Introduction
Bryoria Brodo & D. Hawksw. (Parmeliaceae, Lecanoromycetes) is regarded as one of the taxonomically most difficult genera of macrolichens. The genus is mainly circumboreal, but occurs also in mountainous areas of Asia, Australasia and Africa (Brodo & Hawksworth Reference Brodo and Hawksworth1977). Bryoria species, often referred to colloquially as ‘horsehair lichens’, consist of copiously branched, capillary, whitish grey, brown or blackish branches that vary in habit from erect to pendent or sometimes decumbent. Sexual fruiting structures are uncommon and many species are known only in the sterile condition. As a result, the diagnostic features in species delimitation include characters such as branching pattern, presence and type of soralia, presence and form of pseudocyphellae, growth form, thallus colour and secondary chemistry (see Brodo & Hawksworth Reference Brodo and Hawksworth1977; Krog Reference Krog1980). In some cases, the presence or absence of a single lichen substance has been used to separate taxa which are otherwise anatomically and morphologically more or less indistinguishable. Some authors have treated such taxa as mere chemotypes of one species (Holien Reference Holien1989). Pendent species tend to be especially variable in morphology and chemistry, and putative intermediate forms have occasionally been reported. As a result, the infrageneric relationships and species boundaries are in some cases poorly known. The intermediates found may point to the existence of hybrids via thallus fusion or sexual reproduction, unidentified species, or morphological plasticity caused by environmental factors or photobionts (Brodo & Hawksworth Reference Brodo and Hawksworth1977; Brodo Reference Brodo1978; Holien Reference Holien1989).
Bryoria has been extensively studied by Motyka (Reference Motyka1964), Hawksworth (Reference Hawksworth1971, Reference Hawksworth1972) and, for North American taxa especially, by Brodo & Hawksworth (Reference Brodo and Hawksworth1977), who separated Bryoria from Alectoria Ach. and divided the genus into five sections on the basis of anatomical, chemical and morphological characters. Later, Common & Brodo (Reference Common and Brodo1995) transferred species of the section Subdivergentes (Mot.) Brodo & D. Hawksw. to the genus Nodobryoria Common & Brodo, largely on the basis of cortical structure. Jørgensen (Reference Jørgensen1972, Reference Jørgensen1975), Jørgensen & Ryvarden (Reference Jørgensen and Ryvarden1970) and Jørgensen & Galloway (Reference Jørgensen and Galloway1983) focused on species of the section Divaricatae (DR.) Brodo & D. Hawksw., while Holien (Reference Holien1989, Reference Holien1991, Reference Holien1992) examined the taxonomy of North European species of sections Bryoria and Implexae (Gyeln.) Brodo & D. Hawksw. The only molecular systematic study on Bryoria examined the taxonomy of Bryoria fremontii (Tuck.) Brodo & D. Hawksw. (Velmala et al. Reference Velmala, Myllys, Halonen, Goward and Ahti2009). Currently, Bryoria includes some 80 species (http://www.indexfungorum.org), although many of these ‘species’, viewed from the perspective of the mycobiont, may represent environmental morphotypes of a smaller number of phylospecies. According to recent phylogenetic analyses based on molecular markers Bryoria belongs to the Parmeliaceae (Thell et al. Reference Thell, Feuerer, Stenroos, Kärnefelt and Myllys2004; Crespo et al. Reference Crespo, Lumbsch, Mattson, Blanco, Divakar, Articus, Wiklund, Bawingan and Wedin2007) and is probably related to the genus Alectoria (Halonen et al. Reference Halonen, Myllys, Velmala and Hyvärinen2009). In this paper we have reconstructed a phylogeny for Bryoria using three DNA regions, in addition to some chemical and morphological characters. Based on our results we propose a new infrageneric classification for the genus. We also show that while most species with an erect growth-form are clearly monophyletic, the current species status of many pendent taxa can be questioned.
Materials and Methods
Taxon selection
The analyses included 88 ingroup specimens of 25 species and seven unidentified taxa representing all four currently recognized sections of Bryoria (Tables 1 & 2). We have primarily followed the species concept of Brodo & Hawksworth (Reference Brodo and Hawksworth1977) with the exception of B. trichodes (Michx.) Brodo & D. Hawksw. subsp. trichodes and B. trichodes subsp. americana (Motyka) Brodo & D. Hawksw., which are treated as separate species, and B. pseudofuscescens (Gyeln.) Brodo & D. Hawksw., B. friabilis Brodo & D. Hawksw. and B. vrangiana (Gyeln.) Brodo & D. Hawksw., which are treated as chemotypes of B. implexa (Hoffm.) Brodo & D. Hawksw. following Holien (Reference Holien1989, Reference Holien1994). Bryoria tortuosa (G. Merr.) Brodo & D. Hawksw. is not recognized as separate from B. fremontii based on our molecular phylogeny (Velmala et al. Reference Velmala, Myllys, Halonen, Goward and Ahti2009; but see also Goward Reference Goward2009). When available, three to six specimens for each species (or for each chemotype in case of B. implexa) were included in the analyses to examine the infraspecific variation and species delimitation. Furthermore, we tried to cover the geographical variation by selecting specimens from different continents.
Table 1. List of taxa, their herbarium voucher numbers, sequence ID numbers, secondary chemistry and GenBank Accession numbers

* ale = alectorialic acid, atr = atranorin, bar = barbatolic acid, cfum = confumarprotocetraric acid, fum = fumarprotocetraric acid, gyr = gyrophoric acid, lob = lobaric acid, nsti = norstictic acid, pro = protocetraric acid, pso = psoromic acid, qua = quaesitic acid, sti = stictic acid, usn = usnic acid, vul = vulpinic acid, unk. = unknown, in sor. = present only in soralia, ( ) = present in small amounts, - = tlc not performed.
† sequences of Gowardia arctica are from Halonen et al. (Reference Halonen, Myllys, Velmala and Hyvärinen2009)
Table 2. Sections of Bryoria according to Brodo & Hawksworth (Reference Brodo and Hawksworth1977) and some of their main diagnostic characters. Type species of each section is marked in bold. Species with uncertain section position are marked with question mark

Gowardia arctica Halonen et al. was used as an outgroup taxon based on the phylogenies by Halonen et al. (Reference Halonen, Myllys, Velmala and Hyvärinen2009) and Thell et al. (Reference Myllys, Stenroos and Thell2002, 2004). Nodobryoria abbreviata (Müll. Arg.) Common & Brodo, Pseudephebe pubescens (L.) M. Choisy and Sulcaria isidiifera Brodo were included in the analyses to test the monophyly of the ingroup.
Thin-layer chromatography
Secondary chemistry has played a major role in species identification, especially with regard to the pendent species. Accordingly we analyzed our material using thin-layer chromatography (TLC) for detection of secondary compounds according to the methods described by Orange et al. (Reference Orange, James and White2001). A few specimens were too small to be used for TLC and were directly subjected to DNA extraction. The acetone extracts were spotted with 75 mm / 75 µl Haematocrit glass capillary tubes (Hirschmann Laborgeräten) on 10 × 20 cm Merck silica gel 60 F-254 pre-coated glass plates and run in solvents A and B (formulae from both Culberson Reference Culberson1972 and Mietzsch et al. Reference Mietzsch, Lumbsch and Elix1994, according to Orange et al. Reference Orange, James and White2001, were used for solvent B). When possible, branches without soralia were used for TLC. Soralia were tested separately with Pd reagent for the presence of fumarprotocetraric acid, which is an important diagnostic character, especially in B. fuscescens s. lat. (Brodo & Hawksworth Reference Brodo and Hawksworth1977).
Molecular techniques
Total DNA was extracted using Qiagen's DNeasy Blood & Tissue Kit following the manufacturer's instructions with the following exceptions. Thallus fragments of approximately 0·5–4 cm long were ground with mini-pestles in 40 µl of the lysis buffer, after which 140 µl of the buffer was added. Sometimes the amount of Buffer ATL, Buffer AL and ethanol used was 20 µl less and the amount of proteinase K used was 10 µl less than instructed. The extracted DNA was eluted in 120 µl of the elution buffer so that the first 60 µl of the buffer was added after which the sample was incubated for 5 min and centrifuged. The elution step was repeated once for the same microcentrifuge tube.
We used three DNA regions in our study: 1) ITS regions of the nuclear ribosomal DNA, 2) partial sequences of the small subunit of the mitochondrial ribosomal DNA (mtSSU) and 3) partial sequences from the protein-coding glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The three gene regions were amplified and sequenced from the same extraction. As many Bryoria species tend to grow intermixed, specimens were extracted from the same material already used for the TLC analysis.
Primers used for PCR amplification were as follows: for the ITS region, ITS1-F (Gardes & Bruns Reference Gardes and Bruns1993) together with ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990) or ITS1-LM (Myllys et al. Reference Myllys, Lohtander, Källersjö and Tehler1999) together with ITS2-KL (Lohtander et al. Reference Lohtander, Myllys, Sundin, Källersjö and Tehler1998); for the mtSSU region, mtSSU1-KL together with mtSSU2-KL (Lohtander et al. Reference Lohtander, Oksanen and Rikkinen2002); for the GAPDH region, Gpd1-LM together with Gpd2-LM (Myllys et al. Reference Myllys, Stenroos and Thell2002). PCR amplification was performed as described by Velmala et al. (Reference Halonen, Myllys, Velmala and Hyvärinen2009) using PuReTaq Ready-To-Go PCR beads (GE Healthcare).
The PCR products were viewed under UV light on 1% agarose gel stained with either ethidium bromide or SYBR Safe™ DNA gel stain (Invitrogen). The PCR products were purified according to the manufacturers' protocol with either Qiagen's QIAquick PCR Purification Kit or GE Healthcare illustra's GFX tm PCR DNA and Gel Band Purification Kit, and eluted in 15–50 µl elution buffer or dH2O.
The sequencing reactions were prepared using BigDye Terminator Cycle Sequencing Reaction Kit version 1.1 (Applied Biosystems). The above listed PCR primers were used also for sequencing reactions, except for the ITS region for which the primer ITS5 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990) was used together with ITS2-KL. Ten µl reaction samples containing 3 µl dH2O, 2 µl BigDye, 2 µl sequencing buffer, 1 µl primer at 2·5 µm concentration and 2 µl purified PCR product were run with the following amplification parameters: initial denaturation for 1 min at 96°C or no initial denaturation, followed by 30 cycles of 30 s at 96°C, 15 s at 50°C and 4 min at 60°C.
The post-reaction purification of the samples for ABI PRISM™ DNA Sequencer (Applied Biosystems) was made following the protocol described in Högnabba (Reference Högnabba2006) and for MegaBACE 1000 DNA Analysis System (GE Healthcare) using Montage SEQ96 Cleanup Kit and MultiScreen SEQ384 Filter Plates (Millipore) according to the manufacturer's protocol. In some cases the cleaned PCR products were sequenced by Macrogen Inc., South Korea (www.macrogen.com). The DNA strands were assembled and manually corrected with SeqMan II 4.00 (DNASTAR).
Morphological and chemical characters
In addition to molecular data, 20 morphological or chemical characters were used in our phylogenetic reconstruction. Most of the selected characters have previously been used to delimit sections in Bryoria (see Table 2). The characters were coded primarily according to species morphology and chemistry as given in the literature (Bystrek Reference Bystrek1969; Awasthi Reference Awasthi1970; Jørgensen & Ryvarden Reference Jørgensen and Ryvarden1970; Jørgensen Reference Jørgensen1972, Reference Jørgensen1975; Brodo & Hawksworth Reference Brodo and Hawksworth1977; Jørgensen & Galloway Reference Jørgensen and Galloway1983; Awasthi & Awasthi Reference Awasthi and Awasthi1985; Holien Reference Holien1989, Reference Holien1991, Reference Holien1992, Reference Holien1994; Goward Reference Goward1999; Wang & Harada Reference Wang and Harada2001; Wang et al. Reference Wang, Harada, Narui and Culberson2003, Reference Wang, Harada, Koh and Hur2005, Reference Wang, Harada, Koh and Hur2006; Harada & Wang Reference Harada and Wang2006). Given, however, the wide range of morphological and chemical variation at the infraspecific level, we carefully inspected each specimen used in the analyses. For instance, the literature indicates that pseudocyphellae (character 19) can in some species be either present or absent. For our purposes this character was coded according to the condition actually observed in the specimen. In a few cases our character coding was at variance with that given in the literature, for example one specimen of B. bicolor (Ehrh.) Brodo & D. Hawksw. (S23) contained not only fumarprotocetraric acid but also barbatolic and psoromic acids, these latter two substances hitherto unknown in this species. The data matrix of chemical and morphological characters is presented in the Appendix.
The characters are as follows:
1. Growth form erect to caespitose (0), subpendent (1), pendent (2)
2. Vulpinic acid present (0), only in soralia (1), absent (2)
3. β-orcinol depsidones present (0), absent (1), see characters 4–6
4. Fumarprotocetraric acid present in thallus (0), only in soralia (1), absent (2)
5. Norstictic acid present (0), absent (1)
6. Psoromic acid present (0), absent (1)
7. β-orcinol depsides present (0), absent (1), see characters 8–10
8. Atranorin present (0), absent (1)
9. Alectorialic acid present (0), absent (1)
10. Barbatolic acid present (0), absent (1)
11. Orcinol depsides present (0), absent (1), see characters 12–13
12. Gyrophoric acid present (0), absent (1)
13. Lobaric acid present (0), absent (1)
14. Usnic acid present (0), absent (1)
15. True lateral spinules present (0), absent (1). Brodo & Hawksworth (Reference Brodo and Hawksworth1977) defined true spinules as short branches with constricted base arising at right or slightly acute angles to the main stems. They are typical for the section Divaricatae.
16. Spinulose branches present (0), absent (1). Spinulose branches are not basally constricted (Brodo & Hawksworth Reference Brodo and Hawksworth1977). According to our experience, however, they are sometimes difficult to distinguish from true lateral spinules. In such cases, we followed the information given in the literature.
17. Bicolorous thallus present (0), absent (1). As discussed in Brodo & Hawksworth (Reference Brodo and Hawksworth1977), bicolorous species are typically blackened in the basal parts and pale in the apical portions. Yet some species, for instance B. americana and B. nadvornikiana (Gyeln.) Brodo & D. Hawksw., are also bicolorous but here the blackened areas are not restricted regularly to the basal parts. This character was fairly easy to code with some exceptions: Jørgensen (Reference Jørgensen1972) characterized B. confusa (D.D. Awasthi) Brodo & D. Hawksw. as “uniformly shining brownish,” whereas our specimen was distinctly bicolorous, as already described by Awasthi (Reference Awasthi1970) and Awasthi & Awasthi (Reference Awasthi and Awasthi1985). Unnamed Bryoria specimens L168 and S291 had only slightly paler apices than basal parts but were coded as bicolorous.
18. Soralia present (0), absent (1)
19. Pseudocyphellae present (0), absent (1). For some specimens pseudocyphellae were sometimes difficult to distinguish from young soralia. In such cases the material was coded as missing data.
20. Distribution restricted to SE Asia (0), North America (1), more widely distributed (2). The distribution of each species was used as a basis when coding this character, with one exception: the North American specimens in Clade V representing putative new species were coded as separate taxa. Bryoria indonesica (P. M. Jørg.) Brodo & D. Hawksw. is coded as having a SE Asian distribution, although it has also been reported from New Zealand (Jørgensen & Galloway Reference Jørgensen and Galloway1983).
Sequence alignment and phylogenetic analyses
We were unable to obtain GAPDH and mtSSU sequences from all of the specimens examined. Consequently, two different data matrices were analyzed to examine the effect of missing data: 1) combined ITS, GAPDH and mtSSU data, which included all 92 specimens and 2) the combined ITS, GAPDH and mtSSU data with 71 specimens successfully sequenced in all three gene regions. The first data set will be referred to in Results and Discussion as the large data set and the second as the pruned data set. In addition, we performed an analysis where the morphological and chemical characters were combined with the large data set.
Prior to analysis, the sequences were aligned with MUSCLE, v. 3.6. (Edgar Reference Edgar2004) located at CSC – IT Center for Science (http://www.csc.fi/english) using default parameters. The hypervariable region in the end of the mtSSU was removed from the combined analysis (characters 2328–2498 in the alignment of large data set and characters 2312–2456 in the alignment of pruned data). All the three data sets were subjected to parsimony analysis as implemented in TNT (Goloboff et al. Reference Goloboff, Farris and Nixon2008), using the option traditional search with the following settings: random addition sequence with 100 replicates followed by TBR branch swapping. No more than 10 trees were saved for each replicate. Gaps were treated as missing data in these analyses. Support for each node was estimated using bootstrapping (1000 repetitions; otherwise similar options as in heuristic search).
Results
The combined large data set with 92 specimens and 464 parsimony informative characters from 2637 aligned sites generated six equally parsimonious trees with a length of 1416 steps. In the highly resolved strict consensus (Fig. 1), the monophyly of the genus Bryoria is moderately well supported. Species are divided into five monophyletic groups (Clades I–V, Fig.1). Most of the groups receive moderately high (Clade III) to high (Clades I, II and V) bootstrap values. Only Clade IV remains unsupported.
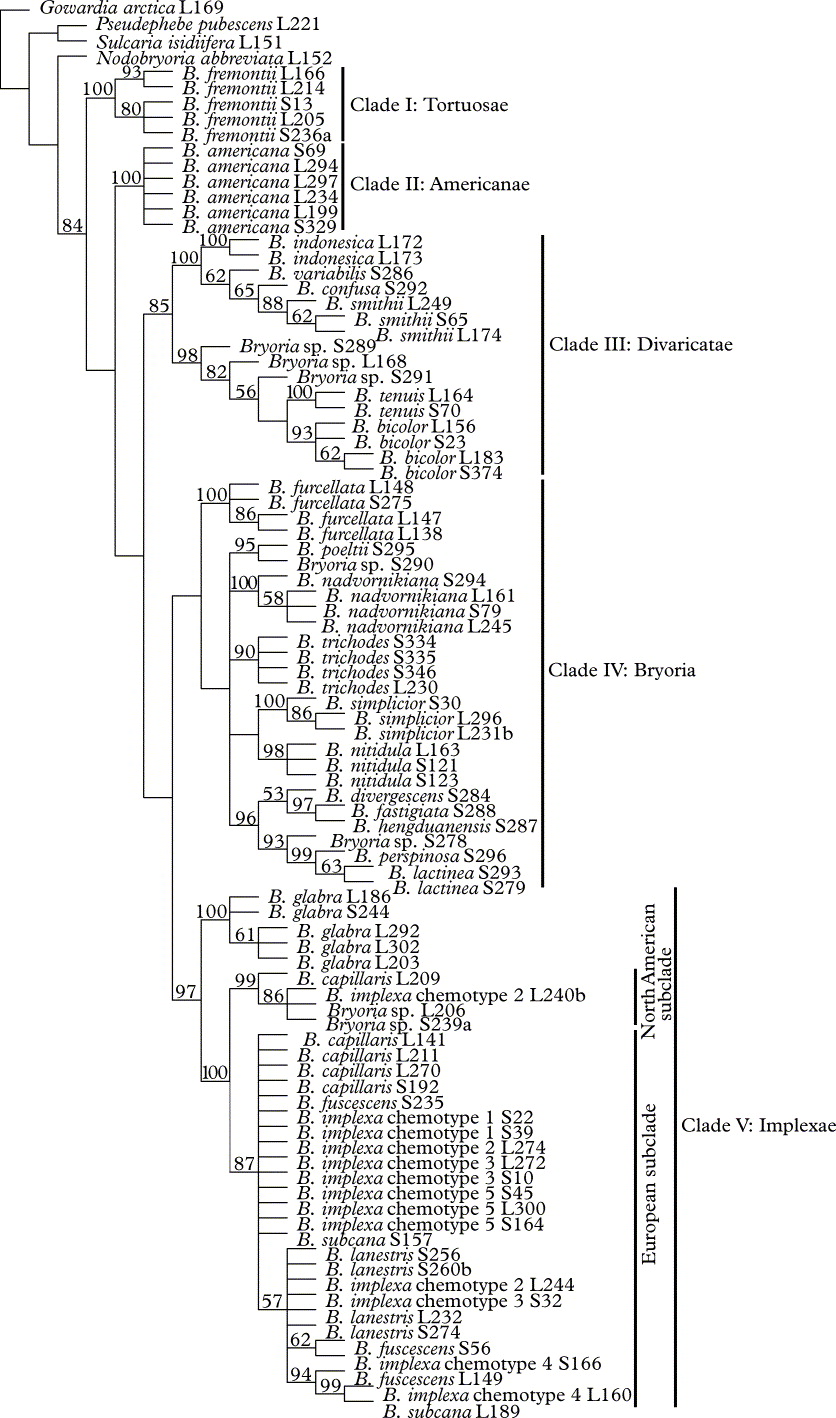
Fig. 1. Strict consensus based on the combined large data set. Bootstrap values are shown at nodes.
Most Bryoria species form strongly supported monophyletic entities, the most notable exceptions being B. capillaris (Ach.) Brodo & D. Hawksw., B. fuscescens (Gyeln.) Brodo & D. Hawksw., B. implexa, B. lanestris (Ach.) Brodo & D. Hawksw. and B. subcana (Nyl. ex Stizenb.) Brodo & D. Hawksw. (Clade V, Fig. 1). These species together form a group in which none of the currently recognized species, or chemotypes in the case of B. implexa, appears as a monophyletic entity. Instead, the specimens are divided into two subclades based on their geographic distribution and partly on secondary chemistry. The first subclade includes only North American specimens of B. capillaris and B. implexa chemotype 2, in addition to two specimens most probably belonging to B. fuscescens s. lat. but not exactly conforming to known species within the complex. The second subclade consists of European and Asian specimens of B. capillaris, B. fuscescens, B. implexa, B. lanestris and B. subcana in addition to North American specimens of B. fuscescens and B. lanestris. Somewhat surprisingly, the morphologically quite similar B. glabra (Motyka) Brodo & D. Hawksw. constitutes a strongly supported sister group to the two subclades.
The large data set combined with morphological and chemical characters gave 20 trees with a length of 1549 steps. The topology of the strict consensus was almost identical to that obtained from the combined molecular data alone; in the consensus all the five major groups were present, but the relationships within the clades were slightly more resolved (Fig. 2).

Fig. 2. Strict consensus based on the combined large data set of molecular, morphological and chemical characters. Bootstrap values are shown at nodes.
The Muscle alignment of the pruned data set produced 2598 aligned sites of which 418 were parsimony informative. The TNT analysis generated three equally parsimonious trees of 904 steps, a strict consensus of which is presented in Figure 3. As the pruned data set included fewer taxa than the large data set, a direct comparison of the tree topologies obtained from different analyses is not possible. Otherwise the topologies were mostly congruent with the following exception: B. americana (Clade I) and B. fremontii (Clade II) form an unsupported group in the pruned data tree while in the tree obtained from the large data set B. fremontii is basal to B. americana. The topologies differ also in the degree of resolution: the pruned data set produced a more resolved tree with generally higher support values, especially for Clade IV.

Fig. 3. Strict consensus based on the combined pruned data set. Bootstrap values are shown at nodes.
Discussion
We detected five major groups within the genus Bryoria (Clades I–V, Figs 1–3). All received strong support values particularly in the pruned data tree (Fig. 3). The lower support values in Clade III and particularly in Clade IV, in trees obtained from large data sets, apparently resulted from the large amount of missing data in these clades (Figs 1 & 2). It should also be noted that in the pruned data tree B. fremontii and B. americana (i.e., lineages I and II) grouped together, but this relationship did not receive any support.
Based on these results, we propose a new infrageneric classification of the genus Bryoria, which we apportion in five sections, that is, section Tortuosae (Bystr.) Brodo & D. Hawksw., section Americanae Myllys & Velmala, section Divaricatae (DR.) Brodo & D. Hawksw., section Bryoria and section Implexae (Gyeln.) Brodo & D. Hawksw. As discussed below, our proposed sections correspond in large part with those described by Brodo & Hawksworth (Reference Brodo and Hawksworth1977) (see Tables 2 and 3).
Section Tortuosae corresponds to Clade I in Figures 1–3, consisting of a single species, B. fremontii. Bryoria fremontii is unique within Bryoria in producing a bright yellow pigment, vulpinic acid. An earlier second species within section Tortuosae, B. tortuosa, was recently synonymized under B. fremontii based on our DNA analyses (Velmala et al. Reference Velmala, Myllys, Halonen, Goward and Ahti2009). Although the presence of vulpinic acid is the only synapomorphy obtained from our chemical and morphological data matrix for Clade I, B. fremontii is also unique within pendent species of Bryoria in its production of proportionately broad, twisted, foveolate and often partly flattened main branches and finer rather terete secondary branches (Fig. 4A; see also Goward Reference Goward2009). The colour of the thallus is typically reddish or yellowish brown depending on the concentration of vulpinic acid. Bryoria fremontii occurs predominantly in western North America and northern Europe (see Velmala et al. Reference Velmala, Myllys, Halonen, Goward and Ahti2009, Fig. 4).

Fig. 4. Bryoria species representing each section of the genus. A, Bryoria fremontii from section Tortuosae (specimen S13 with yellow soralia and foveolate main branches); B, Bryoria americana from section Americanae (specimen L294 with blackened fragmentation regions); C, Bryoria bicolor from section Divaricatae (specimen L183 with bicolorous thallus and lateral spinules); D, Bryoria trichodes from section Bryoria (specimen L230 with oval and white pseudocyphellae); E, Bryoria nadvornikiana from section Bryoria (specimen S79 with spinulose side branches); F, Bryoria implexa from section Implexae (specimen L274 with pseudocyphellae and wide branch angles). Scales: A–F = 1 mm.
Our new section Americanae (Clade II) also consists of a single species, B. americana. None of the morphological and chemical characters examined appeared as synapomorphies for this section. Bryoria americana may indeed be confused with other pendent taxa, especially with B. fuscescens and B. implexa s. str. from which it is distinguished by its small perpendicular side branches, its depressed pseudocyphellae, and its usually fully esorediate stems with blackened fragmentation regions (Fig. 4B). Brodo & Hawksworth (Reference Brodo and Hawksworth1977) treated B. americana and B. trichodes as subspecies within B. trichodes, owing to the presumed existence in North America of intermediate forms. Later, Holien (Reference Holien1994) detected B. americana in Europe and segregated it from the non-European B. trichodes. Our results confirm the latter view and show that B. americana is a distinct species not closely related to B. trichodes. The two taxa are best separated by their pseudocyphellae: fissural, depressed, dark and longer in B. americana versus oval, raised, white and shorter in B. trichodes. Interestingly, our material of B. trichodes from the Russian Far East had pigmented and long (up to 0·85 mm!) pseudocyphellae, but they were never depressed as in B. americana. True B. americana is an oceanic species occurring mainly in humid coastal regions of eastern and western Canada (Brodo & Hawksworth Reference Brodo and Hawksworth1977) and NW Europe. In Europe the species is still probably much overlooked owing to difficulties in identification (see Brodo Reference Brodo1992; Holien Reference Holien1994; Myllys et al. Reference Myllys, Halonen and Velmala2006).
Section Divaricatae has traditionally been considered a well-defined entity characterized by true lateral spinules and the exclusive presence of fumarprotocetraric acid (sometimes absent). Many such species, but not all, are erect or caespitose and have a characteristic bicolorous thallus with blackened basal parts and greyish brown to olive-brown apical branches and spinules (Jørgensen & Ryvarden Reference Jørgensen and Ryvarden1970; Jørgensen Reference Jørgensen1972, Reference Jørgensen1975; Brodo & Hawskworth Reference Jørgensen and Galloway1977). The centre of diversity is in East Asia but some of the species have a wider distribution. In our analyses Divaricatae (Clade III) is more restricted including only taxa with bicolorous thallus, an exclusive synapomorphy for this group (Fig. 4C). Other species with more or less uniformly coloured thalli traditionally included in Divaricatae are now nested in Clade IV.
The section Divaricatae includes two subclades, the first of which consists of B. indonesica, B. variabilis (Bystrek) Brodo & D. Hawksw., B. confusa and B. smithii (Du Rietz) Brodo & D. Hawksw., all characterized by the loss of fumarprotocetraric acid. Our results support Jørgensen (Reference Jørgensen1972) who stated that B. confusa and B. smithii are closely related and represent a typical species pair sensu Poelt (Reference Poelt1970), where B. confusa is fertile and B. smithii sterile bearing isidioid soralia. Bryoria confusa has a more restricted distribution found only in East Asia, while B. smithii is more widespread, occurring even in Fennoscandia.
The species containing fumarprotocetraric acid are restricted to the second subclade of Divaricatae. Bryoria bicolor and B. tenuis (E. Dahl) Brodo & D. Hawksw. have been considered closely related (Jørgensen & Ryvarden Reference Jørgensen and Ryvarden1970; Brodo & Hawksworth Reference Brodo and Hawksworth1977) and our study confirms this view. The two species are fairly easily separated, with B. tenuis having acute branch angles and lacking the tertiary branches typical for B. bicolor. In addition to the two species, the subclade includes three unidentified taxa. They all resemble B. bicolor but differ from this species in branching pattern; for instance specimen S289 collected in China has a distinct main stem and stiffer and coarser habit as compared to B. bicolor. Furthermore, specimens L168 and S291 were only slightly bicolorous. All three taxa probably represent new species although more specimens with similar morphology and chemistry are needed before their status can be discussed further (P. M. Jørgensen, pers. comm.).
Our circumscription of Section Bryoria (Clade IV) differs markedly from that of Brodo & Hawksworth (Reference Brodo and Hawksworth1977), with only the type of the section, B. trichodes, remaining from the original circumscription. All other species in Clade IV were treated within sections Divaricatae and Implexae by Brodo & Hawksworth (Reference Brodo and Hawksworth1977). This result, however, was not unexpected as B. trichodes differs from other species traditionally placed in section Bryoria, (e.g., B. fuscescens) in the production of pseudocyphellae (Fig. 4D). Pseudocyphellae, on the other hand, are lacking in many species in Clade IV, and hence are of little use in circumscribing section Bryoria. The same is true of growth form, which ranges from erect to caespitose, and from subpendent to pendent. Fumarprotocetraric acid is characteristic for the section, though other substances are also present, including lobaric acid [in B. perspinosa (Bystrek) Brodo & D. Hawksw. and B. lactinea (Nyl.) Brodo & D. Hawksw.], barbatolic acid (in B. nadvornikiana), usnic acid (in B. hengduanensis Li S. Wang & H. Harada), and fatty acids [in B. simplicior (Vain.) Brodo & D. Hawksw.]. All species in Clade IV, except B. trichodes have either spinulose branches or true lateral spinules (Fig. 4E). Spinulose branches appear also in Clade II and lateral spinules in Clade III but both characters are absent from Clades I and V.
Virtually all SE Asian species examined in our study belong in Clade IV, the only exceptions being B. confusa and B. variabilis, which have bicolorous thalli and belong in Clade III. Most of the remaining species (i.e., B. divergescens (Nyl.) Brodo & D. Hawksw., B. fastigiata Li S. Wang & H. Harada, B. hengduanensis, B. lactinea, and B. perspinosa) form a strongly supported subclade within section Bryoria. Bryoria poeltii (Bystrek) Brodo & D. Hawksw. also appears to belong in this subclade, especially when the molecular data are amplified with morphological and chemical characters, yet even then without support. The lobaric-acid containing species B. lactinea and B. perspinosa form a smaller group in the SE Asian subclade, although this substance was not observed in the specimens of B. lactinea used in our study. Also belonging in this group is an unidentified specimen (S278), which shares all the characteristic features of B. perspinosa, that is isidioid soralia and presence of lobaric acid, but has a distinctive ITS profile. By contrast, an unidentified specimen S290 shares an almost identical ITS sequence with B. poeltii but differs morphologically in having a pendent thallus (versus caespitose in B. poeltii), dark pseudocyphellae (absent in B. poeltii) and in lacking soralia (isidioid soralia present in B. poeltii). More thorough sampling with multiple specimens of each species is clearly needed before a reliable taxonomy for these species can be achieved. Apparently the mountains of SE Asia are an area of considerable diversity in Bryoria, for which they represent a centre of speciation, as discussed by Jørgensen & Galloway (Reference Jørgensen and Galloway1983).
With the exception of the SE Asian subclade, species relations within section Bryoria remain mostly unresolved. As discussed above, lack of resolution is probably due to missing data in Clade IV. Our study confirms, however, that B. simplicior and B. poeltii are closely related, as suggested by Bystrek (Reference Bystrek1969) and Jørgensen (Reference Jørgensen1972). Bryoria poeltii resembles B. simplicior by having round and wide soralia but has different chemistry containing fumarprotocetraric acid.
Members of section Implexae (Clade V) can be characterized by their pendent growth form and the absence of spinulose branches, although neither character represents a synapomorphy. Most Bryoria species included by Brodo & Hawksworth (Reference Brodo and Hawksworth1977) in sections Bryoria (except B. americana and B. trichodes) and Implexae (except B. nadvornikiana) belong here. Sections Bryoria and Implexae have traditionally been separated on the basis of chemistry and cortical characters (Brodo & Hawksworth Reference Brodo and Hawksworth1977; Holien Reference Holien1989). Species in section Bryoria typically contain fumarprotocetraric acid, at least in the soralia, while section Implexae is defined by the presence of β-orcinol depsidones other than fumarprotocetraric acid. Furthermore, species in the latter section always bear pseudocyphellae (Fig. 4F) and have a characteristic cortical structure with unusually friable branches. As shown by our analyses, however, none of these characters are useful in characterizing infrageneric groupings.
Within section Implexae, only B. glabra is unambiguously supported as a distinct species; it is usually easily distinguished by its obtuse and rounded branches and usually fissural, often regularly oval, white soralia. The systematic position of the remaining species in this section is much more problematic. Our analyses strongly suggest, for example, that B. capillaris (European material only), B. fuscescens, B. implexa, B. lanestris and B. subcana are all conspecific. Low resolution within the ‘European subclade’ (Figs 1–3) reflects a lack of information (i.e. exactly identical sequences among the specimens) rather than incongruence between different gene regions. The few monophyletic groups found within the subclade did not correlate with current taxonomic concepts, nor were corroborating morphological or chemical characters for these smaller groups found.
Unfortunately, no fresh material of B. chalybeiformis (L.) Brodo D. Hawksw. was available to us, though we suspect this taxon also belongs to the ‘European subclade’. Bryoria chalybeiformis is traditionally recognized by its prostrate, stout, shiny, dark thallus and exclusive presence of fumarprotocetraric acid in the soralia (Brodo & Hawksworth Reference Brodo and Hawksworth1977). All specimens originally identified as B. chalybeiformis and preliminarily included in our DNA analyses either contained fumarprotocetraric acid in the whole thallus or turned out to be misidentifications of other, more distantly related species such as B. glabra. Therefore, we suspect that the stout, dark habit may be an adaptation to exposed habitats, and that such specimens largely represent environmental modifications of B. fuscescens s. lat. Indeed, a parallel morphology is seen in the unrelated Alectoria sarmentosa subsp. vexillifera (Nyl.) D. Hawksw. found in similar habitats (I. Brodo, pers. comm.). When material in the European subclade is included, B. chalybeiformis becomes the oldest name at the species level. At this stage, however, we are reluctant to adopt this step given the extreme morphological and chemical variability found within a group. We conclude that more data, including material of B. chalybeiformis and additional gene loci, are needed to solve the relationships of this group.
Our analyses suggest that North American and European B. capillaris and B. implexa chemotype II, respectively, may represent taxonomically distinct entities. This result seems to be upheld by morphological differences, insofar as both B. capillaris and B. implexa are frequently sorediate in Europe, while North American specimens are esorediate in both species (Brodo & Hawksworth Reference Brodo and Hawksworth1977; Holien Reference Holien1989). Two unidentified specimens in the North American subclade resemble B. fuscescens but have pseudocyphellae (specimen L206) or lack secondary substances (specimen S239a); most probably they represent new species. However, as our study included only one North American specimen of each taxon, more data are needed to better understand species delimitation and taxonomy within Clade V.
Table 3. Sections of Bryoria as accepted in this paper and some of their main diagnostic characters. Type species of each section are marked in bold

Nomenclature
Bryoria sect. Americanae Myllys & Velmala, sect. nov
MycoBank: 519583
Thallus pendulus, brunneus, regionibus fragmentationis nigrescentibus. Ramis principales crassi plerumque ramis brevibus perpendiculariter instructis. Soralia rara. Pseudocyphellae brunneolae, fusiformes vel fissurales. Medulla et soralia acidum fumarprotocetraricum continens. Apothecia acidum psoromicum continens.
Type species: Bryoria americana (Motyka) Holien.
(Fig. 4B)
Thallus pendent. Colour brown to dark brown, often with blackened fragmentation areas. Irregularly branched, usually with wide angles, main branches often with perpendicular branches. Soralia absent to sparse, white, tuberculate or fissural, often causing the branch to recurve. Pseudocyphellae sparse, fusiform and depressed, brownish, to 1 mm long.
Ascomata locally common, to 2 mm diam., disc brown, becoming convex; ascospores ellipsoid, 5·5–7 × 4–5 µm.
Conidiomata unknown.
Chemistry. Fumarprotocetraric acid in medulla and soralia (sometimes in low concentrations). Psoromic acid in apothecia.
The section Americanae includes only Bryoria americana.
Conclusions
The phylogenetic analyses presented here identified five main groups in the genus Bryoria (Table 3). Although the groups, here proposed as sections, were defined by molecular characters, we also found some chemical and/or morphological characters to support them. Only two of these characters were synapomorphies: “presence of vulpinic acid” defining section Tortuosae and “presence of bicolorous thallus” characteristic for section Divaricatae. Lateral spinules characterize section Divaricatae and section Bryoria (in part), whereas spinulose branches are present in section Americanae and also partly characterize section Bryoria. Other morphological and chemical characters, including soralia, pseudocyphellae and most secondary substances seem to have little taxonomic value for infrageneric groupings, although they may be useful in delimiting closely related species.
Our study shows the importance of including multiple specimens from each species in phylogenetic studies. For instance, it is apparent that the taxonomic status of species in section Implexae requires further clarification. We have currently started a more comprehensive data sampling to solve the relationships within this group (S. Velmala et al., unpublished). Also the taxonomic status of the unnamed specimens in sections Divaricatae and in Bryoria needs to be examined.
We thank Dr Dmitry Himelbrant, Dr Filip Högnabba and Mr Mohammad Sohrabi for arranging fresh material and Mr Arto Puolasmaa for kind help in field work. We also thank the curators of herbaria H, KUN, OULU, PPU, TRH, TUR, UBC and UPS for providing us with material. We are grateful to Prof. Per Magnus Jørgensen for checking the unidentified specimens of section Divaricatae and Prof. Teuvo Ahti for valuable comments on the manuscript. We would also like to thank EOL for supporting the studies of Parmeliaceae taxonomy. The study was financially supported by the Finnish Ministry of Environment (grant YTB014) and Academy of Finland (grant 133858). National Natural Science Foundation of China and the Research Fund for the Large-scale Scientific Facilities of the Chinese Academy of Sciences provided grants to L.S.W. (Nos. 30870158 and 2009-LSF-GBOWS-01).
Appendix. Data matrix of morphological and chemical characters (see Materials and Methods for explanation of characters). N = missing data










