Introduction
Amino acids are required by all forms of life on Earth, and their adsorption onto minerals has been suggested as an important step for their preconcentration, protection against degradation (e.g., UV-radiation and hydrolysis) and formation of polymers (Bernal Reference Bernal1951). The interaction between amino acids and clay minerals has thus been the focus of many studies of prebiotic chemistry (Lahav & Chang Reference Lahav and Chang1976; Schoonen et al. Reference Schoonen, Smirnov and Cohn2004; Zaia Reference Zaia2004, Reference Zaia2012; Lambert Reference Lambert2008). Amino acids could have been synthesized in several environments on the prebiotic Earth (e.g., atmosphere, hydrothermal vents and mantle) or they could have been delivered to our planet by comets, meteorites or interplanetary dust particles (e.g., Zaia et al. Reference Zaia, Zaia and de Santana2008). Clay minerals could have been synthesized during the interactions between fluid rock and volcanic gasses, which are associated with the formation of the atmosphere and hydrosphere (Hazen et al. Reference Hazen, Papineau, Bleeker, Downs, Ferry, McCoy, Sverjensky and Yang2008).
Previously, studies have shown that the dissolution of clay minerals is dependent on pH and temperature. Specifically, more Si or Al are released from clays at high pH (>8.0 or >11.0, respectively), low pH (<3.0 or <4.0, respectively) and when temperature is >85 °C (Duc et al. Reference Duc, Cartereta, Thomas and Gaboriaud2008; Rozalén et al. Reference Rozalén, Javier Huertas, Brady, Cama, García-Palma and Linares2008, Reference Rozalen, Brady and Javier Huertas2009). The dissolution of clays is high in hydrothermal vent environments, where pH ranges from 2.0 to 13 and temperatures can reach up to 400 °C (Holm & Andersson Reference Holm and Andersson2005).
As reviewed by Zaia (Reference Zaia2012) most prebiotic chemistry experiments that have explored the adsorption of amino acids onto minerals were performed using distilled water or sodium chloride solutions, but these solutions are not the representative of seas of the prebiotic Earth, when the oceans were 1.5–2.0 times more salty than now (Knauth Reference Knauth1998). Only a few studies have assessed the adsorption of amino acids onto minerals in seawater (e.g., Hedges Reference Hedges1977; Benetoli et al. Reference Benetoli, de Souza, da Silva, de Souza, de Santana, Paesano, da Costa, Zaia and Zaia2007), and it has generally been observed that increasing the concentration of chloride salts results in decreased adsorption (Naidja & Huang Reference Naidja and Huang1994; Norén et al. Reference Norén, Loring and Persson2008; Jonsson et al. Reference Jonsson, Jonsson, Estrada, Sverjensky, Cleaves and Hazen2010). This conflicts with the hypothesis presented by Bernal (Reference Bernal1951), because the higher salinities of the prebiotic oceans would have reduced the adsorption of biomolecules onto minerals, thus inhibiting the mechanism of protection and preconcentration. However, minerals are very complex and the exact composition of seawater on the prebiotic Earth is unknown (Schoonen et al. Reference Schoonen, Smirnov and Cohn2004; Zaia Reference Zaia2012).
This study examined the interaction between amino acids found on the prebiotic Earth (i.e., glycine (Gly), α-alanine (α-Ala) and β-alanine (β-Ala); Zaia et al. Reference Zaia, Zaia and de Santana2008) and Na+-montmorillonite at different seawater concentrations (10, 100, 150%) and pH (3.00, 7.20, 10.0). The effect of amino acids on the concentrations of Ca2+, Mg2+ and K+ in different artificial seawaters was measured. The effect of amino acids on the adsorption of Ca2+, Mg2+ and K+ by Na+-montmorillonite in different artificial seawaters was also determined. The effects of salinity, amino acid concentration and pH on the dissolution of Na+-montmorillonite was measured by determining the release of Al and Si from the clay. The amount of amino acids adsorbed onto Na+-montmorillonite was also measured.
Materials and methods
Materials
Montmorillonite
Montmorillonite-KSF (CAS-1318-93-0, Acros Organics) was sieved through 53 μm and saturated with sodium chloride to form Na+-montmorillonite. The cation exchange capacity (CEC) was 280 mmol kg−1 and the concentration of sodium was 406.7 mg kg−1.
Artificial seawater
Artificial seawater was prepared at three different strengths: 10, 100 and 150% (Table 1). Seawater pH (3.00, 7.20, 10.0) was adjusted with NaOH (0.10 mol l−1) and HCl (0.10 mol l−1).
Table 1. Artificial seawater composition
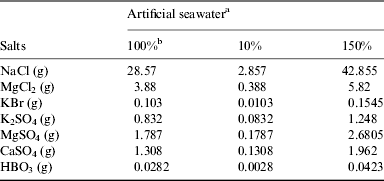
a The amount of salts was dissolved in a 1.0 litre of solution.
b Artificial seawater described by Zaia (Reference Zaia2012).
Amino acids
The amino acids, Gly (Biotec), α-Ala (Fluka) and β-Ala (Sigma-Aldrich) were used without further purification.
Methods
HCl exchange cations (Na+)
In this method, the Na+ cations were exchanged by H+ cations. About 1.0 g of montmorillonite and 10 ml HCl solution (0.05 mol l−1) were added in an Erlenmeyer of 100 ml. The material was mixed for 10 min and left on the bench for 12 h. The cation Na+ was measured using a flame photometer (Williams Reference Williams1984; Rodella & Alcarde Reference Rodella and Alcarde1994). The concentration of sodium was 406.7 mg kg−1.
Cation exchange capacity
In this method, all exchangeable were extracted by Ca2+ and after the Ca2+ was extracted by K+; thus Ca2+ was measured as described below. Montmorillonite (∼1.0 g) was submitted to a sequential saturation/washing procedure (Jaynes & Bigham Reference Jaynes and Bigham1986) in a Centurion multiple vacuum extractor and a buffered (pH 7.0) solution was used to evaluate the CEC using Ca as the index cation primarily adsorbed with a 1.0 mol l−1 Ca-acetate solution at pH 7.0. In the final extraction step, Ca adsorbed was removed with a 1.0 mol l−1 KCl solution and the amount of Ca was determined by atomic absorption spectrometry with GBC equipment. The CEC of montmorillonite was 280.0 mmol kg−1.
Interaction of amino acids in artificial seawater
The amino acids (Gly, α-Ala and β-Ala) were dissolved in 10 ml artificial seawater solutions (10, 100, 150%) at a concentration of 0.010 mol l−1 and at different pH (3.00, 7.20, 10.0). After mixing for 24 h, the solutions were subjected to amino acid (high-performance liquid chromatography (HPLC)) and ion (atomic absorption, UV–vis and flame photometer) analyses.
Adsorption of amino acids onto Na+-montmorillonite
The adsorption of amino acids onto Na+-montmorillonite was determined using two different methods.
Method 1: 10 ml of the amino acid solutions (0.010 mol l−1; see above) were added to 100 mg Na+-montmorillonite. The samples were mixed for 24 h, and then centrifuged for 15 min at 2000 rpm. The supernatant was used for amino acid (HPLC) and ion (atomic absorption, UV–vis and flame photometer) analysis.
Method 2: 7.51 mg of Gly or 8.91 mg of α-Ala/β-Ala were added to 100 mg Na+-montmorillonite. Then 10 ml of artificial seawater (10, 100 or 150%) at different pH (3.00, 7.20 or 10.0) were added. The solutions were then mixed for 24 h and subsequently analysed for amino acid and ions as above. This method mimics the wet branch of the wetting/drying cycles (Lahav et al. Reference Lahav, White and Chang1978).
According to Lambert (Reference Lambert2008) in prebiotic chemistry experiments, buffers should not be used, since they could interfere in the adsorption of biomolecules onto minerals.
Determination of amino acids (HPLC)
In order to remove the cations of the artificial seawater, the samples were treated with BioRad AG-50W-X8 resin cation exchange (Horiuchi et al. Reference Horiuchi, Takano, Ishibashi, Marumo, Urabe and Kobayashi2004). Briefly, resin (50 mg) was mixed with 1.0 ml of the sample in 2 ml Eppendorf tubes. The samples were mixed for 1 h, and then centrifuged for 15 min at 2000 rpm. The supernatants were collected for amino acid analysis. Amino acids were derivatized using naphthalene-2,3-dicarboxaldehyde (NDA) (Siri et al. Reference Siri, Lacroix, Garrigues, Poinsot and Couderc2006). For each analysis, 50 μl of the sample was combined with 100 μl ultra-pure water, 100 μl borate buffer (50 mmol l−1, pH 8.50), 50 μl KCN (5.0 mmol l−1) and 50 μl NDA (1.0 mg l−1). Sample vials were then heated at 65 °C for 25 min. Amino acids were detected by fluorescence (excitation 440 nm and emission 483 nm) after separation using a Perkin Elmer Flexar liquid chromatograph, fitted with a C18-silica column 250×4.6 mm. The mobile phase contained 60% acetonitrile:methanol:water (45 : 45 : 10) and 40% aqueous acetate buffer (25 mmol l−1, pH 6.8), pumped at a rate of 1.3 ml min−1.
Determination of K (flame photometer)
The K concentration was determined by injecting into a flame photometer (Digimed NK-2004). A standard curve was prepared by diluting a K stock solution of 1000 ppm (Merck) to concentrations ranging from 2.0 to 70 mg l−1.
Determination of Ca and Mg (atomic absorption spectrometer)
An atomic absorption spectrometer (AA-7000-Shimadzu) with air/acetylene flame and flow rate of 1.8 ml min−1 was used to measure Ca and Mg concentrations. Stock solutions of 1000 ppm were diluted to generate a standard curve for both Ca (0.05 to 2.0 mg l−1, Merck) and Mg (0.02 to 0.5 mg l−1, Merck). The samples were diluted with distilled water (Milli Q).
Determination of Si and Al (UV–vis spectrophotometer)
Si and Al were measured with a UV–vis spectrophotometer (660 and 534 nm, respectively; UV–vis Genesys 2 Thermo Spectronic) using the ammonium molibdate and aluminon methods as described by AWWA-APHA-WPCI (2006). The standard curve for Si ranged from 0.3 to 5.0 mg l−1 and that for Al ranged from 0.2 to 20.0 mg l−1.
Statistical analysis
The results were submitted to the homogeneity of variances and then were subjected to analysis of variance by (Sisvar) and averages compared by Tukey's test at 5% probability of error.
Results and discussion
We measured the concentration of Ca2+, Mg2+ and K+ in artificial seawaters (10, 100, 150%) at different pH (3.00, 7.20, 10.0) and with or without amino acids (Gly, α-Ala and β-Ala) (Fig. 1). In general, for artificial seawater 10%, amino acids had an effect on the concentration of Ca2+, Mg2+ and K+ (P<0.05). At acidic and basic pH, the 10% seawater solution contained significantly lower concentrations of Ca2+ and K+ when mixed with α-Ala and β-Ala (P<0.05; Fig. 1-a) and at acidic and neutral pH Mg2+ was significantly lower in the 10% seawater solution containing Gly (P<0.05; Fig. 1-a). In summary, our results suggest that α-Ala and β-Ala preferably form complexes with Ca2+ and K+, whereas Gly complexes preferably with Mg2+. Yatsimirskii & Vasilev (Reference Yatsimirskii and Vasilev1960) also observed that the stability constant of Mg2+/Gly was higher than that of Mg2+/α-Ala. Remko and Rode (Reference Remko and Rode2006) calculated that the stability of Gly/Mg2+ was higher than that of Gly/Ca2+. However, Smith et al. (Reference Smith, Motekaitis and Martell1985) observed that the stability constants of Gly or α-Ala/Mg2+ were higher than Gly or α-Ala/Ca2+. According to Shankar et al. (Reference Shankar, Kolandaivela and Senthilkumara2011), the metal-ion affinity for Cys-Mg2+ or Ca2+ follows the order Cys-Mg2+>Cys-Ca2+, suggesting that more stable complexes are formed with Mg2+ than with Ca2+. Smith et al. (Reference Smith, Motekaitis and Martell1985) did not observe differences in the stability constants of Mg2+ bound to unprotonated or monoprotonated amino acids (Gly, α-Ala and β-Ala). It should be pointed out that, in all the experiments cited above, interactions between amino acids and metals were assessed independently, but these were assessed together in our experiments. Our results are showing that α-Ala and β-Ala have a preference for Ca2+ and K+ and Gly for Mg2+. It should be noted that the proteins of living being of today have binding sites for metals and theses sites also show a preference for different metals (Dudev & Lim Reference Dudev and Lim2003).

Fig. 1. Concentration of Ca, Mg and K (mg l−1) in artificial seawaters. The results are presented as mean±standard error of mean. The number of experiments was seven. Means with different letters were statistically different from each other by Tukey's test (P<0.05). Amino acids (0.010 mol l−1) were dissolved in artificial seawater (10, 100, 150%). sw=seawater, sw+Gly=seawater+glycine, sw+α-Ala=seawater+α-alanine, sw+β-Ala=seawater+β-alanine.
For artificial seawaters at 100 and 150% strength, the concentration of Mg2+ was significantly lower when mixed with amino acids (P<0.05), and in most of the cases the concentrations of K+ and Ca2+ were unaffected (Fig. 1-b, c). The effect of amino acids on the concentration of Ca2+, Mg2+ and K+ was likely more significant in the diluted 10% artificial seawater (Fig. 1-a, b, c) due to the low concentrations of amino acids used for all experiments (i.e., 0.010 mol l−1).
Figure 2 shows the concentration of Ca2+, Mg2+ and K+ in 10% artificial seawater (pH=3.00, 7.20 or 10.0) mixed with Na+-montmorillonite and with or without amino acids (Gly, α-Ala and β-Ala). The experiments of the effect of amino acids and Na+-montmorillonite on the Ca2+, Mg2+ and K+ concentrations were performed using two different methods. In method 1, the experiments were carried out as usual, the amino acids were dissolved in the seawater and this solution was added to tubes containing 100 mg of Na+-montmorillonite. In method 2, an amount of solid amino acid (Gly, α-Ala or β-Ala) was added to 100 mg Na+-montmorillonite after 10 ml of seawater were added. Method 2 mimics the wet branch of the wetting/drying cycles (Lahav et al. Reference Lahav, White and Chang1978). For all pH studied, K+ appeared to be adsorbed by Na+-montmorillonite (P<0.05; Fig. 2-a, b, c). On the other hand, the adsorption of Ca2+ and Mg2+ by Na+-montmorillonite occurred only at neutral and basic pH (P<0.05; Fig. 2b, c). Although Ca2+ and Mg2+ were more concentrated than K+ (Table 1), they did not appear to affect the adsorption of K+. This is contrary to the observations of Jalali (Reference Jalali2008), who observed enhanced release of K+ from soils containing Ca2+ and Mg2+.

Fig. 2. Concentration of Ca, Mg and K (mg l−1) after adsorption on Na+-montmorillonite using artificial sea water 10% in several pH. The results are presented as mean±standard error of mean. The number of experiments was seven. Means with different letters were statistically different from each other by Tukey's test (P<0.05). Amino acids (0.010 mol l−1) were dissolved in artificial seawater (10%). sw=seawater, M+sw=Na+-montmorillonite+seawater, M+sw+Gly=Na+-montmorillonite+seawater+glycine, M+sw+α-Ala=Na+-montmorillonite+seawater+α-alanine, M+sw+β-Ala=Na+-montmorillonite+seawater+β-alanine. The experiments were performed using methodology 1 and methodology 2 (see section: Adsorption of amino acids on Na+-montmorillonite).
In general, for the samples at acidic pH, a decrease of the concentrations of Ca2+ and Mg2+ was observed when mixed with Na+-montmorillonite and amino acids (P<0.05; Fig. 2-a). Ca2+ also showed the same effect at basic pH (P<0.05; Fig. 2-c). Fu et al. (Reference Fu, Weckhuysen, Verberckmoes and Schoonheydt1996) also observed that in presence of amino acids (His or Lys), different amounts of Cu2+ were adsorbed onto saponite. However, this effect was generally not observed for K+ (P>0.05; Fig. 2-a, b, c). Amino acids may have prevented the adsorption of Mg2+ onto Na+-montmorillonite at neutral and basic pH (P<0.05; Fig. 2-b, c). For both Ca2+ and Mg2+, differences in the method used to mix amino acids and Na+-montmorillonite were more pronounced at acidic and neutral pH (P<0.05; Fig. 2-a, b). On the other hand, the effect of mixing method on K+ concentrations was generally more pronounced at basic pH (P<0.05; Fig. 2-c).
Figures 3 and 4 show the concentrations of Ca2+, Mg2+ and K+ in 100 and 150% artificial seawaters (pH=3.00, 7.20 or 10.0) when mixed with Na+-montmorillonite and with or without amino acids (Gly, α-Ala and β-Ala). For all samples, Mg2+ was significantly reduced when mixed with Na+-montmorillonite compared to its seawater concentration (P<0.05; Figs. 3 and 4). These results are in good agreement with that observed by Charlet & Tournassat (Reference Charlet and Tournassat2005). According to these authors, in a medium with high chloride concentration such as seawater, montmorillonite has high affinity for Mg. Only the sample of 100% seawater with Na+-montmorillonite and β-Ala (prepared after method 2) exhibited reduced concentrations of Mg2+ compared to the 100% seawater, 100% seawater with Na+-montmorillonite and 100% seawater with Na+-montmorillonite and β-Ala (prepared after method 1) (P<0.05; Fig. 3-c). For all other samples, amino acids did not appear to affect the cation concentration (P>0.05; Figs. 3 and 4).

Fig. 3. Concentration of Ca, Mg and K (mg l−1) after adsorption on Na+-montmorillonite using artificial sea water 100% in several pH. The results are presented as mean±standard error of mean. The number of experiments was seven. Means with different letters were statistically different from each other by Tukey's test (P<0.05). Amino acids (0.010 mol l−1) were dissolved in artificial seawater (100%). sw=seawater, M+sw=Na+-montmorillonite+seawater, M+sw+Gly=Na+-montmorillonite+seawater+glycine, M+sw+α-Ala=Na+-montmorillonite+seawater+α-alanine, M+sw+β-Ala=Na+-montmorillonite+seawater+β-alanine The experiments were performed using methodology 1 and methodology 2 (see section: Adsorption of amino acids on Na+-montmorillonite).

Fig. 4. Concentration of Ca, Mg and K (mg l−1) after adsorption on Na+-montmorillonite using artificial sea water 150% in several pH. The results are presented as mean±standard error of mean. The number of experiments was seven. Means with different letters were statistically different from each other by Tukey's test (P<0.05). Amino acids (0.010 mol l−1) were dissolved in artificial seawater (150%). sw=seawater, M+sw=Na+-montmorillonite+seawater, M+sw+Gly=Na+-montmorillonite+seawater+glycine, M+sw+α-Ala=Na+-montmorillonite+seawater+α-alanine, M+sw+β-Ala=Na+-montmorillonite+seawater+β-alanine The experiments were performed using methodology 1 and methodology 2 (see section: Adsorption of amino acids on Na+-montmorillonite).
Table 2 shows the amount of Si and Al released from Na+-montmorillonite when it was mixed with artificial seawater (10, 100, 150%) with and without amino acids (Gly, α-Ala and β-Ala). For all samples, significantly more Si was released from Na+-montmorillonite at high pH (P<0.05; Table 2). On the other hand, Al was released from Na+-montmorillonite only at acidic pH (Table 2). For all other samples, the amount of Al released from Na+-montmorillonite was under the limit of detection (Table 2). Rozalén et al. (Reference Rozalén, Javier Huertas, Brady, Cama, García-Palma and Linares2008) also observed that the pH of a KNO3 solution, ranging from 1.00 to 13.5, had an effect on the dissolution of montmorillonite. This rate dependence can be described by: R (mol m−2 s−1)=10−12.30a0.40H++10−14.37+10−13.05a0.27OH−. Enhanced release of Al from Na+-montmorillonite was also observed in solution with pH <4.00 or >11.0 (Duc et al. Reference Duc, Cartereta, Thomas and Gaboriaud2008; Rozalén et al. Reference Rozalén, Javier Huertas, Brady, Cama, García-Palma and Linares2008, Reference Rozalen, Brady and Javier Huertas2009).
Table 2. Amount of Si and Al released after Na+-montmorillonite was mixed with seawater and seawater plus amino acid

The results are presented as mean±standard error of mean. Each result is a mean of six experiments in triplicate. ND=not determinate. Lines, for each seawater concentration: means with different lower case letters were statistically different from each other by Tukey's test (P<0.05); for all seawater concentrations: means with different Greek letters were statistically different from each other by Tukey's test (P<0.05). Columns, for each pH: means with different capital letters were statistically different from each other by Tukey's test (P<0.05). M+sw=Na+-montmorillonite+seawater, M+sw+Gly=Na+-montmorillonite+seawater+glycine, M+sw+α-Ala=Na+-montmorillonite+seawater+α-alanine, M+sw+β-Ala=Na+-montmorillonite+seawater+β-alanine.
The dissolution of Na+-montmorillonite could be much higher in hydrothermal environments because these are open systems with pH ranging from 2.00 to 13.0 and temperatures reaching 400 °C (Holm & Andersson Reference Holm and Andersson2005). For the basic seawater (pH 10.0), artificial seawater at 100 or 150% strength exhibited enhanced release of Si from Na+-montmorillonite (P<0.05; Table 2). However, the release of Al from Na+-montmorillonite, the effect was only observed for two samples (P<0.05; Table 2).
For seawater at acidic and neutral pH, amino acids did not have an effect on the release of Si from Na+-montmorillonite (Table 2). On the other hand, for the seawater at basic pH, the addition of amino acid resulted in a significant decrease in the amount of Si released from the Na+-montmorillonite (P<0.05; Table 2). In the high-strength seawater solutions (i.e., 100 and 150%), β-Ala appear to be more protective than other amino acids (P<0.05; Table 2). The protection of Na+-montmorillonite by amino acids could be due to interaction between them. However, the interaction of the cations of the artificial seawater with amino acids (Fig. 1) should also be taken in account, since amino acids decrease the activity of the cations. Kawano & Obokata (Reference Kawano and Obokata2007) and Kawano et al. (Reference Kawano, Hatta and Hwang2009) showed that the amino acids (His, Arg and Lys) interacted strongly with amorphous silica and enhanced the dissolution rates of the mineral, inversely to that observed for the amino acids (Ala, Cys, Asn, Ser, Trp and Thr), which interacted weakly with the mineral. Baú et al. (Reference Baú2012) observed that adenine had a protective effect on the dissolution of zeolites and thymine exhibited the inverse effect. It should be pointed out that for one of the zeolites studied; almost three times more adenine was adsorbed than thymine (Baú et al. Reference Baú2012). Usually most prebiotic chemistry experiments, examine the degradation of biomolecules and the protective role of minerals. The protective role of biomolecules on mineral degradation is rarely considered (Zaia Reference Zaia2012). Thus, experiments performed under mild or extreme conditions (simulating, e.g., hydrothermal environments, acidic lakes and high temperatures) should investigate both possibilities.
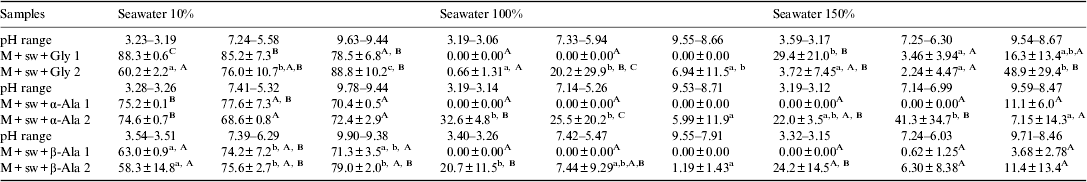
Table 3 shows the adsorption of amino acids (Gly, α-Ala and β-Ala) onto Na+-montmorillonite under different conditions. It should be noted that as reviewed by Zaia (Reference Zaia2012), most of the experiments of adsorption of biomolecules (amino acids and nucleic acid bases) on minerals were performed in distilled water or sodium chloride solutions. Naturally distilled water or sodium chloride solutions are not representative of seawater that existed on the prebiotic Earth. The experiments were also carried out using two methods (1 and 2), as explained above method 1 is the usual and method 2 mimics the wet branch of the wetting/drying cycles (Lahav et al. Reference Lahav, White and Chang1978).
Table 3. Adsorption (%) of glycine, α-alanine and β-alanine on Na+-montmorilonite

The results are presented as mean±standard error of mean. Each result is a mean of six experiments. Lines, for each seawater concentration: means with different lower case letters were statistically different from each other by Tukey's test (P<0.05). Columns, for each pH: means with different capital letters were statistically different from each other by Tukey's test (P<0.05). M+sw=Na+-montmorillonite+seawater, M+sw+Gly=Na+-montmorillonite+seawater+glycine, M+sw+α-Ala=Na+-montmorillonite+seawater+α-alanine, M+sw+β-Ala=Na+-montmorillonite+seawater+β-alanine. 1=method 1 and 2=method 2 (see methodology).
When amino acids (Gly, α-Ala and β-Ala) were dissolved in 10% artificial seawater, the adsorption onto Na+-montmorillonite ranged from 58.3 to 88.8% depending on the mixing method (Table 3). For high concentration of salts, the adsorption of amino acids on Na+-montmorillonite was much higher using method 2 than method 1 (P<0.05). In general, for amino acids dissolved in 10% seawater, the amount adsorbed on Na+-montmorillonite did not depend on the method used (P>0.05; Table 3). However, when seawater 100 or 150% was used, in general, the adsorption of amino acids on Na+-montmorillonite was much higher using method 2 than method 1 (P<0.05; Table 3).
Although the amino acids formed complexes with metals (Fig. 1-a) and Na+-montmorillonite adsorbed metals from seawater (Fig. 2), the formation of these complexes did not appear to diminish the adsorption of amino acids onto Na+-montmorillonite. On the other hand, for the experiments using artificial seawater at 100 or 150% strength, amino acid adsorption was much lower (in the range from 0 to 48.9%; Table 3). In this case, the high concentration of salts likely prevented the adsorption of amino acids onto Na+-montmorillonite, because adsorption sites were occupied by the metals in seawater. Hedges (Reference Hedges1977) observed that montmorillonite adsorbed more Val in seawater than in distilled water; however, kaolinite adsorbed more Val in distilled water. Only 10% or less of the initial Val concentration was adsorbed onto the clays. Naidja & Huang (Reference Naidja and Huang1994) studied the adsorption/desorption of Asp onto montmorillonite, which was completely released from the clay after 1 h in a solution of 0.50 mol l−1 of KCl. Benetoli et al. (Reference Benetoli, de Souza, da Silva, de Souza, de Santana, Paesano, da Costa, Zaia and Zaia2007), using the same artificial seawater used in the current study (100% strength), studied the adsorption of several amino acids (α-Ala, Met, Gln, Asp, His, Lys) onto bentonite and kaolinite over a range of pH (3.00, 6.00, 8.00). They reported that the adsorption of α-Ala onto clays was in the range from 12 to 22%, other amino acids ranged from 2.6% (Met) to 99.5% (Cys) (Benetoli et al. Reference Benetoli, de Souza, da Silva, de Souza, de Santana, Paesano, da Costa, Zaia and Zaia2007). Norén et al. (Reference Norén, Loring and Persson2008) reported no adsorption of sarcosine and decreased adsorption of MDA and EDDA onto Goethite at higher NaCl concentration (from 0 to 0.1 mol l−1). Also when aspartate was dissolved in a NaCl solution (0.01, 0.1, 0.3 mol l−1), a similar trend was observed for its adsorption onto rutile (Jonsson et al. Reference Jonsson, Jonsson, Estrada, Sverjensky, Cleaves and Hazen2010).
The experiments above suggest that the adsorption of amino acids onto minerals is generally reduced in higher salt concentrations. Because oceans on prebiotic Earth may have been 1.5–2.0 times more saline than that of modern oceans (Knauth Reference Knauth1998), the adsorption of amino acids onto clays would have been reduced or may not have occurred. This could be a serious impediment for the Bernal hypothesis (Bernal Reference Bernal1951), in which the adsorption of amino acids onto minerals is proposed as a mechanism for protection against degradation (hydrolysis, UV-radiation) and the formation of polymers. However, as pointed out by several authors, minerals are very complex substances and even similar minerals may exhibit different adsorption behaviours depending on their (Schoonen et al. Reference Schoonen, Smirnov and Cohn2004; Lambert Reference Lambert2008; Zaia Reference Zaia2012).
For several samples, pH influenced the adsorption of amino acids onto Na+-montmorillonite (P<0.05; Table 3). Benetoli et al. (Reference Benetoli, de Souza, da Silva, de Souza, de Santana, Paesano, da Costa, Zaia and Zaia2007) observed that the adsorption of several amino acids (α-Ala, Met, Gln, Asp, His, Lys) onto bentonite and kaolinite was pH dependent, and Ramos & Huertas (Reference Ramos and Huertas2013) also observed the same trend for adsorption of Gly onto montmorillonite. At high pH, Norén et al. (Reference Norén, Loring and Persson2008) and Jonsson et al. (Reference Jonsson, Jonsson, Estrada, Sverjensky, Cleaves and Hazen2010) also observed reduced adsorption of MIDA/EDDA onto Goethite and aspartate onto rutile, respectively.
Conclusions
α-Ala and β-Ala preferentially formed complexes with Ca2+ and K+ and Gly with Mg2+ in artificial seawater.
In the acidic 10% seawater treatment, the amount of K+, Ca2+ and Mg2+ adsorbed onto Na+-montmorillonite was reduced in the presence of amino acids (P<0.05). K+ was significantly adsorbed by Na+-montmorillonite under acidic, neutral and basic pH (P<0.05), whereas Ca2+ and Mg2+ were adsorbed by Na+-montmorillonite only at neutral and basic pH (P<0.05).
For high-salinity seawater (100 and 150% of modern), only Mg2+ concentrations decreased in the presence of Na+-montmorillonite compared to initial concentrations (P<0.05). For method 2, 100% seawater with β-Ala and Na+-montmorillonite had an effect on the concentration of Mg2+ compared to method 1 (P<0.05).
More Si was released from Na+-montmorillonite at basic pH, and more Al was released at acidic pH. In basic solutions, amino acids apparently protected Na+-montmorillonite from the dissolution when the salinity was increased; β-Ala was the most effective. These results suggest that clays may also be protected when complexed with amino acids or the interaction of amino acids with cations of seawater decreased the activity of them.
In the 10% seawater treatment, the adsorption of Gly, α-Ala and β-Ala onto Na+-montmorillonite ranged from 58.3 to 88.8%, which was generally higher than that observed for 100 or 150% seawater (0–48.9%), where the high concentration of salts likely prevented the adsorption of amino acids onto Na+-montmorillonite. Several studies have concluded that amino acid adsorption onto minerals is reduced at high salinity. Because the salinity of seas of the prebiotic Earth could be 1.5–2.0 times more than that of modern seas, the adsorption of amino acids onto minerals may have been prevented. This could seriously conflict with the hypothesis proposed by Bernal (Reference Bernal1951), because adsorption of amino acids onto minerals is an important step for their protection against degradation (hydrolysis, UV-radiation) and formation of polymers.
At high pH, the adsorption of amino acids onto Na+-montmorillonite decreased (P<0.05).
Acknowledgements
APSFF, YST and CEAC acknowledge the PhD fellowship from Fundação Araucária, fellowship from PIBIT/CNPqCNPq and the Post Doc. fellowship from CNPq, respectively. This research was supported by grants from Fundação Araucária (chamada 1, protocolo 23134) and CNPq/Fundação Araucária (Programa de Apoio a Núcleos de Excelência – PRONEX, protocolo 24732).