood-sucking insects, such as tabanids, tsetse flies and Stomoxys calcitrans (Pols, Reference Pols1960; Bigalke, Reference Bigalke1968). The role of arthropods in parasite transmission is also supported by the seasonal incidence of the disease, with a restricted range of spread. Thus far, no cyclic transmission has been suggested because rather close contact is required for successful transmission (Schulz, Reference Schulz1960; Bigalke, Reference Bigalke1968; Liénard et al. Reference Liénard, Salem, Jacquiet, Grisez, Prévot, Blanchard, Bouhsira and Franc2013). Both acutely and chronically infected cattle may serve as reservoirs of the parasite for blood-sucking vectors. First, tachyzoites are thought to be present in blood only during the febrile reaction, and second, bradyzoites released from cysts may be successfully transmitted from long-lived chronically infected cattle that may act as reservoirs for naïve animals (Pols, Reference Pols1960; McCully et al. Reference McCully, Basson, Van Niekerk and Bigalke1966; Bigalke et al. Reference Bigalke, Van Niekerk, Basson and McCully1967). Studies supporting the triad including cattle, wild ruminants and blood-sucking arthropods playing an important role in natural transmission of bovine besnoitiosis have not been conclusive. In recent studies performed in the Pyrenees, where a high density of wild ruminant species, cattle and tabanids is found, specific anti-Besnoitia spp. antibodies were found only in two specimens, a roe and a red deer (Gutiérrez-Expósito et al. Reference Gutiérrez-Expósito, Ortega-Mora, Marco, Boadella, Gortázar, San Miguel-Ayanz, García-Lunar, Lavín and Álvarez-García2013); and, despite the opportunistic feeding behaviour of tabanids, they fed mainly on red deer and cattle, with a preference for red deer (Baldacchino et al. Reference Baldacchino, Gardès, De Stordeur, Jay-Robert and Garros2014). Accordingly it was suggested that two wild ruminant species might serve as an intermediate host, and tabanids might be involved in parasite transmission. However, in both cases parasite detection is not yet confirmed.
In addition, there is epidemiological evidence that supports the hypothesis that horizontal transmission (e.g. direct contact throughout natural mating, wounds and lacerations) could be an important means of parasite dissemination in a herd since an association between the disease prevalence and the animals’ age has been reported. Moreover, Bigalke (Reference Bigalke1968) suggested that the bradyzoite stage might be able to cross mucous membranes. However, this transmission route has not been conclusively proven (reviewed by EFSA Journal, 2010 and Álvarez-García et al. Reference Gutiérrez-Expósito, Ortega-Mora, Marco, Boadella, Gortázar, San Miguel-Ayanz, García-Lunar, Lavín and Álvarez-García2013). Recent reports of the disease in Spain, Italy, Germany and Switzerland have indicated the potential of introducing B. besnoiti into a non-endemic area through the introduction of infected animals into a naïve herd (reviewed by Álvarez-García et al. Reference Gutiérrez-Expósito, Ortega-Mora, Marco, Boadella, Gortázar, San Miguel-Ayanz, García-Lunar, Lavín and Álvarez-García2013). In fact, several studies support the hypothesis that sub-clinical cases may act as reservoirs for infection and spread of the disease in a herd (Basso et al. Reference Basso, Lesser, Grimm, Hilbe, Sydler, Trösch, Ochs, Braun and Deplazes2013; Frey et al. Reference Frey, Gutiérrez-Expósito, Ortega-Mora, Benavides, Marcén, Castillo, Casasús, Sanz, García-Lunar, Esteban-Gil and Álvarez-García2013).
DYNAMICS OF B. BESNOITI INFECTION IN CATTLE
Pathogenesis, clinical signs and pathological findings
Transmission experiments have shown that once bovines are infected with B. besnoiti, the incubation period may vary from 1 to 13 days, depending on the method of the infection with an average of 13 days in natural infections (Bigalke, Reference Bigalke1968; Basson et al. Reference Basson, McCully and Bigalke1970; Bigalke and Prozesky, Reference Bigalke, Prozesky, Coetzer and Tustin2004). Bovine besnoitiosis progresses in two sequential phases (reviewed by Jacquiet et al. Reference Jacquiet, Liénard and Franc2010 and Álvarez-García et al. Reference Gutiérrez-Expósito, Ortega-Mora, Marco, Boadella, Gortázar, San Miguel-Ayanz, García-Lunar, Lavín and Álvarez-García2013): the febrile acute phase, also known as anasarca phase, caused by the fast-replicating tachyzoite stage in endothelial cells, tunica media, adventitia and mononuclear cells, and characterized initially by fever and later by oedema that may last 4–5 weeks post-infection (wpi), followed by the chronic or scleroderma phase, when pathognomonic tissue cysts develop and cause skin lesions (Basson et al. Reference Basson, McCully and Bigalke1970). Subsequently, affected animals remain persistently infected. Oedemas and tissue cysts may be present simultaneously from 11 days post-infection (dpi) until 4–5 wpi; this time interval is also known as the sub-acute phase (Basson et al. Reference Basson, McCully and Bigalke1970).
Febrile acute stage
Initially, the infection is characterized by hyperthermia (40·8 to 41·6 °C), which may cause abortions (Pols, Reference Pols1960; Bigalke, Reference Bigalke1968; Givens and Marley, Reference Givens and Marley2008), and non-specific clinical signs that may go unnoticed, such as depression, swelling of the superficial lymph nodes, weight loss, photophobia, ocular and nasal discharge, and increased heart and respiratory rates (Pols, Reference Pols1960; Schulz, Reference Schulz1960). During this febrile acute stage, which may last for 3 to 10 days, tachyzoites, as obligate intracellular parasites, start invading the endothelial cells of blood vessels, causing degenerative and fibrinoid necrotic vascular lesions, vasculitis, and thrombosis, which subsequently cause congestion, haemorrhages and infarcts (Pols, Reference Pols1960; Basson et al. Reference Basson, McCully and Bigalke1970). It has also been suggested that a possible toxic effect of the parasite might be its ability to cause increased vascular permeability (Bigalke and Prozesky, Reference Bigalke, Prozesky, Coetzer and Tustin2004). These lesions are mainly located in medium-sized and smaller veins as well as some arteries in the skin and testes (McCully et al. Reference McCully, Basson, Van Niekerk and Bigalke1966; Basson et al. Reference Basson, McCully and Bigalke1970). Thus, vascular lesions play a key role in the pathogenesis of bovine besnoitiosis. Rapidly multiplying parasites have been observed up to 10–12 days after infection; at this point, most vascular lesions have already been observed (Basson et al. Reference Basson, McCully and Bigalke1970). Released tachyzoites from endothelial cells and parasitized mononuclear cells are disseminated by the blood circulation (Pols, Reference Pols1960). Free parasites enter new cells to repeat the parasite lytic cycle. Proliferative organisms may be detected in blood from the 3rd up to 12th day of the beginning of the febrile reaction (Bigalke, Reference Bigalke1968; Basson et al. Reference Basson, McCully and Bigalke1970). The rapid multiplication of the parasite in these locations causes increased vascular permeability, and subsequently it produces oedema that initially appears in the head and neck areas, although it may progress to the limbs and ventral parts of the body, such as the breast and scrotum, where it becomes more visible (Pols, Reference Pols1960; Basson et al. Reference Basson, McCully and Bigalke1970). Parasites may cause acute ophthalmitis, likely accompanied by oedema, increased lacrimation and photophobia (McCully et al. Reference McCully, Basson, Van Niekerk and Bigalke1966). Haemorrhages, necrosis, pseudo-membranes, oedemas and erosions may be found in the upper respiratory tract. In severe cases, oedemas in alveolar and interstitial tissues in the lungs, accompanied by pneumonitis and emphysema, may also cause severe respiratory disorders. Oedemas in joints cause painful movements that may lead to permanent posterior lameness.
Tachyzoites, together with a few haemorrhagic and necrotic foci, may also be present in different locations such as the iris, muscles, lymph nodes and lungs with eosinophils and mononuclear infiltration. Interestingly, Dubey et al. (Reference Dubey, van Wilpe, Blignaut, Schares and Williams2013) reported for the first time that acutely infected animals may die of nephrotic syndrome that leads to severe hypoalbuminaemia, hyperproteinuria and mild leucocytosis.
Scleroderma stage
Chronic infection is produced by the slow replication of bradyzoites inside tissue cysts with tropism for connective tissues mainly on the mucous membranes, superficial skin layers and male genital tract (Pols, Reference Pols1960; Bigalke, Reference Bigalke, Ristic and McIntyre1981; Kumi-Diaka et al. Reference Kumi-Diaka, Wilson, Sanusi, Njoku and Osori1981). There is no evidence of parasitaemia in chronically infected cattle, although Besnoit and Robin (Reference Besnoit and Robin1912) suggested the occurrence of periodic parasite release into the blood.
Cyst formation occurs immediately after extracystic proliferation terminates at approximately 11 dpi in blood vessel walls and also extravascularly, and the appearance of cysts is near synchronous (Bigalke, Reference Bigalke1968). Young cysts may contain 1–4 bradyzoites (Dubey et al. Reference Dubey, van Wilpe, Blignaut, Schares and Williams2013). The hypertrophic cells become multinucleated and rapidly enlarge until 4 wpi, when their growth gradually diminishes (Basson et al. Reference Basson, McCully and Bigalke1970). At 4–5 wpi, tissue cysts measuring more than 100 μm are already developed, and cyst development apparently reaches completion at 10 wpi (Basson et al. Reference Basson, McCully and Bigalke1970). In a recent study carried out with different antibody markers, host cyst cells showed features of myofibroblasts (Dubey et al. Reference Dubey, van Wilpe, Blignaut, Schares and Williams2013), which originate from fibroblasts during tissue repair (Hinz, Reference Hinz2007). Interestingly, many tissue cysts may contain dead bradyzoites or even appear empty (Dubey et al. Reference Dubey, van Wilpe, Blignaut, Schares and Williams2013). Multifocal white pinpoint tissue cysts in the scleral conjunctiva are pathognomonic, and, together with those located in the vulvar region, nasal mucous tissues may be detected upon clinical examination as early as 8 wpi (Fig. 2A). Mature cysts are spherical to sub-spherical in shape and measure up to 390 μm in diameter, containing approximately 200 000 packed bradyzoites (Bigalke, Reference Bigalke, Ristic and McIntyre1981). According to Bigalke (Reference Bigalke1968), an estimation of the infection age can be based on cyst size, although it is dependent on the incubation period. Interestingly, tissue cyst disintegration might be observed from 30 dpi onward (Basson et al. Reference Basson, McCully and Bigalke1970). With the disappearance of oedemas, a progressive thickening, folding or wrinkling of the skin and scrotal skin, alopecia, hyperkeratosis, scleroderma, scars and nodules on udders, atrophy and induration of testes are observed (Fig. 2B and C). Skin lesions may be explained by the presence of numerous tissue cysts in the dermal and stratum papillare, accompanied by a granulomatous reaction and fibrosis surrounding the cysts. These events, together with vascular lesions that interfere with the blood supply, are responsible for alopecia (Bigalke, Reference Bigalke1968).

Fig. 2. Clinical signs of chronic bovine besnoitiosis. (A) Tissue cysts in the sclera and palpebral conjunctiva; (B) Elephant skin; (C) Nodules in the udder.
Tissue cysts frequently appear in the same tissues where the initial proliferation in vessels occurred (Neuman, Reference Neuman1962; McCully et al. Reference McCully, Basson, Van Niekerk and Bigalke1966; Kumi-Diaka et al. Reference Kumi-Diaka, Wilson, Sanusi, Njoku and Osori1981; Nobel et al. Reference Nobel, Klopfer, Perl, Nyska, Neumann and Brenner1981). In general terms, tissue cysts are more likely to be found in the skin (dermis and subcutis), sclera, upper respiratory tract, testes and epididymes in males and in the vestibulum vaginae and vagina in females. In accordance with these results, Frey et al. (Reference Frey, Gutiérrez-Expósito, Ortega-Mora, Benavides, Marcén, Castillo, Casasús, Sanz, García-Lunar, Esteban-Gil and Álvarez-García2013) recently assessed the intra-organ parasite distribution in cattle sub-clinically infected with B. besnoiti using histological and molecular tools and determined that the most frequent cyst locations were the upper respiratory tract (rhinarium, larynx/pharynx) followed by the distal genital tract (vulva/vagina) and the skin of the neck. The skin of the face, upper eyelid, tip of the tail and breech harbour the highest parasite load (Basson et al. Reference Basson, McCully and Bigalke1970). Tissue cysts can also be present in other locations, such as connective tissues, skeletal muscles and tendons of the limbs, the periosteum of metatarsal and metacarpal bones, and sometimes in the connective tissue and muscles of the tongue, lymph nodes, spleen, liver, lung and heart (McCully et al. Reference McCully, Basson, Van Niekerk and Bigalke1966; Basson et al. Reference Basson, McCully and Bigalke1970; Nobel et al. Reference Nobel, Neumann, Klopper and Perl1977; Rommel, Reference Rommel1978; Kumi-Diaka et al. Reference Kumi-Diaka, Wilson, Sanusi, Njoku and Osori1981; Rostaher et al. Reference Rostaher, Mueller, Majzoub, Schares and Gollnick2010; Olias et al. Reference Olias, Schade and Mehlhorn2011; Dubey et al. Reference Dubey, van Wilpe, Blignaut, Schares and Williams2013; Frey et al. Reference Frey, Gutiérrez-Expósito, Ortega-Mora, Benavides, Marcén, Castillo, Casasús, Sanz, García-Lunar, Esteban-Gil and Álvarez-García2013).
McCully et al. (Reference McCully, Basson, Van Niekerk and Bigalke1966) studied the distribution of Besnoitia spp. cysts in the cardiovascular system of chronically infected cattle, reporting that small veins of the limbs, head (saphenous, cephalic and facial veins) and tail are the most parasitized blood vessels. Moreover, cysts were frequently observed in the valves and cysts attached to the endocardium. Cysts were also found in the arteries of the limbs. The cysts located under the endothelium may protrude (or not) into the lumen, and, in response to this, the endothelium becomes hyperplasic (McCully et al. Reference McCully, Basson, Van Niekerk and Bigalke1966). Tissue cysts located in the three histological layers of the blood vessels – intima, media and adventitia – may cause sclerosis. Interestingly, no cysts were observed in large veins and arteries (McCully et al. Reference McCully, Basson, Van Niekerk and Bigalke1966). In this study, the presence and position of cysts is reported, which are clearly dependent on the vascularity of the peripheral tissues. Therefore, cysts located in skin appear more numerous in the vascular stratum papillare and subcutis compared with the stratum reticulare. Respiratory system cysts were more frequently found in the nasal mucosa, paranasal sinuses, pharynx and larynx followed by the trachea, bronchi and lungs (McCully et al. Reference McCully, Basson, Van Niekerk and Bigalke1966).
The presence of tissue cysts in different locations of the male genital organs may lead to permanent sterility as a result of several sequential events (Neuman, Reference Neuman1962; Kumi-Diaka et al. Reference Kumi-Diaka, Wilson, Sanusi, Njoku and Osori1981; Dubey et al. Reference Dubey, van Wilpe, Blignaut, Schares and Williams2013). The scrotum and its contents are the most strongly affected organs, in contrast to the vasa deferentia, which are less affected (Neuman, Reference Neuman1962; Kumi-Diaka et al. Reference Kumi-Diaka, Wilson, Sanusi, Njoku and Osori1981). Tissue cysts have tropism for vascular walls, particularly those within the pampiniform plexus, leading to reduction of the blood supply to the testes (Kumi-Diaka et al. Reference Kumi-Diaka, Wilson, Sanusi, Njoku and Osori1981). In addition, scattered cysts are located under the epithelial cells in the parenchyma between the seminiferous tubules and are surrounded by lymphocytic infiltration and extensive fibrosis (Dubey et al. Reference Dubey, van Wilpe, Blignaut, Schares and Williams2013). These cysts cause direct pressure that leads to necrosis and mineralization with subsequent testicular and epididymal atrophy (Kumi-Diaka et al. Reference Kumi-Diaka, Wilson, Sanusi, Njoku and Osori1981) (Fig. 3). Coagulative necrosis with dystrophic calcification may also be found in seminiferous tubules (Basson et al. Reference Basson, McCully and Bigalke1970). Moreover, poor heat exchange through the thickened scrotum can contribute to permanent azoospermia and infertility. The testicles appear fibrotic, and the seminiferous tubules are degenerated or even calcified. Moreover, cysts were also detected in the testes and epididymes (interstitial tissue and seminiferous tubules) as well as in the tunica dartos, vaginalis and albuginea, containing some peritoneal exudate. In addition, in accordance with the observations made by Schulz (Reference Schulz1960), fibrosis and inflammatory cells were detected with diffuse interstitial lymphoplasmacytic orchitis associated with the presence of cysts (Dubey et al. Reference Dubey, van Wilpe, Blignaut, Schares and Williams2013). Consequently, there may be an absence of spermatogenic activity; alternatively, even if the lesions are not very extensive, 50% of the spermatozoa may be dead, and the remainder may be morphologically abnormal. The spermiogram is consequently altered, with an absence of tissue cysts but many giant cells, epithelial cells and medusa cells present in the semen (Kumi-Diaka et al. Reference Kumi-Diaka, Wilson, Sanusi, Njoku and Osori1981). Further investigations are needed to elucidate the incidence of sub-fertile bulls.

Fig. 3. Sequential pathogenic events associated with B. besnoiti infection in the male reproductive tract.
Concerning the female reproductive tract, Nobel et al. (Reference Nobel, Klopfer, Perl, Nyska, Neumann and Brenner1981) assessed the complete genital tract of 16 chronically infected cows with visible tissue cysts in the skin and eyes. However, only six cows with more than 50 cysts per skin/eye section had cysts in their genital tracts, with the uterus being the most affected organ. In this work, granulomas were observed around approximately 30% of the cysts. However, it was stated that neither the presence of cysts nor the development of these granulomas interfered with the normal functioning of the affected organs. Interestingly, cysts were not detected in the placenta. In contrast to the results reported by Nobel et al. (Reference Nobel, Klopfer, Perl, Nyska, Neumann and Brenner1981), Frey et al. (Reference Frey, Gutiérrez-Expósito, Ortega-Mora, Benavides, Marcén, Castillo, Casasús, Sanz, García-Lunar, Esteban-Gil and Álvarez-García2013) were only able to find the parasite in the distal genital tract of sub-clinically infected cows. Moreover, vertical transmission has not been proven. Indeed, chronically infected cows may become pregnant and successfully give birth to healthy calves that develop normally (Shkap et al. Reference Shkap, Pipano, Marcus and Krigel1994), which leads us to hypothesize that vertical transmission is unlikely to occur.
The degree of inflammation surrounding the tissue cysts can be extremely variable, and in some cases, inflammation may not even be detected. Some cysts associated with a significant non-purulent inflammatory infiltrate and normal cysts surrounded by granulomatous reactions without evident degeneration or rupture of the cyst wall can be detected. Inflammation could be observed in all three blood vessel layers, and moreover, necrotizing inflammatory or mild granulomatous reactions were observed mainly around degenerated and/or ruptured cysts, as previously reported by Besnoit and Robin (Reference Besnoit and Robin1912) and Schulz (Reference Schulz1960).
Death may occur in both stages of the disease regardless of the sex of the animal, and animals may be culled due to poor body condition (Pols, Reference Pols1960; Schulz, Reference Schulz1960).
In an infected herd, cattle showing clinical signs either of acute or chronic infection appear to be only the tip of the iceberg in both endemic and epidemic situations. Moreover, only a small portion of infected animals may exhibit macroscopic tissue cysts in the conjunctiva or vulvar region. However, a higher percentage of cattle comprises sub-clinically infected but seropositive animals, thus representing an important risk for disease transmission (Fernández-García et al. Reference Fernández-García, Álvarez-García, Risco-Castillo, Aguado-Martínez, Marcén, Rojo-Montejo, Castillo and Ortega-Mora2010; Basso et al. Reference Basso, Lesser, Grimm, Hilbe, Sydler, Trösch, Ochs, Braun and Deplazes2013; Frey et al. Reference Frey, Gutiérrez-Expósito, Ortega-Mora, Benavides, Marcén, Castillo, Casasús, Sanz, García-Lunar, Esteban-Gil and Álvarez-García2013).
Parasite and host factors associated with B. besnoiti infection
Many fundamental variables related to both the parasite and the host were studied between the 1960s and 1980s under different experimental conditions, making it difficult to compare the results. Indeed, there was a lack of harmonization in experimental design without a commonly accepted procedure for evaluation of the parameters, including experimental infection/inoculations, sampling of animals, number of animals per group (in many cases, only one animal per group was employed) and a variable experimental duration. Moreover, in the past, experiments were mainly based on clinical inspection and histopathology, serological tests were rarely employed, and molecular tests were not available. Despite these issues, experimental inoculations in bovines were carefully described, and the authors pointed out the difficulty of producing a characteristic clinical case of bovine besnoitiosis (Bigalke, Reference Bigalke1968; Basson et al. Reference Basson, McCully and Bigalke1970; Diesing et al. Reference Diesing, Heydorn, Matuschka, Bauer, Pipano, de Waal and Potgieter1988; Shkap and Pipano, Reference Shkap and Pipano1993).
In the studies carried out by Bigalke and co-workers in South Africa, three parameters were considered for infection criteria: detection of a distinct febrile reaction, demonstration of Besnoitia tachyzoites and immunity to challenge, as infected animals seemed to be resistant to re-infections (Bigalke, Reference Bigalke1968).
Parasite-dependent factors
Information available to date concerning parasite-dependent factors, such as virulence of the parasite isolate, parasite stage and inoculation dose, has been obtained from a few previously mentioned studies (summarized in Tables 1 and 2). Most of the acute cases described were characterized by fever and, in a few cases, by localized oedemas in extremities and the head. A few exceptional cases were described by Basson et al. (Reference Basson, McCully and Bigalke1970), in which a generalized and profound oedema restricted to the head and extremities was developed, resulting in the death of animals at 1–2 wpi.
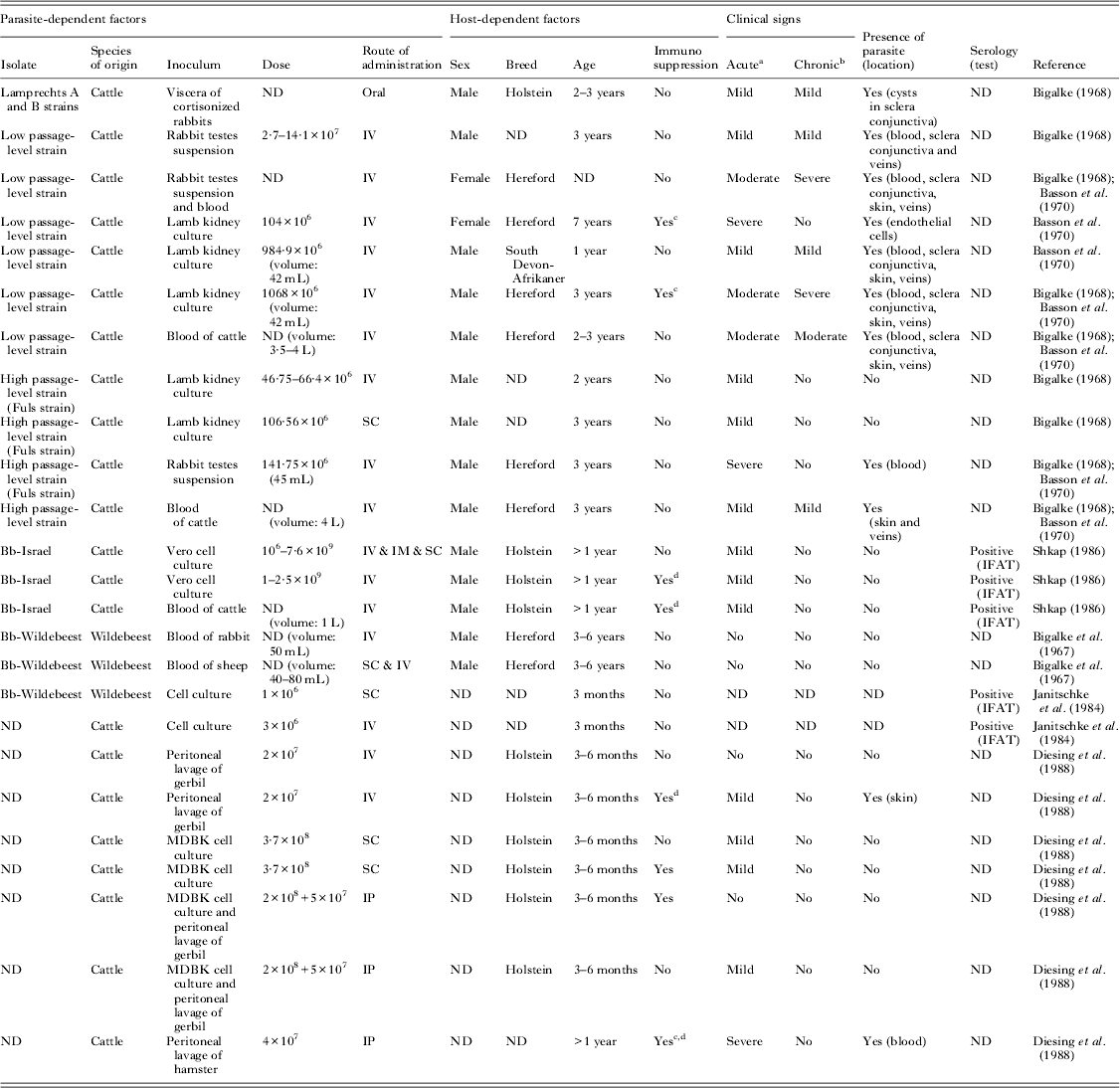
Table 1. Experimental infections with B. besnoiti tachyzoites in cattle

ND: not determined.
a Acute clinical signs: mild (fever)/moderate (fever, oedema, weakness)/severe (death).
b Chronic clinical signs: mild (visible tissue cysts in scleral conjunctiva)/moderate (hyperkeratosis, alopecia and tissue cysts)/severe (loss body condition and death).
c Splenectomy.
d Corticoids.
Table 2. Experimental infections with B. besnoiti bradyzoites in cattle

a Acute clinical signs: mild (fever)/moderate (fever, oedema, weakness)/Severe (death).
b Chronic clinical signs: mild (visible tissue cysts in scleral conjunctiva)/moderate (hyperkeratosis, alopecia and tissue cysts)/severe (loss body condition and death).
c Corticoids.
d Splenectomy.
ND: not determined.
Parasite isolates
Different parasite isolates obtained in various laboratories were employed in various experimental inoculations (between the 1960s and the 1980s) (Tables 1, 2 and 3). They were initially isolated from either acute or chronic cases of besnoitiosis in cattle and were maintained in the laboratory by successive inoculations of blood from donor cattle into rabbits. In some cases, the parasite isolates were maintained in vitro through passaged cell culture systems (Tables 1 and 3). The bovine isolates obtained in Israel and, more recently, in Europe were directly isolated from biopsies from chronically infected cattle and further maintained and propagated in cell cultures (Table 3; Fig. 1A).
Table 3. B. besnoiti isolates

a At present not available isolates.
The isolate appears to be a key parasite-dependent factor according to differences in virulence observed among isolates. Two B. besnoiti isolates were obtained from cattle and other ruminant species, including chronically infected blue wildebeest and impala antelopes in South Africa. At the time of the study, it was assumed that these isolates were B. besnoiti species due to the similarities of the clinical signs and tissue cysts observed. In addition, it was possible to induce cross-immunity between the cattle and antelope isolates. Moreover, differences in virulence were reported, and it was speculated that the higher virulence observed in cattle could be explained by a recent adaptation of the parasite to the host, compared with a more ancient form of besnoitiosis in antelopes. In particular, when the impala and blue wildebeest strains were inoculated into rabbits, they produced milder infections compared with the bovine isolates (Basson et al. Reference Basson, McCully and Bigalke1970). The blue wildebeest strain was not virulent in cattle and showed viscerotropism compared with dermatotropism of bovine strains and was later employed as a live vaccine (Bigalke et al. Reference Bigalke, Van Niekerk, Basson and McCully1967, Reference Bigalke, Schoeman and McCully1974). Furthermore, a different topographical distribution of tissue cysts was observed between the bovine and antelope strains: the antelope strains were usually found in the jugular veins compared with cattle strains, whose cysts were more abundant in superficial veins of the head (McCully et al. Reference McCully, Basson, Van Niekerk and Bigalke1966). Another significant difference was the position of the tissue cysts of cattle strains in the three histological layers of blood vessels whereas in the antelope tissue cysts were attached to the intima (McCully et al. Reference McCully, Basson, Van Niekerk and Bigalke1966). However, whether it is a strain- or host-dependent finding remains to be elucidated. The Israeli bovine and the South African wildebeest strain were compared using isoenzyme electrophoresis to clarify the relationship between cattle and antelope strains. The banding patterns of six enzymes were used to distinguish between the two strains. The results indicated clear genetic differences, thus supporting the hypothesis of Bigalke et al. (Reference Bigalke, Van Niekerk, Basson and McCully1967) and Tadros and Laarman (Reference Tadros and Laarman1982) that cattle and antelope strains are distinct (Le Blancq et al. Reference Le Blancq, Desser, Shkap and Pipano1986). The bovine isolate of B. besnoiti employed by Basson et al. (Reference Basson, McCully and Bigalke1970) was more pathogenic to rabbits than the isolate obtained from an antelope, which was unable to cause lesions in the skin and testes. Either tachyzoites or bradyzoites isolated from natural bovine cases or even isolates maintained in vitro for 2–13 passages in cell culture were able to induce the formation of a high number of cysts in rabbits (Pols, Reference Pols1954, Reference Pols1960; Bigalke et al. Reference Bigalke, Van Niekerk, Basson and McCully1967, Bigalke, Reference Bigalke1968). This finding contrasts with the finding that a high passage-level rabbit isolate (the Fuls isolate) was considerably less pathogenic, and it was hypothesized that this strain may have lost its cyst-forming capacity (Basson et al. Reference Basson, McCully and Bigalke1970). In the experimental infections carried out by Shkap, a bovine isolate from Israel proved to be of low virulence and has been employed as a live vaccine for the last two decades (Shkap, Reference Shkap1986). The degree of pathogenicity of various B. besnoiti isolates has not yet been addressed and therefore requires further research. Basson et al. (Reference Basson, McCully and Bigalke1970) suggested that differences in the parasite isolate or bovine host might influence the cyst growth rate and the parasite load. Intra-specific strain/isolate variability should be further investigated, as the panel of available B. besnoiti isolates might differ and is likely to have changed from the 1960s to the present day (Table 3).
Parasite stage
Both parasite stages described to date – tachyzoite and bradyzoite – have been used for inoculations, and the results showed that both stages are infective for both cattle and rabbits (Pols, Reference Pols1960; Bigalke et al. Reference Bigalke, Van Niekerk, Basson and McCully1967, Bigalke, Reference Bigalke1968). However, different inoculation routes and variable ranges of inoculation doses and types of inoculum were employed (Tables 1 and 2).
The clinical outcome of experimental infection in cattle varied from complete absence of clinical signs to severe acute cases with respiratory disorders and even death with parasitaemia. There were also infected animals that showed early development of tissue cysts and skin lesions. Tissue cysts were only found in the veins of the extremities in the mildest chronic cases, while as the severity of the disease increased, cysts were widely distributed and were observed in the conjunctiva, upper respiratory tract and subcutis. Severely affected chronic cases showed thickened skin and partial alopecia, mainly around the muzzle, ears, eyes and neck. In general, most infected animals developed mild disease characterized by fever with no oedema development; a few cases developed tissue cysts. Infections with bradyzoites were less severe, and the most successful infections were obtained upon immunosuppression treatments with cortisone (Diesing et al. Reference Diesing, Heydorn, Matuschka, Bauer, Pipano, de Waal and Potgieter1988). However, further comparative experimental infections are needed to determine the ability to induce clinical signs when bradyzoites vs tachyzoites from the same isolate are inoculated in cattle.
There are several concerns that need to be addressed with regard to the inocula employed for both parasite stages. Acutely and chronically infected animals usually served as parasite donors throughout infection via blood transfusions and skin tissues, respectively. On some occasions, blood from other acutely infected species (e.g. rabbits and sheep) as well as rabbit testes suspensions was employed in the inoculations to provide proliferating organisms (tachyzoites). The main limitations included the variable inoculation volumes, undetermined parasite doses, number of in vivo rabbit passages, preparation of inocula that could compromise parasite viability and unknown virulence of the isolates. Moreover, parasites propagated in cell culture were also inoculated. However, on some occasions, the number of in vitro passages and the parasite viability prior to inoculations were not determined (Tables 1 and 2).
Dose
A variable range of tachyzoite- and bradyzoite-based inocula doses were employed (Tables 1 and 2). The dose is expected to play an important role in producing disease. Based on the clinical findings in experimentally infected cattle, the inocula dose might be highly dependent on the parasite viability and virulence of the isolate. According to the results obtained, it appears that this variable might be an important determinant in prioritizing a severe vs a mild infection.
Host-dependent factors
The most robust data on host-dependent factors have been obtained from natural infections. The limitations of the experimental infections mentioned above make it difficult to infer conclusive results regarding sex, aptitude and breed, age and immunity. Moreover, the animals employed in the experimental inoculations were not previously serologically tested in most cases, and immunity to re-infections has been reported.
Host species
Regarding ruminant species, other similar Besnoitia spp. infections have also been reported in reindeer and caribou, caused by B. tarandi (Dubey et al. Reference Dubey, Sreekumar, Rosenthal, Vianna, Nylund, Nikander and Oksanen2004), and in goats, caused by B. caprae (Njenga et al. Reference Njenga, Bwangamoi, Mutiga, Kangethe and Mugera1993; Oryan and Azizi, Reference Oryan and Azizi2008). The ability of these species to infect cattle has not yet been investigated; thus, it is not known whether these species provide cross-immunity to bovine B. besnoiti. This important issue deserves further attention to determine the possible role of these ruminants in the epidemiology of bovine besnoitiosis. With regard to this question, Pols (Reference Pols1960) showed that sheep and goats were susceptible to B. besnoiti infections. When these small ruminant species received parasitized blood from rabbits, both developed fever, and skin tissue cysts could be detected in goats.
Concerning laboratory animals, rabbits were inoculated repeatedly with B. besnoiti because both the acute and chronic stages of the disease could be successfully reproduced in this animal species, which served as a source of parasites for experimental inoculations in cattle. These studies also provided some clues about the role of parasite-dependent factors in the pathogenesis of the disease (Pols, Reference Pols1960; Bigalke, Reference Bigalke1968). Unfortunately, many other rodent species, such as Syrian hamsters (Mesocricetus auratus), guinea pigs (Cavia porcellus), gerbils (Meriones unguiculatus), common voles (Microtus arvalis) and NMRI mice have also been inoculated with either B. besnoiti tachyzoites or bradyzoites; however, the persistence of the parasite could only be demonstrated in voles, and none of the species developed clinical signs (Bigalke, Reference Bigalke1968; Basso et al. Reference Basso, Schares, Gollnick, Rütten and Desplazes2011). Thus, information regarding other potential intermediate hosts aside from bovids is still scarce. Moreover, the possible role of different mammal species, including cats, different wild felid species, and dogs, in addition to birds and snakes as definitive hosts was investigated by administering tachyzoites and tissue cysts containing bradyzoites from chronically infected cattle via an oral route; however, oocysts were not shed in the faeces (Diesing et al. Reference Diesing, Heydorn, Matuschka, Bauer, Pipano, de Waal and Potgieter1988; Basso et al. Reference Basso, Schares, Gollnick, Rütten and Desplazes2011).
Inoculation route
The inoculation route is also a critical factor in infection. In fact, the incubation period in cattle is dependent on the route of infection, and the shortest period (less than 2 days) was observed when tachyzoites were intravenously inoculated. Consequently, the route of inoculation is associated with the time required to develop mature tissue cysts (Bigalke, Reference Bigalke1968; Basson et al. Reference Basson, McCully and Bigalke1970). Subcutaneous and intravenous inoculations appear to more properly mimic the natural transmission route of the parasites, as they were the most successful with respect to inducing the characteristic clinical signs of bovine besnoitiosis (Tables 1 and 2).
Interestingly, the oral and nasal routes were also employed in experimental cattle infections (Bigalke, Reference Bigalke1968). When inoculations were successful, the incubation period lasted between 9 and 14 days. When viscera of experimentally infected rabbits harbouring most likely only tachyzoites were given orally to an ox, a pronounced febrile reaction lasting for 5 days was observed and a few tissue cysts were detected at 80 dpi. In contrast, when cysts were administered via an oral route, the typical bovine besnoitiosis signs were absent. Finally, the author suggested that bradyzoites are able to penetrate mucous membranes when cysts are administered via a nasal route, causing a mild infection.
Breed
All breeds of cattle appear to be susceptible to besnoitiosis. The infection has been described in a wide variety of breeds, such as Africander, Hereford, Shorthorn, Bonsmara, Nguni, Jersey, Holstein, Alpine brown, Pyrenean, Cross-breeds, Charolaise and Limousine (among others), and significant differences appear to be related with breed aptitude and, consequently, husbandry conditions that may facilitate parasite transmission, as higher prevalence rates have been detected in beef cattle breeds (Bigalke, Reference Bigalke1968; Goldman and Pipano, Reference Goldman and Pipano1983; Liénard et al. Reference Liénard, Salem, Grisez, Prévot, Bergeaud, Franc, Gottstein, Alzieu, Lagalisse and Jacquiet2011; Álvarez-García et al. Reference Álvarez-García, Fernández-García, Gutiérrez-Expósito, Ruiz-Santa Quinteria, Aguado-Martínez and Ortega-Mora2014).
Aptitude
Differences in prevalence have been detected between beef and dairy cattle, which appear to be associated with different management systems rather than different breed susceptibilities. In Israel, a seroprevalence rate of 10% for dairy cattle vs 50% for beef cattle was reported when 1700 animals were tested. Moreover, the highest antibody titres corresponded to beef cattle (Goldman and Pipano, Reference Goldman and Pipano1983). Similarly, a recent study conducted in a traditionally endemic northern province in Spain (Navarra) with mountainous areas showed a significant association between the prevalence of B. besnoiti infection and cattle management systems, reporting 16% seroprevalence in beef cattle vs 0% in dairy cattle (Álvarez-García et al. Reference Álvarez-García, Fernández-García, Gutiérrez-Expósito, Ruiz-Santa Quinteria, Aguado-Martínez and Ortega-Mora2014). In beef cattle systems, parasite transmission might be favoured by different factors, such as natural mating, communal pastures outdoors, and exposure to wild ruminants (red deer and roe deer) and blood-sucking arthropods, which may explain the differences in prevalence rates observed between beef and dairy cattle (Frank et al. Reference Frank, Pipano and Rosenberg1977; Goldman and Pipano, Reference Goldman and Pipano1983; Fernández-García et al. Reference Fernández-García, Álvarez-García, Risco-Castillo, Aguado-Martínez, Marcén, Rojo-Montejo, Castillo and Ortega-Mora2010; Álvarez-García et al. Reference Álvarez-García, Fernández-García, Gutiérrez-Expósito, Ruiz-Santa Quinteria, Aguado-Martínez and Ortega-Mora2014).
Nevertheless, bovine besnoitiosis has also been described in dairy cattle. Liénard et al. (Reference Liénard, Salem, Grisez, Prévot, Bergeaud, Franc, Gottstein, Alzieu, Lagalisse and Jacquiet2011) carried out a longitudinal study in an affected dairy herd located in a region of France, where the disease is endemic and clinical cases have been recorded for many years. An increase in seroprevalence from 30 to 89·5% over a period of 14 months was reported, similar to findings reported in beef cattle in other regions (Cortes et al. Reference Cortes, Vidal and Reis2004). The prevalence of scleral conjunctiva cyst detection did not vary between the first and last clinical inspections (10·53 and 12·3%, respectively), with a peak in the middle of study (22·81%) lasting for more than a year. Most risky practices (e.g. natural mating and communal pastures outdoors) carried out in extensive husbandry systems are irrelevant under intensive husbandry, and animal trade is considered to be the main entryway for the infection into dairy herds. Moreover, because artificial insemination is predominant in dairy herds, the most important consequences of the infection might be loss of weight, decreased milk production and occasional abortion in pregnant dams, likely caused by the high fever of short duration that occurs in the acute phase of the disease (unpublished result in a B. besnoiti-infected dairy herd in northern Spain).
Age
The disease has been mainly reported in animals older than 6 months. Moreover, an increase in seropositivity with age has been reported, which might be explained by the efficient horizontal transmission. This was also observed when bulls from Israel were serologically examined. Older animals have a greater chance of becoming infected due to longer periods of exposure; these animals are more often serologically positive and clinically diseased (Goldman and Pipano, Reference Goldman and Pipano1983; Fernández-García et al. Reference Fernández-García, Álvarez-García, Risco-Castillo, Aguado-Martínez, Marcén, Rojo-Montejo, Castillo and Ortega-Mora2010; Gutiérrez-Expósito et al. in press).
Clinical disease is more often observed among 2–4-year-old adults than among those older than 4 years (Legrand, Reference Legrand2003; Alzieu, Reference Alzieu2007). This could indicate that many of the infected animals are able to develop an immunity (most likely non-sterile) during the years following the infection, thus reducing the severity of clinical signs of a chronic infection. The clinical incidence was lower in calves and heifers compared with bulls and cows (Bigalke, Reference Bigalke1968). Schulz (Reference Schulz1960) reported a peak between 3–4 years and noticed that calves are susceptible from the age of 6 months. Later, Bigalke (Reference Bigalke, Ristic and McIntyre1981) and Janitschke et al. (Reference Janitschke, De Vos and Bigalke1984) reported that clinical cases rarely occur in calves under 6 months of age. It has been speculated that calves younger than 6 months might be more resistant to the infection or even less exposed to the infection; however these issues remain to be clarified. The most feasible explanation could be related with the presence of colostral antibodies that might be protective (Shkap et al. Reference Shkap, Pipano, Marcus and Krigel1994). However, the disease was recently confirmed in a 4-month-old calf (unpublished data), demonstrating that animals younger than 6 months can also be infected (Ferrié, Reference Ferrié1984; Legrand, Reference Legrand2003; Alzieu, Reference Alzieu2007).
Sex
Regarding clinical signs, Pols (Reference Pols1960) pointed out that there was no sex susceptibility. Data obtained from several prevalence studies, initially reported in Israel (Goldman and Pipano, Reference Goldman and Pipano1983) and more recently in Spain, showed that both sexes may be equally infected. In Spain, Fernández-García et al. (Reference Fernández-García, Álvarez-García, Risco-Castillo, Aguado-Martínez, Marcén, Rojo-Montejo, Castillo and Ortega-Mora2010) did not observe significant differences during an outbreak in central Spain. In accordance with a recent study carried out in an area where the disease is endemic – the Spanish Pyrenees – both sexes were similarly infected, with 49·2 and 52·3% prevalence rates in males and females, respectively (Gutiérrez-Expósito et al. in press).
With regard to the prevalence of clinically affected animals, several authors have reported that males were more susceptible than females, as they appeared to show more acute signs and a higher mortality rate; these results were in agreement with local reports provided by veterinarians in Spain (Schulz, Reference Schulz1960; reviewed by Jacquiet et al. Reference Jacquiet, Liénard and Franc2010). However, these observations might be biased by the fact that breeding bulls are the most valuable animals in a herd and, consequently, may attract the attention of the veterinarians more easily (only based on clinical inspection).
Immune response
There are many unanswered questions concerning acquired humoral and cellular immune responses elicited against B. besnoiti infection in cattle. Nevertheless, the most striking finding is the field evidence showing that cattle that have contracted the disease, either clinically or sub-clinically, become immune against re-infections. This finding was supported by Bigalke (Reference Bigalke1968), who conducted a cohabitation experiment with chronically infected and naïve cattle. Cohabitation led to a 100% transmission rate with unapparent infections. This finding set the basis for later vaccination studies. The efficacy of the first live vaccine based on a blue wildebeest strain with naturally low pathogenicity was assessed by Bigalke et al. (Reference Bigalke, Schoeman and McCully1974). The field trial showed that vaccination induced protective immunity against the clinical form of the disease in 100% of vaccinated animals. Subsequently, vaccinations with tachyzoites of bovine isolates from Israel passaged in vitro for years produced variable increases in specific antibody levels at 30–40 dpi without any clinical signs. Immunized calves did not develop a clinical response to tachyzoite challenge with large numbers (>2×109 per dose) of parasites (Shkap, Reference Shkap1986). Interestingly, following infection, partial clinical recovery with an appreciable decrease in visible cysts has been observed in chronically infected animals (Frey et al. Reference Frey, Gutiérrez-Expósito, Ortega-Mora, Benavides, Marcén, Castillo, Casasús, Sanz, García-Lunar, Esteban-Gil and Álvarez-García2013).
Although the role of the humoral response during B. besnoiti infection remains to be clarified, it is known that animals develop specific antibody levels at 2–3 wpi that remain detectable for a long period of time, and the majority of chronically infected cattle with tissue cysts are seropositive (Fernández-García et al. Reference Fernández-García, Álvarez-García, Risco-Castillo, Aguado-Martínez, Marcén, Rojo-Montejo, Castillo and Ortega-Mora2010). In fact several authors reported higher antibody levels in animals with tissue cysts in their scleral conjunctiva than in animals without cysts (Liénard et al. Reference Liénard, Salem, Grisez, Prévot, Bergeaud, Franc, Gottstein, Alzieu, Lagalisse and Jacquiet2011; Schares et al. Reference Schares, Langenmayer, Scharr, Minke, Maksimov, Maksimov, Schares, Bärwald, Basso, Dubey, Conraths and Gollnick2013). Interestingly, Frey et al. (Reference Frey, Gutiérrez-Expósito, Ortega-Mora, Benavides, Marcén, Castillo, Casasús, Sanz, García-Lunar, Esteban-Gil and Álvarez-García2013) described a significant increase in antibody levels related to the presence of ruptured cysts, which may have favoured the re-exposure of the immune system to parasite antigen. The existence of parasite reactivation and subsequent recrudescence of infection remains to be clarified.
The critical question of whether colostral antibodies are protective requires further research. It is known that colostral antibodies may still persist at 4 months of age. These specific anti-B. besnoiti antibodies were observed by indirect immunofluorescence in four calves born from two chronically infected dams with IFAT titres ranging from 1 : 64 to 1 : 1024. Although the protective effect against infection was not studied, these calves did not contract the infection and developed normally (Shkap et al. Reference Shkap, Pipano, Marcus and Krigel1994).
The cell-mediated immune response is likely to play a crucial role similar to its role in the defence against other intracellular obligate and closely related parasites (N. caninum and T. gondii). Indeed, a T-cell response has been detected around several tissue cysts. Immunohistochemical studies have provided details regarding the immune reaction around the cysts in naturally infected cattle. Frey et al. (Reference Frey, Gutiérrez-Expósito, Ortega-Mora, Benavides, Marcén, Castillo, Casasús, Sanz, García-Lunar, Esteban-Gil and Álvarez-García2013) showed that cysts were associated with a significant non-purulent inflammatory infiltrate consisting predominantly of T lymphocytes and activated cells of the monocyte/macrophage lineage. Interestingly, some cysts appeared to be degenerated, as previously indicated by others (Bigalke et al. Reference Bigalke, Van Niekerk, McCully and Basson1966; Basson et al. Reference Basson, McCully and Bigalke1970). Bradyzoites were not clearly identified, and some inflammatory cells were infiltrating the degenerated cyst. The effect of cyst rupture has been studied by Frenkel (Reference Frenkel1955) in B. jellisoni infection. This study showed that organisms liberated by cyst rupture were found to be poorly stained and subject to phagocytosis. The lesions were circumscribed, resembling a granuloma; however, there was no evidence that Besnoitia liberated from cysts entered new cells and proliferated (Frenkel, Reference Frenkel1955). Nevertheless, during immunosuppression, B. besnoiti proliferation might be facilitated. Indeed, the administration of high doses of corticosteroids to hamsters chronically infected with B. jellisoni triggered relapse in 7–14 days, with an increase in parasites (Frenkel and Lunde, Reference Frenkel and Lunde1966). Regarding the role of immunosuppression during B. besnoiti infection, Bigalke (Reference Bigalke1968) described a severe reaction with fatal termination in a 3-year-old splenectomized ox inoculated intravenously with 1×109 tachyzoites from cell culture. Shkap (Reference Shkap1986) showed that cattle infected intravenously with different numbers of tachyzoites from cell culture (Table 1) did not develop tissue cysts under treatment with high levels of corticosteroids (0·6 mg kg−1/24 days), which may be explained by the fact that the isolate employed had naturally low pathogenicity. In contrast, when Diesing et al. (Reference Diesing, Heydorn, Matuschka, Bauer, Pipano, de Waal and Potgieter1988) inoculated bradyzoites in cattle clinical signs were observed upon immunosuppression treatment with cortisone.
CONCLUDING REMARKS
Bovine besnoitiosis is a re-emergent disease in Europe, and this parasitic disease is expected to have a noticeable economic impact in beef cattle raised under extensive conditions but this impact has not yet been quantified. Moreover, there are still many gaps related to the pathogenesis of the disease that deserve further research. In this context, the development of a well-established experimental bovine model should be prioritized to explore the role of host- and parasite-dependent factors that may be involved in parasite virulence and to evaluate the safety and efficacy of tools for parasite control. Thus, harmonized experimental designs and validated serological, histological and molecular techniques should be employed to successfully reproduce the disease and to study the elicited immune response in depth.
ACKNOWLEDGEMENTS
We thank the veterinarians for their collaborative fieldwork, in particular to Javier Zabala and Mikel Serrano.
FINANCIAL SUPPORT
This work was partially supported by a research grant from the Spanish Ministry of Economy and Competitiveness (AGL 2010-20561) and CYTED (Thematic Network 113RT0469 Protozoovac). Daniel Gutiérrez Expósito and Paula García Lunar have been financially supported by the Ministry of Economy and Competitiveness (grant no. BES-2011-043753) and Complutense University of Madrid, respectively.