Introduction
Copepods parasitic on fishes belong to two orders, the Siphonostomatoida and the Cyclopoida which now includes the Poecilostomatoida (Khodami et al., Reference Khodami, McArthur, Blanco-Bercial and Martinez Arbizu2017).
The order Siphonostomatoida Thorell, 1859 currently consists of 39 families that are mostly marine and infect invertebrate as well as vertebrate hosts (Walter & Boxshall, Reference Walter and Boxshall2018). Most of the copepods using teleost and elasmobranch fishes as hosts are members of the Siphonostomatoida (Dippenaar, Reference Dippenaar2004). Ten siphonostomatoid families have been reported as parasites of elasmobranchs (Kabata, Reference Kabata1979; Benz, Reference Benz1994; Boxshall & Halsey, Reference Boxshall and Halsey2004; Dippenaar, Reference Dippenaar2016).
Chondrichthyan fishes are considered one of the most ancient and successful vertebrate lineages dating back about 400 million years, near the Devonian–Silurian boundary (Corrigan & Beheregaray, Reference Corrigan and Beheregaray2009). Their lineage has survived four mass extinction events (Raup & Sepkoski, Reference Raup and Sepkoski1982). Therefore, the elasmobranchs are of particular interest with regards to host-parasite co-evolutionary relationships (Henderson et al., Reference Henderson, Reeve and Tang2013). Despite the large amount of literature that exists on the copepod parasites of teleost fishes, studies of parasites of elasmobranchs are scarcer and geographically patchy: little is known of the diversity of parasitic copepods of chondrichthyan fishes (Henderson et al., Reference Henderson, Reeve and Tang2013).
Tunisia has a rich diversity of elasmobranchs, with more than 61 reported species (Bradaï et al., Reference Bradaï, Saiidi and Enajjar2012). However, investigations into their parasites in Tunisian waters are rare (Essafi, Reference Essafi1975; Youssef et al., Reference Youssef, Benmansour, Ben Hassine and Zouari-Tlig2016) and our understanding of species distributions and host-parasite specificity is incomplete. Thus, knowledge of the host–parasite associations will enhance our future understanding of the dynamics between siphonostomatoids and their hosts and of their co-evolutionary history. The aim of this study is to provide new data on the parasitic copepod species that infect chondrichthyan fishes off the Tunisian coast, as well as to provide data on the host associations of these species.
Materials and methods
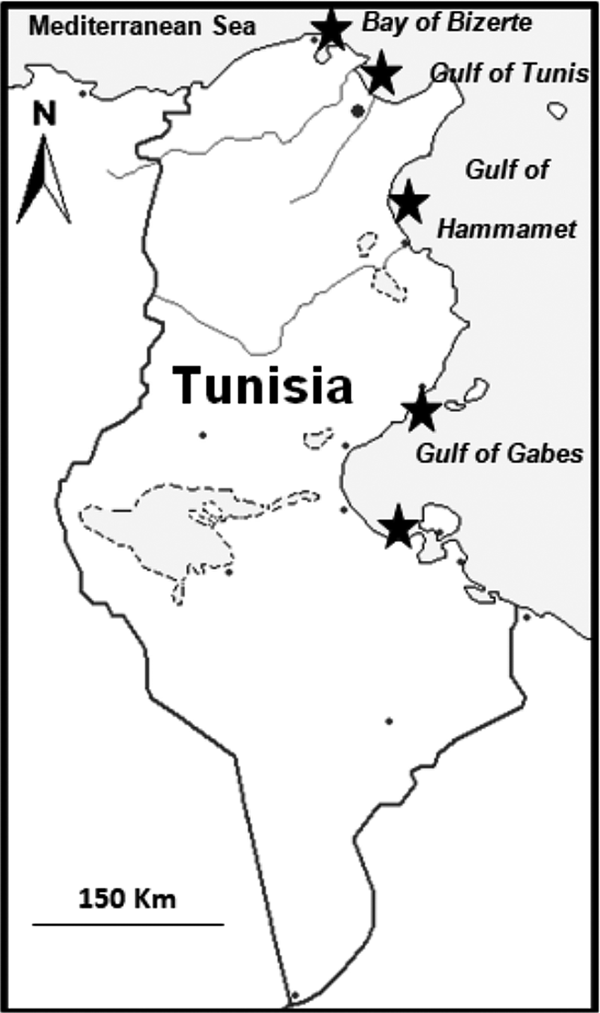
Between 2013 and 2015, 2092 fish belonging to eight species of chondrichthyan fishes were examined for parasitic copepods. Samples were collected along the Tunisian coast, focusing especially on the Bay of Bizerte, the Gulf of Tunis, the Gulf of Hammamet and the Gulf of Gabes (Figure 1).
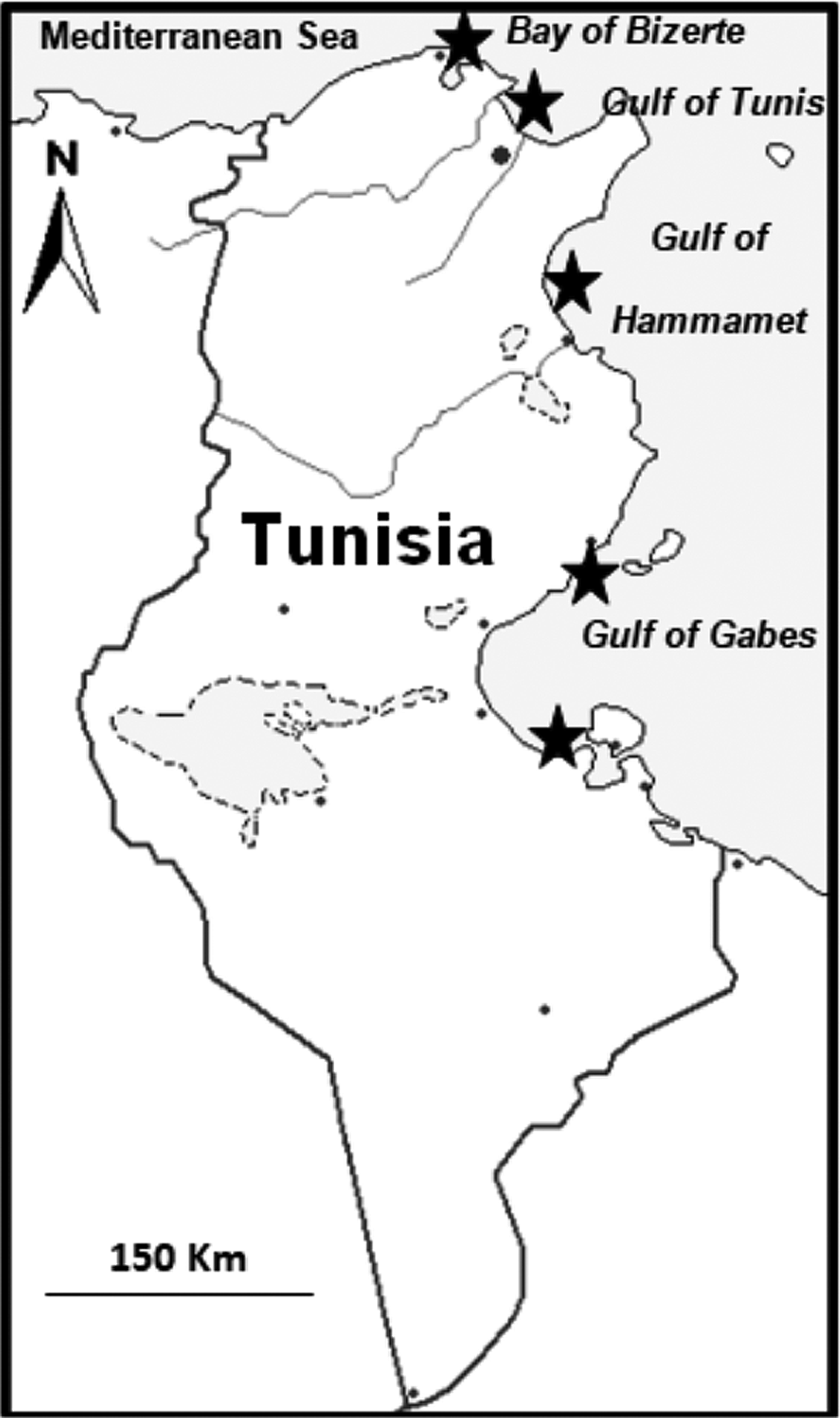
Fig. 1. Sampling sites, indicated by black stars.
The collected fish were immediately transported to the laboratory for analysis. For each fish, the total length and the standard length were measured and the weight was taken using a digital balance. The host species were identified using Fischer et al. (Reference Fischer, Bauchot and Schneider1987) and Séret (Reference Séret2006). Host nomenclature is according to Froese & Pauly (Reference Froese and Pauly2018).
All body parts (skin, fins, gills, mouth, cloaca) were carefully examined. Gills were removed and placed in Petri dishes containing seawater. Each holobranch was individually examined. Copepods were removed from the hosts and preserved in 70% ethanol. The date, sampling area, name and size of host fish and the microhabitat of the parasite were noted. Subsequently, specimens were cleared in lactic acid for 2 h prior to examination by stereo and light microscopy. Specimens were dissected on glass slides and mounted as temporary preparations in lactophenol. Parasite species identification was done at the Natural History Museum of London and based on morphological features following Kabata (Reference Kabata1964, Reference Kabata1979), Cressey (Reference Cressey1967), Deets (Reference Deets1994) and Boxshall & Halsey (Reference Boxshall and Halsey2004).
Rates of infestation were evaluated using prevalence and mean intensity as defined by Margolis et al. (Reference Margolis, Esche, Holmes, Kuris and Schrad1982) and modified by Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997).
Three indices (specific richness (SR), the Shannon–Weaver index (H′) and Simpson index (D)) were calculated using Microsoft Excel 2007 software to explore copepod diversity on the different hosts.
Results
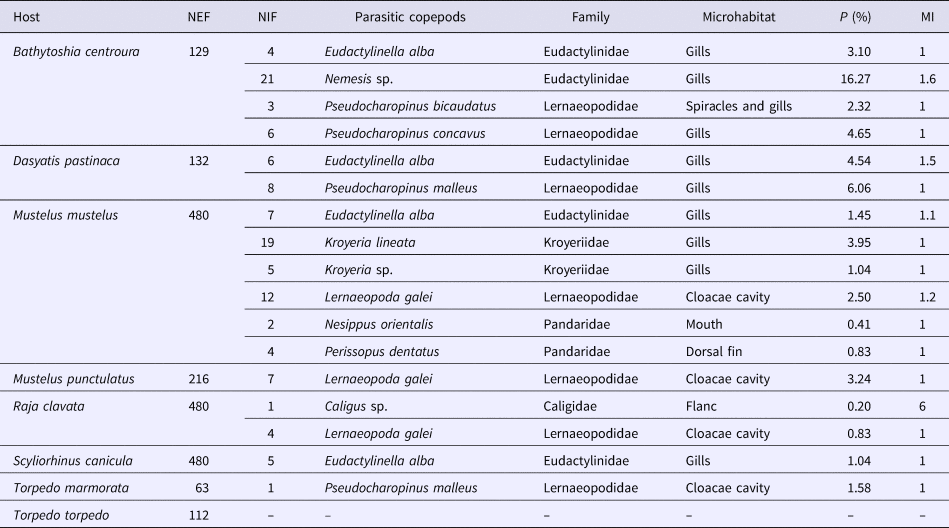
Eleven different species of copepods were collected, namely: Eudactylinella alba Wilson, 1932, Nemesis sp., Pseudocharopinus concavus (Wilson, 1913), Pseudocharopinus malleus (Rudolphi in Nordmann, 1832), Pseudocharopinus bicaudatus (Krøyer, 1837), Kroyeria sp., Kroyeria lineata Van Beneden, 1853, Perissopus dentatus Steenstrup & Lütken, 1861, Nesippus orientalis Heller, 1865, Lernaeopoda galei Krøyer, 1837 and Caligus sp.
Parasitological indices of the collected siphonostomatoids
Our analysis shows that prevalence is low for most species studied. Nemesis sp. on Bathytoshia centroura (Mitchill, 1815) has the highest prevalence recorded (P = 16.27%). Pseudocharopinus malleus found on Dasyatis pastinaca (Linnaeus, 1758) also showed a relatively high prevalence (P = 6.06%) (Table 1).
Table 1. List of siphonostomatoid parasites and their hosts

NEF, Number of Examined Fishes; NIF, Number of Infested Fishes; P (%), Prevalence; MI, Mean Intensity; –, Absent.
Caligus sp. found on Raja clavata Linnaeus, 1758 has the lowest prevalence recorded during this survey (P = 0.20%) (Table 1).
The mean intensity of the different species was relatively low and did not exceed 1.61 except for Caligus sp. on R. clavata with the highest mean intensity in our study (MI = 6) (Table 1).
Eudactylinella alba was collected from four different host species (B. centroura, D. pastinaca, Mustelus mustelus (Linnaeus, 1758) and Scyliorhinus canicula (Linnaeus, 1758)). This copepod exhibits a higher prevalence on host species from the family Dasyatidae (P = 3.10%), compared with its prevalence on M. mustelus (P = 1.45%) and S. canicula (P = 1.04%) (Table 1).
Lernaeopoda galei was also found on multiple hosts (Mustelus punctulatus (Risso, 1826), M. mustelus and Raja clavata). The highest prevalence of this parasite was recorded on M. punctulatus (P = 3.24%), whereas it showed a low prevalence on M. mustelus (P = 2.50%) and on R. clavata (P = 0.83%) (Table 1).
Parasitic richness per host species
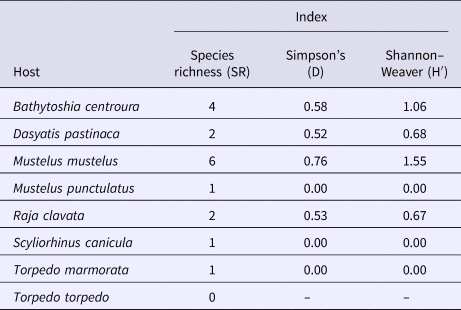
Among the eight different species of elasmobranchs, seven species were infested by parasitic copepods. Mustelus mustelus had the highest richness (SR) with six different species of copepods (Table 2). Bathytoshia centroura displayed the second highest specific richness (SR) with four different species (Table 2). Dasyatis pastinaca and R. clavata were each infested by two species of copepods. Mustelus punctulatus, S. canicula and Torpedo marmorata (Risso, 1810) each hosted a single copepod (Table 2). While Torpedo torpedo (Linnaeus, 1758) was the only species in our survey that was not parasitized by any copepod species (RS = 0) (Table 2).
Table 2. Measures of the parasites biodiversity for the different host species

The Simpson index (D) varied between 0.00 and 0.76. Mustelus mustelus presented the highest value (D = 0.76), while the lowest value was noted for three different hosts (M. punctulatus, S. canicula and T. marmorata) (D = 0.00) (Table 2).
The highest value of the Shannon–Weaver index (H′) was observed for the parasite community of M. mustelus (H′ = 1.55) followed by the parasitic community of B. centroura (H′ = 1.06). The Shannon–Weaver indices of D. pastinaca (H′= 0.68) and R. clavata (H′ = 0.67) were almost identical (Table 2). The value of the Shannon-Weaver index was 0.00 for M. punctulatus, S. canicula and T. marmorata (Table 2).
Parasitic richness per family
The 11 species collected during our survey belong to five families Caligidae, Eudactylinidae, Kroyeriidae, Lernaeopodidae and Pandaridae (Table 1).
Two copepod species belong to the family Eudactylinidae (E. alba and Nemesis sp.) and two are Kroyeriidae species (Kroyeria sp. and K. lineata). The Pandaridae is also represented by two species, Perissopus dentatus and Nesippus orientalis. The highest diversity was for the Lernaeopodidae with four species (Pseudocharopinus bicaudatus, P. concavus, P. malleus and Lernaeopoda galei). The Caligidae has the lowest richness and was only represented by a single species (Caligus sp.).
Microhabitat of the collected parasites
The copepods occupied several different microhabitats (Table 1). The majority were found on the gills of their hosts, but some species were exclusively gathered from the cloaca (Pseudocharopinus malleus and Lerneaeopoda galei), while others were found on the external surface of the host, such as Caligus sp. and Perissopus dentatus. Nesippus orientalis was the only species found in the mouth of its host, Mustelus mustelus.
Discussion
Among the eight fish species examined, only Torpedo torpedo was not parasitized by any copepod. In the Mediterranean Sea, only Pseudocharopinus malleus was reported from this fish (Raibaut et al., Reference Raibaut, Combes and Benoit1998; Benkirane et al., Reference Benkirane, Coste and Raibaut1999). This suggests that T. torpedo presents low parasitic copepod richness.
Analysis of the three calculated indexes of copepod diversity (the specific richness (SR), Shannon-Weaver index (H′) and Simpson index (D)) shows that Mustelus mustelus has the highest specific richness among the different host species studied in Tunisian waters. This may be due to the difference in the life traits of the host species. Mustelus mustelus is an active, strong-swimming epibenthic shark (Smale & Compagno, Reference Smale and Compagno1997). Trawl catches often revealed individuals of similar size in the same net which suggests some schooling, or at least aggregations, for at least some of the time (Smale & Compagno, Reference Smale and Compagno1997). This behaviour would ease the transmission of parasites and increase the specific richness (Combes, Reference Combes1995). Indeed, the size of the parasite community (parasite species richness) may increase because aggregated hosts provide a collectively larger habitat for parasites through effects analogous to island biogeography (Morand, Reference Morand, Poulin, Morand and Skorping2000). Moreover, it is worth considering specific richness on the host on a geographic scale (Poulin et al., Reference Poulin, Krasnov and Mouillot2011). According to Raibaut et al. (Reference Raibaut, Combes and Benoit1998), Carcharhinidae (Prionace glauca (Linnaeus, 1758)) and Triakidae (Mustelus mustelus and M. punctulatus) show the highest richness among elasmobranch species in the Mediterranean.
Mustelus mustelus and M. punctulatus are demersal species that have the same diet (teleosts and cephalopods) (Compagno, Reference Compagno1984). According to Bradaï (Reference Bradaï2000), Mustelus punctulatus is common along the Tunisian coasts, however it still rarer than M. mustelus. Indeed, during this study, we were able to examine 216 specimens of M. punctulatus over a three-year period while M. mustelus was more abundant and 480 fish were examined in just one year. Mustelus mustelus has the highest parasitic richness with six species, while M. punctulatus was host to only Lernaeopoda galei. These results may be induced by the low population density of this host species. Indeed, Kamiya et al. (Reference Kamiya, O'Dwyer, Nakagawa and Poulin2014) identified host population density as one of the key universal determinants of interspecific variation in parasite species richness.
The family Lernaeopodidae has the highest diversity with four different species (Table 1). These results are consistent with the findings of Raibaut et al. (Reference Raibaut, Combes and Benoit1998) on elasmobranch species in the Mediterranean.
Lernaeopoda galei was found on three hosts (Mustelus mustelus, M. punctulatus and Raja clavata). It is a common parasite of elasmobranchs and has been reported from several host species (Raibaut et al., Reference Raibaut, Combes and Benoit1998; Henderson et al., Reference Henderson, Flannery and Dunne2003; Dippenaar, Reference Dippenaar2004; Karaytug et al., Reference Karaytug, Sak and Alper2004; Gaevskaya, Reference Gaevskaya2012), although it seems to display a preference for Triakidae since it was reported by Raibaut et al. (Reference Raibaut, Combes and Benoit1998), Dippenaar (Reference Dippenaar2004) and Karaytug et al. (Reference Karaytug, Sak and Alper2004) from M. mustelus and by Raibaut et al. (Reference Raibaut, Combes and Benoit1998) from M. punctulatus. We collected this parasite on Raja clavata, and this is a new host record.
Three different species belonging to the genus Pseudocharopinus (Kabata, Reference Kabata1964) were collected. P. bicaudatus seems to prefer members of the Squalidae as hosts. In fact, this copepod has been collected on Squalus acanthias (Linnaeus, 1758) (Raibaut et al., Reference Raibaut, Combes and Benoit1998; Benkirane et al., Reference Benkirane, Coste and Raibaut1999; Henderson et al., Reference Henderson, Flannery and Dunne2002), S. acutipinnis (Regan, 1908) (Dippenaar, Reference Dippenaar2004) and S. megalops (Macleay, 1881) (Dippenaar & Molele, Reference Dippenaar and Molele2015). We found P. bicaudatus on Bathytoshia centroura and this is the first report on this host and on any Dasyatidae species.
We, also, report for the first time Pseudocharopinus concavus from Bathytoshia centroura. The relatively high prevalence (P = 4.65%) suggests that its presence on this host is neither accidental nor opportunistic.
We collected Pseudocharopinus malleus on two hosts (Dasyatis pastinaca and Torpedo marmorata). Raibaut et al. (Reference Raibaut, Combes and Benoit1998) reported this parasite on a variety of hosts in the Mediterranean namely D. pastinaca, Myliobatis aquila (Linnaeus, 1758), Rhinoptera marginata (Geoffroy Saint-Hilaire, 1817), T. marmorata and Torpedo torpedo. We note that this species had the second highest prevalence on D. pastinaca (P = 6.06%). In contrast, its prevalence on T. marmorata is relatively low (P = 1.58%) which may suggest that the preferred host in Tunisian waters is D. pastinaca.
A single species of the family Caligidae was found on Raja clavata. This host was sampled for three years, but Caligus sp. was found only during the summer season and it had the lowest prevalence recorded during this study (P = 0.20%). Only adult males of a Caligus sp. were found. This host is known to harbour two caligids, Caligus coryphaenae (Steenstrup & Lütken, 1861) and Lepeophtheirus pectoralis (O.F. Müller, 1776) (Kabata, Reference Kabata1979). However, morphological features of the collected Caligus are different from those two species.
Two species of the Family Eudactylinidae were recovered from the gills of four species of Tunisian elasmobranchs.
Eudactylinella alba exhibited the highest host diversity. It was found on B. centroura, D. pastinaca, M. mustelus and S. canicula. This parasite seems to prefer members of the Dasyatidae as hosts and has higher prevalence on D. pastinaca (P = 3.30%) and on B. centroura (P = 3.10%).
Eudactylinella alba has previously been reported from two Japanese dasyatid rays, Taeniura meyeni (Müller & Henle, 1841) and Dayastis akajei (Müller & Henle, 1841) (Izawa, Reference Izawa2011). However, this is the first record on B. centroura, D. pastinaca, M. mustelus and S. canicula.
Two Nemesis species (Nemesis lamna Risso, 1826 and N. robusta (Van Beneden, 1851)) were found on numerous hosts in the Mediterranean (Raibaut et al., Reference Raibaut, Combes and Benoit1998). During our survey, we found Nemesis sp. only on B. centroura. It was attached to the gills of its host and showed the highest prevalence recorded in our study (P = 16.27%). This is the first record of Nemesis sp. on B. centroura from Tunisia.
We collected two Kroyeriidae species, Kroyeria lineata and Kroyeria sp. Both were found on the gills of M. mustelus. Kroyeria lineata was previously reported on M. mustelus by Deets (Reference Deets1994) and Raibaut et al. (Reference Raibaut, Combes and Benoit1998). The other Kroyeria sp. appears to be a new species.
Kroyeria species are considered to have a high parasite load on their hosts, similar to Nemesis (Risso, 1826) species and in contrast to Eudactylina (Van Beneden, 1853) species (both Eudactylinidae) (Deets, Reference Deets1994). However, in the present work Kroyeria species display relatively low prevalence. Indeed, the prevalence of K. lineata is 3.12% which is slightly higher than the prevalence of Kroyeria sp. (P = 1.04%). In contrast we note that Nemesis sp. exhibits the highest prevalence (P = 16.27%) recorded in our study. Furthermore, species of Kroyeria and Eudactylina, like many other parasitic copepods, typically exhibit a high degree of host specificity (Deets, Reference Deets1994). However, we found Kroyeria lineata on M. mustelus and Eudactylinella alba on B. centroura, D. pastinaca, M. mustelus and S. canicula.
Copepods of the Pandaridae are typically ectoparasites of elasmobranchs and, less commonly, species of Actinopterygian fishes (Bernot & Boxshall, Reference Bernot and Boxshall2017). Two species belonging to this family were found, both on M. mustelus with a fairly low prevalence. Nesippus orientalis was attached in the mouth of its host and Perissopus dentatus on the dorsal fin.
Nesippus orientalis is very common, having been reported from a number of sharks, but appears to be restricted to inshore species and is usually found in the mouth and on the gill arches of the host (Cressey, Reference Cressey1967, Reference Cressey1970). In the South African coasts, Dippenaar & Jordaan (Reference Dippenaar and Jordaan2012) reported this species on 12 different host species (Carcharodon carcharias (Linnaeus, 1758); Isurus oxyrinchus Rafinesque, 1810; Alopias vulpinus (Bonnaterre, 1788); Carcharias taurus Rafinesque, 1810; Carcharhinus brevipinna (Müller & Henle, 1839); C. brachyurus (Günther, 1870); C. leucas (Müller & Henle, 1839); C. limbatus (Müller & Henle, 1839); C. obscurus (Lesueur, 1818); Sphyrna lewini (Griffith & Smith, 1834); S. mokarran (Rüppell, 1837) and S. zygaena (Linnaeus, 1758)). In the Mediterranean, Raibaut et al. (Reference Raibaut, Combes and Benoit1998) reported N. orientalis on M. mustelus.
Perissopus dentatus was found on M. mustelus in the Mediterranean (Raibaut et al., Reference Raibaut, Combes and Benoit1998) and on Carcharhinus limbatus, C. sealei (Pietschmann, 1916), C. leucas, C. obscurus, Mustelus mosis Hemprich & Ehrenberg, 1899 and M. mustelus off the South African coast (Dippenaar & Jordaan, Reference Dippenaar and Jordaan2007). This is the first record of these two parasites in Tunisian waters where M. mustelus seems to be their preferred host.
Host and microhabitat or site selection is exhibited to a varying degree between parasite species and groups (Rohde, Reference Rohde1979; Kabata, Reference Kabata1981). The morphological and physiological factors that determine the selection of a specific site by a specific copepod are still unknown for most species (Kabata, Reference Kabata1981). Siphonostomatoids are found attached to virtually all external body surfaces of their hosts (Benz et al., Reference Benz, Kabata and Bullard2000). In general, all copepods exhibit a high degree of host and attachment site specificity (Kabata, Reference Kabata1979; Benmansour, Reference Benmansour2001). We note that the majority of the parasites collected were found on the gills of their hosts. This is probably due to the richness of this habitat as a food source (the host's blood), ease of access and the relative protection that gills offer from the external environment.
Acknowledgements
The authors thank Professor Geoff A. Boxshall (Natural History Museum, London) for his more than valuable advice, help and guidance that allowed the identification of the different parasitic species and for his comments and corrections on this paper.
Authors ORCID
Feriel youssef http://orcid.org/0000-0002-3400-2560.
Financial support
Ministry of Higher Education and Scientific Research, Tunisia.