Introduction
Within the last eight years, the number of known lichenicolous fungal species has risen by about one quarter, roughly from 1300 to 1700 [Lawrey & Diederich Reference Lawrey and Diederich2003; author's database, derived from Culberson et al. (Reference Culberson, Egan and Esslinger2011)], which corresponds to a long-established opinion that the diversity of this hostal group of fungi is yet to be revealed. However, specific estimates of their species richness vary from 2000 (Hawksworth Reference Hawksworth1991) to 5000–7000 (Lawrey & Diederich Reference Lawrey and Diederich2003). Taking into consideration that 1) the value of the Lichenicolous Index (ratio of species of lichenicolous fungi per species of lichens within an area) for the best known mycobiotas of the world is about 0·2 (Zhurbenko Reference Zhurbenko2007), 2) the known and estimated numbers of lichen species in the world vary from 17 500 to 28 000 (Feuerer & Hawksworth Reference Feuerer and Hawksworth2007; Lücking Reference Lücking2008), 3500 to 5500 species of lichenicolous fungi could be expected worldwide, which means that approximately 30–50% of them are presently described. This corresponds with the results of studies of fungi inhabiting particular lichen genera, such as Pilophorus, Stereocaulon, Tephromela or Thamnolia, where about 15–35% of the species occurring are new to science (Zhurbenko & Triebel Reference Zhurbenko and Triebel2005; Hafellner Reference Hafellner2007; Zhurbenko Reference Zhurbenko2010a; present study). Such studies are also optimal to show variousaspects of ‘host-parasite’ relationships, for instance, how many fungi can invade one lichen species. The world record in that respect seems to belong to Peltigera rufescens (Weiss) Humb., supporting 54 species of lichenicolous fungi (author's database).
The oroarctic lichen genus Thamnolia consists of three species: a widely distributed, cosmopolitan T. vermicularis (Sw.) Schaer., T. papelillo R. Sant., known from South America, and T. juncea R. Sant., known from Papua New Guinea (Santesson Reference Santesson2004). Until now, 13 species of lichenicolous fungi were known to occur on Thamnolia, four of which were specifically treated in Norway (Ihlen Reference Ihlen1995). The results of the revision of fungi found on Thamnolia vermicularis within the Holarctic (with one find on T. papelillo from the Neotropics) are presented here. They nearly double the number of thamnoliicolous fungi species now reaching 23, including eight (35%) described herewith. A new anamorphic lichenicolous fungal genus and a synonym within Cercidospora are introduced. Since no key to fungi growing on Thamnolia has been published previously, one is provided here, along with notes on their taxonomy, hosts, and geography.
Materials and Methods
The study is based on 190 samples of lichenicolous fungi on Thamnolia vermicularis, obtained from no less than 1500–2000 host specimens examined from the Holarctic, and one sample on T. papelillo from South America. Furthermore, 37 LE herbarium specimens were revised. The material was examined and photographed using Zeiss microscopes Stemi 2000–CS and Axio Imager A1 equipped with Nomarski differential interference contrast optics. Microscopical examination was carried out on material mounted in water, 10% KOH (K), Lugol's iodine, directly (I) or after a KOH pre-treatment (K/I), or Brilliant Cresyl blue (BCr). The length, breadth, and length/breadth ratio (l/b) of asci and ascospores are given (where possible) as: (min−){x −SD}−{x +SD}(−max), where min and max are the extreme values, x the arithmetic mean, and SD the corresponding standard deviation. Measurements of the ascus sizes were rounded to the nearest 1 µm, those of the ascospores and conidia to the nearest 0·5 µm. The Ascomycota classification follows Lumbsch & Huhndorf (Reference Lumbsch and Huhndorf2007). Terms for simple plane shapes, used for describing asci and ascospores, mostly follow Stearn (Reference Stearn1992: 539). The estimation of total diversity of thamnoliicolous fungi in the Holarctic was based on a simple estimator proposed by Turing (Chao & Shen Reference Chao and Shen2003), based on the proportion of single occurrences (singletons) in the sample![]() , where f is the number of singletons and S is the number of known species. All examined specimens are housed in the mycological herbarium of the V. L. Komarov Botanical Institute in St. Petersburg (LE).
, where f is the number of singletons and S is the number of known species. All examined specimens are housed in the mycological herbarium of the V. L. Komarov Botanical Institute in St. Petersburg (LE).
The following specimens have been examined and compared with the species studied: Cercidospora lecidomae Zhurb. & Triebel: LE 207636 (paratype), LE 260308, LE 260555. Cercidospora parva Hafellner & Ihlen: LE 260155, LE 260054, LE 233214. Cercidospora punctillata (Nyl.) R. Sant.: LE 206999, LE 207003, LE 207007, LE 207012, LE 207779, LE 233253, LE 260095, LE 260166, LE 260177, LE 260245, LE 260276, LE 260296.
The Taxa
Capronia thamnoliae Zhurb. sp. nov
MycoBank no.: MB 563049
Fungus lichenicola in thallis lichenum generis Thamnolia crescens. Similis speciei Capronia normandinae, sed differt praecipue ascosporis minoribus, (11–)13–18(–29) × (5–)6–8(−8·5) µm, et hospite diverso.
Typus: USA, Alaska, Great Kobuk Sand Dunes, 67°05′ N, 159°00′ W, 45 m elev., Dryas-lichen-moss vegetation among sparse Picea glauca, on moribund bases of Thamnolia vermicularis var. subuliformis thalli, 13 August 2000, M. Zhurbenko 003 (LE 260400—holotypus).
(Fig. 1)

Fig. 1. Capronia thamnoliae. A, ascoma (holotype); B, asci with spores in K/I (LE 260410); C, setae in K/I (holotype). Scales: A = 100 µm; B & C = 10 µm.
Vegetative hyphae pale brown, cylindrical to torulose, 2–3 µm wide, septate, with cells 4–15 µm long, scarcely branched, originating from the base of ascomata, mostly superficial.
Ascomata perithecioid, black, subglobose to occasionally pyriform, (70–)90–160 µm diam., with an ostiole c. 15 µm diam., usually densely setose throughout, semi-immersed to mostly sessile, arising singly, dispersed. Setae dark brown, subulate, the tips rounded, 15–75 µm tall, 2–3 µm wide, base swollen 6–9 µm wide, thick-walled, the edges smooth or occasionally crenate, non-septate or rarely with 1(–2 ?) septa, unbranched. Exciple medium brown, in surface view composed of angular pseudoparenchymatous cells (textura angularis) 4–13 µm across, K+ acquiring a vague grey shade. Hymenial gel I+ coral to rose, K/I+ blue. Hamathecium not observed. Asci clavate to subcylindrical, with strongly thickened apical wall, often penetrated by an internal apical beak (best developed in immature asci), (56–)60–72(–76) × (13–)14–18(–21) µm (n = 29), 8-spored, I−, K/I−. Ascospores initially hyaline, then pale to medium grey (septa sometimes with a brown shade), mostly elliptic to narrowly elliptic, occasionally very narrowly elliptic, broadly elliptic, rotund, narrowly ovate, or narrowly obovate, the apices attenuated or occasionally rounded, (11–)13·5–18·0(–29) × (5–)6– 8(–12) µm, l/b = (1·3–)1·8–2·8(–5·8) (n = 154), mostly submuriform, with (0–)3–5(–7) transverse septa and as a rule with longitudinal or oblique septa in 1–3(–4) segments (very rare, probably when immature, non-septate), not or rarely constricted at the septa (when overmature), smooth-walled, non-halonate, often guttulate, arranged biseriate to partly overlapping and uniseriate in the ascus.
Anamorph not observed.
Notes. Thirteen of c. 60 previously known species of Capronia Sacc. (Herpotrichiellaceae, Chaetothyriales) grow on lichens (Untereiner Reference Untereiner2000; Lawrey & Diederich Reference Lawrey and Diederich2011) [Capronia triseptata (Diederich) Etayo does not belong to Capronia (P. Diederich, pers. comm., Reference Diederich, Lawrey, Sikaroodi, van den Boom and Ertz2011)], all being exclusively lichenicolous. Nearly all of them are restricted to a particular lichen host genus [with the exception of Capronia hypotrachynae Etayo & Diederich known on Hypotrachyna and Menegazzia (Etayo & Diederich Reference Etayo and Diederich1998; von Brackel Reference von Brackel2010b) and perhaps C. epilobarina S. Y. Kondr. & D. J. Galloway, which was described from Lobaria and was reported (with ‘cf.’) also on Erioderma (Etayo Reference Etayo2002)]; none has been found on Thamnolia yet. Ascospore colour, size, and septation are among the most reliable morphological characters for the species delimitation within the genus. Seven lichenicolous Capronia species also have submuriform (or muriform) ascospores, characteristic of Capronia thamnoliae. Of these, Capronia epilobarina differs from the latter in its greyish brown, narrower ascospores up to 7 µm wide (Kondratyuk & Galloway Reference Kondratyuk and Galloway1995; Etayo & Diederich Reference Etayo and Diederich1996b); C. guatemalense Etayo & van den Boom has larger ascospores (22–27 × 9·0–11·5 µm), which are brownish from the beginning and have more septa (Etayo & van den Boom Reference Etayo and van den Boom2006); C. hypotrachynae has slightly narrower ascospores (12–19 × 5·5–7·5 µm) and aseptate and much shorter setae, measuring 5–35 × 3–4 µm, often less than 10 µm long (Etayo & Diederich Reference Etayo and Diederich1998); C. leopoldiana Etayo has longer and narrower ascospores [(15–)17–22(–24) × 4–5·5 µm], shorter setae (15–40 × 3–4 µm), and smaller ascomata (70–100 µm diam.) (Etayo & Sancho Reference Etayo and Sancho2008); C. magellanica Etayo differs in its shorter and narrower ascospores [(11–)13–16(–17·5) × 4–6 µm], which only occasionally have 1 longitudinal septum in some cells, shorter setae (20–45 × 4–5 µm), and the I−, K/I− reaction of the hymenial gel (Etayo & Sancho Reference Etayo and Sancho2008); C. normandinae R. Sant. & D. Hawksw. has pale olivaceous brown, slightly larger ascospores [(13–)15–21(–27) × 7·5–9·0 µm], tending to appear densely dictyosporous due to guttules and putative distosepta, and non-septate setae, mostly developing around the ostiole (Hawksworth Reference Hawksworth1990); C. pseudonormandinae Diederich has pale brown, smaller and less septate ascospores (12·5–16 × 6–7·5 µm), and non-septate, shorter setae, measuring 10–30 × 2·5–4 µm (Aptroot et al. Reference Aptroot, Diederich, Sérusiaux and Sipman1997).
Distribution and host. Circumpolarly known in the Holarctic from arctic and alpine tundras and northern boreal forests. Saprotrophic or commensalistic on old or moribund portions of Thamnolia vermicularis thalli.
Additional specimens examined [all on Thamnolia vermicularis var. subuliformis (Ehrh.) Schaer.]. Canada: Canadian Arctic Archipelago: Axel Heiberg Island, Bunde Fjord, 80°30′ N, 94°36′ W, 30 m, 1999, N. Matveeva (LE 260410).—Norway: Troms County: Skibotndalen Valley, 69°15′ N, 20°23′ E, 950 m, 2003, M. Zhurbenko (LE 260458).—Russia: Taimyr Peninsula: Byrranga Mts., Levinson-Lessinga Lake, 74°30–33′ N, 98°26–33′ E, 120–300 m, 1994, M. Zhurbenko (LE 207477c); ibid., 1995, M. Zhurbenko (LE 260390, LE 260598).
Cercidospora epithamnolia Zhurb. sp. nov
MycoBank no.: MB 563050
Fungus lichenicola in thallis lichenum generis Thamnolia crescens. Similis speciei Cercidospora solearispora, sed differt praecipue ascosporis minoribus, (12–)13–16(–19) × (3–)3·5–4·5(–5) µm, et hospite diverso.
Typus: Russia, Severnaya Zemlya, Bolshevik Island, W coast of Mikoyana Bay, 79°18′ N, 101°55′ E, 10 m elev., polar desert, on thalli of Thamnolia vermicularis var. subuliformis, 21 July 1996, M. Zhurbenko 96908 (LE 232954—holotypus).
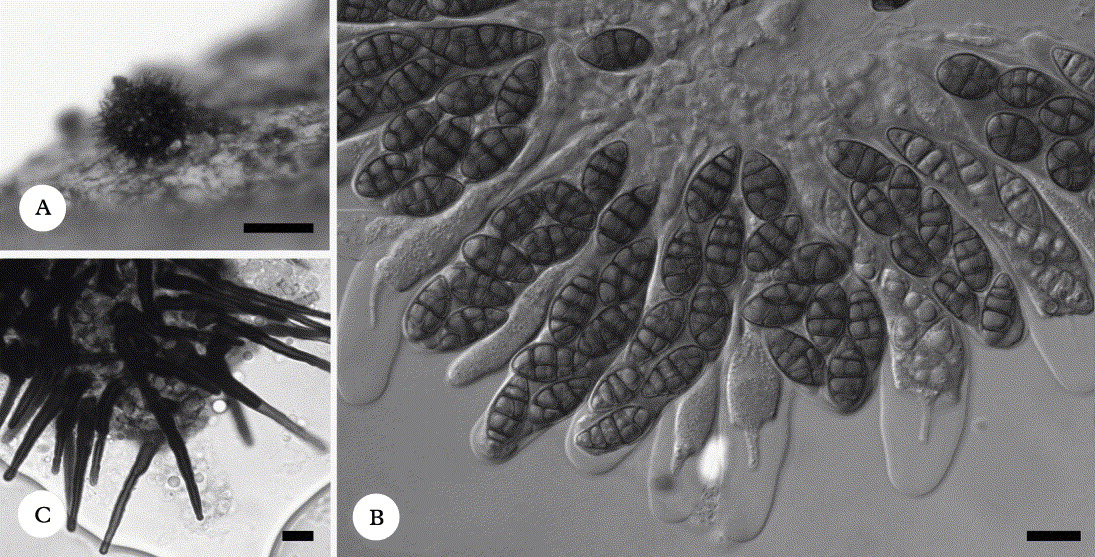
(Fig. 2A, B, D & F)

Fig. 2. Cercidospora epithamnolia A, B, D & F; A, infection habitus (LE 260574); B, ascomata (LE 233044) D, ascus in water (LE 260590); F, ascospores in K (LE 260590). Cercidospora thamnogalloides (holotype) C, E & G; C, infection habitus, note gall; E, asci in I; G, ascospore in water, note halo. Scales: A = 1 mm; B = 200 µm; C = 500 µm; D−G = 10 µm.
Vegetative hyphae indistinct.
Ascomata perithecioid, black, often with a greenish or bluish grey shade when slightly shielded by host tissues, smooth, subglobose, 90–120 µm diam., with an ostiole of 10–25 µm diam., immersed to slightly protruding in the ostiolar area, arising singly or occasionally contiguous, aggregated into groups of up to 100 or more. Exciple dark bluish green with a grey shade above, hyaline below, in surface view resembling textura angularis (cells mostly 3–5 µm across), K−. Hymenial gel I−, K/I−. Hamathecium of sparse or sometimes indistinct paraphysoids, non-capitate, c. 1·5 µm wide, septate, scarcely branched. Asci subcylindrical or moderately thickened in lower part, wall 1–2 µm thick apically and 0·5–1·5 µm laterally, sometimes with a small ocular chamber, with an indistinct or short stalk, (40–)44–60(–63) × 10–13(–14) µm (n = 12; in K elongated up to 76 µm), 8-spored, I−, K/I−. Ascospores hyaline, strongly heteropolar, narrowly soleiform or skittle-shaped with narrower to attenuated lower part and rounded ends, (12–)13–16(–19) × (3–)3·5–4·5(–5) µm, l/b = (2·9–)3·1–4·1(–5·0) (n = 40), with (0–)1 central septum, constricted at the septum only in K, smooth-walled, halo not observed, sometimes with a few conspicuous guttules per cell, arranged irregularly biseriate in the ascus.
Anamorph not observed.
Notes. Sixteen of 27 Cercidospora Körb. species previously described have exclusively or mainly 1-septate ascospores. Of these, the following five species are most similar to C. epithamnolia by colour of exciple and mainly 8-spored asci: C. caudata Kernst. s. lat. (grows on Caloplaca and Xanthopeltis), C. epipolytropa (Mudd) Arnold (on Lecanora), C. parva (on Baeomyces), C. solearispora Calat., Nav.-Ros. & Hafellner (on Aspicilia and Circinaria) and C. verrucosaria (Linds.) Arnold (on Megaspora) (Ihlen Reference Ihlen1998; Navarro-Rosinés et al. Reference Navarro-Rosinés, Calatayud, Hafellner, Nash, Ryan, Diederich, Gries and Bungartz2004, Reference Navarro-Rosinés, Calatayud and Hafellner2009; specimens of Cercidospora parva were examined for comparison). Cercidospora caudata is distinguished from C. epithamnolia by its larger ascospores [(14–)16–24(–30) × (3–)4–6(–7) µm], which are usually strongly heteropolar, with the lower cells curved and attenuated as a tail; C. epipolytropa has larger ascospores [(14–)15–19(–22) × (4·5–)5–6(–7) µm]; C. parva has a more green exciple, 4–8-spored asci and is a weak parasite; C. solearispora differs in its larger ascospores [(15–)17–21(–22) × (4·5–)5–6(–7) µm] with much shorter and narrower lower cells; C. verrucosaria has a more brown exciple and larger ascospores, measuring (14·5–)15·5–19·5(–23) × (4·5–)5–6(–7) µm. Moreover, all of them grow on different host genera (most Cercidospora species are restricted to a particular host genus) and have better developed paraphysoids. Comparison with C. thamnogalloides Zhurb., which also has 1-septate ascospores, is provided in Table 1. Though scarce interascal filaments are not typical for Cercidospora, they have been reported for C. cladoniicola Alstrup, C. macrospora (Uloth) Hafellner & Nav.-Ros. (type species of the genus) and C. thamnoliicola Ihlen (Ihlen Reference Ihlen1995; Alstrup Reference Alstrup1997; Navarro-Rosinés et al. Reference Navarro-Rosinés, Calatayud, Hafellner, Nash, Ryan, Diederich, Gries and Bungartz2004).
Table 1 Distinguishing characteristics of Cercidospora species growing on Thamnolia (based on the author's own data).

Distribution and host. Known from polar desert and arctic tundra of Asia. Commensalistically growing throughout healthy-looking thalli of Thamnolia vermicularis.
Additional specimens examined (all on Thamnolia vermicularis var. subuliformis). Russia: Severnaya Zemlya: Bolshevik Island, Akhmatova Bay, 79°04′ N, 102°45′ E, 10 m, 1996, M. Zhurbenko (LE 260574); ibid., Shokalskogo Strait, 79°18′ N, 101°40′ E, 20 m, 1996, M. Zhurbenko (LE 233044). Taimyr Peninsula: Byrranga Mts., Levinson-Lessinga Lake, 74°33′ N, 98°26′ E, 100 m, 1995, M. Zhurbenko (LE 260490). Wrangel Island: Naskhok River, 71°25′ N, 178°20′ W, 10 m, 1998, S. Kholod (LE 260590, LE 260530).
Cercidospora thamnogalloides Zhurb. sp. nov
MycoBank no.: MB 563051
Fungus lichenicola in thallis lichenum generis Thamnolia crescens. Similis speciei Cercidospora galligena, sed differt praecipue ascomatibus minoribus, 80–120 µm diam., ascosporis angustioribus, (12–)14–17(–19) × (3·5–)4–5(–5·5) µm, et hospite diverso.
Typus: Russia, Komi Republic, Northern Ural, Pechora-Ilych State Reserve, headwaters of Pechora River, Yanypupuner Range, Mt. “981”, 62°05′ N, 59°06′ E, 800 m elev., dwarf shrub alpine tundra, on thalli of Thamnolia vermicularis var. subuliformis, 30 June 1997, M. Zhurbenko 97394 (LE 260510—holotypus).
(Fig. 2C, E & G)
Vegetative hyphae indistinct.
Ascomata perithecioid, black, smooth, subglobose, 80–120 µm diam., with an ostiole mostly of 10–20 µm diam., immersed to slightly protruding in the ostiolar area, arising singly, aggregated in groups of up to 100 or more. Exciple dark bluish grey and 15–20 µm thick above, hyaline and 10–15 µm thick below, in surface view resembling textura angularis (cells 5–12 µm across), K−. Hymenial gel I−, K/I−. Hamathecium of sparse or sometimes indistinct paraphysoids, non-capitate, 1·5–2·0 µm wide, septate, scarcely branched. Asci obclavate to narrowly elliptic, occasionally subcylindrical, apical wall strongly thickened only in immature asci, rarely with an indistinct ocular chamber, with an indistinct or short stalk, (41–)46–60(–72) × (10–)12–16(–18) µm (n = 34; in K elongated up to 85 µm), 8-spored, I−, K/I−. Ascospores hyaline, distinctly heteropolar, narrowly soleiform, skittle-shaped, or narrowly oblanceolate, lower cell narrower than the upper one, usually with rounded ends, (12–)14–17(–19) × (3·5–)4–5(–5·5) µm, l/b = (2·7–)3·0–3·8(–4·1) (n = 57), with 1 central septum, not constricted at the septum, smooth-walled, with distinct halo 1·5–2·0 µm thick, usually with a few conspicuous guttules per cell best seen in K, arranged biseriate to partly diagonally uniseriate in the ascus.
Anamorph not observed.
Etymology. The specific epithet reflects external similarity of the new species to Thamnogalla crombiei (Mudd) D. Hawksw.
Notes. Just two previously known species of Cercidospora regularly induce galls: C. cecidiiformans Grube & Hafellner (on Rhizocarpon) and C. galligena Hafellner & Nav.-Ros. (on Aspicilia and Circinaria). However, occasional production of cecidia was also observed in C. macrospora [on the Lecanora muralis (Schreb.) Rabenh. group], C. solearispora, and C. stereocaulorum (Arnold) Hafellner (on Stereocaulon) (Hafellner Reference Hafellner1993; Navarro-Rosinés et al. Reference Navarro-Rosinés, Calatayud, Hafellner, Nash, Ryan, Diederich, Gries and Bungartz2004, Reference Navarro-Rosinés, Calatayud and Hafellner2009; Zhurbenko Reference Zhurbenko2010a). Cercidospora cecidiiformans differs from C. thamnogalloides in its larger ascomata (130–150 µm diam.), olive-brown exciple, wider ascospores (13–19 × 5–8 µm), and the I+ red, K/I+ blue reaction of the hymenial gel; C. galligena has larger ascomata (mainly 120–190 µm diam.), a brown to violet-black exciple, (4–)6–8-spored asci, and occasionally simple, somewhat wider ascospores [(13·5–)14–19 × 5–6(–7) µm], being only slightly heteropolar; C. macrospora has larger ascomata (150–250 µm diam.), 4(–8)-spored asci, and longer, rarely slightly heteropolar, ascospores [(19–)20–25(–30) × 4–6(–7) µm]; C. solearispora differs in its larger ascomata (160–230 µm diam.) and larger ascospores [(15–)17–21(–22) × (4·5–)5·0–6·0(–7·0) µm] with a very different shape and size of lower and upper cells; C. stereocaulorum has larger, sometimes superficial ascomata [100–200(–300) µm diam.], (2–)4(–8)-spored asci, and (1–)3(–6)-septate, larger ascospores, measuring (13–)18·5–25·5(–30) × (4–)5–7(–8) µm. Moreover, all these species grow on different hosts and all, except C. macrospora, have abundant paraphysoids. Galls induced by the new species strongly resemble those caused by Thamnogalla crombiei. Curiously, the latter species was also found in the type collection.
Distribution and host. Known from alpine tundra in the type locality in the Northern Ural at the boundary between Europe and Asia. The species induces gall-like swellings on healthy-looking thalli of Thamnolia vermicularis. The swellings are concolorous with the host thalli or pale brownish vinaceous. Host thalli are sometimes contorted under infection.
Additional specimen examined. Russia: Northern Ural: headwaters of Pechora River, Yanypupuner Range, 62°05′ N, 59°06′ E, 800 m, on Thamnolia vermicularis var. subuliformis, 1997, M. Zhurbenko (LE 260560).
Cercidospora thamnoliae Zhurb. sp. nov
MycoBank no.: MB 563052
Fungus lichenicola in thallis lichenum generis Thamnolia crescens. Similis speciei Cercidospora ochrolechiae, sed differt praecipue ascosporis (1–)3(–4)-septatis, et hospite diverso.
Typus: Russia, Taimyr Peninsula, Byrranga Mts., N of Levinson-Lessinga Lake, 74°33′ N, 98°26′ E, 180 m elev., arctic tundra, on thalli of Thamnolia vermicularis var. subuliformis, 26 August 1995, M. Zhurbenko 95571 (LE 260533—holotypus).
(Fig. 3A–D)

Fig. 3. Cercidospora thamnoliae A–D; A, ascomata (LE 260570); B, exciple in surface view in K (LE 260600); C, interascal filaments in K/I (LE 260600); D, asci with spores in I (LE 232882). Cercidospora thamnoliicola E, asci with spores in water (LE 260420). Scales: A = 100 µm; B–E = 10 µm.
Vegetative hyphae indistinct.
Ascomata perithecioid, black, smooth, subglobose, (60–)90–130(–200) µm diam. (ascomata of more than 150 µm diam. were observed only in LE 260528), with an ostiole of 15–30 µm diam., almost always immersed, rarely slightly protruding in the ostiolar area or exceptionally 1/3 exposed (only in LE 260528), arising singly, dispersed. Exciple dark bluish green often with a grey shade and 30–40 µm thick above, hyaline below, in surface view resembling textura epidermoidea or textura angularis (cells 3–10 µm across), K−. Hymenial gel I−, K/I−. Hamathecium of abundant paraphysoids, non-capitate, 1·5–2·0 µm wide, septate with individual cells 6–15 µm long, rather frequently branched and anastomosed. Asci subcylindrical or usually moderately thickened in lower or sometimes central part, wall 1·5–5·0 µm thick apically and 0·5–1·5 µm laterally, often with a small ocular chamber, with an indistinct or short stalk, (41–)53–69(–80) × (10–)11–15(–18) µm (n = 100; in K elongated up to 112 µm), (4–)8-spored, I−, K/I−. Ascospores hyaline, usually distinctly heteropolar, narrowly soleiform with a narrower to attenuated lower part, rarely almost narrowly elliptic, upper or both ends often rather acute, (12·5–)16·5–21·0(–27·5) × (4–)4·5–5·5(–8) µm, l/b = (2·5–)3·2–4·2(–5·4) (n = 308), with (1–)3(–4) septa (4-septate spores occurred only in LE 260528), usually not or only slightly constricted at the septa (markedly constricted only when overmature or in K), smooth walled, with many small guttules best seen in K, arranged irregularly (often diagonally) uni- or more commonly biseriate in the ascus; a halo, 1·0–1·5 µm thick, was sometimes observed around spores within the asci.
Anamorph not observed.
Notes. The following eight previously described Cercidospora species regularly have ascospores with three or more septa: C. alpina Ihlen & Wedin (on Stereocaulon), C. cladoniicola (on Cladonia), C. decolorella (Nyl.) O. E. Erikss. & J. Z. Yue (on terricolous algal films, Mycobilimbia, Peltigera, Protopannaria, and Solorina), C. ochrolechiae Zhurb. (on Ochrolechia and Pertusaria), C. pluriseptata (Nyl.) Zopf (on Lecanora), C. punctillata (on Biatora, Buellia, Cladonia, Lecanora, Micarea, Mycobilimbia, Peltigera, Phaeorrhiza, Pilophorus, Protopannaria, Psoroma, Solorina, and Sphaerophorus), C. soror Obermayer & Triebel (on Arthrorhaphis), and C. stereocaulorum (on Stereocaulon) (Nylander Reference Nylander1866; Alstrup et al. Reference Alstrup, Christensen, Hansen and Svane1994; Hafellner & Obermayer Reference Hafellner and Obermayer1995; Zhurbenko et al. Reference Zhurbenko, Santesson, Walker, Auerbach and Lewis1995; Zhurbenko & Santesson Reference Zhurbenko and Santesson1996; Alstrup Reference Alstrup1997; Zhurbenko Reference Zhurbenko2002, Reference Zhurbenko2008, Reference Zhurbenko2009a, b, Reference Zhurbenko2010b; Zhurbenko & Alstrup Reference Zhurbenko and Alstrup2004; Zhurbenko & Triebel Reference Zhurbenko and Triebel2005; Ihlen & Wedin Reference Ihlen and Wedin2007; von Brackel Reference von Brackel2010a; specimens of Cercidospora punctillata examined for comparison). Cercidospora alpina differs from C. thamnoliae in its much larger (200–350 µm diam.), semi-immersed ascomata and longer ascospores [(18–)19·5–33(–43) × (4–)4·5–6·5(–7) µm] with up to 7 septa; C. decolorella has somewhat larger ascomata (100–250 µm diam.) and ascospores often with 4–5 septa; C. cladoniicola readily differs in its olive-brown exciple; C. ochrolechiae is most similar to the new species, differing from the latter mainly in the occasional presence of 5-septate ascospores; C. pluriseptata has a brown exciple and ascospores with up to 7 septa; C. punctillata differs in its somewhat larger and markedly erumpent ascomata, larger ascospores [(14–)18·5–25·0(–33) × (4–)4·5–6·5(–9) µm] with (1–)3–5(–6) septa, and distinct pathogenicity; C. soror is clearly distinguished by its (2–)4-spored asci; C. stereocaulorum has larger, sometimes almost superficial ascomata [100–200(–300) µm diam.], (2–)4(–8)-spored asci, and larger ascospores [(13–)18·5–25·5(–30) × (4–)5–7(–8) µm] with up to 6 septa. Additionally, all these species grow on different host genera. The comparison with C. thamnoliicola, which also has many 3-septate ascospores, is provided in Table 1.
Features such as markedly exposed ascomata of more than 150 µm diam., the occurrence of 4-septate ascospores, and the clear bleaching of host tissues were observed only in LE 260528, the inclusion of which significantly broadened the new species concept. That deviating sample resembles some specimens of Cercidospora punctillata agg., which thus might be the fifth Cercidospora species growing on Thamnolia. However, the latter species is the only one in the genus with an extremely wide host range, which suggests that it might be heterogenous. It is noteworthy in this connection that 20 of 27 Cercidospora species are confined to one host genus, the exceptions being, apart from C. punctillata [syn. C. decolorella var. lichenicola (Zopf) O. E. Erikss. & J. Z. Yue], C. decolorella (actually indistinguishable from the latter), C. caudata, C. galligena, C. ochrolechiae, C. solearispora, and C. werneri Nav.-Ros., Calat. & Hafellner. Another consideration is that in LE 260528 Cercidospora thamnoliae occurred on an abnormally robust host thallus, which might be the reason for developing comparatively large and exposed ascomata. Further material is needed to confirm the suggested broad species concept or make it narrower.
Specimens examined of Cercidospora thamnoliae also fit the protologue of C. lecidomae Zhurb. & Triebel, the species distinguished from the earlier described C. punctillata (Nyl.) R. Sant. mainly by its smaller and less septate ascospores (Zhurbenko & Triebel Reference Zhurbenko and Triebel2003). However, careful comparison of additional material of Cercidospora lecidomae and C. punctillata revealed that ascospores of both species are (1–)3–5(–6)-septate and almost indistinguishable in size: (15·0–)18·5–27·0(–35·5) × (4·5–)5·0–6·5(–8·0) µm (n = 92) vs. (14–)18·5–25·0(–33) × (4–)4·5–6·5(–9) µm (n = 479). No additional discriminating characters between the two species were found. As a consequence, Cercidospora lecidomae Zhurb. & Triebel [Bibliotheca Lichenologica 86: 206 (2003)] is reduced here to synonymy (syn. nov.) with Cercidospora punctillata (Nyl.) Sant. [Lichen forming and Lichenicolous fungi of Fennoscandia 83 (2004); basionym: Verrucaria punctillata Nyl. Flora 67: 223 (1884)]. Cercidospora lecidomae has been reported on Thamnolia vermicularis by Kukwa & Flakus (Reference Kukwa and Flakus2009). Although I did not examine this material, it is likely to belong to Cercidospora thamnoliae.
Distribution and host. Known from polar deserts and arctic tundra of the American and Russian Arctic. Mostly found on old or decaying thalli of Thamnolia vermicularis, sometimes possibly causing local bleaching of host tissues (their distinct discoloration was observed only in LE 260528).
Additional specimens examined [on Thamnolia vermicularis var. subuliformis (15), var. vermicularis (Sw.) Schaer. (2)]. Canada: Canadian Arctic Archipelago: Ellef Ringnes Island, Isachsen Bay, 78°47′ N, 103°33′ W, 40 m, 2005, N. Matveeva (LE 260316, LE 260526).—Russia: Nenets Region: Malozemel'skaya Tundra, Seduiyakha River, 68°23′ N, 53°15′ E, 1998, O. Lavrinenko (LE 232884). Sverdrupa Island in Kara Sea: 74°33′ N, 79°25′ E, 10 m, 1992, Yu. Kozhevnikov (LE 260483). Severnaya Zemlya: Bolshevik Island, Mikoyana Bay, 79°18′ N, 101°55′ E, 10 m, 1996, M. Zhurbenko [LE 210306, erroneously reported as Cercidospora thamnoliicola in Zhurbenko (Reference Zhurbenko2008); LE 260573]; ibid., Shokalskogo Strait, 79°16′ N, 101°40′ E, 20 m, 1996, M. Zhurbenko (LE 233034, LE 232844, LE 232813, LE 260583, LE 260528); Sedova Archipelago, 79°25′ N, 91°40′ E, 1930, V. Savicz [LE 207056, erroneously reported as C. thamnoliicola in Zhurbenko & Santesson (Reference Zhurbenko and Santesson1996)]. Novosibirskie Islands: Zhokhova Island, 76°08′ N, 152°45′ E, 1989, M. Samarskii [LE 207223, erroneously reported as C. thamnoliicola in Karatygin et al. (Reference Karatygin, Nezdoiminogo, Novozhilov and Zhurbenko1999)]. Wrangel Island: Klark River, 71°07′ N, 178°14′ W, 100 m, 1998, S. Kholod (LE 232882); Naskhok River, 71°24′ N, 178°08′ W, 5 m, 1998, S. Kholod (LE 260570, LE 260600); Neozhidannaya River, 71°02′ N, 179°10′ E, 110 m, 1992, S. Kholod (LE 260374a).
Cercidospora thamnoliicola Ihlen
Graphis Scripta 7(1): 18 (1995); type: Norway, Nordland, on Thamnolia vermicularis var. subuliformis, Norman (O—holotype, n. v.).
Notes. Considering that the newly examined specimens reveal some discrepancies with the protologue of this insufficiently known species (Ihlen Reference Ihlen1995), its full description is provided here.
Vegetative hyphae indistinct.
Ascomata perithecioid, black, smooth, subglobose to broadly ovate, 50–125 µm diam., with an ostiole of 15–20(–40) µm diam., immersed to slightly protruding in the ostiolar area, arising singly or sometimes contiguous, aggregated in groups of up to 100 or more at the apices or central parts of the host thalli. Exciple medium to dark olive-brown to olivaceous buff and 17–25 µm thick above, hyaline and 8–12 µm thick below, in surface view resembling textura epidermoidea or textura angularis (cells 3–8 µm across), K+ yellowish brown. Hymenial gel I−, K/I−. Hamathecium of sparse or sometimes indistinct, non-capitate, c. 1·5 µm wide, septate, scarcely branched paraphysoids. Asci cylindrical or slightly thickened in the central or lower part, wall 1–3 µm thick apically and 0·5–1·5 µm laterally, ocular chamber not observed (with shallow ocular chamber from the protologue), (35–)46–62(–65) ×(7–)8–10 µm (n = 46; in K elongated up to 76 µm), (2–)4(–6)-spored (almost always with 4 well-developed spores, very rare with 2–3 normal and 1–2 abortive spores or with 4 normal and 2 abortive spores) [37–41 ×6–8(–12) µm, 4(–6)-spored taken from the protologue], I−, K/I− (Fig. 3E). Ascospores hyaline, usually distinctly heteropolar, narrowly elliptic, mostly with narrower to attenuated lower or occasionally upper part and often with rather acute ends, (11–)14·0–17·5(–21) × (4·0–)4·5–5·5(–6·5) µm, l/b = (2·2–)2·6–3·4(–4·2) (n = 158), with (1–)2–3 septa (3-septate according to the protologue), not or sometimes slightly constricted at the septa, smooth-walled, distinct halo not observed (halonate in the protologue), often with a few conspicuous guttules per cell best seen in K, uniseriate or diagonally arranged in the ascus.
Anamorph not observed. Infected host parts usually are slightly greyish and ‘scurfy’, sometimes also somewhat curved or swollen.
Poorly developed paraphysoids (also stressed in the protologue), asci with an indistinct ocular chamber, and K+ reaction of the exciple are not typical for Cercidospora. This evidently rare species has been confused with the more frequent C. thamnoliae (see above under the latter species) and thus was in fact formerly known only from two finds in the Norwegian mountains (Ihlen Reference Ihlen1995). Here it is confirmed for this type region and also reported new to the Asian Arctic.
The occurrence of four Cercidospora species on one host species (Table 1) is remarkable for lichenicolous fungi in general, but not for this particular lichenicolous fungal genus, where almost half of the host lichen genera support more than one Cercidospora species. For instance, three Cercidospora species are known on Aspicilia and Circinaria (C. galligena, C. solearispora and C. werneri) and another three on Caloplaca [C. caudata, C. epicallopisma Arnold and C. epicarphinea (Nyl.) Grube & Hafellner] (Navarro-Rosinés et al. Reference Navarro-Rosinés, Calatayud, Hafellner, Nash, Ryan, Diederich, Gries and Bungartz2004, Reference Navarro-Rosinés, Calatayud and Hafellner2009). It may also reflect the high heterogeneity of Thamnolia vermicularis populations (Nelsen & Gargas Reference Nelsen and Gargas2009a, b). It is also possible that three Cercidospora species with indistinct paraphysoids, treated above (Table 1), might be better disposed in another genus.
Specimens examined [all in arctic or alpine tundras on Thamnolia vermicularis var. subuliformis (2), var. vermicularis (1)]. Norway: Troms County: Skibotndalen Valley, 69°16′ N, 20°23′ E, 950 m, 2003, M. Zhurbenko (LE 260460).—Russia: Taimyr Peninsula: Byrranga Mts., Levinson-Lessinga Lake, 74°24′ N, 98°38′ E, 150 m, 1995, M. Zhurbenko (LE 260420). Chukchi Peninsula: Provideniya, 64°27′ N, 173°11′ W, 100 m, 2001, M. Zhurbenko (LE 260440).
Cladosporium licheniphilum Heuchert & U. Braun
Herzogia 19: 12 (2006); type: Russia, Altai, on Pertusaria alpina Hepp ex H. E. Ahles, 1999, Davydov (LE—holotype!).
Notes. New to the Arctic. Thamnolia is a new host genus.
Specimen examined. Russia: Taimyr Peninsula: Byrranga Mts., Levinson-Lessinga Lake, 74°32′ N, 98°33′ E, 250 m, arctic tundra, on damaged portions of Thamnolia vermicularis var. subuliformis thalli, 1995, M. Zhurbenko (LE 260309, HAL 2444 F; det. U. Braun).
Dacampia thamnoliicola Zhurb. ad int
(Fig. 4)

Fig. 4. Dacampia thamnoliicola (LE 207531b). A, ascomata; B, ascospore in water, note halo; C, immature ascus in water; D, interascal filaments in water; E, asci with spores in water. Scales: A = 100 µm; B–E = 10 µm.
Ascomata perithecioid, black, subglobose, 100–200 µm diam., ostiolate, erumpent to sessile. Exciple brown, in surface view similar to textura angularis, composed of thick-walled, angular, pseudoparenchymatous cells. Hamathecium formed by well-developed interascal filaments, which are 1·5–3·5 µm wide, septate, and branched. Asci fissitunicate, clavate, with a distinct foot, 70–100 × 22–24 µm (n = 5), 8-spored. Ascospores initially pale, then dark brown, elliptic, with rounded or rather acute ends, (23–)25–29(–31·5) × (10·5–)11–13(–14) µm, l/b = (1·8–)2·1–2·5(–2·7) (n = 45), muriform when mature with 5 − 7 transverse septa and a longitudinal or oblique septum in central cells, slightly constricted at the septa, wall ornamented (×1000), with halo 1·5–2·5 µm thick, guttules not observed, arranged diagonally uniseriate to biseriate in the ascus. On ± decaying podetia of the host.
Notes. As noted by Halici & Hawksworth (Reference Halici and Hawksworth2008), the lichenicolous species in the Dacampiaceae, with the exception of Pyrenidium actinellum Nyl., are almost always host-specific. None of the 13 previously known Dacampia A. Massal. species has been found on Thamnolia. Among them, ascospores with up to 7 trans-septa were known in D. cyrtellae Brackel (on Lecania), D. lecaniae Kocourk. & K. Knudsen (on Lecania), D. leptogiicola (D. Hawksw.) D. Hawksw. (on Collema, Leptogium, and Pannaria), and D. muralicola Halici & D. Hawksw. (on Protoparmeliopsis) (Hawksworth Reference Hawksworth1975; Etayo & Breuss Reference Etayo and Breuss1996; Halici & Hawksworth Reference Halici and Hawksworth2008; Lopez de Silanes et al. Reference López de Silanes, Etayo and Paz-Bermúdez2009; von Brackel Reference von Brackel2010b; Kocourková & Knudsen Reference Kocourková and Knudsen2010). Dacampia cyrtellae differs from D. thamnoliicola in its smaller ascomata (110–160 µm diam.) and smaller ascospores [(19·5–)21·2–24·9(–26·0) × (6·5–)6·8–8·3(–9) µm]; D. lecaniae seems to be the closest to this presumably new species, including the presence of a conspicuous gelatinous sheath, but has somewhat smaller ascospores, measuring (21–)22·8–26·5(–28) × (8–)9·5–12·0 µm; D. leptogiicola has smaller ascospores (21–25 × 5·0–6·5 µm); and D. muralicola has somewhat smaller ascospores [21–26(–31·5) × (7·0–)9·0–12·5(–14·5) µm] and smaller ascomata (95–120 µm diam.). Ascospores of Dacampia thamnoliicola are not that typical for the genus in being halonate and ornamented; the first feature also shared by D. lecaniae and D. rubra Halici, Candan & Calat. and the second one by D. rhizocarpicola D. Hawksw., D. rubra, and D. xanthomendozae Etayo & Halici (Halici & Hawksworth Reference Halici and Hawksworth2008; Halici et al. Reference Halici, Candan and Calatayud2009a, b).
Specimen examined. Russia: Severnaya Zemlya: Sedova Archipelago, 79°25′ N, 91°40′ E, on Thamnolia vermicularis var. vermicularis, 1930, V. Savicz 2017b (LE 207531b).
Epithamnolia Zhurb. gen. nov
MycoBank no.: MB 563053
Genus lichenicola ad Coelomycetes pertinens. Conidiomata plus minusve nigra, subglobosa ad cupuliformia, cum muris textura globulosa vel porrecta, subimmersa ad superficialia, singularia, dispersa. Conidiophora desunt. Cellulae conidiogenae enteroblasticae, phialidicae, non prolifericae, hyalinae, lagenulatae vel anguste ellipticae. Conidia acrogena, hyalina, cylindrica, (0–)1(–2?)-septata, laevia.
Typus: Epithamnolia karatyginii Zhurb.
Genus lichenicolous, belonging to the Coelomycetes.
Conidiomata pycnidial, blackish, rugose, irregularly subglobose, but becoming cupuliform with age, initially almost closed, then with an irregular opening, subimmersed in thalli of Thamnolia species to finally superficial, arising singly, dispersed. Exciple in cross-section dark to medium brown throughout, in surface view resembling textura angularis/globulosa or textura porrecta, K−. Conidiophores absent. Conidiogenous cells lining the pycnidial cavity, enteroblastic, phialidic, with indistinct collarette and periclinal thickening, not proliferating, hyaline, lageniform to narrowly elliptic, smooth-walled. Conidia acrogenous, arising singly, not catenate, hyaline, cylindrical, with obtuse to sometimes slightly truncated ends, ± straight, (0–)1(–2?)-septate, not constricted at the septum, smooth-walled, sometimes with sparce inconspicuous guttules.
Notes. Amongst the non-lichenicolous pycnidial fungal genera, the new genus mainly recalls Ascochyta Lib. agg., Clypeopycnis Petr., Didymochaeta Sacc. & Ellis, Gelatinopycnis Dyko & B. Sutton, Pocillopycnis Dyko & B. Sutton emend. DiCosmo, Pseudocenangium P. Karst., and Septopatella Petr. (Dyko & Sutton Reference Dyko and Sutton1979; DiCosmo Reference DiCosmo1980; Sutton Reference Sutton1980; Mel'nik Reference Mel'nik2000). However, there are significant differences. In Ascochyta agg. the conidiomata are non-cupulate; in Clypeopycnis the conidiomata are non-cupulate, and conidiophores are present; in Didymochaeta Sacc. & Ellis the conidiomata are non-cupulate, and the conidiogenous cells have a distinct collarette and a periclinal thickening; in Gelatinopycnis the conidiomata are closed and have a complex wall, and conidiophores are present; in Pocillopycnis conidiophores are present, the conidiogenous cells are sympodially proliferating, and the conidia are holoblastic, lunate to sigmoid, 3–10-septate; in Pseudocenangium conidiophores are present and the conidiogenous cells are annelidic; in Septopatella conidiophores are present, and the conidiogenous cells are sympodially proliferating. Considering the coelomycetes known to occur on lichens, Epithamnolia karatyginii, the type species of this monotypic genus, resembles species of Hainesia Ellis & Sacc. in having finally cupulate conidiomata and hyaline, cylindrical, septate conidia (Etayo & Diederich Reference Etayo and Diederich1996a; von Brackel Reference von Brackel2009). However, the latter are easily distinguished from the new species by their branched conidiophores (Punithalingam & Spooner Reference Punithalingam and Spooner1997). Epithamnolia karatyginii may also resemble the lichenized Woessia fusarioides D. Hawksw., Poelt & Tsch.-Woess., which differs from the former in having white, shallow cupuliform to disc-like conidiomata, falcate, non-septate conidia and in the lignicolous life habit (Hawksworth & Poelt Reference Hawksworth and Poelt1986).
Epithamnolia karatyginii Zhurb. sp. nov
MycoBank no.: MB 563054
Fungus lichenicola in thallis lichenum generis Thamnolia crescens. Conidiomata 110–220 µm diam. Cellulae conidiogenae (5–)6·5–9(–10) × (1·5–)2–3 µm. Conidia (14–)18·5–27(–32) × (1–)1·5–2(–2·5) µm.
Typus: Canada, British Columbia, Wells Gray Provincial Park, Mt. Raft, 51°44′ N, 119°50′ W, 2100 m elev., alpine tundra with sparse Picea engelmannii, on moribund thalli of Thamnolia vermicularis var. subuliformis, 3 August 2002, M. Zhurbenko 02343 (LE 260498—holotypus).
(Fig. 5)

Fig. 5. Epithamnolia karatyginii (holotype). A, conidiomata; B, exciple in water; C, conidiogenous cells and conidia in water; D, conidia in water. Scales: A = 100 µm; B–D = 10 µm.
Vegetative hyphae pale brown, somewhat flexuose, 2·5–5·0 µm wide, scarcely septate, constricted at the septa, immersed.
Conidiomata pycnidial, blackish, glossy, rugose, irregularly subglobose at first, becoming cupuliform with age, 110–220 µm diam., initially almost closed, then with an irregular opening (20–)40–60(–100) µm across, exposing the pale brown interior, subimmersed to finally superficial, arising singly, dispersed. Exciple in cross-section dark to medium brown throughout, basally of 3–5 cell layers, in surface view resembling textura angularis/globulosa (cells 3·5–10·0 µm across) or textura porrecta, K−. Conidiophores absent. Conidiogenous cells lining the pycnidial cavity, enteroblastic, phyalidic, with indistinct collarette and periclinal thickening, not proliferating, hyaline, lageniform to narrowly elliptic, (5–)6·5–9·0(–10) × (1·5–)2–3 µm (n = 39), smooth-walled. Conidia abundant, acrogenous, arising singly, not catenate, hyaline, cylindrical, with obtuse to sometimes slightly truncated ends, ± straight, (14–)18·5–27·0(–32) × (1·0–)1·5–2·0(–2·5) µm, l/b = (6·0–)9·8–17·4(–21·3) (n = 122), (0–)1(–2?)-septate, not constricted at the septum, smooth-walled, sometimes with sparce inconspicuous guttules.
Etymology. The species is named after the eminent Russian mycologist Dr Igor V. Karatygin, the main author of the first catalogue of the Russian Arctic fungi (Karatygin et al. Reference Karatygin, Nezdoiminogo, Novozhilov and Zhurbenko1999).
Distribution and host. Known from polar deserts, arctic and alpine tundras of North America and Eurasia. On ± damaged basal or apical parts of Thamnolia vermicularis thalli, sometimes intermixed with Sphaerellothecium thamnoliae Zhurb. or Stigmidium frigidum (Th. Fr. ex Sacc.) Alstrup & D. Hawksw.
Additional specimens examined [on Thamnolia vermicularis var. subuliformis (5), var. vermicularis (1)]. Russia: Severnaya Zemlya: Bolshevik Island, Shokalskogo Strait, 79°16′ N, 101°40′ E, 20 m, 1996, M. Zhurbenko (LE 232933). Northern Ural: headwaters of Pechora River, Yanypupuner Range, 62°05′ N, 59°06′ E, 800 m, 1997, M. Zhurbenko (LE 260538a, intermixed with Sphaerellothecium thamnoliae var. thamnoliae). Kola Peninsula: Barents Sea coast, Voron'ya River mouth, 69°09′N, 35°50′ E, 20 m, 1997, M. Zhurbenko (LE 260448). Taimyr Peninsula: Byrranga Mts., Bolshaya Bootankaga River, 74°30′ N, 97°40′ E, 350 m, 1995, M. Zhurbenko (LE 232994b, intermixed with Stigmidium frigidum). Yakutiya: Laptev Sea coast, Tiksi, 71°40′ N, 128°40′ E, 50 m, 1998, M. Zhurbenko (LE 260444b, intermixed with Stigmidium frigidum).
Geltingia associata (Th. Fr.) Alstrup & D. Hawksw
Meddel. Grønl., Biosc. 31: 33 (1990); type: Spitsbergen, Danskøn, 1861, Malmgren (UPS—lectotype, n. v.).
Note. This fungus mostly grows on Ochrolechia spp., but has also been recorded from Thamnolia (Walker Reference Walker1970; Zhurbenko & Santesson Reference Zhurbenko and Santesson1996; Diederich et al. Reference Diederich, Ertz and Etayo2010).
Specimen examined. Russia: Taimyr Peninsula: Byrranga Mts., Levinson-Lessinga Lake, 74°31′ N, 98°36′ E, 250 m, arctic tundra, on moribund thalli of Thamnolia vermicularis var. subuliformis, 1995, M. Zhurbenko (LE 260468).
Lichenoconium usneae (Anzi) D. Hawksw
Persoonia 9(2): 185 (1977); type: Italy, on Usnea filipendula Stirt. agg. (K—isotype, n. v.).
Note. This common coelomycete has been recorded from no less than 29 lichen genera, including one previous record on Thamnolia (Hafellner & Türk Reference Hafellner and Türk1995).
Specimens examined. Russia: Taimyr Peninsula: Byrranga Mts., Levinson-Lessinga Lake, 74°32–33′ N, 98°26–33′ E, 180–300 m, arctic tundra, on partly moribund thalli of Thamnolia vermicularis var. subuliformis, 1994, M. Zhurbenko (LE 207532c); ibid., 1995, M. Zhurbenko (LE 260544).
Lichenopeltella thamnoliae R. Sant
Thunbergia 28: 7 (1998); type: Colombia, Boyacá, on Thamnolia vermicularis var. vermicularis, 1972, Cleef (UPS—holotype, n. v.).
Notes. Previously known from Colombia, Venezuela, Ecuador, and Peru (Santesson Reference Santesson1998; Etayo Reference Etayo2010). New to Bolivia. Thamnolia papelillo var. subsolida (M. Satô) R. Sant. is a new host variety.
Specimen examined. Bolivia: 25 km S of Sorata, 1 km NE of Laguna Ajuyani, 15°53′ S, 68°39′ W, 4250 m, on Thamnolia papelillo var. subsolida, 2000, A. Elvebakk (LE).
Merismatium thamnoliicola Alstrup & E. S. Hansen
Graphis Scripta 12: 43 (2001); type: Greenland, Thule District, on Thamnolia vermicularis, 1992, Alstrup (C—holotype, n. v.).
Vegetative hyphae pale to medium brown, 1·5–2·5 µm wide.
Ascomata perithecioid, black, subglobose, often with a small papilla, 100–150 µm diam., 1/4–3/4 protruding (superficial according to the protologue). Hymenial gel I+ brownish orange, K/I+ blue. Ascospores initially hyaline, then pale to medium olivaceous buff, snuff brown, or greyish brown, at maturity with contrasting darker septa, elliptic to oblanceolate (Fig. 6), constricted lower ends noted in the protologue were observed only in some overmature spores and are evidently the result of spore destruction, (14–)16·5–24·0(–34) × (6–)6·5–8·5(–9) µm, l/b = (1·6–)2·1–3·3(–4·3) (n = 89) (22–30 × 8·5–9·5 µm according to the protologue), with (3–)5–7(–8) transverse septa and a longitudinal or oblique septum in central or all cells, smooth-walled.

Fig. 6. Merismatium thamnoliicola, old ascospores in I (LE 260554). Scale = 10 µm.
Notes. Mostly found on moribund bases of host thalli, pathogenicity not observed. The species was described and known previously from two collections in Greenland (Alstrup & Hansen Reference Alstrup and Hansen2001) and thus is new to Eurasia. Examination of new material revealed some discrepancies with its protologue, described above.
Specimens examined (all in polar desert, arctic or alpine tundras on Thamnolia vermicularis var. subuliformis). Russia: Severnaya Zemlya: Bolshevik Island, Akhmatova Bay, 79°04′ N, 102°45′ E, 10 m, 1996, M. Zhurbenko (LE 260554); Sedova Archipelago, 79°25′ N, 91°40′ E, 1930, V. Savicz (LE 260430, LE 260450). Taimyr Peninsula: Byrranga Mts., Bolshaya Bootankaga River, 74°20′ N, 98°05′ E, 400 m, 1991, V. Kuvaev (LE 260473). Eastern Sayan Mts.: Kryzhina Range, headwaters of Belyi Kitat River, 53°59′ N, 95°32′ E, 1500 m, 2009, M. Zhurbenko (LE 260373).
Odontotrema santessonii Zhurb., Etayo & Diederich
Lichenologist 34: 495; type: Russia, Chukchi Peninsula, on Thamnolia vermicularis var. subuliformis, 1971, Yurtsev (LE 207696—holotype!; hb. Diederich—isotype!).
Notes. Ascomata up to 450 µm diam., finally sometimes almost sessile. Hymenium I+ brownish red to coral throughout. Ascospores hyaline, narrowly oblanceolate, (15–)18–23(–25) × (3–)3·5–4·5(–5) µm, l/b = (3·8–)4·7–5·9(–6·6) (n = 39), with (3–)7–8 transverse septa and often a few longitudinal or oblique septa in central cells, arranged ± in two 4-spored fascicles in the ascus. Pathogenicity not observed. New to North America.
Specimens examined [all in polar desert or arctic tundra on Thamnolia vermicularis var. subuliformis (5), var. vermicularis (4)]. Canada: Canadian Arctic Archipelago: Axel Heiberg Island, Bunde Fjord, 80°30′ N, 94°36′ W, 30 m, 1999, N. Matveeva (LE 260543).—Russia: Severnaya Zemlya: Bolshevik Island, Akhmatova Bay, 79°04′ N, 102°45′ E, 10 m, 1996, M. Zhurbenko (LE 232853). Taimyr Peninsula: Byrranga Mts., Bolshaya Bootankaga River, 74°30′ N, 97°40′ E, 200 m, 1995, M. Zhurbenko 95309 (LE 232814). Yakutiya: Laptev Sea coast, Tiksi, 71°39–40′ N, 128°40–45′ E, 50 m, 1998, M. Zhurbenko (LE 232854, LE 260524, LE 260324); Lena River delta, Stolb Island, 72°24′ N, 126°40′ E, 50 m, 1998, M. Zhurbenko (LE 260364). Wrangel Island: Neizvestnaya River, 71°11′ N, 179°15′ W, 170 m, 1987, S. Kholod (LE 232962). Chukotka: Gil'mymlineiveem River, 65°48′ N, 173°15′ W, 1977, I. Makarova (LE 233094).
Odontotrema thamnoliae Zhurb., Diederich & Etayo
Lichenologist 34: 497; type: Russia, Chukchi Peninsula, on Thamnolia vermicularis var. vermicularis, 1971, Makarova (LE 207695—holotype!).
Notes. Hymenium I+ orange except the uppermost part, which is I+ blue. Ascal wall I+ blue at the apex, I− below. Sometimes slightly bleaching the host tissues. New to North America.
Specimens examined [all in arctic or alpine tundras on Thamnolia vermicularis var. subuliformis (3), var. vermicularis (3)]. USA: Alaska: Seward Peninsula, 7 km NE of Nome, 64°33′ N, 165°21′ W, 220 m, 2001, M. Zhurbenko (LE 260376).—Russia: Kola Peninsula: Barents Sea coast, Voron'ya River mouth, 69°09′ N, 35°50′ E, 20 m, 1997, M. Zhurbenko (LE 260464). Western Sayan Mts.: Kulumys Range, 52°51′ N, 93°17′ E, 1700 m, 2010, M. Zhurbenko (LE 260588). Taimyr Peninsula: Byrranga Mts., Levinson-Lessinga Lake, 74°24′ N, 98°38′ E, 150 m, 1995, M. Zhurbenko (LE 260597). Yakutiya: Laptev Sea coast, Tiksi, 71°37′ N, 128°54′ E, 70 m, 1998, M. Zhurbenko (LE 233074). Chukotka: Kolyuchinskaya Bay, Yuniveem River mouth, 66°40′ N, 173°54′ W, 20 m, 1980, A. Katenin & M. Zhurbenko (LE 232864).
Phaeospora arctica Horáková & Alstrup
Graphis Scripta 6: 61 (1994); type: Greenland, Disco Island, on Arctocetraria andreievii (Oxner) Kärnefelt & A. Thell, 1983, Poelt (PRM 842918—holotype, n. v.).
Ascomata black, subglobose, c. 120 µm diam., semi-immersed. Hymenial gel appears I+ pale red. Hamathecium not observed. Asci subcylindrical to obclavate, with a long internal apical beak, c. 55–60 × 11–14 µm, 6–8-spored, I−. Ascospores hyaline to pale cinnamon-brown, narrowly elliptic (mostly) to elliptic and often slightly narrower below, (10–)15·5–20·5(–22) × (4·5–)5–6(–8·0) µm, l/b = (1·7–)2·7–3·9(–4·7) (n = 51), (1–)3-septate, not constricted at the septa, smooth-walled, without halo, guttulate, biseriate in the ascus. Growing on the bleached, possibly moribund base of the host's podetia.
Notes. The specimen fits well the species concept of Phaeospora arctica, except staining of the hymenial gel with I, not reported before (Horáková & Alstrup Reference Horáková and Alstrup1994; Zhurbenko & Santesson Reference Zhurbenko and Santesson1996). The species was formerly known on Allocetraria, Arctocetraria, and Arctoparmelia, thus Thamnolia being a new host genus.
Specimen examined. USA: Alaska: Great Kobuk Sand Dunes, 67°05′ N, 158°58′ W, 50 m, on Thamnolia vermicularis var. subuliformis, 2000, M. Zhurbenko (LE 260359).
Phoma thamnoliae Zhurb. ad int
(Fig. 7)

Fig. 7. Phoma thamnoliae (LE 260349). A, squashed peridium in water; B, conidia in water. Scales: A & B = 10 µm.
Vegetative hyphae pale brown, 3–5(–7) µm diam., septate, constricted at the septa.
Conidiomata pycnidial, black, subglobose, indistinctly papillate, 75–110 µm diam., with an ostiole of c. 10 µm diam., subimmersed to almost sessile. Pycnidial wall brown, darker around the ostiole, in surface view composed of angular pseudoparenchymatous cells (textura angularis) 4–10 µm across, K−. Pycnidial gel I−, K/I−. Conidiogenous cells not observed clearly, probably ampulliform, c. 5 × 2 µm. Conidia hyaline, oblong to occasionally elliptic, with rounded ends, (3·5–)4–5(–5·5) × (1·5–)2·0(–2·5) µm, l/b = (1·6–)2·0–2·6(–2·8) (n = 135), non-septate, smooth-walled, with 2 − 3 conspicuous, usually apical, guttules, abundant, arising singly. Infected parts of the host thalli are bleached or pale red.
Notes. The rather scant material does not permit a formal description of this evidently new Phoma Sacc. species. Most (c. 70%) of the 22 currently recognized lichenicolous species of Phoma are restricted to a single host genus, and none of them has been reported on Thamnolia (Icmadophilaceae, Pertusariales). The only other Phoma species hosted by a Pertusariales genus (Dibaeis) is P. maculiformans Ihlen (Ihlen Reference Ihlen1998), which readily differs from P. thamnoliae in its larger pycnidia (120–200 µm diam.) and non-guttulate, longer conidia, measuring 5–6(–7) × 2·0–2·5 µm. The shape and size of the conidia are among the most important diagnostic morphological characters in Phoma taxonomy. Of the other lichenicolous species referred to the genus, Phoma thamnoliae is most similar in these characters to P. aggregata Calat. & Etayo, P. cladoniicola Diederich, Kocourk. & Etayo, P. grumantiana Zhurb. & Diederich, P. lecanorina Diederich, P. melanohaleicola D. Hawksw. & Earl.-Benn., P. peltigerae (P. Karst.) D. Hawksw., and P. rozziana Etayo (Hawksworth Reference Hawksworth1981; Diederich Reference Diederich1986; Etayo Reference Etayo1996; Calatayud & Etayo Reference Calatayud and Etayo2001; Hawksworth & Cole Reference Hawksworth and Cole2004; Earland-Bennett et al. Reference Earland-Bennett, Hitch and Hawksworth2006; Diederich et al. Reference Diederich, Kocourková, Etayo and Zhurbenko2007; Etayo & Sancho Reference Etayo and Sancho2008). However, Phoma aggregata (on Diploschistes) is clearly separated from the new species by the minute pycnidia (30–50 µm diam.), aggregated in dense groups of up to 200; P. cladoniicola (on Cladonia) has larger conidia [(4–)4·5–6·0(–7·5) × (2–)2·5–3·0(–3·5) µm]; P. grumantiana is very similar to the studied material in the sizes of pycnidia (50–100 µm diam.) and conidia [(3–)4–5(–6) × 1·5–2·0(–3) µm], but could be distinguished by its host selection (Cladonia, Lecanorales); P. lecanorina (on Lecanora and Flavoparmelia) differs in its smaller pycnidia (15–60 µm diam.), the peridium, being dark green above and subhyaline below, and narrower conidia (3·2–5·0 × 1·2–1·6 µm); P. melanohaleicola (on Melanohalea) has slightly longer conidia (5–5·5 × 2–2·5 µm); P. peltigerae (on Peltigera) has larger pycnidia (up to 200 µm diam.) and conidia [(4–)4·5–6·0(–7) × 2·0–2·5(–3) µm]; and P. rozziana (on Pseudocyphellaria) has smaller pycnidia, measuring 50–80 µm diam. Phoma thamnoliae might also be compared with Briancoppinsia cytospora (Vouaux) Diederich, Ertz, Lawrey & van den Boom [syn. Phoma cytospora (Vouaux) D. Hawksw.], growing on a wide range of different host genera (Diederich et al. Reference Diederich, Lawrey, Sikaroodi, van den Boom and Ertz2012). However, that species differs in its smaller pycnidia (40–80 µm diam.), the peridium reacting K+ dark olivaceous, pycnidial gel reacting I+ and K/I+ red, and basally truncate, slightly curved, longer conidia, measuring 5–7 × 1·5–2 µm.
Specimens examined (both on upper parts of podetia of Thamnolia vermicularis var. subuliformis). USA: Alaska: Great Kobuk Sand Dunes, 67°05′ N, 158°58′–159°00′ W, 50 m, Dryas-lichen-moss vegetation among sparse Picea glauca, 2000, M. Zhurbenko 00226, 00468 (LE 260369, LE 260349).
Polycoccum vermicularium (Linds.) D. Hawksw
Bull. Br. Mus. nat. Hist., Bot. 14(2): 172 (1985); type: Falkland Isles, on Thamnolia vermicularis, 1842, Hooker (E—holotype, n. v.).
Rich material now available of this species permits us to improve or complete its previous descriptions (Hawksworth Reference Hawksworth1985; Hawksworth & Diederich Reference Hawksworth and Diederich1988; Ihlen Reference Ihlen1995).
Ascomata perithecioid, black, subglobose, 50–120 µm diam., ostiolate, initially completely immersed (sometimes in few layers), then mostly protruding in the ostiolar area, arising singly, sometimes adjacent or a few confluent, aggregated in dense groups. Hamathecium of well-developed interascal filaments, which are 1·5–3·5 µm wide, septate, branched, composed of individual cells 4–12 µm long. Asci elongate-clavate, often with a distinct ocular chamber, with a short foot, (55–)61–75(–80) × 20–26(–30) µm (n = 30), 8-spored, I− (except plasma becoming red-brown). Ascospores long hyaline, then olive and finally dark brown, walls and septum of young pale olive spores markedly darker than lumina, narrowly obovate to elliptic, upper cell usually wider than lower one, sometimes equally-celled, (13–)15·5–20·0(–28) × (6–)7–9(–12) µm, l/b = (1·5–)1·9–2·5(–3·3) (n = 165), (0–)1-septate, not to markedly constricted at the septum, wall smooth, not verruculose as stated in Hawksworth (Reference Hawksworth1985), occasionally with halo 1–2 µm thick (mostly around young hyaline spores), often with one big guttule in each cell, arranged irregularly biseriate in the ascus.
Conidiogenous cells ± pyriform, 6–8 × 2–3 µm. Conidia hyaline, ± oblong, straight to slightly bent, (3–)4–6(–7·5) × (1–)1·5 µm (n = 63), non-septate.
Notes. Infected host tissues become grey, then black, sometimes exhibit brown gall-like swellings up to 2 mm diam., or disintegrate. Macroscopically the infections are similar to those of Stigmidium frigidum. New to Asia (Russia and Mongolia).
Specimens examined [all in polar desert, arctic or alpine tundras, or northern boreal forest on Thamnolia vermicularis var. subuliformis (22), var. vermicularis (7)]. USA: Alaska: Great Kobuk Sand Dunes, 67°05′ N, 158°58′ W, 50 m, 2000, M. Zhurbenko (LE 260339).—Norway: Troms County: Skibotndalen Valley, 69°16′ N, 20°23′ E, 950 m, 2003, M. Zhurbenko (LE 260306).—Russia: Kola Peninsula: Barents Sea coast, Voron'ya River mouth, 69°09′ N, 35°50′ E, 20 m, 1997, M. Zhurbenko (LE 260414). Nenetz Region: Bolshoi Tsinkovyi Island, 70°27′ N, 58°40′ E, 1997, V. Shevchenko (LE 232924). Altai Mts.: Ukok Tableland, 49°19′ N, 87°36′ E, 2500 m, 1996, T. Lunke (LE 260453). Western Sayan Mts.: Chernoe Lake, 52°49′ N, 94°07′ E, 1570 m, 2010, M. Zhurbenko (LE 260320); Kulumys Range, 52°51–52′ N, 93°15–17′ E, 1700 m, 2010, M. Zhurbenko (LE 260326, LE 260360); Oiskii Range, headwaters of Olen'ya River, 52°48′ N, 93°15′ E, 1650 m, 2010, M. Zhurbenko (LE 260380a). Eastern Sayan Mts.: Kryzhina Range, headwaters of Belyi Kitat River, 53°59′–54°01′ N, 95°27′–95°33′E, 1800 m, 2009, M. Zhurbenko (LE 260403b, LE 260393). Taimyr Peninsula: Byrranga Mts., Levinson-Lessinga Lake, 74°34–37′ N, 98°33–47′ E, 150–250 m, 1995, M. Zhurbenko (LE 233054, LE 260424); ibid., Bolshaya Bootankaga River, 74°30′ N, 97°40′ E, 350 m, 1995, M. Zhurbenko (LE 260593); ibid., Krasnaya River, 74°35′ N, 98°08′ E, 200 m, 1994, M. Zhurbenko (LE 232803, LE 260563 − anamorph); Mt. “217” near junction of Pravaya Uboinaya and Uboinaya Rivers, 73°25′ N, 82°51′ E, 150 m, M. Zhurbenko (LE 260363). Yakutiya: Laptev Sea coast, Tiksi, 71°37–39′ N, 128°45–54′ E, 70 m, 1998, M. Zhurbenko (LE 232833, LE 260344a, LE 260348); Lena River delta, 3 km E of Cape Krest-Tumsa, 72°22′ N, 126°42′ E, 50 m, 1998, M. Zhurbenko (LE 232914). Wrangel Island: Naskhok River, 71°24′ N, 178°08′ W, 5 m, 1998, S. Kholod (LE 260580); Neizvestnaya River, 71°20′ N, 179°29′ W, 1986, A. Dobrysh (LE 260397 − anamorph). Komandorskie Islands: Bering Island, Nikolskoe, 55°12′ N, 165°57′ E, 1980, A. Dombrovskaya (KPABG, LE 232804). Chukotka: Baranikha, 68°30′ N, 168°16′ E, 1971, A. Galanin (LE 260553); ibid., 1971, I. Makarova (LE 260319 − anamorph); Chaplinskie hot springs, 64°25′ N, 172°30′ W, 1957, V. Gavrilyuk & P. Gagarin (LE 232934); Inchoun, 66°15′ N, 170°20′ W, 1975, I. Makarova (LE 232874).—Mongolia: Bayan-Khongor Region: N of Khukh-Nur Lake, 47°31′ N, 98°33′ E, 3000 m, 1972, L. Biazrov 5804 (LE 260474). Dzabkhan Region: Tarbagatai Range, Solongotuin-Daba Pass, 48°15′ N, 98°54′ E, 2650 m, 1973, L. Biazrov 2134 (LE 260494).
Sphaerellothecium thamnoliae Zhurb. sp. nov
MycoBank no.: MB 563055
Fungus lichenicola in thallis lichenum generis Thamnolia crescens. Similis speciei Stigmidium frigidum, sed differt hyphis semper copiosis, bene visibilibus, ascomatibus minoribus, (30–)50(–80) µm diam., sessilibus vel semi-immersis, dispersis, ascosporis interdum 2–3-septatis et halonatis.
Typus: USA, Alaska, Kobuk Valley Wilderness, junction of Kobuk River and Kavet Creek, 67°07′ N, 159°03′ W, 50 m elev., Dryas-lichen-moss vegetation among sparse Picea glauca, on thalli of Thamnolia vermicularis var. subuliformis, 13 August 2000, M. Zhurbenko 00212 (LE 260567—holotypus).
(Fig. 8)

Fig. 8. Sphaerellothecium thamnoliae. A, C & D, var. thamnoliae; A, infection habitus (holotype); C, asci in water (LE 260558); D, ascospores in I, note halo (holotype); B & E, var. taimyricum; B, infection habitus (LE 232894); E, ascospores in water, note halo (holotype). Scales: A & B = 500 µm; C–E = 10 µm.
Vegetative hyphae medium brown, with sculptured surface, composed of elongate cells 3·5–9·0 µm wide, septate, markedly constricted at the septa, branched (often more or less rectangularly) and anastomosed, BCr+ blue-green, I−, mostly superficial, abundant, forming a conspicuous dense reticulum.
Ascomata perithecioid, black, subglobose to somewhat conical above, (30–)50(–80) µm diam., with an ostiole of 5–15 µm diam., without appendices or projections, sessile to semi-immersed, dispersed. Exciple in surface view brown throughout, sculptured, similar to textura angularis, composed of a few layers of angular, pseudoparenchymatous cells (3–)5–8(–12) µm across, BCr+ blue-green, K−. Hymenial gel I−, K/I−. Hamathecium not observed. Asci elliptic to pyriform, occasionally with a short foot, endoascus thickened above, distinct ocular chamber not observed, size significantly differing in the two varieties described below, 8-spored, BCr−, I−, K/I− (except plasma reacting BCr+ blue, I+, and K/I+ yellow to orange). Ascospores colourless and smooth-walled or rarely (when over-mature ?) pale to medium brown and with a rough wall, obskittle-shaped to narrowly obovate, usually with a wider and sometimes shorter upper cell, occasionally located upside down in the ascus, size significantly differing in the two varieties described below, almost always 1-septate, but non-septate hyaline and 2–3-septate brown spores were also observed, mostly constricted at the septa, sometimes with halo 0·5–2·0 µm thick, usually with a few big guttules in each cell, irregularly arranged in 2–3 rows in the ascus, wall and septum BCr−, plasma BCr+ blue.
Conidiomata pycnidial. Conidia hyaline, bacilliform, 2·0–4·5 × 0·5 µm.
Notes. The material examined (42 specimens) exhibits significant variability in the development of vegetative hyphae, size of the ascomata, degree of their immersion in the host thalli, and size of asci and ascospores. The species is typified on the specimen representing the group with abundantly developed superficial hyphae, comparatively small, mostly sessile ascomata, and small ascospores. It can be opposed to the group distinguished by the larger asci and ascospores. However, there is no sharp delimitation between the two groups (Table 2), which are therefore recognized here as varieties.
Table 2 Distinguishing characteristics of Sphaerellothecium and Stigmidium taxa growing on Thamnolia (based on the author's own data).

In spite of the absence of visible interascal filaments, the species is disposed within Sphaerellothecium Zopf vs. Stigmidium Trevis. due to its brown, thick-walled, sculptured, comparatively thick and short-celled vegetative hyphae with swollen individual cells, forming a conspicuous superficial reticulum, and non-pseudotetrablastic ascospores (generic delimitation according to: Triebel Reference Triebel1989; Roux & Triebel Reference Roux and Triebel1994; Calatayud & Triebel Reference Calatayud and Triebel1999, Reference Calatayud and Etayo2001). Up to now, no Sphaerellothecium species have been reported from Thamnolia. Together with Sphaerellothecium araneosum (Arnold) Zopf, S. icmadophilae (R. Sant.) Zhurb. and S. stereocaulorum Zhurb. & Triebel, the new species belongs to the group with 1(–3)-septate, hyaline ascospores, occasionally becoming brown with age (Santesson Reference Santesson1984; Roux & Triebel Reference Roux and Triebel1994; Zhurbenko & Triebel Reference Zhurbenko and Triebel2008). Sphaerellothecium araneosum differs from both varieties of S. thamnoliae in its sometimes distinct interascal and ostiolar filaments and different sizes of asci (34–45 × 14–16 µm) and ascospores [(12·5–) 13·5–17·0 (–22·0) × (3·5–) 5–7 (–7·5) µm], S. icmadophilae has non-halonate ascospores of intermediate size (14–19 × 4·5–5·5 µm), and S. stereocaulorum has slightly smaller ascomata (20–50 µm diam.), smaller asci [(23–)25–34(–38) × (10–)11–16(–19) µm], non-halonate, smaller ascospores [(9–)10–13(–16) × 3·0–4·5(–6·0) µm] and grows in the epinecral layer of the host thallus. Characters distinguishing Sphaerellothecium thamnoliae from Stigmidium frigidum are summarized in Table 2.
Distribution and host. Circumpolarly known in the Holarctic from polar deserts, arctic and alpine tundras. Mainly growing on bases of Thamnolia vermicularis thalli; occasionally throughout whole thallus, particularly when they are prostrate. Also found on galls induced in the host by Thamnogalla crombiei. Evidently pathogenic as infected host parts are bleached.
Sphaerellothecium thamnoliae var. thamnoliae Zhurb
(Fig. 8A, C & D)
Notes. The type variety of the species is mainly characterized by comparatively small asci, measuring (26–)31–41(–55)× (10–)14–20(–21) µm (n = 129) and ascospores, measuring (8·5–)11·0–13·5(–16·0)× (3·5–)4·5–5·5(–6·5) µm, l/b = (1·8–)2·3–2·9(–3·9) (n = 502; including free overmature spores).
Specimens of Sphaerellothecium thamnoliae var. thamnoliae examined [all in arctic or alpine tundras or northern boreal forest on Thamnolia vermicularis var. subuliformis (16), var. vermicularis (5)]. USA: Alaska: Great Kobuk Sand Dunes, 67°02–06′ N, 158°50′–159°01′ W, 50 m, 2000, M. Zhurbenko (LE 260518, LE 260318, LE 260338, LE 260387).—Canada: Canadian Arctic Archipelago: Prince Patrick Island, Mould Bay, 76°14′ N, 119°18′ W, 2004, D. Walker (LE 260388).—Greenland: Mellem Land: Skovfjorden, Qinngua River mouth, 61°15′ N, 45°30′ W, 150 m, 2005, M. Zhurbenko (LE 260368).—Svalbard: Dickson Land: W coast of Billefjorden, 78°37′ N, 16°20′ E, 200 m, 2003, M. Zhurbenko (LE 260577).—Norway: Troms County: Skibotndalen Valley, 69°15′ N, 20°23′ E, 950 m, 2003, M. Zhurbenko (LE 260478).—Russia: Kola Peninsula: Barents Sea coast, Voron'ya River mouth, 69°09′ N, 35°50′ E, 20 m, 1997, M. Zhurbenko (LE 260398). Northern Ural: headwaters of Pechora River, Yanypupuner Range, 62°05′ N, 59°06′ E, 800 m, 1997, M. Zhurbenko (LE 260538b). Taimyr Peninsula: Byrranga Mts., Levinson-Lessinga Lake, 74°30–32′ N, 98°30–33′ E, 120–300 m, 1994, M. Zhurbenko (LE 207477a); ibid., 1995, M. Zhurbenko (LE 260428); Enisei Gulf, Sibiryakova Island, 72°55′ N, 79°00′ E, 10 m, 1989, V. Kuvaev (LE 260558). Yakutiya: Laptev Sea coast, Tiksi, 71°37′ N, 128°54′ E, 70 m, 1998, M. Zhurbenko (LE 232964, LE 260344b); Lena River delta, Stolb Island, 72°24′ N, 126°40′ E, 50 m,1998, M. Zhurbenko (LE 260378). Wrangel Island: Klark River, 71°05′ N, 178°16′ E, 110 m, 1998, S. Kholod (LE 260508); Neizvestnaya River, 71°14′ N, 179°23′ W, 130 m, 1987, S. Kholod (LE 260548). Chukotka: Baranikha, 68°30′ N, 168°16′ E, 1971, I. Makarova (232944b, LE 260599); Yanrakynnot, 64°53′ N, 172°30′ E, 1976, A. Sytin (LE 260408).
Additional specimens examined of Sphaerellothecium thamnoliae var. thamnoliae, formerly published as Stigmidium frigidum (Zhurbenko & Santesson Reference Zhurbenko and Santesson1996; Zhurbenko & Pospelova Reference Zhurbenko and Pospelova2001; Zhurbenko Reference Zhurbenko2008): LE 210405, LE 207538, LE 207540, LE 207541, LE 207543, LE 207544.
Sphaerellothecium thamnoliae var. taimyricum Zhurb. var. nov
MycoBank no.: MB 563057
Differt ab varietate Sphaerellothecium thamnoliae var. thamnoliae ascis et ascosporis majoribus.
Typus: Russia, Taimyr Peninsula, Byrranga Mts., N of Levinson-Lessinga Lake, 74°34′ N, 98°47′ E, 250 m elev., arctic tundra, on thalli of Thamnolia vermicularis var. subuliformis, 20 August 1995, M. Zhurbenko 95586 (LE 260438—holotypus).
(Fig. 8B & E)
Etymology. The specific epithet refers to Taimyr Peninsula in Siberia, where the holotype was found.
Notes. This variety is mainly distinguished from the type variety by its larger asci, measuring 47–64(–85) × (14–)17–27(–33) µm (n = 15), and ascospores, measuring (12·5–)14·0–19·5(–24·0) × (4·5–)5–8(–9·0) µm, l/b = (1·7–)2·2–3·0(–3·8) (n = 159). Furthermore, the variety taimyricum seems to differ from the variety thamnolia in having a more immersed mycelium, mostly semi-immersed and slightly larger ascomata, and a better developed halo around the ascospores. It is so far also known only from the Arctic.
Specimens of Sphaerellothecium thamnoliae var. taimyricum examined (all in polar desert or arctic tundra on Thamnolia vermicularis var. subuliformis). Russia: Severnaya Zemlya: Bolshevik Island, Akhmatova Bay, 79°01′ N, 102°43′ E, 60 m, 1996, M. Zhurbenko (LE 232894); Oktyabr'skoi Revolutsii Island, Cape Nekrasova, 80°02′ N, 99°19′ E, 2007, M. Gavrilo (LE 260418). Taimyr Peninsula: Byrranga Mts., Levinson-Lessinga Lake, 74°24–31′ N, 98°36–46′ E, 150–300 m, 1995, M. Zhurbenko (LE 260328, LE 260568); Uboinaya River mouth, 73°39′ N, 82°22′ E, 20 m, 1990, M. Zhurbenko (LE 260503). Novosibirskie Islands: Zhokhova Island, 76°08′ N, 152°45′ E, 50 m, 1989, M. Samarskii (LE 232974). Wrangel Island: Neozhidannaya River, 71°01′ N, 179°09′ E, 100 m, 1992, S. Kholod (LE 260488). Chukotka: Iskaten' Pass near km 32 Egvekinot−Iul'tin Highway, 66°35′ N, 179°10′ W, 1971, I. Makarova (LE 260578).
Additional specimens examined of Sphaerellothecium thamnoliae var. taimyricum, formerly published as Stigmidium frigidum (Zhurbenko & Santesson Reference Zhurbenko and Santesson1996; Karatygin et al. Reference Karatygin, Nezdoiminogo, Novozhilov and Zhurbenko1999): LE 207531a, LE 207534, LE 207535, LE 207536, LE 207542, LE 207545, LE 207546.
Stigmidium frigidum (Th. Fr. ex Sacc.) Alstrup & D. Hawksw
Meddel. Grønl., Biosc. 31: 67 (1990); type: Canada, British Columbia, Rocky Mountains, Hector, c. 51°25′ N, 116°21′ W (I. Brodo, pers. comm.), on Thamnolia vermicularis var. subuliformis, 27 July 1885, J. Macoun (E—neotype, photographs examined).

Fig. 9. Stigmidium frigidum. A, infection habitus (LE 260444); B, ascomata (LE 232994); C, asci with spores in water, note dark over-mature ascospores (LE 260354). Scales: A = 1 mm; B = 200 µm; C = 10 µm.
(Fig. 9A & B)
Vegetative hyphae pale to medium brown, composed of elongate cells 2·5–7·0 µm wide, septate, constricted at the septa, scarcely branched, BCr+ blue-green, I−, immersed to occasionally superficial, rather sparse, usually inconspicuous.
Ascomata perithecioid, black, subglobose, (30–)50–80(–120) µm diam., sometimes with an indistinct papilla, with an ostiole of 10–20 µm diam., initially completely immersed (sometimes in a few layers), then mostly protruding in the ostiolar area or occasionally up to half erumpent, arising singly or sometimes adjacent or a few confluent, aggregated in groups of up to 500. Exciple in surface view brown, smooth, similar to textura angularis, composed of a few layers of thick-walled, angular, pseudoparenchymatous cells (3–)5–8(–12) µm across, BCr+ blue-green, K+ olive. Hymenial gel I−, K/I−. Hamathecium not observed. Asci elliptic to pyriform, endoascus thickened above, sometimes with a small ocular chamber, foot not observed, (33–)40–52(–60) × (13–)15–21(–33) µm (n = 62), 8-spored, BCr−, I−, K/I− (except plasma reacting BCr+ blue, I+ and K/I+ yellow to orange). Ascospores colourless or occasionally pale to medium brown (when over-mature ?) (Fig. 9C), obskittle-shaped to narrowly obovate, usually with a wider and shorter upper cell, occasionally located upside down in the ascus, (11–)13·5–16·5(–20) × (4·0–)5·0–6·5(–7·5) µm, l/b = (1·7–)2·3–2·9(–4·1) (n = 214), 1-septate, usually constricted at the septum, smooth-walled, halo not observed, usually with a few big guttules in each cell, irregularly diagonally arranged in 2–3 rows in the ascus, wall and septum BCr−, plasma BCr+ blue.
Anamorph not observed.
Notes. The species was originally described from Greenland as “Sphaeria n. 10” by Fries (Reference Fries1879: 370) and later formally introduced as “Epicymatia frigida Sacc. ex Fr. Hedw. 1881, p. 60 (Sphaeria n. 10)” (Saccardo Reference Saccardo1882: 572). According to this description, the type specimen had dark torulose superficial hyphae and ascospores of 15–18 × 5–7 µm, and thus may well represent Sphaerellothecium thamnoliae var. taimyricum. According to Alstrup & Hawksworth (Reference Alstrup and Hawksworth1990: 67), this original material was lost and the authors neotypified the species on John Macoun's “Canadian Lichens” no. 180 (E—neotype) and combined it into Stigmidium. The locality on the neotype label (“Hector, B.C.”) is indistinctly handwritten, which was evidently the reason for erroneously referring it to Alaska (Hawksworth Reference Hawksworth1980: 180). The examination of the neotype by R. Yahr (pers. comm.), together with the notes of Hawksworth (op. cit.), leave little doubt that it fits the species concept presented here (see below). This concept also corresponds to the description of Stigmidium frigidum in Ihlen (Reference Ihlen1995). The only deviating character of the species reported by the latter author is the 4(–6)-spored asci, not typical for Stigmidium in general and not observed in the examined material.
Usually growing on the upper parts or even tips of Thamnolia vermicularis thalli, but occasionally observed on their middle or basal parts. Evidently pathogenic, as causing bleaching or even destruction of the host tissues; heavy infections also induce various malformations of the host thalli which, for example, become bent, twisted and/or somewhat swollen (Fig. 9A). Superficially the infections are similar to those of Polycoccum vermicularium.
Typical Stigmidium frigidum can be readily distinguished from Sphaerellothecium thamnoliae, even with a dissecting microscope, by its numerous, more or less immersed ascomata, crowded at the tips of the host thalli, and the absence of a visible mycelium; malformations of the host thalli are also diagnostic (Table 2). Confusion can be caused by the occasional development of superficial vegetative hyphae. However, in well-developed specimens it is clearly seen that such hyphae are associated only with some groups of ascomata and not forming a reticulum.
New to Mongolia; a first verified report from the USA.
Specimens examined [all in polar deserts, arctic or alpine tundras on Thamnolia vermicularis var. subuliformis (13), var. vermicularis (8)]. USA: Alaska: Brooks Range, headwaters of Atigun River near mile 248 Dalton Highway, 68°07′ N, 149°28′ W, 1400 m, 2001, M. Zhurbenko (LE 260356); Kotzebue, 66°53′ N, 162°31′ W, 30 m, 2000, M. Zhurbenko (LE 260406).—Russia: Severnaya Zemlya: Bolshevik Island, Mikoyana Bay, 79°18′ N, 101°55′ E, 10 m, 1996, M. Zhurbenko (LE 232943). Taimyr Peninsula: Byrranga Mts., Bolshaya Bootankaga River, 74°30′ N, 97°40′ E, 350 m, 1995, M. Zhurbenko (LE 232994a); ibid., Levinson-Lessinga Lake, 74°24–34′ N, 98°26–47′ E, 60–300 m, 1994, M. Zhurbenko (LE 207612); ibid., 1995, M. Zhurbenko (LE 232984, LE 232923, LE 232823, LE 232863, LE 260523, LE 260334, LE 260564, LE 260354); Dikson Island, 73°30′ N, 80°20′ E, 30 m, 1990, M. Zhurbenko (LE 260353); Ragozinka River mouth, 72°48′ N, 80°53′ E, 30 m, 1990, M. Zhurbenko (LE 260413). Yakutiya: 60 km E of Cherskii, Belaya Strelka Mts., 68°40′ N, 162°40′ E, 400 m, 1975, A. Egorova (LE 260454); Laptev Sea coast, Tiksi, 71°39–40′ N, 128°40–45′ E, 50–70 m, 1998, M. Zhurbenko (LE 260484, LE 260444a). Chukotka: Baranikha, 68°30′ N, 168°16′ E, 1971, I. Makarova (LE 233084); Gil'mymlineiveem River, 65°48′ N, 173°15′ W, 1977, I. Makarova (LE 232953).—Mongolia: Dzabkhan Region: Mt. Otgon-Tenger, 47°35′ N, 97°32′ E, 3250 m, 1976, L. Biazrov 7513 (LE 260384).
Additional examined specimens of Stigmidium frigidum published in Zhurbenko & Santesson (Reference Zhurbenko and Santesson1996): LE 207533, LE 207537, LE 207539.
Thamnogalla crombiei (Mudd) D. Hawksw
Notes Roy. Bot. Gard. Edinburgh 38(1): 178 (1980); type: Scotland, Ben Lawers, on Thamnolia vermicularis, 1864, Crombie (BM—lectotype, n. v.).
Notes. Ascomata perithecioid, 75–125 µm diam., immersed or rarely erumpent. Exciple in cross-section orange-brown throughout, K+ becoming more intensively red-orange. Hamathecium of filiform, non-capitate, c. 1 µm wide, septate, scarcely branched interascal filaments. Asci cylindrical, with a long foot, (50–)70–90 × 5–6 µm (n = 17), 8-spored. Ascospores hyaline, mostly narrowly elliptic to narrowly oblong, with rounded ends, (7–)8–11·5(–15) × (2–)2·5–3·0(–4) µm, l/b = (2·1–)2·8–4·0(–5·2) (n = 113) [(7–)9·6(–12) ×(2·5–)3·7(–5·0) µm, l/b = 2·6 according to Hoffmann & Hafellner (Reference Hoffmann and Hafellner2000)], non-septate, smooth-walled, non-halonate, often with a few guttules, arranged uniseriate and overlapping to biseriate in the ascus. Inducing concolorous or pale coloured (cinnamon, pinkish buff, peach, flesh pink, or grey), finally bullate and basally constricted galls throughout the host thalli. Otherwise pathogenicity not observed.
This is a frequent and cosmopolitan thamnoliicolous fungus yet is reported here for the first time from Greenland and Svalbard.
Specimens examined [all in polar deserts, arctic or alpine tundras, or northern boreal forest on Thamnolia vermicularis var. subuliformis (26), var. vermicularis (14)]. USA: Alaska: Kotzebue, 66°53′ N, 162°31′ W, 30 m, 2000, M. Zhurbenko (LE 260386, LE 260493); ibid., 1961, B. Neiland (LE 260466); Great Kobuk Sand Dunes, 67°06′ N, 159°01′ W, 50 m, 2000, M. Zhurbenko (LE 260336); Seward Peninsula, Bering Land Bridge National Preserve, 65°50′ N, 164°32′ W, 612 m, 2002, J. Roth (LE 260423); ibid., 66°17′ N, 165°19′ W, 22 m, 2002, J. Roth (LE 260383, LE 260433).—Canada: British Columbia: Wells Gray Provincial Park, Mt. Raft, 51°44′ N, 119°50′ W, 2100 m, 2002, M. Zhurbenko (LE 260456).—Greenland: Mellem Land, Skovfjorden, Qinngua River mouth, 61°15′ N, 45°30′ W, 150 m, 2005, M. Zhurbenko (LE 260370); Tugtugtooq Island SW of Narssaq, 2 km SW of Blue Moon Lake, 60°51′ N, 46°25′ W, 15 m, 2005, M. Zhurbenko (LE 260340, LE 260330).—Svalbard: Bünsow Land: Norddammen Lake, 78°38′ N, 16°44′ E, 10 m, 2003, M. Zhurbenko (LE 260350).—Norway: Troms County: Skibotndalen Valley, 69°13–19′ N, 20°21–29′ E, 70–650 m, 2003, M. Zhurbenko (LE 260416, LE 260366).—Russia: Kola Peninsula: Barents Sea coast, Voron'ya River mouth, 69°09′ N, 35°50′ E, 20 m, 1997, M. Zhurbenko (LE 260534); ibid., 4 km SSE of Dal'nie Zelentsy, 69°05′ N, 36°07′ E, 100 m, 1997, M. Zhurbenko (LE 260594); ibid., 17 km N of Tumannyi, 69°01′ N, 35°48′ E, 100 m, 1997, M. Zhurbenko (LE 260584); Khibiny Mts., headwaters of Kaskasnyunjok Creek, 67°46′ N, 33°49′ E, 2007, M. Zhurbenko (LE 233042). Nenets Region: Dolgii Island, 69°18′ N, 58°52′ E, 1998, V. Shevchenko (LE 232824). Northern Ural: headwaters of Pechora River, Yanypupuner Range, Mt. “981”, 62°05′ N, 59°06′ E, 800 m, 1997, M. Zhurbenko (LE 260404, LE 260504). Western Sayan Mts.: Kulumys Range, 52°51′ N, 93°17′ E, 1700 m, 2010, M. Zhurbenko (LE 260310); Oiskii Range, headwaters of Olen'ya River, 52°48′ N, 93°15′ E, 1650 m, 2010, M. Zhurbenko (LE 260380b). Eastern Sayan Mts.: Kryzhina Range, headwaters of Belyi Kitat River, 54°01′ N, 95°27′ E, 1800 m, 2009, M. Zhurbenko (LE 260403a). Severnaya Zemlya: Bolshevik Island, Akhmatova Bay, 79°04′ N, 102°45′ E, 10–20 m, 1996, M. Zhurbenko (LE 233064, LE 260314, LE 260514); ibid., Mikoyana Bay, 79°18′ N, 101°55′ E, 10 m, 1996, M. Zhurbenko (LE 232904); ibid., Shokalskogo Strait, 79°18′ N, 101°40′ E, 20 m, 1996, M. Zhurbenko (LE 232834). Taimyr Peninsula: Byrranga Mts., Levinson-Lessinga Lake, 74°33′ N, 98°43′ E, 100 m, 1995, M. Zhurbenko (LE 260434); Ragozinka River mouth, 72°48′ N, 80°53′ E, 25 m, 1990, M. Zhurbenko (LE 260513); Uboinaya River mouth, 73°36′ N, 82°22′ E, 10 m, 1990, M. Zhurbenko (LE 260463). Yakutiya: Lena River delta, 3 km E of Cape Krest-Tumsa, 72°22′ N, 126°42′ E, 50 m, 1998, M. Zhurbenko (LE 233014). Magadan Region: Magadan, Mt. Nagaevskaya, 59°34′ N, 150°45′ E, 400 m, 2003, N. Sazanova (LE 260443). Wrangel Island: Gusinaya River, 71°08′ N, 179°10′ E, 1991, V. Shtrik (LE 232982); Naskhok River, 71°24′ N, 178°08′ W, 5 m, 1998, S. Kholod (LE 232932); Neozhidannaya River, 71°02′ N, 179°10′ E, 110 m, 1992, S. Kholod (LE 260374b); Tundrovaya River, 71°30′ N, 179°45′ W, 1996, S. Kholod (LE 233004). Chukotka: Baranikha, 68°30′ N, 168°16′ E, 1971, I. Makarova (LE 232944a); Chaplinskie hot springs, 64°25′ N, 172°30′ W, 1957, V. Gavrilyuk & P. Gagarin (LE 232843).
Discussion
To date, 23 species representing 18 genera of lichenicolous fungi are known on Thamnolia worldwide, of which 18 species (see key below; c. 80%) and two genera (Epithamnolia and Thamnogalla D. Hawksw.; c. 10%) have been reported only from this host genus. All these species inhabit Thamnolia vermicularis, six of them (Endococcus thamnoliae, Lichenopeltella thamnoliae, Odontotrema santessonii, Polycoccum vermicularium, Stigmidium frigidum, and Thamnogalla crombiei) are also known on T. papelillo (Etayo Reference Etayo2010), and none has yet been found on T. juncea. Of the 190 newly studied fungal specimens growing on Thamnolia vermicularis, 144 (76%) were found on its var. subuliformis and 46 (24%) on var. vermicularis. This disproportion might merely reflect the fact that the former variety is more abundant in the Northern Hemisphere than the latter (Thomson Reference Thomson1984).
The genus Thamnolia has no species of lichenicolous fungi in common with the other genera of the lichen family Icmadophilaceae and is much more ‘hospitable’ compared to other genera in the family: Dibaeis (hosts 5 species of lichenicolous fungi), Icmadophila (5), Pseudobaeomyces (0), Siphula (1 ?), Siphulella (0) (author's database).
In the richness of lichenicolous mycobiota which it supports, Thamnolia vermicularis ranks 15th worldwide after Peltigera rufescens (hosts 54 species of lichenicolous fungi), Cladonia pyxidata (L.) Hoffm. (35), Xanthoria parietina (L.) Th. Fr. (34), Peltigera didactyla (With.) J.R. Laundon (32), P. canina (L.) Willd. (30), P. praetextata (Flörke ex Sommerf.) Zopf (29), Lobaria pulmonaria (L.) Hoffm. (28), Parmelia saxatilis (L.) Ach. (28), P. sulcata Taylor (27), Cladonia pocillum (Ach.) Grognot (26), Peltigera polydactylon (Necker) Hoffm. (26), P. aphthosa (L.) Willd. (25), Physcia stellaris (L.) Nyl. (25), and Circinaria calcarea (L.) A. Nordin, S. Savić & Tibell (24) (author's database). It is noteworthy that 14 of these 15 most hospitable lichen species of the world occur in the Arctic, and at least 11 of them thrive there (Kristinsson et al. Reference Kristinsson, Zhurbenko and Hansen2010).
The following taxa of Thamnolia-inhabiting fungi were the most common in the Holarctic: Thamnogalla crombiei (21% of 190 specimens examined), Polycoccum vermicularium (15%), Sphaerellothecium thamnoliae var. thamnoliae (12%), Stigmidium frigidum (11%), Cercidospora thamnoliae (9%), and Odontotrema santessonii (5%).
The study ends in formally describing six, and introducing another two, new species of thamnoliicolous fungi, which is about 35% of their known total number. However, some of their specimens still remain unnamed. Among these are a Lasiosphaeriopsis-like pyrenomycete with brown, 4 − 6-septate ascospores, c. 33–38 × 10–13 µm (1996, M. Zhurbenko 96910); a fungus with a closed fruit body containing filiform, hyaline, 4–5-septate diaspores, c. 58–69 × 1·5–2 µm (1998, S. Kholod); a gall-inducing hyphomycete (1989, A. Katenin); and a rather common fungus with thick dark superficial hyphal strands and conspicuous black discoid sterile fruit-like structures, resembling species of Lichenostigma Hafellner subgenus Lichenogramma Nav.-Ros. & Hafellner. One more unidentified coelomycete on Thamnolia with pleurogenous conidiogenous cells was mentioned by Hawksworth (Reference Hawksworth1980: 180). Four of 21 named Thamnolia-dwelling fungal species found in the Holarctic were represented by single finds. According to a Turing estimator, this suggests that about 80% of these fungi were detected during the survey, or that their real diversity in the Holarctic is no less than 26 species.
Three of the 23 fungal species that have been reported from Thamnolia were not found in the Holarctic lichen collections examined: Cornutispora intermedia Punith. & D. Hawksw., Endococcus thamnoliae Etayo & R. Sant., and Odontotrema intermedium Diederich, Zhurb. & Etayo (Diederich et al. Reference Diederich, Zhurbenko and Etayo2002; Etayo Reference Etayo2010); all are known only from a single or a few finds, which makes it futile to speculate about their geography. On the other hand a fourth, Lichenopeltella thamnoliae, seems to be rather common in South America (Etayo Reference Etayo2010) and thus its absence in the Holarctic might not be accidental. More observations from the extra-holarctic regions are needed to answer questions about the distribution patterns of thamnoliicolous fungi and their infrageneric preferences within Thamnolia.
Key to the lichenicolous fungi growing on Thamnolia
Based on data in Hawksworth (Reference Hawksworth1977), Santesson (Reference Santesson1998), Diederich et al. (Reference Diederich, Zhurbenko and Etayo2002, Reference Diederich, Ertz and Etayo2010), Punithalingam (Reference Punithalingam2003), Heuchert & Braun (Reference Heuchert and Braun2006), Etayo (Reference Etayo2010), and the present publication. Species of lichenicolous fungi not specific to Thamnolia are given in parentheses.
1 Anamorphic fungus ... 2
Teleomorphic fungus ... 8
2(1) Hyphomycete, conidiophores usually with divergent terminal branches, conidiogenous cells integrated, mostly terminal, with a single or few distant enteroblastic-percurrent proliferations, conidia pale brown or yellowish brown, usually in branched acropetal chains, subglobose or limoniform to ellipsoid-subcylindrical, aseptate (3·5–8·0 × 3–5 µm) or 1-septate (7–13 × 5–7 µm) ... ... (Cladosporium licheniphilum)
Coelomycete ... 3
3(2) Conidia dark brown, subglobose, aseptate, (2·5–)3·0–4·0(–5·0) µm diam., ornamented (× 1000), pycnidia (40–)50–80(–110) µm diam. ... ... (Lichenoconium usneae)
Conidia hyaline ... 4
4(3) Conidia with (1–)3(–4) divergent arms ... (Cornutispora intermedia)
Conidia without arms ... 5
5(4) Conidia (0–)1(–2?)-septate, cylindrical, (14–)18·5–27·0(–32) × (1·0–)1·5–2·0(–2·5) µm ... Epithamnolia karatyginii
Conidia aseptate ... 6
6(5) Conidia 2·0–4·5 × 0·5 µm, bacilliform ... ... anamorph of Sphaerellothecium thamnoliae
Conidia over 0·5 µm wide ... 7
7(6) Conidia (3·0–)4–6(–7·5) × (1–)1·5 µm, oblong ... ... anamorph of Polycoccum vermicularium
Conidia (3·5–)4–5(–5·5) × (1·5–)2·0(–2·5) µm, oblong to occasionally elliptic ... ... Phoma thamnoliae ad int.
8(1) Hymenium at least partly exposed at maturity, ascospores hyaline ... 9
Hymenium not exposed at maturity ... 12
9(8) Ascospores aseptate, subglobose to shortly ellipsoid, 6–10 × 5·0–7·5µm ... ... (Geltingia associata)
Ascospores septate, ascomata urceolate ... 10
10(9) Ascospores with (3–)6–8(–9) transverse septa and often a few longitudinal or oblique septa in central cells, (12·5–)18–23(–25·0) × (3–)3·5–4·5(–5) µm ... ... Odontotrema santessonii
Ascospores with less septa, never submuriform ... 11
11(10) Ascospores 1(–2)-septate, (9–)11–15(–18) × 2·5–3·0(–3·5)µm ... ... Odontotrema thamnoliae
Ascospores 3-septate, (10·0–)10·5–12·5(–13·5) × (3–)3·5–4·0(–4·5)µm ... ... Odontotrema intermedium
12(8) Ascomata catathecia, non-setose, asci 4-spored, ascospores hyaline, 1-septate, 11·5– 16 × 2·5–5 µm, without setulae ... Lichenopeltella thamnoliae
Ascomata perithecioid, ascospores hyaline or pigmented ... 13
13(12) Ascomata setose, ascospores initially hyaline, then pale to medium grey, with (0–)3– 5(–7) transverse septa and usually longitudinal or oblique septa in central cells, (11 –)13·5–18·0(–29) × (5–)6–8(–12) µm ... Capronia thamnoliae
Ascomata without setae ... 14
14(13) Ascospores pigmented, at least when mature ... 15
Ascospores not or only occasionally pigmented ... 19
15(14) Ascospores muriform ... 16
Ascospores only transversely septate ... 17
16(15) Interascal filaments present, ascospores ornamented (×1000), (23–)25–29(–31·5) × (10·5–)11–13(–14) µm ... Dacampia thamnoliicola ad int.
Interascal filaments absent, ascospores smooth-walled, (14–)16·5–24·0(–34) × (6–) 6·5–8·5(–9) µm ... Merismatium thamnoliicola
17(15) Ascospores (1–)3-septate, hyaline to pale cinnamon-brown, (10–)15·5–20·5(–22)× (4·5–)5–6(–8·0) µm ... (Phaeospora arctica)
Ascospores (0–)1-septate ... 18
18(17) Interascal filaments absent, ascospores 9·0–12·5 × 3·5–5·0 µm ... ... Endococcus thamnoliae
Interascal filaments well developed, ascospores (13–)15·5–20·5(–28) × (6–)7–9(–12) µm ... Polycoccum vermicularium
19(14) Ascomata immersed in distinct bullate galls ... 20
Ascomata not associated with bullate galls ... 21
20(19) Exciple orange-brown throughout, interascal filaments well developed, ascospores aseptate, (7–)8·0–11·5(–15) × (2–)2·5–3·0(–4) µm, without halo ... ... Thamnogalla crombiei
Exciple bluish grey, interascal filaments sparse to indistinct, ascospores 1-septate, (12–)14–17(–19) × (3·5–)4–5(–5·5) µm, halonate ... ... Cercidospora thamnogalloides
21(19) Exciple bluish green or olive-brown above, hyaline below, vegetative hyphae inconspicuous, ascomata subimmersed, interascal filaments indistinct or abundant ... 22
Exciple brown throughout, vegetative hyphae conspicuous or not, ascomata subimmersed to sessile, interascal filaments absent ... 24
22(21) Exciple olive-brown above, asci mainly 4-spored, interascal filaments sparse to indistinct, ascospores (11–)14·0–17·5(–21) × (4·0–)4·5–5·5(–6·5) µm, (1–)2–3- septate, without halo ... Cercidospora thamnoliicola
Exciple bluish green above, asci only/mainly 8-spored ... 23
23(22) Interascal filaments abundant, ascospores (1–)3(–4)-septate, (12·5–)16·5–21·0(–27·5) × (4–)4·5–5·5(–8) µm, occasionally halonate ... ... Cercidospora thamnoliae
Interascal filaments sparse to indistinct, ascospores (0–)1-septate, (12–)13–16(–19) × (3–)3·5–4·5(–5) µm, without halo ... Cercidospora epithamnolia
24(21) Ascospores (1–)3-septate, hyaline to pale cinnamon-brown, (10–)15·5–20·5(–22) × (4·5–)5·0–6·0(–8·0) µm ... (Phaeospora arctica)
Ascospores only or mainly 1-septate ... 25
25(24) Vegetative hyphae usually inconspicuous, ascomata (30–)50–80(–120) µm diam., mostly subimmersed and aggregated on tips of host thalli, ascospores 1-septate, (11–)13·5–16·5(–20) × (4·0–)5·0–6·5(–7·5) µm, without halo ... ... Stigmidium frigidum
Vegetative hyphae forming a conspicuous dark reticulum, ascomata (30–)50(–80) µm diam., semi-immersed to sessile, dispersed, usually on bases of host thalli, ascospores (0–)1(–3)-septate, sometimes halonate ... 26
26(25) Asci (26–)31–41(–55) × (10–)14–20(–21) µm, ascospores (8·5–)11·0–13·5(–16·0)× (3·5–)4·5–5·5(–6·5) µm ... ... Sphaerellothecium thamnoliae var. thamnoliae
Asci 47–64(–85) × (14–)17–27(–33) µm, ascospores (12·5–)14·0–19·5(–24·0)× (4·5–)5– 8(–9) µm ... Sphaerellothecium thamnoliae var. taimyricum
I am indebted to Irina Makarova, Sergei Kholod, Nadya Matveeva, Adrian Katenin, J. Roth, Mikhail Samarskii, Vladimir Shevchenko, Olga Lavrinenko, Nina Sazanova, Thomas Lunke, Arve Elvebakk, Donald Walker and Lev Biazrov for putting their undetermined lichen collections at my disposal. Wolfgang von Brackel, Paul Diederich, Dagmar Triebel, Karen Dillman, Vadim Mel'nik, Ernie Brodo, and Brian Coppins are thanked for their various help, as well as Uwe Braun for the revision of Cladosporium licheniphilum and Rebecca Yahr and Louise Olley for the examination of the Stigmidium frigidum neotype.