Introduction
The genus Silphium (L.) is a part of the Heliantheae (sunflower) tribe of the Asteraceae family and originated in the prairies of North America. The native range is from the Rocky Mountains eastward to Appalachia, and from Canada to the Gulf of Mexico (USDA – NRCS, 2019). Silphium integrifolium Michx. is considered a candidate for domestication to generate a new perennial oilseed crop due to its large seeds and resistance to drought and heat stress (Vilela et al., Reference Vilela, González-Paleo, Turner, Peterson, Ravetta, Crews and Van Tassel2018). In the early 2000s, work on S. integrifolium was initiated independently in Kansas, USA, for oilseed production and Poland for biomass production (Kowalski and Wiercinski, Reference Kowalski and Wiercinski2004; Van Tassel et al., Reference Van Tassel, Asselin, Cox, Sideli and Cattani2014). Despite the fact that domestication of S. integrifolium began just a few breeding cycles ago, recent reports show advances towards domestication (Van Tassel et al., Reference Van Tassel, Albrecht, Bever, Boe, Brandvain, Crews, Gansberger, Gerstberger, González-Paleo, Hulke, Kane, Johnson, Pestsova, Picasso Risso, Prasifka, Ravetta, Schlautman, Sheaffer, Smith, Speranza, Turner, Vilela, von Gehren and Wever2017). However, intensified research over the last several years did not include analysis of the natural variation in seed size and seed oil traits in this species.
Increased seed size is a prominent part of the domestication of many crops, likely because larger seeds are better adapted to deeper sowing depths facilitated by mechanical tillage, and deep sowing provides more consistent access to available moisture in the soil to begin germination. Seed morphology traits can also play a role in the ease of threshing (Fuller, Reference Fuller2007). In the contribal relative wild sunflower (Helianthus annuus L.) as well as wild relatives of the confamilial safflower (Carthamus oxyacanthus Bieb.), heritable variation in seed size correlated with the geographical region of origin of the plants (Cantamutto et al., Reference Cantamutto, Alvarez, Presotto, Fernandez-Moroni and Velasco2008; Sabzalian et al., Reference Sabzalian, Mirlohi, Saeidi and Rabbani2009; Nooryazdan et al., Reference Nooryazdan, Serieys, Baciliéri, David and Bervillé2010; Majidi and Zadhoush, Reference Majidi and Zadhoush2014). The differences in seed size among accessions seem to be the effect of local adaptation to different environments to increase survival of seedlings (Metz et al., Reference Metz, Liancourt, Kigel, Harel, Sternberg and Tielbörger2010).
Oil content and composition are essential to an oilseed crop's utility. Reports from a diverse set of plant species, including domesticated sunflower, indicate that the total seed oil content and seed oil composition of a plant accession are affected by the climate of the environment of origin as a result of adaptation to each environment (Sanyal and Decocq, Reference Sanyal and Decocq2016) and thus, are explained mostly by plant genotype. For instance, higher proportions of unsaturated fats allow for faster germination at colder temperatures (Linder, Reference Linder2017). However, ambient temperature during seed development is also known to have effects on the seed oil content and composition in several species, with high temperatures during seed development reducing total oil content and linoleic acid in sunflower, so some environmental plasticity occurs in these traits (Harris et al., Reference Harris, McWilliam and Mason1978; Kizil et al., Reference Kizil, Çakmak, Kirici and İnan2008). Thus, it is important to study accessions collected from diverse environments by growing them in a common environment.
Our objective was to assess the phenotypic distribution of seed size and seed oil traits for a wild S. integrifolium collection in a common environment to guide future collection activities as well as structured crosses in breeding programmes.
Material and methods
Plant material
Seeds from wild S. integrifolium populations were obtained from several sources. First, The Land Institute employees collected seeds from plants found along roadsides and in other public places. Second, an appeal was made to supporters of The Land Institute to look for native Silphium on their private property and lands operated by their organizations. These volunteers were given instructions about when seeds are mature, and asked to harvest seeds from a single stalk per clump and to sample only clumps about 1 m apart from each other, to ensure that each sample was from an individual genotype. Volunteers recorded the location of the population or individual plants by taking photos of them using smart phones. The seeds and photos were sent to The Land Institute and the GPS coordinates of the populations were extracted from the photo metadata. Finally, some seeds were donated from organizations that had made similar collections previously and kept remnant seeds in cold storage. Information about the populations used in this study is presented in Fig. 1 and online Supplementary Table S1.
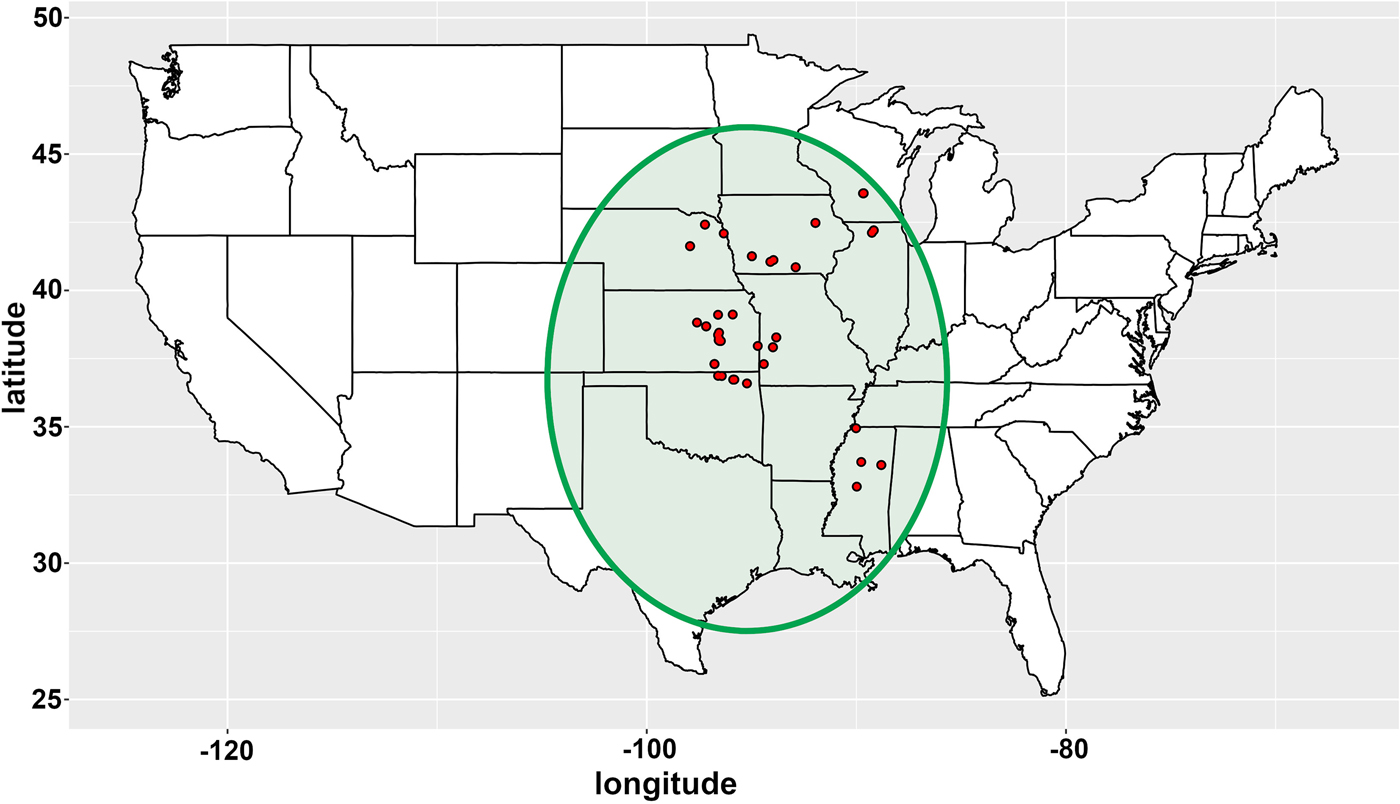
Fig. 1. Map of the USA adjusted in scale to fit on a latitude/longitude grid, with collection site overlay for the 53 accessions of Silphium integrifolium Michx. The green shaded area is its native range.
Heads from collected samples were threshed using a BT14 belt thresher (Almaco, Nevada, IA, USA) in December 2016 and cleaned using an STS-WM2 aspirator (SeedTech Systems, Wilton, CA, USA) and hand sieves. In February 2017, achenes were placed on germination paper that was laid on top of a cotton towel on a tray. The paper and towel were moistened, and trays were placed in a cooler (4°C) for 6 weeks. At the end of this cold-moist stratification treatment, seeds were allowed to germinate in a warm place (approximately 24°C). Trays were kept moist and covered, and checked daily. As soon as radicles could be seen emerging from an achene, it was placed in the dimple of a hydrated 50 × 95 mm peat pellet (Jiffy Products of America, Lorain, OH, USA) and covered with turface (Turface MVP, Profile Products LLC, Buffalo Grove, IL, USA). Seedlings were raised in an unheated space and watered as often as necessary to keep the pellets wet with Miracle-Gro Water Soluble All Purpose Plant Food (The Scotts Miracle-Gro Company, Marysville, OH, USA) diluted according to the label, weekly or as necessary to maintain dark green foliage. Seedlings were transplanted to the field in May 2017. The field had been prepared by tillage and the seeding of tall fescue (Festuca arundinacea, Scotts Sta-green, Scotts Miracle-Gro Company), as a ground cover. The fescue had germinated prior to transplanting, so shallow furrows were made in a 1 m × 2 m grid to kill the grass within the furrow and to indicate where seedlings would be planted. Pellets were buried at the intersection of the furrows. Transplants were hand watered immediately after transplanting and every few days for the next two weeks, as needed. The grass intercrop was managed by mowing with standard lawn equipment. Annual weeds were controlled by manual pulling. The whole field was fertilized each June with 50 kg/ha nitrogen in the form of urea.
In 2018, the plants flowered for the first time, beginning in late June. Plants from the same population were flagged and bagged with cotton bags to allow controlled pollination as they began to flower. Any heads with florets in anthesis or older were removed prior to bagging. Generally, two related plants were treated as females and a third plant from the same population was used as a pollen parent. Pollen parents were also bagged with cotton or nylon mesh bags to protect the pollen from predation. Heads from the pollen parents were detached, if they had a lot of fresh pollen visible, and the detached heads were rubbed on the female parent heads (after temporarily removing the cotton bags). In September, the seed was harvested from the bagged heads after heads had matured (turned brown) and further dried at room temperature. Crossed seed was threshed and cleaned as described above. Seed from these plant materials is not kept in stock, but can be requested from The Land Institute with ample notice to allow for seed production.
Phenotyping
Ten seeds sampled from several sib-mated plants of each accession were laid out in a straight line and scanned using an Epson Perfection V800 Photo flatbed scanner (Seiko Epson Corporation, Suwa, Japan). The seeds were scanned as negatives to increase the contrast between hull wings and actual seed. Image processing was done with a custom modification of functions within the R package GiNA (online Supplementary File 1; Diaz-Garcia et al., Reference Diaz-Garcia, Covarrubias-Pazaran, Schlautman and Zalapa2016). Briefly, background extraction and object (e.g. kernel) identification was performed using threshold-based segmentation in the blue colour channel. Next, for each seed, we performed an additional round of thresholding to identify the wings and the achene boundaries, and then used functions in GiNA to measure various size parameters of the achene anatomy (Fig. 2).

Fig. 2. Example of the seed scan used for seed size trait phenotyping. The kernel is highlighted in blue and the wing (hull) of the seed in red. The kernel and wing together form the achene.
For the oil content analysis, 8 ml of clean Silphium seeds (with empty hulls and chaff removed) from several sib-mated plants of each accession were weighed in an 18 mm glass cuvette and subjected to nuclear magnetic resonance (NMR) analysis on an Oxford MQC NMR fitted with a 18 mm probe (Oxford Instruments, Abingdon, UK). The calibration curve used for this instrument and probe size is specific for Silphium spp. and was developed from a continuum of samples with different oil contents as quantified with the AOAC method 2003.05 analysis with petroleum ether as solvent (Minnesota Valley Testing Labs, New Ulm, MN, USA; Joel Sieh, pers. comm., 2017). Oil content is expressed in g oil per kg total achene mass.
The seed fatty acid composition was analysed according to the sunflower method described by Hulke et al. (Reference Hulke, Miller, Gulya and Vick2010), with the following adaptations. Each Silphium accession was analysed in a subsample of 20 seeds harvested from several sib-mated plants of each accession. Subsamples were pulverized using an IKA Tube Mill (IKA Works, Inc, Wilmington, NC, USA). The samples were then derivatized in 3 ml of hexane-chloroform-0.5 M sodium methoxide in methanol (75:20:5, v/v). After 10 s of vortexing, 1 ml of the sample solution was transferred to a 2 ml gas chromatograph autosampler vial. To confirm the identity of detected fatty acids, we analysed the molecules of five samples using gas chromatography-mass spectrometry (GC-MS) at the North Central Soil Conservation Research Laboratory (USDA-ARS, Morris, MN, USA; Russ Gesch, pers. comm., 2019). Samples were analysed according to the procedure described by Walia et al. (Reference Walia, Forcella, Wyse, Gardner, Wells, Cubins and Gesch2018). Fatty acid composition fractions are expressed as g fatty acid per kg total oil.
Statistical analysis
For each trait, observations were averaged across technical replications to produce mean values. On the mean values, we calculated the Pearson correlation for kernel length (KL), kernel width (KW), kernel area (KA), wing area (WA), achene area (AA), kernel area-wing area ratio (KA/WA), seed oil content (Oil), myristic acid (14:0), palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1) and linoleic acid (18:2), by using the ‘rcorr’ function implemented in the Hmisc R package (R Core Team, 2018).
We conducted a regression analysis using the GLM procedure implemented in SAS v. 9.4 (SAS Institute, Cary, NC) that included the geographical coordinates (latitude and longitude) of the collection sites for each of the 53 genotypes to estimate clines in phenotypic variation across the native range of the species. From the linear variables, two-dimensional contour plots were developed to graphically show the geographical clines for each of the traits.
Results
To identify variation in the seed size traits, seed oil content and the seed oil composition of S. integrifolium, we analysed 53 genotypes from a wild collection in a common garden experiment, collected across the native range in North America. The phenotypic values of seed size and seed oil traits revealed considerable variation among analysed genotypes (Fig. 3, online Supplemental Table S1). For seed size traits, the ranges were: KW (3.3 ± 0.2 mm to 6.7 ± 0.5 mm), KL (6.4 ± 0.2 mm to 13.9 ± 0.3 mm), KA (17.8 ± 1.1 mm2 to 67.2 ± 4.1 mm2), WA (12.6 ± 1.0 mm2 to 39.9 ± 1.9 mm2), AA (31.4 ± 1.4 mm2 to 97.8 ± 4.1 mm2) and KA/WA (1.1 ± 0.1 to 3.0 ± 0.2). Similarly, large variation had been detected for seed oil traits with oil (118–253 g/kg), 14:0 (17–44 g/kg), 16:0 (77–116 g/kg), 18:0 (14–44 g/kg), 18:1 (156–280 g/kg) and 18:2 (562–699 g/kg.
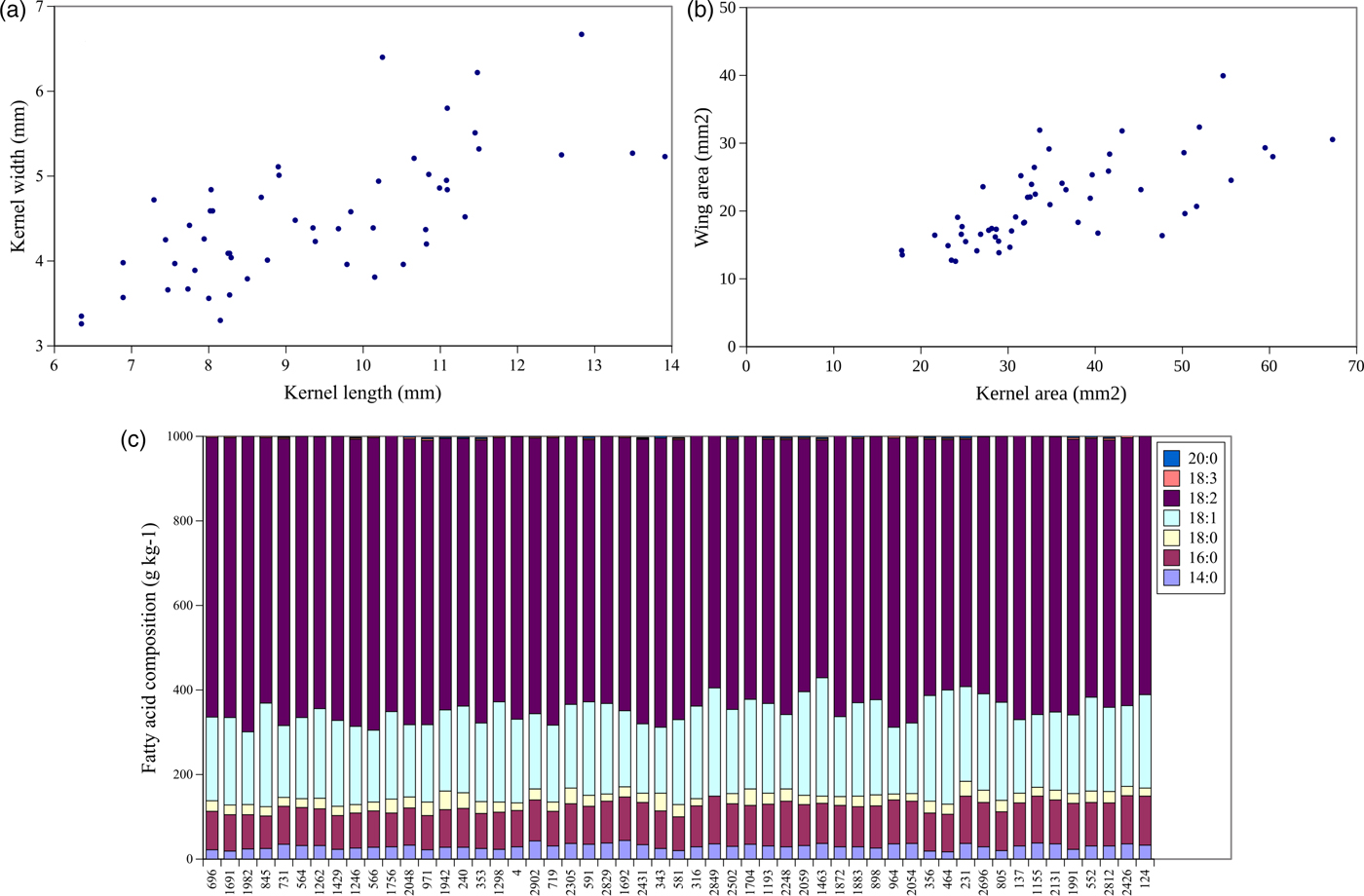
Fig. 3. Graphical representations of seed trait data from 53 wild accessions of Silphium integrifolium. (a) Scatterplot of accession mean kernel length versus kernel width, (b) scatterplot of mean kernel area versus wing area, (c) cumulative bar plot of fatty acid composition organized by the longitude of collection site, west (left) to east (right).
In order to analyse the effects of biogeography, we performed regression analyses. We incorporated the latitude and longitude as linear variables in the regression analysis, which revealed significant differences among seed and oil traits due to latitude and/or longitude (Table 1). Among the 12 traits, eight traits were significantly influenced by the origin of the genotype. Longitude affected seed size and seed oil traits more often than latitude. To graphically demonstrate these effects, we developed contour plots scaled to latitude/longitude coordinates for traits which were significantly influenced by latitude and/or longitude (Fig. 4). Kernel area was affected by latitude and longitude, which generally results in increased kernel area in genotypes from the southwestern regions. The average estimated effects of latitude and longitude on the KA were −1.2 mm2/°latitude and −1.6 mm2/°longitude, respectively (Table 1). Traits KW and KA/WA were only affected by latitude, where genotypes collected in the southern areas of the origin of S. integrifolium showed an increase in KW. The effect of latitude on KW was −0.1 mm (Table 1) for each latitudinal degree north. The other seed size traits (KL, WA and AA) were influenced by longitude only. Genotypes from collection sites in the western regions of S. integrifolium were larger in size for these traits compared to genotypes collected in eastern regions. For each longitudinal degree east, KL, WA and AA varied by −0.4 mm (KL), −1.2 mm2 (WA) and −2.8 mm2 (AA) (Table 1). However, among oil traits, only longitude influenced phenotypes, with significant effects for 16:0 and 18:2. The share of the oil composition for 16:0 decreased but 18:2 increased with more westerly collection site. The average effect estimates for the oil traits were 2 g/(kg · °longitude) (16:0) and −4 g/(kg · °longitude) (18:2, Table 1).
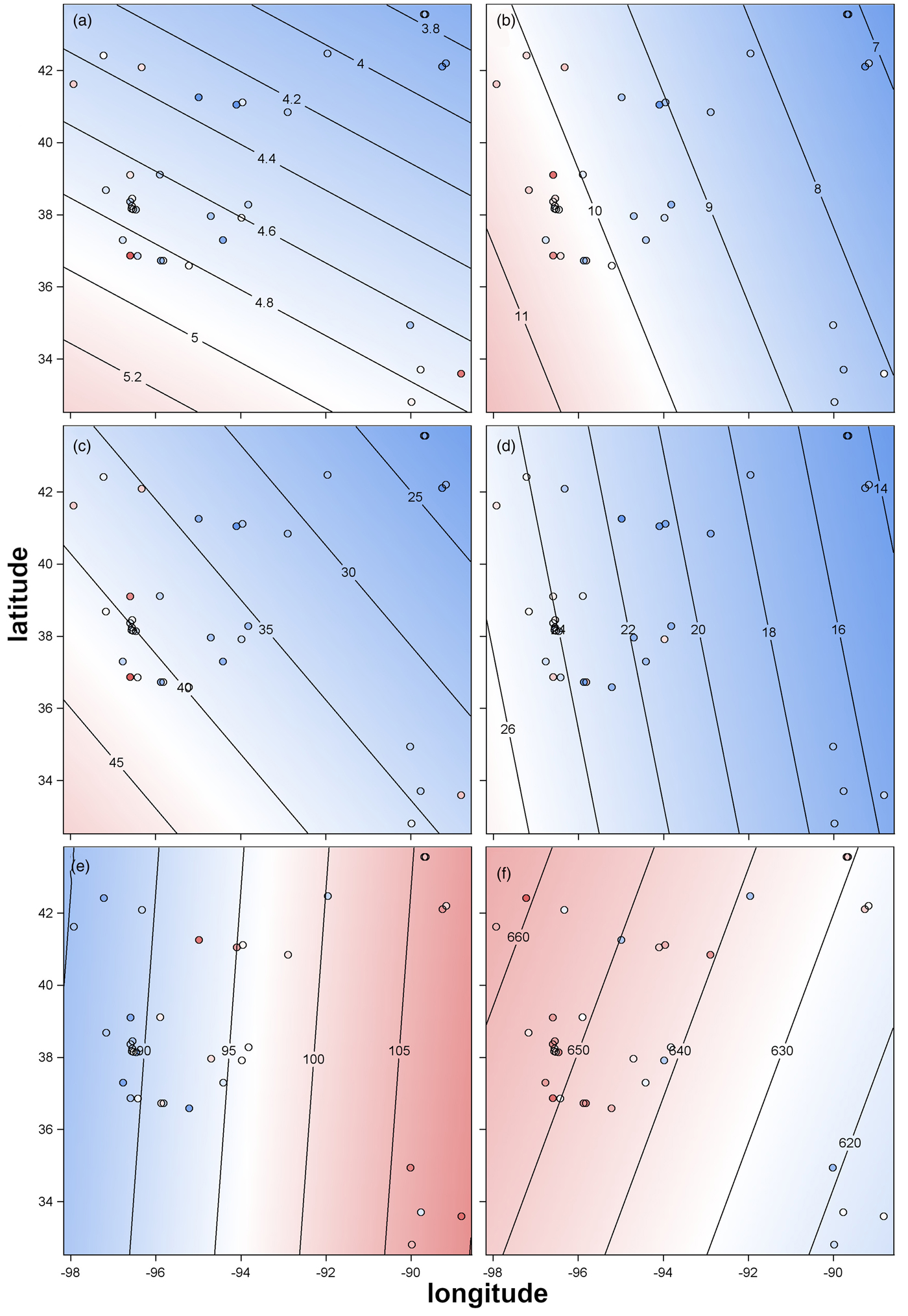
Fig. 4. Contour plots showing the trends of seed traits over the latitude/longitude gradient of the collection site. Small circles denote an accession data point based on latitude/longitude coordinates, and the colour within each circle indicates the phenotypic value of the accession on the gradient colour scale. (a) Kernel width, (b) kernel length, (c) kernel area, (d) wing area, (e) palmitic acid and (f) linoleic acid.
Table 1. ANOVA and effect estimate table for regression analyses of seed and oil traits using geographical coordinates of the origin of analysed genotypes as linear covariates

Long, longitude; Lat, latitude; ns, not significant.
Further, we identified correlations between seed size and oil traits, which were supported by the biogeographic distribution (Table 2). Interestingly, the correlation analysis revealed a moderate, positive correlation of 18:2 with KL (r = 0.31) and WA (r = 0.28) but negative, moderate correlations of 16:0 with KW (r = −0.35), KL (r = −0.55), KA (r = −0.44), WA (r = −0.54) and AA (r = −0.51). Among all measured oil traits, only 14:0 (r = −0.31) and 16:0 (r = −0.37) revealed a correlation with oil content. The negative correlation between 16:0, the dominant saturated fat, and 18:2, the dominant unsaturated fat, while not surprising, could be beneficial for optimizing for either saturated fat or unsaturated fat content in Silphium breeding. The seed dimensional traits are generally moderately to highly correlated with one another, with the noteworthy exception that KA/WA ratio is not correlated with WA, indicating that change in KA largely drives that ratio.
Table 2. Pearson correlations among seed size traits, oil content and oil composition for a wild Silphium integrifolium collection
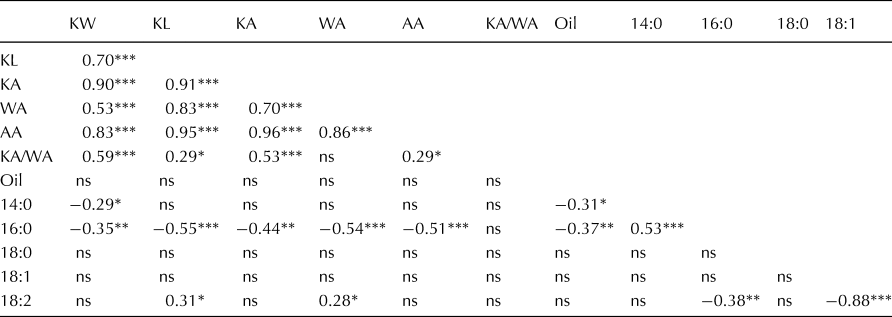
KW, kernel width; KL, kernel length; KA, kernel area; WA, wing area; AA, achene area; KA/WA, kernel area to wing area ratio; Oil, total seed oil content; 14:0, myristic acid; 16:0, palmitic acid; 18:0, stearic acid; 18:1, oleic acid; 18:2, linoleic acid.
Significance thresholds indicated as ns = not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Phenotypic variation for seed traits such as seed size and seed oil can vary among wild-collected accessions due to natural selection for adaptation (Sabzalian et al., Reference Sabzalian, Mirlohi, Saeidi and Rabbani2009; Safavi et al., Reference Safavi, Pourdad, Taeb and Khosroshahli2010). Agronomically, seed size, oil content and composition are also critical traits for the success of S. integrifolium as a sustainable oilseed crop. Therefore, the analysis of the natural variation for these traits in a common environment is necessary to guide plant collection and breeding.
Kernel size, and whole seed size in general, has increased during domestication of sunflower and most other crops. This is generally understood to be the effect of either direct or indirect selection for larger seeds, which are more adapted to the deeper sowing that occurred in primitive agricultural systems versus wild conditions (Fuller, Reference Fuller2007). Deeper sowing of seeds is still encouraged today in general agronomic recommendations because access to soil-water is more consistent, resulting in consistent germination. Increasing kernel size and achene size also improves threshing and mechanical separation of seeds from the chaff (Fuller, Reference Fuller2007). The wing area itself in Silphium can be almost the same size as the kernel area, which increases surface area and can dramatically reduce efficiency in separating filled from unfilled seeds using the forced air columns in commercial harvesting equipment. Reducing wing area while increasing kernel area (and accordingly, kernel mass) should improve harvestability and separation. Kernel area to wing area ratio is positively correlated to achene area and, to a greater extent, kernel area and kernel width (Table 2). A closer look at correlations indicates that wing area is also positively correlated with achene area, kernel area and kernel width, but not kernel area to wing area ratio, indicating that while larger seeds also have larger wing areas than smaller seeds, the wing size increases at a lesser rate than kernel size as achene size increases. This is a favourable result, and due mainly to increases in kernel width over kernel length.
Interestingly, seed size traits in Silphium, including the kernel area to wing area ratio, are significantly affected by a southwestern cline, such that we identified seeds with increased kernel size and high kernel area to wing area ratios in the southwestern part of the range of S. integrifolium (Table 1, Fig. 4). Similar to our findings, Sabzalian et al. (Reference Sabzalian, Mirlohi, Saeidi and Rabbani2009) showed in wild safflower species, Carthamus oxyacanthus Bieb., that agro-morphological traits such as seed size were correlated with the site of collection. It is noteworthy to mention that yearly average precipitation decreases from east to west and temperature increases from north to south, such that the hottest, driest conditions occur in the southwest. These differences among sites may have encouraged natural selection for seeds with increased reserves during the sensitive germination and seedling stages.
Similarly, the oil composition is influenced by longitude, where the palmitic acid content decreases and the linoleic acid content, on average, increases with each degree longitude west. For a domestication goal to generate an oilseed crop for human consumption similar to oilseed sunflower, a seed oil profile with low palmitic acid content should be achieved. Arslan (Reference Arslan2007) showed that differences in seed oil content and seed oil composition among safflower cultivars as well as wild safflower relatives can be explained by their geographic origin. He revealed differences in oil composition, ranging from 41 to 79 g/kg total oil for palmitic acid and 72 to 773 g/kg total oil for linoleic acid, are influenced by biogeography. Moreover, it has been shown that palmitic acid is positively correlated to the total seed oil content in safflower (Arslan, Reference Arslan2007). In contrast to safflower, in S. integrifolium, total oil content and palmitic acid are negatively correlated, which is in line with correlations of oil content and palmitic acid in sunflower (Petakov et al., Reference Petakov, Ivanov and Nikolova1993). Taxonomically, the Silphium genus is more closely related to sunflower than safflower, which may explain such trends.
A surprising result is the presence of myristic acid in meaningful quantities in Silphium seed oil. Given the close relationship to sunflower, we expected to see a very similar oil composition to wild-type sunflower, which lacks myristic acid and is rich in palmitic, stearic, oleic and linoleic acids, particularly the latter two (White, Reference White and Chow2000). While in both Helianthus and Silphium, linoleic acid is the dominant fatty acid in wild-type plants, the total saturated fat content of Silphium can be nearly twice as high as sunflower, with the majority of the saturated fat as palmitic acid, a fatty acid positively correlated with myristic acid in this study. Increased consumption of myristic, palmitic and stearic acids in the human diet has been shown to increase the risk of cardiovascular disease in long-term dietary studies (Zong et al., Reference Zong, Li, Wanders, Alssema, Zock, Willett, Hu and Sun2016). Higher saturated fat content compared to similar vegetable oils reduces the desirability of wild-type Silphium oil as a vegetable oil for human consumption, based solely on health virtues, and the oil product appears to be too heterogeneous to fit into other potential uses independent of the health virtues. However, two strategies can provide hope through selective breeding. First, considerable variation exists for fatty acid content among accessions and oil composition is affected by biogeography, indicating that careful parent selection and monitoring of fatty acid content in breeding lines can reduce total saturated fats in favour of linoleic acid, a polyunsaturated fat considered to be a healthy substitute for saturated fatty acids in the human diet (Maki et al., Reference Maki, Eren, Cassens, Dicklin and Davidson2018). Second, mutation breeding on favourable parental lines could radically change the fatty acid composition with alterations in single genes. This has been the case in sunflower, where FAD2-1 was mutated to reduce saturated fats and convert most of the linoleic acid to oleic acid, in an effort to balance oxidative stability of the oil with health characteristics (Schuppert et al., Reference Schuppert, Tang, Slabaugh and Knapp2006). Similar work has also increased stearic acid content in sunflower to around 180 g/kg, which is used as an identity-preserved palm oil substitute from which a solid or semi-solid fat at room temperature can be produced for certain food applications where solid fats are required (Anushree et al., Reference Anushree, André, Guillaume and Frédéric2017). Determining an ideal fatty acid composition for a crop is a moving target, but many prominent oilseeds have multiple, identity-preserved oil types suited to different end markets, usually optimizing attributes such as price, healthiness, oxidative stability, flavour and whether it is solid or liquid at room temperature, depending on end use. In the current vegetable oil market, a heterogeneous oil such as wild-type Silphium will need to be directed through breeding into one or more desirable profiles, with the most obvious one to increase linoleic acid for a household cooking or commercial food preparation oil. Conversely, increasing palmitic or stearic acid may also be useful for developing a solid fat alternative to palm oil.
An interesting result is the correlation between seed size traits (KW, KL, KA, WA, AA) and seed oil traits (16:0, 18:2). The present study revealed negative correlations of palmitic acid to KW, KL, KA, WA, AA and linoleic acid. Genotypes collected in more southwesterly regions possess a lower palmitic acid content but a higher linoleic acid content as well as increased KW, KL, KA, WA, AA and KA/WA. Similar to oilseed sunflower, higher yield, higher kernel to wing ratio, lower palmitic and higher linoleic acid will likely be the preferred direction of improvement in Silphium. To obtain resources for such a breeding goal, we should focus on genotypes from southwestern regions. But focusing on southwestern genotypes solely could generate genetic bottlenecks due to limited diversity in small populations. These bottlenecks will increase the proportion of deleterious variants, negatively impacting plant fitness (Makino et al., Reference Makino, Rubin, Carneiro, Axelsson, Andersson and Webster2018). More importantly, we could lose genetic variation for other traits of agronomic importance by eliminating northeasterly accessions from breeding programmes, such as the biotic stress resistance that has been seen in such accessions. Acknowledging the strengths and weaknesses of potential parent stocks will be the key to developing robust breeding programmes that adequately balance necessary traits.
We identified the phenotypic variation of seed size and seed oil traits in wild S. integrifolium. The variation among accessions is affected by geographical clines, with the east-west cline producing the most important variation for oilseed suitability. This study is the first assessment of seed size and seed oil variation in the domestication candidate S. integrifolium and is a starting point to understanding how variation in the wild accessions can be best utilized for domestication. Future analyses of flowering time and disease resistance, for example, are needed to obtain sufficient information to guide future plant collection and improvement objectives.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262119000248
Acknowledgements
The authors would like to thank Michael Grove of USDA-ARS in Fargo for conducting the gas chromatography analyses, Russ Gesch at USDA-ARS in Morris, MN, and Terry Isbell from USDA-ARS, Peoria, IL, for assistance with mass spectroscopy of rare fatty acid species, Joel Sieh of Minnesota Valley Testing Laboratories in New Ulm, MN, for excellent help in developing calibration curves for non-destructive evaluation of Silphium seed oil content, and Luis Diaz-Garcia of the Instituto Nacional de Investigaciones Forestales, Agricolas y Pecuarias in Aguascalientes, Mexico, for comments and assistance in modify functions in the R GiNA package.