INTRODUCTION
Myxosporeans are mainly parasites of fishes, both freshwater and marine, with a few species parasitizing amphibians, reptiles and rarely invertebrates (Kent et al. Reference Kent, Margolis and Corliss1994). Coelozoic species mainly parasitize the gall bladder or urinary ducts, whereas histozoic species may be found in most of the soft body tissues. Originally classified with protistan taxa, myxosporeans are now accepted as being metazoan organisms related to the Cnidaria (Smothers et al. Reference Smothers, von Dohlen, Smith and Spall1994; Siddall et al. Reference Siddall, Martin, Bridge, Desser and Cone1995; Nesnidal et al. Reference Nesnidal, Maximilian, Helmkampf, Bruchhaus, El-Matbouli and Hausdorf2013). Until the publication of the seminal paper of Markiv and Wolf (Reference Markiv and Wolf1983), the myxosporean life cycle was thought to be of the direct single host type. These authors proved this assumption to be wrong by showing that a myxosporean parasite of a freshwater fish actually had an indirect two-stage life cycle, with a myxosporean stage in the fish host and an actinosporean stage in an oligochaeta. Previously the Myxosporea and Actinosporea had been classified as separate phyla. Since then many more similar life cycles have been described for myxosporeans infecting freshwater fishes, but to date only six complete life cycles of marine species are known, all of them involving polychaetes as invertebrate alternate hosts (Køie et al. Reference Køie, Whipps and Kent2004, Reference Køie, Karlsbakk and Nylund2007, Reference Køie, Karlsbakk and Nylund2008, Reference Køie, Karlsbakk, Einen and Nylund2013; Rangel et al. Reference Rangel, Santos, Cech and Székely2009; Karlsbakk and Køie, Reference Karlsbakk and Køie2012). Evidence of direct fish-to-fish transmission has also been reported for some marine species (Diamant, Reference Diamant1997; Redondo et al. Reference Redondo, Palenzuela, Riaza, Macías and Álvarez-Pelliterro2002; Yasuda et al. Reference Yasuda, Ooyama, Iwata, Tun, Yokoyama and Ogawa2002).
Some marine myxosporeans have been reported as pathogens of fishes, both in the wild and in mariculture (Sindermann, Reference Sindermann1957; Kabata and Whitaker, Reference Kabata and Whitaker1985; Maeno and Sorimachi, Reference Maeno, Sorimachi and Svrjcek1992; Álvarez-Pelliterro and Sitjá-Bobadilla, Reference Álvarez-Pelliterro and Sitjá-Bobadilla1993; Feist, Reference Feist1997; Moran et al. Reference Moran, Whitaker and Kent1999; Kent et al. Reference Kent, Andree, Bartholomew, El-Matbouli, Desser, Devlin, Feist, Hedrick, Hoffmann, Khattra, Hallett, Lester, Longshaw, Palenzuela, Siddall and Xiao2001; MacKenzie et al. Reference MacKenzie, Kalavati, Gaard and Hemmingsen2005; Palenzuela, Reference Palenzuela2006). In addition, some histozoic species are important as spoilage agents because they produce unsightly macroscopic cysts in the fish flesh and/or cause the condition known as post-mortem myoliquefaction, resulting in considerable economic losses (Henning et al. Reference Henning, Hoffman and Manley2013). They have also been used as biological tags in population studies of commercially important species of marine fish (Kabata, Reference Kabata1967; Khan and Tuck, Reference Khan and Tuck1995; Larsen et al. Reference Larsen, Hemmingsen, MacKenzie and Lysne1997; Campbell, Reference Campbell2005). Their use as biological tags, however, is limited by lack of knowledge on the duration of infections in their fish hosts.
The purpose of this review is to collate the information available on myxosporeans infecting marine fishes and to analyse them in relation to their occurrence in different oceans and in different taxonomic groups of marine fishes. We have done our best to trace all relevant records, but with such a wide and diverse amount of literature to search it is possible that we may have missed some. We apologize in advance for any such omissions.
MATERIALS AND METHODS
The records of myxosporeans from marine fish in this paper were collected from a search of the relevant literature. These records are scattered throughout the parasitological and fish-related literature, but certain text-books and reviews proved particularly useful (Lom and Dyková, Reference Lom and Dyková1992, Reference Lom and Dyková2006; Moran et al. Reference Moran, Whitaker and Kent1999; Eiras, Reference Eiras2002, Reference Eiras2006; Eiras et al. Reference Eiras, Molnár and Lu2005, Reference Eiras, Saraiva, Cruz, Santos and Fiala2011, Reference Eiras, Lu, Gibson, Fiala, Saraiva, Cruz and Santos2012, Reference Eiras, Saraiva and Cruz2014a , Reference Eiras, Zhang and Molnar b ; Zhao et al. Reference Zhao, Zhou, Kent and Whipps2008; Alama-Bermejo et al. Reference Alama-Bermejo, Cuadrado, Raga and Holzer2009; Kaur and Singh, Reference Kaur and Singh2012; Rangel et al. Reference Rangel, Gibson and Santos2014). Four recent publications have proposed important changes to the taxonomy of myxosporeans: a revised classification of the order Multivalvulida (Whipps et al. Reference Whipps, Grossel, Adlard, Yokoyama, Bryant, Munday and Kent2004), the replacement of the preoccupied genus Davisia with a new genus Myxodavisia (Zhao et al. Reference Zhao, Zhou, Kent and Whipps2008), the removal of the long-established genus Leptotheca Thélohan, 1895, and the reassignment of its species to other genera (Gunter and Adlard, Reference Gunter and Adlard2010), the re-establishment of the families Coccomyxidae (see Heiniger et al. Reference Heiniger, Gunter and Adlard2011) and Myxobilatidae (see Whipps, Reference Whipps2011) and the creation of the new genera Ellipsomyxa (see Køie, Reference Køie2003a ), Latyspora (see Bartošova et al. Reference Bartošova, Freeman, Yokoyama, Caffara and Fiala2011) and Sigmomyxa (see Karlsbakk and Køie, Reference Karlsbakk and Køie2012). We have accepted the changes proposed in these publications, but we noted that Heiniger and Adlard (Reference Heiniger and Adlard2014) questioned the validity of Sigmomyxa.
In this paper we have divided the world's oceans into five regions: North Atlantic, South Atlantic, North Pacific, South Pacific and Indian Ocean. The North Atlantic includes the Arctic section of the Atlantic and the White, Barents, Baltic, Black, Mediterranean and Caspian Seas. The South Atlantic and South Pacific regions include the Antarctic section of each ocean. The Indian Ocean includes the Red Sea and Persian Gulf.
RESULTS
Geographical distribution of myxosporeans in marine fishes (Tables 1 and 2)
Order Bivalvulida
Family Ceratomyxidae, with four genera in marine fishes
Ceratomyxa is the genus with the largest number of named species (252) infecting marine fishes. More species have been reported from the North Atlantic than from any other region, with fewest reports from the South Atlantic and Indian Oceans. Eight species were reported from two of our designated regions. Eight named species of the genus Ellipsomyxa have been described, four each from the North Atlantic and the South Pacific. The genus Sigmomyxa is represented by two named species, from the North Atlantic and the Indian Ocean (Karlsbakk and Køie, Reference Karlsbakk and Køie2012; Heiniger and Adlard, Reference Heiniger and Adlard2014), and the genus Meglitschia by only one named species, from the South Pacific (Kovaleva, Reference Kovaleva1988).
Table 1. Numbers of species of different myxosporean genera reported from marine fish in different regions. Note that some species have been reported from more than one region

Table 2. Reports of different myxosporean families from five oceanic regions expressed as percentages of the total number of reports of each family

Family Myxidiidae, with three genera in marine fishes
Seventy-six named species of the genus Myxidium have been reported from marine fishes, all infecting the gall bladder except for two that infect the urinary bladder and one that infects the intestine. Of these, eight species have been reported from more than one region. The most cosmopolitan species appear to be Myxidium coryphaenoideum Noble, 1966, which has been reported from all regions except the Indian Ocean, and Myxidium incurvatum Thélohan, 1892, which has been reported from three regions. Approximately two-thirds of the marine Myxidium species described to date have been reported from the North Atlantic and North Pacific. Fifty named species of the genus Zschokkella have been reported from marine fishes, most infecting the gall bladder, but with about one-quarter infecting the urinary tract. They are more evenly divided than the Myxidium species between the oceanic regions, but with slightly more reported from the North Atlantic than from the other regions. The third genus in this family – Enteromyxum – consists of three named species, all of which parasitize the intestine of marine fishes in the North Atlantic, North Pacific and Indian Ocean (Red Sea).
Family Myxobolidae, with four genera in marine fishes
Sixty-four named species of the genus Myxobolus have been reported from marine fishes, infecting a variety of tissues; most have been reported from the North Atlantic. Twenty-seven named species of the genus Henneguya have been reported from marine fishes, also infecting a variety of tissues; just over half were reported from the North Atlantic. Thelohanellus is mainly a parasite of freshwater fishes, but three named species have been reported from marine fishes, two from the gall bladder and one from the urinary tract; two were reported from the Indian Ocean and one from the North Pacific.
Family Sphaeromyxidae, with one genus in marine fishes
Forty-five named species of the genus Sphaeromyxa have been reported from marine fishes, all infecting the gall bladder. Most have been reported from the northern hemisphere. Two species were reported from two regions.
Family Sinuolineidae, with eight genera in marine fishes
Twenty-three named species of Sinuolinea have been reported from marine fishes. Most are parasites of the urinary tract, with only four species infecting the gall bladder. Reports of Sinuolinea species are mostly from the North Atlantic and North Pacific, with three species reported from the Indian Ocean and one each from the South Atlantic and South Pacific. Twenty-three named species of Myxoproteus have been reported from marine fishes. Seven species infect the gall bladder, while the remainder infect the urinary tract. Most species have been reported from the North Atlantic, and one species, Myxoproteus abyssus Yoshino & Noble, 1974, has been reported from the North and South Pacific and the North Atlantic. Thirty-one named species of Myxodavisia have been reported from marine fishes, most of them infecting the urinary tract, with only six infecting the gall bladder. This genus was formerly Davisia, but the name was changed by Zhao et al. (Reference Zhao, Zhou, Kent and Whipps2008) because Davisia was preoccupied. Most reports are from the northern hemisphere.
Eight named species of Schulmania have been reported, all infecting the urinary tract. All but one was reported from the northern hemisphere. Seven named species of Bipteria have been reported from marine fishes. Two species infect the gall bladder and five the urinary tract. The South Pacific is the only region from which Bipteria has not been reported. The genera Neobipteria, Noblea, Paramyxoproteus and Latyspora are all parasites of the urinary tract. Neobipteria is represented by two named species infecting marine fishes, and each of the others by one. The Neobipteria species were described from the North Pacific and Indian Ocean, Noblea and Paramyxoproteus from the North Atlantic, and Latyspora from the Indian Ocean.
Family Sphaerosporidae, with five genera in marine fishes
Twenty-nine named species of the genus Sphaerospora have been reported from marine fishes, most infecting the urinary tract, but with four species infecting the gall bladder, one infecting the testes and one systemic species. Reports are predominantly from the northern hemisphere (26). Five named species of Palliatus have been reported from marine fishes, all infecting the gall bladder except for one that infects the pancreas. The South Pacific is the only region from which no Palliatus species has been reported. Two named species of the genus Polysporoplasma have been reported from the urinary tract of marine fishes in the North Atlantic.
Family Chloromyxidae, with one genus in marine fishes
Thirty-six named species of the genus Chloromyxum have been reported, mostly infecting the gall bladder but with seven infecting the urinary tracts of marine fishes.
Family Alatasporidae, with three genera in marine fishes
Eighteen named species of the genus Alataspora have been reported, all from marine fishes, all infecting the gall bladder, and with most reported from the North Atlantic. Thirteen named species of the genus Pseudalataspora have been described, all infecting the gall bladder, and most from the North Atlantic. The genus Renispora is represented by a single species from the gall bladder of a fish in the South Atlantic.
Family Coccomyxidae, with three genera in marine fishes
Eleven named species of the genus Coccomyxa have been reported from marine fishes, all infecting the gall bladder except for Coccomyxa hoffmani, which infects the gill cartilage. Ten named species of Auerbachia have been described from marine fishes, all infecting the gall bladder. Most reports are from the South Pacific and Indian Ocean. One species, Auerbachia pulchra, has been reported from the North Atlantic and North Pacific. One named species of the genus Globospora was described from the gall bladder of a fish in the Southwest Atlantic.
Family Ortholineidae, with one genus in marine fishes
Thirteen named species of the genus Ortholinea have been reported from the urinary tract of marine fishes.
Family Parvicapsulidae, with three genera in marine fishes
Eleven named species of the genus Parvicapsula have been reported from marine fishes. Eight species infect the urinary tract, one the gall bladder and one the wall of the intestine. Seven species were reported from the North Atlantic. Two named species of Neoparvicapsula have been reported from marine fishes, both infecting the urinary tract and both from the South Atlantic. Three named species of Gadimyxa have been reported from marine fishes, all infecting the urinary tract and all from the North Atlantic.
Family Myxobilatidae, with two genera in marine fishes
Three named species of Myxobilatus have been reported from marine fishes, two from the North Atlantic infecting the urinary tract and one from the South Atlantic infecting the gall bladder. A single named species of the genus Hoferellus has been reported infecting the urinary tract of a fish in the North Atlantic (Caspian Sea).
Family Fabesporidae, with one genus in marine fishes
Only one named species of Fabespora has been reported from the gall bladder of marine fishes in the North Atlantic (Black Sea).
Order Multivalvulida
This sub-order was recognized as having five families by Lom and Dyková, (Reference Lom and Dyková1992), but Whipps et al. (Reference Whipps, Grossel, Adlard, Yokoyama, Bryant, Munday and Kent2004) questioned this classification on the basis of their comparative ribosomal DNA sequence analysis. We accept the proposal of the latter authors that the families Pentacapsulidae, Hexacapsulidae and Septemcapsulidae be synonymized with Kudoidae to accommodate all myxozoans having four or more shell valves and polar capsules.
Family Kudoidae, with one genus in marine fishes
One hundred and two named species of the genus Kudoa have been reported from marine fishes. Most of them infect the musculature, but they have also been reported from other soft tissues. More than half of the reports are from the northern hemisphere, with one cosmopolitan species, Kudoa thyrsites, reported from all four regions.
Family Trilosporidae, with three genera in marine fishes
Eleven named species of the genus Unicapsula have been reported from marine fishes, most from the musculature, but one infecting the urinary bladder and one the gills. The South Atlantic is the only region from which no Unicapsula species has been reported. Four named species of the genus Trilospora have been reported from marine fishes, three infecting the gall bladder and one the musculature. All reports are from the northern hemisphere. One named species of Trilosporoides has been reported from the gall bladder of a marine fish in the North Atlantic.
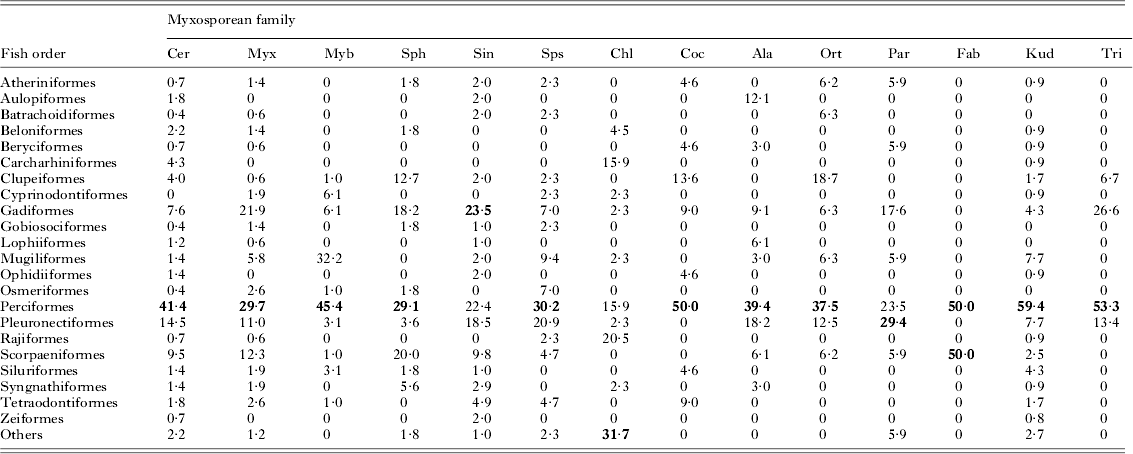
Occurrence of myxosporeans in different orders of marine fishes (Tables 3 and 4, Fig. 1)
Perciform fishes host the largest number of myxosporean species, followed by the Pleuronectiformes and Gadiformes. Forty per cent or more of species in the myxosporean families Ceratomyxidae, Myxobolidae, Coccomyxidae, Fabesporidae, Kudoidae and Trilosporidae infect perciforms. Families Myxidiidae, Sinuolineidae and Trilosporidae are particularly common parasites of gadiform fishes, and the Parvicapsulidae of pleuronectiforms. Of the major myxosporean families, the Sphaeromyxidae are best represented in clupeiformes, the Myxidiidae and Sinuolineidae in gadiformes, the Myxobolidae in mugiliformes, and the Sphaerosporidae in perciforms, while the Ceratomyxidae dominate the myxosporean fauna of the Chondrichthyes fishes.

Fig. 1. Species of different myxosporean families infecting major taxa of marine fishes, shown as proportions of the total numbers of species infecting each host order of the Class Osteichthyes, but with the orders in the Class Chondrichthyes pooled. Myxosporean families: Cer, Ceratomyxidae; Myx, Myxidiidae; Myb, Myxobolidae; Sph, Sphaeromyxidae; Sin, Sinuolineidae; Sps, Sphaerosporidae; Ala, Alatasporidae; Kud, Kudoidae. Fish orders: Clu, Clupeiformes; Gad, Gadiformes; Mug, Mugiliformes; Per, Perciformes; Ple, Pleuronectiformes; Sco, Scorpaeniformes; Cho, Chondrichthyes.
Table 3. Numbers of species of different myxosporean families reported from different orders of marine fishes. Cer, Ceratomyxidae; Myx, Myxidiidae; Myb, Myxobolidae; Sph, Sphaeromyxidae; Sin, Sinuolineidae; Sps, Sphaerosporidae; Chl, Chloromyxidae; Coc, Coccomyxidae; Ala, Alatasporidae; Ort, Ortholineidae; Par, Parvicapsulidae; Fab, Fabesporidae; Kud, Kudoidae; Tri, Trilosporidae. Note that some species have been reported from more than one host order. Host orders from which less than five myxosporean species have been reported are grouped under ‘others’

Table 4. Species of different myxosporean families infecting different orders of marine fishes, expressed as percentages of the total numbers of species of each myxosporean family. The host orders with most species of each myxosporean family are shown in bold font. Abbreviations as in Table 3

DISCUSSION
Although myxosporeans have been described from marine fishes for more than a century, it is clear that only a small fraction of the total number of species infecting marine fishes have been reported to date. The fact that most have been described from the northern hemisphere is probably mainly due to the greater research effort in this region, and the greater number of myxosporean specialists working in Europe, North America and Japan. This has changed somewhat in recent years, however, with South American and Australian parasitologists making significant contributions to our knowledge of the group. For example, since 2008 67 new species of myxosporean have been described from fishes of the Great Barrier Reef in Australia, 46 of them of the family Ceratomyxidae (Gunter and Adlard, Reference Gunter and Adlard2008, Reference Gunter and Adlard2009; Heiniger et al. Reference Heiniger, Gunter and Adlard2008, Reference Heiniger, Gunter and Adlard2011, Reference Heiniger, Cribb and Adlard2013; Gunter et al. Reference Gunter, Whipps and Adlard2009, Reference Gunter, Burger and Adlard2010; Burger and Adlard, Reference Burger and Adlard2010; Gleeson et al. Reference Gleeson, Bennett and Adlard2010; Gleeson and Adlard, Reference Gleeson and Adlard2011, Reference Gleeson and Adlard2012; Heiniger and Adlard, Reference Heiniger and Adlard2013a , Reference Heiniger and Adlard b , Reference Heiniger and Adlard2014; Miller and Adlard, Reference Miller and Adlard2013). This means that more than half of the total number of ceratomyxid species described from the South Pacific region have been described in the last 7 years. The South Atlantic and Indian Oceans remain largely unexplored with regard to myxosporeans, so more research is needed in these regions. Most records from the Indian Ocean have been by Indian workers from fishes caught in the Bay of Bengal, but there are very few reports from the rest of the region. The geographical distribution of myxosporeans described in this paper therefore merely provides a snapshot of the situation at the time of writing and is likely to change considerably in the near future.
The orders of marine fishes that contain the largest numbers of fish species are predictably those infected with the largest numbers of myxosporean species, while the distribution of myxosporean families in different orders of marine fishes has undoubtedly been influenced by specialist research interests in particular taxa of myxosporeans or fishes. However, it is likely that the distribution shown in our analyses is closer to the real situation than is the case with the geographical distribution. These distributions can indicate phylogenetic relationships between parasite and host and suggest the origins of different myxosporean taxa. For example, our analyses show that in the marine environment the family Myxobolidae are common parasites of euryhaline fish such as members of the Cyprinodontiformes, Mugiliformes and Osmeriformes. This, together with the fact that the Myxobolidae are predominantly parasites of freshwater fishes (Lom and Dyková, Reference Lom and Dyková1992), suggests a freshwater origin for the Myxobolidae. Species in the family Ceratomyxidae, on the other hand, are predominantly parasites of marine fish and are the most abundant myxosporean parasites of the primitive Chondrichthyes, suggesting their origin as parasites of early cartilaginous fishes.
We have followed the traditional myxosporean classification based on morphological features, but new molecular information on phylogenetic relationships within the Myxozoa shows that similarities in spore morphology do not necessarily indicate close phylogenetic relationships (Fiala, Reference Fiala2006; Fiala and Bartošová, Reference Fiala and Bartošová2010; Gunter et al. Reference Gunter, Burger and Adlard2010). There is no doubt that the coming years will see major changes to the classification of myxozoans and descriptions of many new species. New analyses based on an amended classification will thus shed more light on phylogenetic relationships between myxosporean parasites and their fish hosts.
ACKNOWLEDGEMENTS
The authors are grateful to the anonymous reviewers whose suggestions helped to improve the original version of this paper.