Introduction
Ecosystem engineers are organisms capable of modifying an environment through their physical presence (autogenic engineers) or biological activity (allogenic engineers), by modulating, directly or indirectly, the availability of resources for other species with which they have no direct trophic relationship (Jones et al., Reference Jones, Lawton and Shachak1994; Jones & Gutiérrez, Reference Jones, Gutiérrez, Cuddington, Byers, Wilson and Hastings2007). Ecosystem engineering, an ecological concept proposed during the 1990s (see Jones et al., Reference Jones, Lawton and Shachak1994), has been widely discussed (Wright & Jones, Reference Wright and Jones2006; Jones & Gutiérrez, Reference Jones, Gutiérrez, Cuddington, Byers, Wilson and Hastings2007; Jones et al., Reference Jones, Gutiérrez, Byers, Crooks, Lambrinos and Talley2010) and contested by a number of ecologists (Power, Reference Power1997; Wright & Jones, Reference Wright and Jones2006). Many studies have validated this concept, however, given its potential for the interpretation of the role of certain species in the organization of the community, and the provision of important insights for further synthesis, integration and generalization of the approach (Jones & Gutiérrez, Reference Jones, Gutiérrez, Cuddington, Byers, Wilson and Hastings2007; Jones et al., Reference Jones, Gutiérrez, Byers, Crooks, Lambrinos and Talley2010).
In the benthic domain, ecosystem engineers may have a range of different impacts, either stabilizing or destabilizing the substrate, altering its texture, or creating biostructures (Reise, Reference Reise2002; Bouma et al., Reference Bouma, Olenin, Reise and Ysebaert2009). Endobenthic species are able to alter the characteristics of the substrate through processes of bioturbation and bioirrigation, that is, by reworking the sediment and altering both its physical structure and chemical characteristics, increasing flow rates and altering water–sediment interfaces (Meysman et al., Reference Meysman, Middelburg and Heip2006; Bouma et al., Reference Bouma, Olenin, Reise and Ysebaert2009). Other benthic organisms are also known to have the ability to construct biogenic structures that influence the composition and organization of benthic communities, and thus facilitate the occurrence of organisms by offering new habitats, increasing protection against abiotic and predation pressures, or contributing to the availability of food (Bouma et al., Reference Bouma, Olenin, Reise and Ysebaert2009; Jones et al., Reference Jones, Gutiérrez, Byers, Crooks, Lambrinos and Talley2010).
Many species of the family Sabellariidae form large conglomerates of sandy tubes, referred to as ‘reefs’, in the mesolittoral and infralittoral zones of coastal areas. These reefs provide habitats for a wide range of organisms (Dubois et al., Reference Dubois, Retiere and Olivier2002, Reference Dubois, Commito, Olivier and Retière2006; Eeo et al., Reference Eeo, Chong and Sasekumar2017; Jones et al., Reference Jones, Dubois, Desroy and Fournier2018) and may interfere in the hydrodynamics and composition of the sediment in the areas they occupy (Gram, Reference Gram1968; Noernberg et al., Reference Noernberg, Fournier, Dubois and Populus2010; Desroy et al., Reference Desroy, Dubois, Fournier, Ricquiers, Le Mao, Guerin, Gerla, Rougerie and Legendre2011). Due to the significant changes in abiotic factors, and consequently in the biological community, provoked by reef-building sabellariids, these organisms are considered to be important ecosystem engineers (Dubois et al., Reference Dubois, Commito, Olivier and Retière2006; Ataide et al., Reference Ataide, Venekey, Rosa Filho and dos Santos2014; Jones et al., Reference Jones, Dubois, Desroy and Fournier2018).
In temperate regions, sabellariid reefs have been shown frequently to be environments with a high diversity of benthic species, generally richer and more productive than adjacent, unconsolidated substrates (Mettam, Reference Mettam1992; Gherardi & Cassidy, Reference Gherardi and Cassidy1994; Hiscock, Reference Hiscock2004; Jones et al., Reference Jones, Dubois, Desroy and Fournier2018) or hard, rocky bottoms without reefs (George & Warwick, Reference George and Warwick1985). The associated benthic communities have also been compared among patches of reef at different stages of the construction cycle, showing that the assemblages present at each stage have distinct characteristics (Porras et al., Reference Porras, Bataller, Murgui and Torregrosa1996; Dias & Paula, Reference Dias and Paula2001; Dubois et al., Reference Dubois, Retiere and Olivier2002; Gravina et al., Reference Gravina, Cardone, Bonifazi, Bertrandino, Chimienti, Longo, Marzano, Moretti, Lisco, Moretti, Corriero and Giangrande2018; Jones et al., Reference Jones, Dubois, Desroy and Fournier2018). There is also evidence that different areas of the same reef may be colonized by different assemblages, especially when comparing more protected areas with those more exposed to wave action (Gruet, Reference Gruet1971).
While studied widely in temperate European waters, sabellariid reefs in tropical and subtropical regions are poorly known (Fournier, Reference Fournier2010; Eeo et al., Reference Eeo, Chong and Sasekumar2017). Sabellaria wilsoni Lana & Gruet, Reference Lana and Gruet1989 is endemic to the Atlantic Ocean, occurring in the shallow waters of estuaries and the continental shelf (Lana & Gruet, Reference Lana and Gruet1989; Lana & Bremec, Reference Lana, Bremec, Dauvin, Laubier and Reish1994; Lomônaco et al., Reference Lomônaco, Santos and Martin2011). This species usually forms small aggregates on rocky substrates (Lana & Gruet, Reference Lana and Gruet1989), and has been reported to form reefs only when associated with other sabellariids (Lomônaco et al., Reference Lomônaco, Santos and Martin2011).
On Algodoal-Maiandeua Island, located on the Brazilian Amazon Coast, S. wilsoni builds extensive reefs on rocky outcrops in the shallow infralittoral and lower mesolittoral of sandy beaches. Ataide et al. (Reference Ataide, Venekey, Rosa Filho and dos Santos2014) highlighted the effects of these reefs on the benthic meiofauna, including an increase in the number of taxa and shifts in the composition of the community, depending on the morphology of the reef and its location on the island. The present study tested two main hypotheses: (i) the reefs sustain benthic assemblages that are structurally distinct from those inhabiting adjacent sandy sediments, and (ii) the fauna that occupies the more eroded portion of the reef is distinct from that of the central portion, which is more protected from direct wave action.
Materials and methods
Study area
Algodoal-Maiandeua Island is located on the Brazilian Amazon coast (00°36′ S 047°34′ W). The island is surrounded on three sides by rivers and estuarine channels, while its northern coast faces the Atlantic Ocean (Figure 1). The climate is humid tropical with a mean annual temperature of 27.7 ± 1.1°C (Martorano et al., Reference Martorano, Pereira, Cézar and Pereira1993) and annual rainfall (30-year series) ranging from 2200 to 2800 mm (Moraes et al., Reference Moraes, Costa, Costa and Costa2005). Rainfall rates vary considerably over the year, with a well-marked rainy season from January to July, with total precipitation of ~1657 mm, and a dry season from August to December, with total rainfall of just 490 mm (Moraes et al., Reference Moraes, Costa, Costa and Costa2005). The region is dominated by semidiurnal macrotides with amplitudes of 4–7 m (Silva et al., Reference Silva, Pereira, Gorayeb, Vila-Concejo, Sousa, Asp and da Costa2011a). The island's beaches are covered with fine sand and there is a wide mesolittoral zone of 200–400 m (Rosa Filho et al., Reference Rosa Filho, Gomes, Almeida and Silva2011) with some rocky outcrops (lateritized sandstone) which are often colonized by S. wilsoni.

Fig. 1. Map showing the location of Algodoal-Maiandeua Island in northern Brazil (A, B, C), the sampling layout (d1 = 10 m; d2 = 5 m) (D) and aspect of sampling zones in the Sabellaria wilsoni reef (E).
Sampling
Samples were collected in November 2010 (the dry season) from a continuous patch of reef (~800 m2) on Farol beach, a semi-exposed, low tide sandflat (Rosa Filho et al., Reference Rosa Filho, Gomes, Almeida and Silva2011). As the reef is established on a large rocky outcrop, it is essentially composed of a cohesive cluster of hummocks. Two sampling zones were defined on the reef (Figure 1D): (i) the exposed zone (within 5 m of the outer margin of the reef that faces the sea, and is visibly more eroded by wave action – Figure 1E), and (ii) the protected zone (central portion of the reef – Figure 1E). Samples were also collected in the ‘lower zone’ (same level of exposed reef zone) and ‘upper zone’ (same level of protected reef zone) (Figure 1D) of the sandy beach (bare sediment adjacent to the reef, with minimum distance of 5 m from the reef margins).
A total of eight biological samples, plus four samples for substrate characterization (granulometry and organic matter content) were collected randomly within each zone. A 10-cm diameter cylindrical sampler was inserted into the substrate to a depth of 20 cm. Samples of the macrofauna were extracted using a sieve with a 0.3 mm mesh, and fixed in 4% formalin saline. A 0.3 mm mesh was used here, rather than the more traditional 0.5 mm mesh, because it is more effective for the retention of juvenile organisms, in particular polychaetes (Bemvenuti, Reference Bemvenuti1994). The samples for sediment analyses were cooled in the field and frozen in the laboratory.
In the laboratory, the fauna samples were disaggregated, and the organisms were identified and counted. A total of 100 sabellariids were selected randomly from each reef zone for the measurement of the opercular crown diameter. For the abiotic analysis, reef fragments of about 100 g were disaggregated manually and the macrofauna (sabellariids and all other organisms) were removed. These samples were dried in an oven at 60°C. To quantify the organic matter, ~5 g of the sediment was macerated, weighed and calcined in a muffle furnace at 550°C for 5 h (Ball, Reference Ball1964). The pre-treatment protocol proposed by Naylor & Viles (Reference Naylor and Viles2000) was used for granulometry. In contrast with the results obtained by Lisco et al. (Reference Lisco, Moretti, Moretti, Cardone, Corriero and Longo2017) for Sabellaria spinulosa (Leuckart, 1849), the treatment of the samples of the S. wilsoni reef with potassium hydroxide (10% solution) followed by hydrogen peroxide (6% solution), resulted in a satisfactory disintegration of the grains. The samples were then dried once again. While rare, fragments of shell larger than 2 mm were removed (sieved using a mechanical shaker) before the samples were processed by physical (ultrasound) and chemical (solution of sodium hexametaphosphate) dispersion. Grain sizes were measured using a laser particle size analyser (Fritsch Analysette 22), with a reading scale ranging from 0.04 µm to 2 mm.
Statistical analysis
Density (ind. m−2), richness (total number of taxa), diversity (Shannon–Wiener's index), and evenness (Pielou J’) were calculated for each biological sample. The statistical parameters of the sediments were determined based on the method of Folk & Ward (Reference Folk and Ward1957). A two-way analysis of variance (ANOVA) was used to compare the fauna and sediment descriptors between environments (reef and bare sediment, two levels, fixed factors and orthogonal design) and zones (upper and lower, two levels, nested design, zone nested in environment). The Student–Newman–Keuls (SNK) test was also used for an a posteriori comparison. The density of S. wilsoni was analysed separately from that of the macrofauna and the data were compared between reef zones using a one-way ANOVA. Prior to the ANOVA, the data were tested for normality (Shapiro–Wilk test) and homoscedasticity of variance (Cochran's test), and when required (richness and abundance), the values were log (x + 1) transformed.
Non-metric Multidimensional Scaling (NMDS) and a Permutational Multivariate Analysis of Variance (PERMANOVA) were used to represent and compare benthic community structure between environments and sampling zones. These analyses were run using the similarity matrices calculated from the density estimates for each taxon (fourth-root transformed), based on the Bray–Curtis index. The design of the PERMANOVA was the same as that described above for the ANOVAs. The contribution of each taxon to the similarity and dissimilarity between environments and zones were assessed using the similarity percentage (SIMPER) routine. Species represented occurring in only a single sample were excluded from the analyses.
Results
Sediment characteristics
The beach and reef sediments were significantly different from one another (Figure 2). Although fine sand was dominant in both environments, the percentages of silt (F = 72.9; P < 0.01), clay (F = 110.1; P < 0.01), and medium (F = 35.0; P < 0.05) and coarse sand (F = 10.3; P < 0.05) were significantly higher on the reef. The organic matter content was also significantly higher (F = 28.4; P < 0.01) on the reef. No significant differences were found between zones for any of the sediment parameters, although the protected zone of the reef had a higher concentration of fine grains and greater organic matter content (Figure 2).

Fig. 2. Granulometric composition of the sediments and organic matter content of the Sabellaria wilsoni reef and adjacent sandy substrate on Algodoal-Maiandeua Island in northern Brazil.
Macrobenthic community
A total of 89 taxa were recorded during the present study, of which 81 were associated with the reef, and 10 were found in the bare sediment of the adjacent beach (Supplementary Material). Only two taxa (Nemertea and Armandia sp.) occurred in both environments. The reef was inhabited by a taxonomically diverse fauna, with a variety of life forms (see supplementary Appendix). Density, richness and diversity were all significantly higher on the reef (Figure 3). Density was the parameter that varied most between environments, ranging from 11,013 to 159,494 ind. m−2 on the reef, in comparison with 127–1519 ind. m−2 on the beach. Significant differences between zones were only found in the reef samples. The density, richness and diversity of the associated fauna were all significantly higher in the exposed zone (Figure 3), while the protected zone was characterized by a higher density of sabellariids (F = 32.4; P < 0.01) and worms of a larger mean size (F = 18.9; P < 0.01), which were mostly assigned to larger opercular crown size classes (Figure 4).

Fig. 3. Biotic variables (mean ± SE) of the macrofauna associated with the Sabellaria wilsoni reef and the adjacent sandy beach on Algodoal-Maiandeua Island in Pará, northern Brazil. Abundance (A), richness (B), diversity (C) and evenness (D).

Fig. 4. Sabellaria wilsoni size-class histogram by reef zone, based on the measurement of the opercular crown.
The PERMANOVA confirmed the differences in the macrofaunal structure between environments and reef zones (Table 1).
Table 1. Results of the PERMANOVA and pairwise tests for the structure of the benthic macrofauna between environments and zones

df, degrees of freedom; MS, mean squares.
* Significant differences (P < 0.05).
The results of the SIMPER (Table 2) indicated the taxa which most contributed to the dissimilarity between the environments (total dissimilarity of 99.7%) and reef zones (total dissimilarity of 62.8%). Three infaunal polychaetes (Armandia sp., Orbinia sp. and Nephtys simoni Perkins, 1980) were the most common species in the sandy sediment. The reef was dominated by mobile – e.g. Syllis garciai (Campoy, 1982) and Eulalia viridis (Linnaeus, 1767) – and sedentary worms (e.g. Capitella capitata (Fabricius, 1780) and Mediomastus sp.), as well as anemones and molluscs typical of hard substrates, such as Sphenia fragilis (H. Adams & A. Adams, 1854) and Hiatella arctica (Linnaeus, 1767). For dissimilarity between reef zones, most of the indicated species (worms and molluscs) by SIMPER were more abundant in the exposed zone, except for the Tubificinae, the anemone Bunodosoma cangicum Belém & Preslercravo, 1973, and the crabs Panopeus americanus Saussure, 1857 and Menippe nodifrons Stimpson, 1859, which were more common in the protected zone.
Table 2. Mean dissimilarities between the samples from the reef and beach, and between zones, with the contribution from the species

The species that contributed ~50% of dissimilarities are organized in order of in decreasing contribution.
Abund., indicating where the highest values abundances; Av. Diss, Average dissimilarity; SD, standard deviation; Contrib., contribution for average dissimilarity; Cumul., cumulative contribution.
Discussion
Effect of the presence of ecosystem engineers
The Sabellaria wilsoni reef, in addition to creating a marked topographic alteration to the landscape, provided a substrate with characteristics quite distinct from those of the adjacent sandy beach on Algodoal-Maiandeua Island. The higher textural heterogeneity (coarse and fine grains) and organic matter content on the reef result from the selective behaviour of the reef-building organisms and the deposition of materials during the construction of the reef. Sabellariids are capable of selecting sand grains and other objects of the same size (e.g. shell fragments, foraminiferan valves) and gluing them together using a highly cohesive proteinic cement (Fournier et al., Reference Fournier, Etienne and Le Cam2010). Sabellariids may use a wide spectrum of grain sizes to build their tubes, which tend to vary according to the age and size of the constructor organ of the worms (Gruet, Reference Gruet1984). While fine grains (silt and clay) and organic matter are not used in the tubes, they can be rather sediment in the tube, as the tube-building activity continues, and even from the accumulation of the faeces or pseudo-faeces of the sabellariids themselves (Vovelle, Reference Vovelle1965; Gruet, Reference Gruet1984; Naylor & Viles, Reference Naylor and Viles2000). Vovelle (Reference Vovelle1965) and Naylor & Viles (Reference Naylor and Viles2000) observed that fine material gets stuck in the cracks and the tube lumen of Sabellaria alveolata (Linnaeus, 1767) reefs, but are not part of the cemented matrix, and would thus be available for colonization by other organisms.
The macrofauna of S. wilsoni reefs was completely different from that of the adjacent sandy sediment, and from that found on other Amazon beaches (Rosa Filho et al., Reference Rosa Filho, Almeida and Aviz2009, Reference Rosa Filho, Gomes, Almeida and Silva2011), with significant differences in composition, a higher density of organisms, and greater taxonomic and functional diversity. Our results are similar to the findings of other comparisons between sabellariid reefs and their adjacent substrates (Mettam, Reference Mettam1992; Gherardi & Cassidy, Reference Gherardi and Cassidy1994; Hiscock, Reference Hiscock2004; Jones et al., Reference Jones, Dubois, Desroy and Fournier2018), and also to the findings of Ataíde et al. (Reference Ataide, Venekey, Rosa Filho and dos Santos2014) for the meiofauna of S. wilsoni reefs in Algodoal-Maiandeua Island. These findings, once again, stress the contribution of sabellarid reefs to local species abundance and diversity.
Diversity is much greater on the sabellariid reefs, given that these bioconstructions accumulate species typical of both soft and hard bottoms (Dubois et al., Reference Dubois, Retiere and Olivier2002, Reference Dubois, Commito, Olivier and Retière2006). In sabellariid reefs, consolidated tubes provide a hard substrate that permits the settlement of encrusting organisms (Achary, Reference Achary1969; Dubois et al., Reference Dubois, Commito, Olivier and Retière2006; Gravina et al., Reference Gravina, Cardone, Bonifazi, Bertrandino, Chimienti, Longo, Marzano, Moretti, Lisco, Moretti, Corriero and Giangrande2018). While most of the substrate is formed by consolidated tubes, crevices and empty tubes, as well as sand and mud deposited in these features, are colonizable environments. This infauna includes deposit-feeders (i.e. annelids and peracarids), which consume allochthonous organic matter, dead organisms, faeces and pseudo-faeces, suspension feeders (i.e. crabs, bivalves), for which the reefs provide shelter, and carnivores, which prey on the associated fauna and even on the sabellariids themselves (Gore et al., Reference Gore, Scotto and Becker1978; Porras et al., Reference Porras, Bataller, Murgui and Torregrosa1996). The relatively high chlorophyll-a (~20 µg cm−2) concentrations found in these reefs (Ataide et al., Reference Ataide, Venekey, Rosa Filho and dos Santos2014) also indicate the availability of periphyton for herbivore scrapers, such as small, epifaunal gastropods.
For example, while the fauna recorded on the S. wilsoni reef was quite distinct from that found on the adjacent sandy beach, it contained many species that have been recorded on other types of hard bottom (Aviz et al., Reference Aviz, Mello and Silva2009; Beasley et al., Reference Beasley, Fernandes, Figueira, Sampaio, Melo, Barros, Saint-Paul and Schneider2010; Morais & Lee, Reference Morais and Lee2013) and unconsolidated substrates (Beasley et al., Reference Beasley, Fernandes, Gomes, Brito, dos Santos and Tagliaro2005, Reference Beasley, Fernandes, Figueira, Sampaio, Melo, Barros, Saint-Paul and Schneider2010; Braga et al., Reference Braga, Monteiro, Rosa-Filho and Beasley2011; Silva et al., Reference Silva, Rosa Filho, Souza and Souza-Filho2011b) on the Amazon coast (Table 3). The most common species found in these environments include those typical of muddy habitats (C. capitata, Mediomastus sp., Lumbrineris sp. and Halmyrapseudes spaansi Bacescu & Gutu, 1975), species that are common in sandy-muddy substrates (e.g. Alitta succinea (Leuckart, 1847), Laeonereis culveri (Webster, 1879) and Sigambra sp.) and on rocky outcrops, i.e. Clibanarius symmetricus (Randall, 1840), Thaisella coronata (Lamarck, 1816), P. americanus, Petrolisthes armatus (Gibbes, 1850) and Alpheus armillatus H. Milne Edwards, 1837. The association of organisms with different ecological adaptations resulted in a much higher diversity of organisms compared with other substrates found on the Amazon coast (Table 3). A similar combination of lifestyles has been reported from environments constructed by other sabellariids (Gruet, Reference Gruet1971; Gherardi & Cassidy, Reference Gherardi and Cassidy1994; Dias & Paula, Reference Dias and Paula2001; Dubois et al., Reference Dubois, Commito, Olivier and Retière2006; Gravina et al., Reference Gravina, Cardone, Bonifazi, Bertrandino, Chimienti, Longo, Marzano, Moretti, Lisco, Moretti, Corriero and Giangrande2018; Jones et al., Reference Jones, Dubois, Desroy and Fournier2018).
Table 3. Total richness and most common species observed in the Sabellaria wilsoni reef and other habitats on the Brazilian Amazon Coast and in sabellariid reefs in various coastal regions worldwide
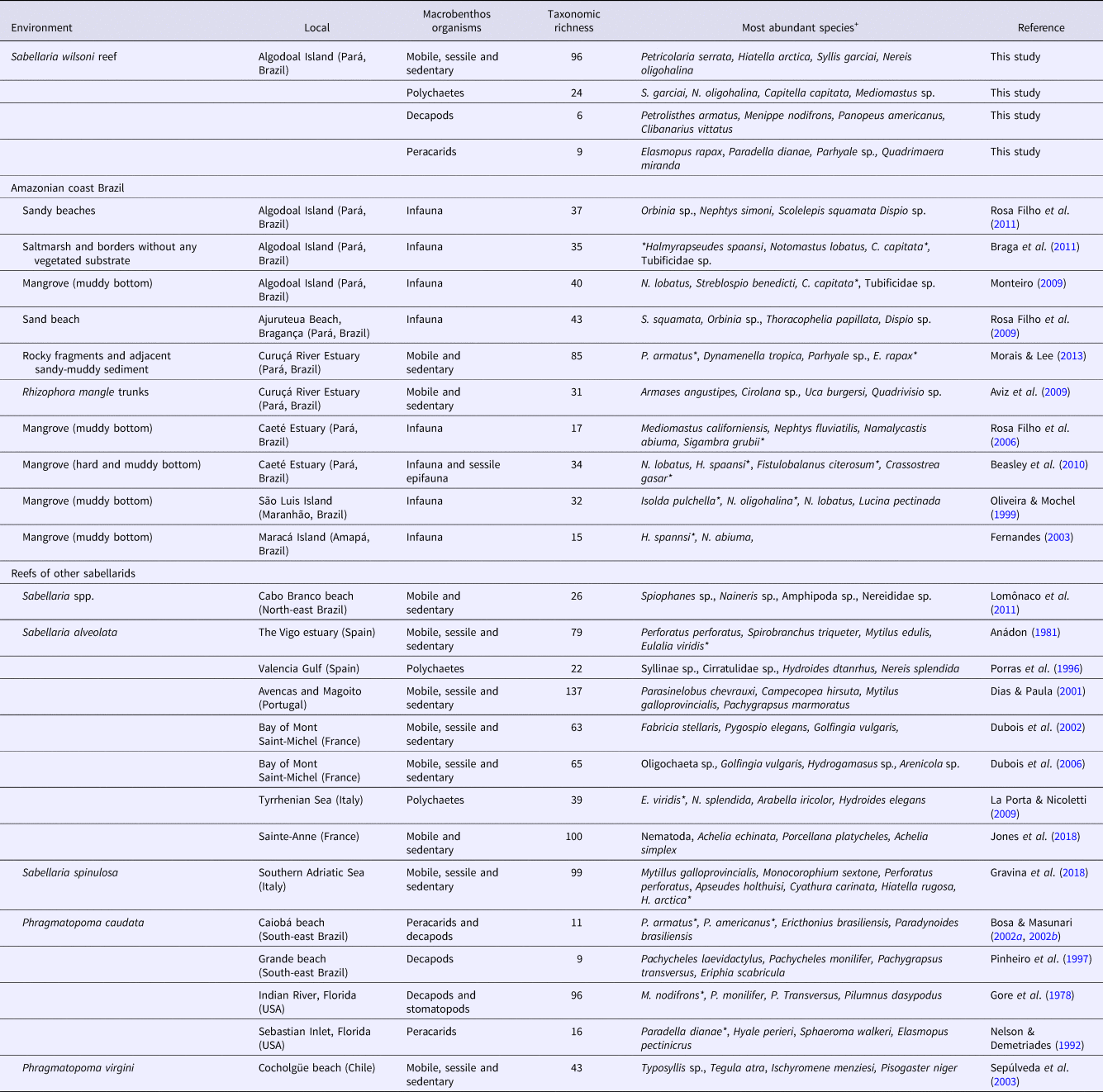
+The four most abundant species cited in each study; *Indicate species recorded in Sabellaria wilsoni reefs.
Like other reef-building sabellariids, S. wilsoni is an ecosystem engineer, that is, an organism capable of modifying the environment by mechanically transforming materials (sand) from one state (disaggregated grains) to another (reef), resulting in marked alterations of the distribution of other species (Jones et al., Reference Jones, Lawton and Shachak1994, Reference Jones, Gutiérrez, Byers, Crooks, Lambrinos and Talley2010). Jones et al. (Reference Jones, Lawton and Shachak1994) originally divided ecosystem engineers into autogenic (in which the structure of the species itself alters the environment, e.g. trees) and allogenic species, which engineer habitats that they do not occupy directly, e.g. beavers. While useful, this dichotomy does not fully embrace the diverse mechanisms and pathways through which engineers influence ecosystems. Berke (Reference Berke2010), for example, considers organisms that create or modify structural elements of the habitat to be ‘structural engineers’, including reef-builders, tube-builders, macroalgae, seagrasses and mangroves. In general, highly diverse benthic assemblages are expected to occupy habitats dominated by structural engineers, which create relatively complex environments (Holt et al., Reference Holt, Rees, Hawkins and Seed1998; Bouma et al., Reference Bouma, Olenin, Reise and Ysebaert2009; Berke, Reference Berke2010). In addition to the increased structural complexity of the habitat, the resident fauna may benefit from a reduction of pressures, such as thermal hydrodynamic stress, and an increase in the availability of resources, including oxygen, food and shelter (Bouma et al., Reference Bouma, Olenin, Reise and Ysebaert2009; Berke, Reference Berke2010; Kovalenko et al., Reference Kovalenko, Thomaz and Warfe2012; Jones et al., Reference Jones, Dubois, Desroy and Fournier2018).
The quantitative and qualitative differences found between the macrofauna of the reef and the adjacent sediment (bioengineering absent) in the present study were more extreme than those recorded in other sabellariid species (Gherardi & Cassidy, Reference Gherardi and Cassidy1994; Jones et al., Reference Jones, Dubois, Desroy and Fournier2018). The mean density and richness of the fauna associated with the S. wilsoni reef were 79,929 ± 12,316 ind. m−2 and 27 ± 0.3 taxa per sample, respectively, in contrast with 593 ± 123 ind. m−2 and 2 ± 0.3 taxa per sample in the adjacent sandy sediment – about 100 (density) and 13 (richness) times smaller than the reef. In sand flats located in protected bays, for example, Gherardi & Cassidy (Reference Gherardi and Cassidy1994) and Jones et al. (Reference Jones, Dubois, Desroy and Fournier2018) observed an increase of only two to eight times the mean density of macrobenthic organisms, and double the richness in sabellariid reefs, when compared with control sediments (no engineer). In the Bristol Channel and adjacent estuaries, Mettam (Reference Mettam1992) found that, in areas of strong currents that had been defaunated by sediment mobility and tidal scour, the presence of a Sabellaria reef permitted the development of a benthic community.
The impact of ecosystem engineering tends to increase in stressful environments, where diversity is kept at low levels by the specific adaptations required for survival (Bouma et al., Reference Bouma, Olenin, Reise and Ysebaert2009). The intertidal areas of sandy beaches are stressful marine environments, in which physical factors are the primary regulators of benthic populations (Defeo & McLachlan, Reference Defeo and Mclachlan2005). The sandy beaches of the Amazon coast have a relatively low richness of macrofauna in comparison with beaches that have similar morphodynamic stages in temperate and tropical regions (Rosa Filho et al., Reference Rosa Filho, Almeida and Aviz2009, Reference Rosa Filho, Gomes, Almeida and Silva2011). This lower richness probably results from the considerable periodic (daily and seasonal) variation in environmental characteristics, resulting from the semidiurnal macrotidal regime (tidal range of 4–11 m) and the equatorial climate of the Amazon region (Rosa Filho et al., Reference Rosa Filho, Pereira, Aviz, Braga, Monteiro, da Costa, Asp, Beasley, Lana and Bernardino2018). Consolidated bottoms are naturally scarce on the Amazon coast, where biological reefs represent an important type of habitat that reduces physical stress and increases biodiversity.
The data from studies on reef-building sabellariids confirm that their reefs typically have an associated macrofauna that is quite complex, being composed of assemblages of a variety of taxonomic groups (Table 3). The composition of the associated fauna is nevertheless similar among reefs, given that these structures offer similar habitats and resources. A considerable proportion of the diversity of sabellarid reefs is composed of infaunal organisms (Anádon, 1981; Dias & Paula, Reference Dias and Paula2001; Dubois et al., Reference Dubois, Retiere and Olivier2002; Sepúlveda et al., Reference Sepúlveda, Moreno and Carrasco2003; Dubois et al., Reference Dubois, Commito, Olivier and Retière2006; Lomônaco et al., Reference Lomônaco, Santos and Martin2011; Gravina et al., Reference Gravina, Cardone, Bonifazi, Bertrandino, Chimienti, Longo, Marzano, Moretti, Lisco, Moretti, Corriero and Giangrande2018; Jones et al., Reference Jones, Dubois, Desroy and Fournier2018). These organisms are favoured by the conditions of the rigid and stable reef architecture and the refuges it offers, as well as the availability of resources typical of soft substrates, as discussed above. The worms include errant polychaetes, such as those of the families Neredidae (Nereis spp.), Phyllodocidae (E. viridis) and Syllidae (Syllis garciai and Typosyllis sp.), and sedentary species, such as cirratulids and spionids (Table 3). The most common and abundant crustaceans include cryptic species of peracarids and crabs, such as brachyurans and porcelanids (Table 3). Epifaunal organisms are also common on these reefs, including many substrate-generalist encrusting species, such as oysters (Crassostrea spp.), barnacles (Balanus spp., Perforatus perforates and Fistulobalanus citerosum), mussels (Mytilus spp. and Modiolus spp.), and annelids (sabellids and serpulids).
Difference between exposed and protected areas
No differences in the structure of the macrofauna were found between the beach sampling zones, due to the small sampling scale (stretches of the lower mesolittoral). On Algodoal-Maiandeua Island, the fauna tends to vary along morphodynamic gradients (from exposed to protected beaches) and coastal levels, i.e. from upper to lower levels (Rosa Filho et al., Reference Rosa Filho, Gomes, Almeida and Silva2011). Within the reef, by contrast, distinct assemblages were found in the exposed and protected zones. The more exposed area of the reef, which is eroded by waves, had a more diverse and denser associated fauna, even though the density of S. wilsoni was lower. A significant decrease in the density of sabellariids and enrichment of the associated fauna have also been observed in temperate reefs in the destruction phase (Porras et al., Reference Porras, Bataller, Murgui and Torregrosa1996; Dias & Paula, Reference Dias and Paula2001; Dubois et al., Reference Dubois, Retiere and Olivier2002; Gravina et al., Reference Gravina, Cardone, Bonifazi, Bertrandino, Chimienti, Longo, Marzano, Moretti, Lisco, Moretti, Corriero and Giangrande2018; Jones et al., Reference Jones, Dubois, Desroy and Fournier2018), which indicates that the areas exposed to wave action, as observed in the present study, may have similar faunistic conditions to declining reefs.
In addition to a reduced density of sabellariids, the exposed zone had worms of smaller body size. Hydrodynamic exposure is considered to be a modulating factor for sabellariid reefs, affecting the biology (McCarthy et al., Reference McCarthy, Young and Emson2003), distribution and growth of its constructors (Lomônaco et al., Reference Lomônaco, Santos and Martin2011), the morphology of its aggregates (Gruet, Reference Gruet1986) and the settlement of the associated fauna (La Porta & Nicoletti, Reference La Porta and Nicoletti2009; Lomônaco et al., Reference Lomônaco, Santos and Martin2011). A number of hypotheses might explain the lower density and size of sabellariids in more hydrodynamic areas, including (i) in exposed areas, settlement and recruitment may be hampered by the constant erosive process, resulting in higher mortality and shorter life expectancy, with resident organisms being constantly eliminated and replaced by new juveniles (Gruet & Lassus, Reference Gruet and Lassus1983), and (ii) survival in exposed areas entails higher energetic costs for the capture of grains and reconstruction of tubes, resulting in a reduced amount of energy available for conversion into body mass (Lomônaco et al., Reference Lomônaco, Santos and Martin2011). Taking the limitations of our sample effort (in time and space) into account, and the fact that recruits settle in a highly gregarious fashion, resulting in extremely patchy recruitment patterns, any such conclusions should be treated with caution, although they may be validated through the collection of additional data in future studies.
The elimination of the constructor worms represents a reduction in competition and an increase in the space available for the associated fauna. Sabellariids are competitors, capable of suppressing other species (Sveshnikov, Reference Sveshnikov1985), either by direct overlap during reef development (Gruet, Reference Gruet1972) or competition for food (Porras et al., Reference Porras, Bataller, Murgui and Torregrosa1996). The disturbance caused by the waves on the structure of the reef may also contribute to the differences between reef zones, given that wave-induced erosion at the reef margins would also tend to increase structural complexity by creating crevices and eroding the structure blocks (Gruet, Reference Gruet1972; Dubois et al., Reference Dubois, Retiere and Olivier2002). Environments with a greater surface area, and more variation in the number and size of spaces, may be suitable for organisms of an ample range of body sizes and different degrees of motility, contributing to an increase in the diversity of the fauna (Bell, Reference Bell1985; Tokeshi & Arakaki, Reference Tokeshi and Arakaki2012; St Pierre & Kovalenko, Reference St Pierre and Kovalenko2014). Substrate heterogeneity may also alter hydrodynamics during high tide and affect shading and wind intensity during low tide (Benedetti-Cecchi & Cinelli, Reference Benedetti-Cecchi and Cinelli1997; Araújo et al., Reference Araújo, Bárbara, Sousa-Pinto and Quintino2005). Jones et al. (Reference Jones, Dubois, Desroy and Fournier2018) also suggested that the spatial continuity of platform reefs and engineered sediments with a ‘good ecological status’ contribute to an increase in the dispersal potential of mobile predators (i.e. decapods, gastropods and errant polychaetes), which decreases species richness and beta diversity.
Wave action may also remove resources from the reef, including excrement, grains and food items (Dias & Paula, Reference Dias and Paula2001). The greater proportion of fine sediments found in the protected zone indicates higher deposition and/or reduced washing, which would favour organisms such as tubificine oligochaetes (Table 2), opportunistic organisms typical of muddy areas (Caspers, Reference Caspers, Brinkhurst and Cook1980). In addition to tubificines, a number of epifaunal organisms (anemones, pagurans, snails and mussels), and porcellanid (Petrolisthes armatus) and xanthid crabs (Menippe nodifrons and Panopeus americanus), were either more abundant or occurred only in the central portion of the reef. These organisms may benefit from the greater shelter from waves, and the more stable conditions. Crabs typically seek out well-developed areas in sabellariid reefs to excavate their cavities (Gore et al., Reference Gore, Scotto and Becker1978).
As in other sabellariids in temperate waters, then, S. wilsoni is capable of modifying, maintaining and creating habitats, which support highly diverse macrofaunal assemblies. The results of the present study, in addition to the findings of previous studies, indicate clearly that the associated macrobenthic community is influenced by the presence and structure of the bioconstructions. However, the influence of the abundance of the reef-building worms on the internal conditions of the reef and the associated fauna requires further investigation.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315418001157.
Author ORCID
Daiane Aviz Masters, 0000-0002-7828-3229.
Acknowledgements
We are grateful to the late Dr André Souza dos Santos for his assistance with species identification. We would like to thank Daniela Tannus and Stephen Ferrari for language revision of the manuscript. Thanks also to two anonymous reviewers for their comments, which helped us to improve the manuscript.
Financial support
Financial support was provided by the National Council for Scientific and Technological Development (CNPq-Brazil) through Universal Project no. 486204/2007. The first and second authors were also awarded scholarships by CNPq-Brazil.









