Introduction
The World Health Organization (WHO) identified bipolar disorder as the sixth leading cause of disability-adjusted life years in the world among people aged 15–44 years (Murray & Lopez, Reference Murray and Lopez1996). The true prevalence and incidence rates (IRs) of (hypo)manic disorder remain unclear. Lifetime prevalence estimates vary from around 1–2% (Regier et al. Reference Regier, Boyd, Burke, Rae, Myers, Kramer, Robins, George, Karno and Locke1988; Kessler et al. Reference Kessler, McGonagle, Zhao, Nelson, Hughes, Eshleman, Wittchen and Kendler1994; Weissman et al. Reference Weissman, Bland, Canino, Faravelli, Greenwald, Hwu, Joyce, Karam, Lee, Lellouch, Lepine, Newman, Rubio-Stipec, Wells, Wickramaratne, Wittchen and Yeh1996) to 5–8% (Carlson & Kashani, Reference Carlson and Kashani1988; Lewinsohn et al. Reference Lewinsohn, Klein and Seeley1995; Angst, Reference Angst1998; Szádóczky et al. Reference Szádóczky, Papp, Vitrai, Ríhmer and Füredi1998; Judd & Akiskal, Reference Judd and Akiskal2003) whereas IRs vary between 4 and 33/105 per year (Bebbington & Ramana, Reference Bebbington and Ramana1995).
The reported variability in bipolar population morbidity rates may be caused by several factors. First, nearly all incidence studies on bipolar disorder are based on participants in clinical care, which probably results in a substantial underestimation of rates (Spicer et al. Reference Spicer, Hare and Slater1973; Leff et al. Reference Leff, Fischer and Bertelsen1976; Rasanen et al. Reference Rasanen, Tiihonen and Hakko1998; Lloyd et al. Reference Lloyd, Kennedy, Fearon, Kirkbride, Mallett, Leff, Holloway, Harrison, Dazzan, Morgan, Murray and Jones2005; Kennedy et al. Reference Kennedy, Everitt, Boydell, van Os, Jones and Murray2005b) because many cases either have not sought help or are not diagnosed correctly (Ghaemi et al. Reference Ghaemi, Ko and Goodwin2002).
Second, previous work suggests that the age of the study population may be crucial, as there are indications that much of the population lifetime risk for bipolar disorder is consumed in adolescence. Thus, Lewinsohn et al. (Reference Lewinsohn, Seeley and Klein2003) showed that, in a population sample stratified by the age categories <9, 19–23 and 24–29 years, the first lifetime onset of bipolar disorder and subthreshold bipolar disorder almost always occurred in adolescence. Therefore, a young study population as used in the Early Developmental Stages of Psychopathology (EDSP) Study is most appropriate (Wittchen et al. Reference Wittchen, Mühlig and Pezawas2003). The issue of age is also important in view of the considerable psychopathological, longitudinal and familial/genetic overlap between bipolar disorder and childhood disorders, in particular attention deficit hyperactivity disorder (ADHD), but also oppositional–defiant disorder (ODD) and conduct disorder (CD) (Nierenberg et al. Reference Nierenberg, Miyahara, Spencer, Wisniewski, Otto, Simon, Pollack, Ostacher, Yan, Siegel and Sachs2005; Henin et al. Reference Henin, Biederman, Mick, Hirschfeld-Becker, Sachs, Wu, Yan, Ogutha and Nierenberg2007). Studying the onset of (hypo)manic symptoms in adolescents allows for quantification of the amount of the bipolar population morbidity rate that can be traced to childhood disorders.
Third, diagnostic criteria used have a major impact on population rates (Akiskal et al. Reference Akiskal, Bourgeois, Angst, Post, Möller and Hirschfeld2000; Angst et al. Reference Angst, Gamma, Benazzi, Ajdacic, Eich and Rössler2003). Many people in the general population display subthreshold bipolar disorder (Merikangas et al. Reference Merikangas, Akiskal, Angst, Greenberg, Hirschfeld, Petukhova and Kessler2007). Therefore, widening criteria for bipolar disorder will naturally increase the number of cases.
An informative way of describing the bipolar population morbidity rate is to replace dichotomous criteria with dimensional measures; the possible use of dimensional measures in bipolar disorder is currently being examined in DSM-V (First, Reference First2006). Angst & Marneros (Reference Angst and Marneros2001) suggest that a natural continuum may exist on which all (hypo)manic manifestations of varied length, frequency and severity can be represented. This dimensional approach may be more sensitive and informative in the search for determinants of onset and change, making it easier to monitor onset and progression of psychiatric phenotypes (Cougnard et al. Reference Cougnard, Marcelis, Myin-Germeys, de Graaf, Vollebergh, Krabbendam, Lieb, Wittchen, Henquet, Spauwen and van Os2007; van Os et al. Reference van Os, Linscott, Myin-Germeys, Delespaul and Krabbendam2009). Furthermore, it allows for fuller examination of the impact of symptoms on well-being and functioning, severity and distress (Regeer et al. Reference Regeer, Krabbendam, de Graaf, ten Have, Nolen and van Os2006), and may facilitate recognition of at-risk states and early intervention (Egeland et al. Reference Egeland, Hostetter, Pauls and Sussex2000; Hanssen et al. Reference Hanssen, Bak, Bijl, Vollebergh and van Os2005; Birmaher & Axelson, Reference Birmaher and Axelson2006). For bipolar disorder, it has the additional advantage of allowing for the separate study of manic and depressive dimensions, the co-occurrence of which in the same mood episode is common in clinical practice and therefore represents an important parameter for study in epidemiological and taxonomic investigations. Finally, it can increase statistical power without loss of clinical utility.
Therefore, the aim of the current study was to investigate dimensional (hypo)manic categories, independent of receipt of mental health care, in a large representative cohort of adolescents followed over a period of up to 10 years.
Method
Sample
This examination is part of the EDSP study, a prospective longitudinal cohort study. Detailed information about the design, sample, instruments, procedures and statistical methods of the EDSP study is presented elsewhere (Wittchen et al. Reference Wittchen, Perkonigg, Lachner and Nelson1998b; Lieb et al. Reference Lieb, Isensee, von Sydow and Wittchen2000). Data were collected in a representative population sample of adolescents and young adults living in the Munich area (Germany), aged 14–24 years at baseline. The study sample was drawn randomly from the 1994 government population registers. Fourteen to 15-year-olds were sampled at twice the rate of 16- to 21-year-olds, and 22- to 24-year-olds were sampled at half this rate.
Study design
The present study is based on a subset of EDSP respondents, aged 14–17 years at baseline (T0, n=1395, response rate 75%), thus ensuring a population at risk of developing incident bipolar disorder. Participants completed a baseline survey (T0, n=1395) and three follow-up investigations (T1, T2, T3), covering a time period of approximately 1.6 (T0–T1, s.d.=0.2), 3.4 (T0–T2, s.d.=0.3) and 8.3 years (T0–T3, range 7.4–10.6 years, s.d.=0.7) respectively. Response rates (conditional on T0 completion) were 88% at T1 (n=1228), 83% at T2 (n=1169) and 73% at T3 (n=1022).
Instruments
Interviews were conducted using the computerized version of the Munich-Composite International Diagnostic Interview (DIA-X/M-CIDI; Wittchen & Pfister, Reference Wittchen and Pfister1997), an updated version of the WHO's CIDI version 1.2 (WHO, 1990). The DIA-X/M-CIDI is a comprehensive, fully standardized diagnostic interview and assesses symptoms, syndromes and diagnoses of various mental disorders in accordance with definitions and criteria of the DSM-IV. Its features have been developed and tested in several methodological studies with the CIDI or modifications thereof, including the deletion of many of the CIDI's skipping rules to allow for the study of subthreshold conditions. High inter-rater and test–retest reliability of the CIDI have been established (Wittchen et al. Reference Wittchen, Robins, Cottler, Sartorius, Burker and Regier1991; Wittchen, Reference Wittchen1994), in addition to validity (Reed et al. Reference Reed, Gander, Pfister, Steiger, Sonntag, Trenkwalder, Hundt and Wittchen1998). Test–retest reliability (κ) of the DIA-X/M-CIDI was reported to be 0.68 (p<0.001) for major depressive disorder and 0.64 (p<0.001) for bipolar disorder (Wittchen et al. Reference Wittchen, Lachner, Wunderlich and Pfister1998a). To ensure reliability of the assessments, fully trained and experienced psychologists who were allowed to probe with follow-up questions conducted the interviews. At baseline, the lifetime version of the DIA-X/M-CIDI was used, and the interval version for subsequent investigations. By using the lifetime version of the DIA-X/M-CIDI at baseline, which includes questions regarding the time period from birth until the interview, it was possible to take into account the possible onset of bipolar disorder before the baseline interview.
Mania categories based on DSM-IV algorithms
Using M-CIDI/DSM-IV diagnostic algorithms (Pfister & Wittchen, Reference Pfister and Wittchen1995), participants were divided into four groups, those experiencing: (1) neither hypomanic nor manic episodes; (2) DSM-IV manic episodes; (3) DSM-IV hypomanic episodes; and (4) either manic or hypomanic episodes [hereafter: (hypo)manic episodes]. The last three groups were subsequently subdivided into participants (a) with lifetime co-morbid depressive episodes and (b) without lifetime co-morbid depressive episodes.
Mania categories based on symptom score
(Hypo)manic symptoms were assessed using 11 items of the DIA-X/M-CIDI mania section, and concerned items regarding an increase in goal-directed activity, psychomotor agitation, spending sprees, sexual indiscretions, increased talkativeness, flight of ideas, increased self-esteem or grandiosity, decreased need for sleep, and distractibility. These items were rated yes (1) or no (0) and were only rated if: (a) at least one of the core symptoms ‘unusual happiness or excitement’ or ‘unusual irritability’ was present; (b) core symptoms were either noticed by others or caused participants problems; (c) symptoms were present for at least four successive days; (d) symptoms were not a result of alcohol/drugs use. Guided by previous work (Regeer et al. Reference Regeer, Krabbendam, de Graaf, ten Have, Nolen and van Os2006), a sum score of symptom ratings was formed (range 0–11 symptoms). Five progressively stricter and overlapping subcategories of this sum score were created (I, no symptoms; II, ⩾1 symptom; III, ⩾4 symptoms; IV, ⩾7 symptoms; V, ⩾10 symptoms), which represented the underlying continuous score of (hypo)manic symptoms.
Mania categories based on distress
In participants with ⩾4 (hypo)manic symptoms, a division was made based on the level of distress, as reported in the DIA-X/M-CIDI mania section. Distress was assessed by asking participants if, at the moment the symptoms were at their worst, they interfered with life, work or leisure activities, and was coded (1) no interference, (2) some interference, (3) considerable interference and (4) much interference.
Grouping by mental health care
In participants fulfilling criteria for at least one of the above categories, grouping was applied based on whether or not mental health care had been received at the respective assessment. First, participants were asked if they had ever been treated in a hospital or had spoken to a professional because of (hypo)manic symptoms. Second, participants were shown a list on which several types of out-patient, in-patient or day-patient institutions for mental health problems were mentioned, after which they were asked if they had ever sought help at any of these institutions because of mental health problems. All participants who responded positively to either one of these questions were considered to have received mental health care.
Childhood disorders
Between T0 and T1, face-to-face interviews were carried out with respondents' parents to collect information regarding ODD, CD and ADHD (hereafter collectively referred to as ‘childhood disorders’). These childhood disorders were assessed with questions covering the criteria defined by the DSM-IV. Information was mostly based on maternal responses (97.4%). The response rate of parents was 86% (n=1053).
Statistical analysis
Cumulative lifetime incidence (CLI) and person-year IRs
Weighting occurred to account for differences in sampling probabilities and also systematic non-response at baseline according to age, gender and geographical location (Lieb et al. Reference Lieb, Isensee, von Sydow and Wittchen2000). Cumulative lifetime incidence up to T3 (CLI) of the (hypo)manic categories was calculated at T3.
Survival analysis was conducted to determine IRs between T0 and T3 using the ST commands in Stata, version 9.2 (StataCorp, 2005). CLI and IR estimates were calculated, grouping by receipt of mental health care. The IR is defined as the number of new cases of disease during a given time period divided by the sum of time that each person remains under observation and is free from disease (the total person-time of observation). After defining appropriate risk sets, IRs were calculated for each category. The risk set is defined as the set of individuals at risk of belonging to a certain category for the first time during the study. Therefore, participants with past or current evidence of this category at baseline were excluded from analysis, in which the strictest possible exclusion criteria were used [e.g. all participants experiencing ⩾1 symptom were excluded for analyses of incidence of (hypo)manic symptoms]. The total person-times of observation of the individual risk sets thus defined are presented in Table 1.
Table 1. Cumulative lifetime incidence up to T3 and incidence rates (T0–T3) of (hypo)manic episodes, stratified by care

CLI, Cumulative lifetime incidence; IR, incidence rate; MHC+, episodes in combination with mental health care; DEP+, with depressive episode; DEP–, without depressive episode.
a Values are expressed as percentage (number) of cases.
b Values are expressed as number of cases per 100 000 person-years (denominator is population of person-years).
c Total group, independent of having lifetime depressive episodes.
d (Hypo)manic episode: either hypomanic or manic episode.
Childhood morbidity sensitivity analyses
To assess how much of the incidence of bipolar categories could be traced to childhood morbidity, co-morbidity with childhood disorders was assessed and childhood morbidity sensitivity analyses (CMSAs) performed. First, lifetime co-morbidity between T0 lifetime (hypo)manic episodes and childhood disorders was assessed using logistic regression. Second, Cox regression was used to calculate associations between childhood disorders and incidence of new (hypo)manic episodes between T0 and T3. Third, CLI and IRs for (hypo)manic episodes were recalculated with exclusion of individuals with these childhood disorders. Similar sensitivity analyses were performed for other (hypo)manic categories.
Demographic risk factors
IRs were calculated stratified by age group as a time-varying variable (15–16, 17–18, 19–21, 22–24 and 25–28 years; age=age during any moment of the study), sex and urbanicity [living in city or rural area at baseline; city area=city of Munich (population density 4061 persons per square mile) and rural area=the Munich surrounding area (population density 553 persons per square mile)] (Spauwen et al. Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2006). Statistical differences in IRs within age, sex or urbanicity categories were tested using Cox regression analysis yielding hazard ratios (HRs), using the 15-year age group, male sex, and rural area as reference categories (Table 2). To assess whether any association with demographic factors was independent of the others and unconfounded by co-morbid current or childhood psychopathology, HRs of all categories were adjusted for age, sex, urbanicity, presence of depression (diagnosis of a lifetime DSM-IV depressive episode) and presence of childhood disorders (diagnosis of either ODD, CD or ADHD according to DSM-IV criteria) using the Stata strata option for adjustment by stratification in Cox regression. HRs of the distress categories were additionally adjusted for number of (hypo)manic symptoms (as indexed by the continuous sum score of symptom ratings). As part of the CMSA, HRs were calculated similarly after exclusion of participants suffering from childhood disorders.
Table 2. Predictors of incident (hypo)manic categories
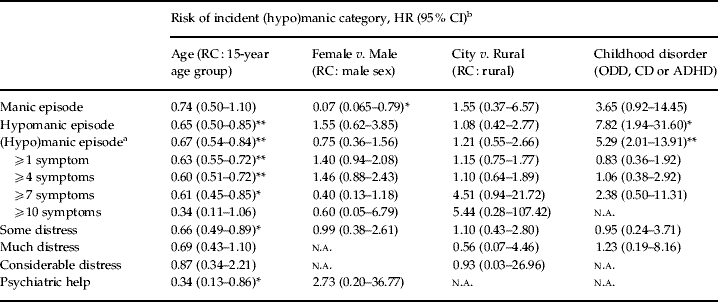
HR, Hazard ratio; CI, confidence interval; RC, reference category; ODD, oppositional–defiant disorder; CD, conduct disorder; ADHD, attention deficit hyperactivity disorder; n.a., not applicable.
a (Hypo)manic episode: either hypomanic or manic episode.
b Results adjusted for age, sex, urbanicity, depression and childhood disorders. In case of distress/psychiatric help category: results also adjusted for number of (hypo)manic symptoms.
* p⩽0.05, ** p⩽0.001.
Results
Analyses are based on 1395 adolescents (51% male). The mean age at baseline was 15.1 years (s.d.=1.1). Four-hundred and fifteen adolescents (30%) were living in a rural area. Of the 1395 adolescents, 1022 completed T3. Attrition rates were almost equal for sex (28.8% females v. 24.8% males), urbanicity (25.3% rural v. 27.3% city), and age (22.4% 13-year-olds v. 23.8% 14-year-olds v. 29.6% 15-year-olds v. 25.7% 16-year-olds v. 29.5% 17-year-olds).
Incidence of DSM-IV (hypo)manic episodes
CLI rates varied between 1.3% and 7.6% for the different DSM-IV episodic groups, declining after restriction to episodes plus mental health care (Table 1). Approximately a third of participants had co-morbid depressive episodes. IRs ranged from 104/105 to 714/105 person-years, declining after restriction to episodes plus mental health care (Table 1).
Incidence of (hypo)manic symptoms
The number of participants steadily declined with increasing level of symptoms (Table 3). For participants experiencing ⩾1 symptom, the CLI was six times higher before restriction of the group to participants with symptoms plus mental health care (Table 3). This discrepancy between CLI rates decreased as the number of symptoms increased.
Table 3. Cumulative lifetime incidence up to T3 and incidence rates (T0–T3) of (hypo)manic symptoms, stratified by care

CLI, Cumulative lifetime incidence; IR, incidence rate; MHC+, episodes in combination with mental health care.
a Values are expressed as percentage (number) of cases.
b Values are expressed as number of cases per 100 000 person-years (denominator is population of person-years).
Incidence of distress
CLI and IRs are presented in Table 4. Higher rates of distress were experienced by fewer people. The majority of people with ‘some distress’ remained outside care, and the majority of people with ‘much distress’ were in care.
Table 4. Cumulative lifetime incidence up to T3 and incidence rates (T0–T3) of distress, stratified by careFootnote a

CLI, Cumulative lifetime incidence; IR, incidence rate; MHC+, episodes in combination with mental health care.
a In participants with ⩾4 (hypo)manic symptoms.
b Values are expressed as percentage (number) of cases.
c Values are expressed as number of cases per 100 000 person-years (denominator is population of person-years).
Association with childhood disorders
Seventy-six participants (7.2%) were diagnosed with any childhood disorder, mostly with ADHD (4.1%). Being diagnosed with a childhood disorder did not increase the risk of belonging to any (hypo)manic category at T0 (for detailed results see Tables 1-B, 1-D and 1-E at www.mania.homestead.com). The association between the incident (hypo)manic categories and childhood morbidity was large and significant [HR 5.29, 95% confidence interval (CI) 2.01–13.91, p=0.001 for (hypo)manic episode] (Table 2). Thus, CMSAs reduced incidence rates (at most) by a factor of 2 according to the (hypo)manic category (see Table 1-C at www.mania.homestead.com).
Age, sex and urbanicity
A strong association existed between age and IRs for all (hypo)manic categories (Table 2, Figs 1–3), with IRs decreasing as age increased. Post-hoc analyses showed that the IRs of episodic categories, compared to the age group 15–21 years, decreased very strongly after the age of 21 years [HR 0.021, 95% CI 0.0024–0.18, p=0.000 for hypomanic episode; HR 0.031, 95% CI 0.0050–0.19, p=0.000 for (hypo)manic episode], with a similar decline (albeit statistically inconclusive) after restriction to episodes plus mental health care. Subsequent CMSAs showed a similar pattern of associations (see Table 1-C, Figs 2-B and 3-B at www.mania.homestead.com).

Fig. 1. Incidence of (hypo)manic disorder, stratified by age and care. ––▪––, (Hypo)manic episodea,b; - -▪- -, (hypo)manic episodea,b, MHC+. MHC+, episodes in combination with mental health care. a (Hypo)manic episode: either hypomanic or manic episode. b Independent of having lifetime depressive episodes.

Fig. 2. Hazard ratios of incident (hypo)manic categories, stratified by age group. ––▪––, (Hypo)manic episodea,b,d; · · ·▵· · ·, ⩾1 symptomd; - -◆- -, some distressc,e. Reference category: the 19–21 years age group. a (Hypo)manic episode: either hypomanic or manic episode. b Independent of having lifetime depressive episodes. c In participants with ⩾4 (hypo)manic symptoms. d Results adjusted for sex, urbanicity, depression and childhood disorders [oppositional–defiant disorder (OD), conduct disorder (CD) or attention-deficit/hyperactivity disorder (ADHD)]. e Results adjusted for sex, urbanicity, depression, childhood disorders (ODD, CD or ADHD) and number of (hypo)manic symptoms.

Fig. 3. Incidence of experienced distress in participants with ⩾4 (hypo)manic symptoms, stratified by age and care. ––▴––, Some distress; · · ·▴· · ·, some distress, MHC+. MHC+, distress in combination with mental health care.
The incidence of manic episodes was 14 times lower in women compared to men (HR 0.072, 95% CI 0.065–0.79, p=0.031). However, no sex differences were present for other (hypo)manic categories and there was no association with urbanicity. In the CMSA, a male preponderance in incidence of manic episodes remained (HR 0.08, 95% CI 0.070–0.86, p=0.037), whereas a female preponderance was seen in the category with ⩾1 symptom (HR 1.51, 95% CI 1.01–2.26, p=0.045).
Discussion
Findings
In this large prospective study of over 1000 adolescents and young adults, the results show that IRs of both (hypo)manic episodes and (hypo)manic symptoms are much higher than those reported previously, and that the risk of developing a disorder is very low after the age of 21 years, independent of childhood disorders such as ADHD. In addition, the results demonstrate a continuous distribution of (hypo)manic symptoms and distress, thus supporting the hypothesis that a dimensional representation may usefully describe the (hypo)manic phenotype (Allardyce et al. Reference Allardyce, Suppes and van Os2007). Only a small fraction of adolescents and young adults experiencing these phenomena were receiving psychiatric care and the co-occurrence of (hypo)manic episodes with depression was low compared to most of the literature. The incidence of the (hypo)manic categories, in particular categories at the level of clinical morbidity, was strongly associated with previous childhood disorders and male sex. In conclusion, this study showed, for the first time, that experiencing (hypo)manic symptoms is a common adolescent phenomenon that infrequently predicts mental health care use. The findings suggest that the onset of bipolar disorder can be elucidated by studying the pathway from non-pathological phenotypic expression to disability.
Limitations
Several limitations need to be considered. First, although a prospective design was used, the study became partly retrospective by implementing questions regarding time intervals between waves. Therefore, the possibility of recall bias cannot be excluded, although arguably this would be likely to contribute more to false negatives than false positives (Simon & VonKorff, Reference Simon and VonKorff1995).
Second, exclusion of individuals at T0 and exclusion of the older cohort means that the results are based on a limited age range with an associated decrease in statistical power. This could have caused the incidence to fall after the age of 21 years. However, similar results were found after the oldest two age groups were collapsed, thus increasing statistical power.
Third, the age range of participants was limited as follow-up of participants did not begin until the age of 14 years. Future studies should examine whether adolescent bipolar symptoms, relevant to adult clinical morbidity, are present also in younger samples.
Fourth, the analyses of childhood disorders were all based on retrospective parental report. This may have influenced the reliability of the results. However, Faraone et al. (Reference Faraone, Biederman and Milberger1995) have shown that maternal reports of their children's psychopathology by 1-year recall provided a reliable and accurate means of assessment. In addition, the rates found for the childhood disorders analysed in this study are comparable to the rates found in other studies (Costello et al. Reference Costello, Mustillo, Erkanli, Keeler and Angold2003), which likewise attests to their validity.
Fifth, the predictive validity of the broad category of (hypo)manic symptoms may be enhanced by testing whether (hypo)manic symptoms can also predict depression. Testing this in a post-hoc analysis revealed that participants who had at least two (hypo)manic symptoms once at T0, T1 or T2 had a nearly twofold higher risk of ever experiencing a depressive episode compared to participants with less than two (hypo)manic symptoms at T0/T1/T2.
Cumulative incidence and person-year incidence rates
IRs in this study are much higher than those reported previously (Bebbington & Ramana, Reference Bebbington and Ramana1995). A partial explanation for this discrepancy may be the use of clinical samples in previous work. The effect of sample type on observed IRs is clearly shown in the current data, in which grouping for mental health care decreased IRs. However, the elevated IRs cannot be explained entirely by sample type because even for participants receiving mental health care, IRs were still higher than previously reported estimates, whereas CLI estimates did yield estimates comparable to those in previous reports (Lish et al. Reference Lish, Dime-Meenan, Whybrow, Price and Hirschfeld1994). One reason for the higher IRs may be that the current sample consisted of adolescents, who display the highest risk of developing mental disorders (Kessler et al. Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005). The focus on clinical samples might also explain the low co-occurrence of depressive episodes with (hypo)manic episodes because both types of episodes, independently of each other, increase need for care and help-seeking, resulting in more ‘co-morbid’ psychopathology at the level of mental health care (‘Berkson's bias’) (Regeer et al., in press). Accordingly, the co-occurrence of depressive episodes in the current study was higher in participants receiving mental health care.
Bipolar disease as a developmental disorder
The greatest risk of developing (hypo)manic episodes was before age 22 years, after which it decreased to the point of almost disappearing. This finding is supported by other studies in which the most common age of onset for bipolar disorder was reported to be between 15 and 19 years (Szádóczky et al. Reference Szádóczky, Papp, Vitrai, Ríhmer and Füredi1998). Findings similarly concur with studies in which substantial numbers of adult patients retrospectively reported first experiencing symptoms in childhood or adolescence (Joyce, Reference Joyce1984). The findings in the current sample show low levels of cross-sectional co-morbidity of childhood disorders with (hypo)manic categories at T0, but very high longitudinal co-morbidity with new, incident bipolar categories, in particular at the level of clinical morbidity, over time. The CMSA in which childhood disorders were excluded did not change the pattern of association with age. The pattern of findings therefore suggests that the ontogenesis of (hypo)manic symptoms and (hypo)manic disorder may be traced to the adolescent developmental period and that expression of certain childhood disorders may increase the risk for later expression of bipolar morbidity.
Continuity
The current results suggest that (hypo)manic symptoms may represent a common phenomenon in the general population. Symptoms and clinical morbidity showed dose–response relationships, in that more cases of clinical morbidity arose as the number of symptoms increased, supporting continuity between subclinical and clinical categories. Evidence for continuity has been provided by others (Angst et al. Reference Angst, Gamma, Benazzi, Ajdacic, Eich and Rössler2003; Lewinsohn et al. Reference Lewinsohn, Seeley and Klein2003; Regeer et al. Reference Regeer, Krabbendam, de Graaf, ten Have, Nolen and van Os2006). However, as evidenced by findings in the current study, the vast majority of these individuals with symptomatic expression never develop bipolar disorder. Thus, (hypo)manic symptoms may be conceived partially as pertaining to normal adolescent development. If, however, symptoms persist over time, individuals may be at risk of transition to bipolar disorder (Cougnard et al. Reference Cougnard, Marcelis, Myin-Germeys, de Graaf, Vollebergh, Krabbendam, Lieb, Wittchen, Henquet, Spauwen and van Os2007). Thus, future work should investigate whether adolescents with persistent (hypo)manic symptoms are at risk of making the transition to bipolar disorder, and which factors drive such transitions. Possible factors are symptom factors (intrusiveness, frequency and co-morbidity of symptoms), personal and cultural factors (coping, illness behaviour, societal tolerance and the development of functional impairments), and known bipolar risk factors (a positive family history, exposure to life events, or an interaction between these factors) (Lapalme et al. Reference Lapalme, Hodgins and LaRoche1997; van Os & Verdoux, Reference van Os, Verdoux, Murray, Jones, Susser, van Os and Cannon2003; Hillegers et al. Reference Hillegers, Burger, Wals, Reichart, Verhulst, Nolen and Ormel2004; van Os et al. Reference van Os, Linscott, Myin-Germeys, Delespaul and Krabbendam2009).
Risk factors
Male sex was a risk factor for the onset of manic episodes. This is inconsistent with studies finding equal sex distributions (Lloyd et al. Reference Lloyd, Kennedy, Fearon, Kirkbride, Mallett, Leff, Holloway, Harrison, Dazzan, Morgan, Murray and Jones2005). However, the finding does concur with several studies in which it was suggested that male sex is associated with earlier onset of mania (Carlson et al. Reference Carlson, Bromet and Sievers2000; Kennedy et al. Reference Kennedy, Boydell, Kalidindi, Fearon, Jones, van Os and Murray2005a). Male preponderance in incidence was not seen for subclinical bipolar categories, suggesting that male sex specifically increases the risk for clinical morbidity. The link between male sex and poor outcome is well known for other types of psychotic illness, in particular schizophrenia (Castle & Murray, Reference Castle and Murray1991).
Urbanicity generally did not increase the risk for (hypo)manic categories. This concurs with previous findings (Krabbendam & van Os, Reference Krabbendam and van Os2005). A recent study showed any association between (hypo)manic disorder and urbanicity is probably mediated by positive psychotic symptoms (Kaymaz et al. Reference Kaymaz, van Os, de Graaf, ten Have, Nolen and Krabbendam2007).
Clinical implications
Several clinical implications are suggested by this study. First, given the possible wide distribution of low-grade bipolar experiences, the suggested specific developmental phase of expression and evidence that subclinical expression of bipolarity increases the risk for later bipolar disorder in a dose–response fashion (Regeer et al. Reference Regeer, Krabbendam, de Graaf, ten Have, Nolen and van Os2006), a public health approach focusing on targeted early identification may merit further investigation. Second, work focusing on psychotic disorder has indicated that subclinical phenotypes may be more likely to make the transition to the fully developed disorder if there is persistence over time (Cougnard et al. Reference Cougnard, Marcelis, Myin-Germeys, de Graaf, Vollebergh, Krabbendam, Lieb, Wittchen, Henquet, Spauwen and van Os2007; Dominguez et al., in press). Thus, examining patterns and determinants of persistence of subclinical expression of bipolar experiences may be similarly instructive. Third, the findings suggest that greater symptom load and greater levels of distress are associated with a higher probability of the outcome of mental health care. Understanding the dynamics between symptoms, distress and help-seeking is necessary to develop early interventions. Fourth, the findings suggest that only a minority of those with bipolar experiences are in care, similar to findings in depression and anxiety disorders. Given that, in depression, subsyndromal expression is associated with a substantial amount of disability (Judd et al. Reference Judd, Schettler and Akiskal2002), individuals with subclinical bipolar experiences not receiving care may similarly have a considerable degree of disability that could be reduced if recognized and treated.
Acknowledgements
This work is part of the EDSP study, which is funded by the German Federal Ministry of Education and Research (BMBF; project nos. 01EB9405/6, 01EB9901/6, EB01016200, 01EB0140 and 01EB0440). Part of the fieldwork and analyses were also supported by grants from the Deutsche Forschungsgemeinschaft (DFG; project nos. LA1148/1-1, WI2246/1-1, WI 709/7-1 and WI 709/8-1). M. Höfler provided helpful comments and suggestions on earlier drafts of this article. K. von Sydow, G. Lachner, A. Perkonigg, P. Schuster, H. Sonntag, T. Brückl, E. Garczynski, B. Isensee, A. Nocon, C. Nelson, H. Pfister, M. Höfler, V. Reed, B. Spiegel, A. Schreier, U. Wunderlich, P. Zimmermann, K. Beesdo and A. Bittner are current or past core staff members of the EDSP group and managed field and data work. J. Angst (Zurich), J. Margraf (Basel), G. Esser (Potsdam), K. Merikangas (NIMH, Bethesda), R. Kessler (Harvard, Boston) and J. van Os (Maastricht) provided scientific advice on the design of the EDSP study.
Declaration of Interest
J. van Os is/has been an unrestricted research grant holder with, or has received financial compensation as an independent symposium speaker from, Eli Lilly, BMS, Lundbeck, Organon, Janssen-Cilag, GSK, AstraZeneca, Pfizer and Servier. H.-U. Wittchen is in receipt of research support from Eli Lilly and Company, Novartis, Pfizer and Schering-Plough, and is a consultant for Eli Lilly, GlaxoSmithKline Pharmaceuticals, Hoffmann–La Roche Pharmaceuticals, Novartis, Pfizer and Wyeth. Speaking honoraria have been received by H.-U. Wittchen from Novartis, Schering-Plough, Pfizer and Wyeth, by R. Lieb from Wyeth, and by K. Beesdo from Pfizer.









