Mastitis is considered the most persistent disease in dairy cows and is of great economic importance as it is associated with decreased milk production, expensive treatment costs, extra labour and an increased rate of culling (Klaas et al. Reference Klaas, Wessels, Rothfuss, Tenhagen, Heuwieser and Schallenberger2004). Treatment of the disease accounts for the most common cause of antibiotic use in dairy cows, where it can lead to antibiotic residues in milk with concomitant economic losses due to discarding milk during and post treatment (Kaneene & Miller, Reference Kaneene and Miller1992; Costello, Reference Costello2004). In addition, some studies suggest that antibiotics may be relatively ineffective for the treatment of pathogens like Staphylococcus aureus, which can internalize in eukaryotic host cells, evading the immune system (Barkema et al. Reference Barkema, Schukken and Zadoks2006). An effective, non-antibiotic mastitis treatment could reduce costs associated with antibiotic therapy and would also relieve some of the pressures facing the agricultural and veterinary sectors to limit the use of antibiotics. Several alternative approaches have therefore been used in the treatment of mastitis, including milking the infected quarter several times a day (Roberson et al. Reference Roberson, Warnick and Moore2004), hydrotherapy, intramammary infusions of glucose solutions (Reinhold et al. Reference Reinhold, Schulz, Beuche and Jaekel1986) and the use of lysostaphin (Oldham & Daley, Reference Oldham and Daley1991) and nisin (Cao et al. Reference Cao, Wu, Xie, Hu and Mo2007; Wu et al. Reference Wu, Hu and Cao2007). Other remedies have included the use of casein hydrolysates (Silanikove et al. Reference Silanikove, Iscovich and Leitner2005), hot and cold packing, ultrasonic therapy, topically applied liniments (Knight et al. Reference Knight, Fitzpatrick, Logue and Platt2000), drugs such as the milk ejection hormone oxytocin (Guterbock et al. Reference Guterbock, Van Eenennaam, Anderson, Gardener, Cullor and Holmberg1993), antihistamines, steroids, non-steroidal anti-inflammatories and homoeopathic preparations (Varshney & Naresh, Reference Varshney and Naresh2004). Furthermore, the intramammary treatment of subclinical mastitis with Lactobacillus species through competitive elimination has been studied by Greene et al. (Reference Greene, Gano, Smith, Hogan and Todhunter1991).
In the animal trials reported in this paper, a novel non-antibiotic treatment, consisting of a live culture suspension of Lactococcus lactis DPC3147, was examined for its ability to treat naturally occurring mastitis. Lc. lactis DPC3147 belongs to a family of bacteria known collectively as the lactic acid bacteria. These organisms are widely used in the dairy industry as starters for fermentative processes and, as such, are food grade. Lc. lactis DPC3147 produces the bacteriocin lacticin 3147, a two-component lantibiotic (Ryan et al. Reference Ryan, Jack, Josten, Sahl, Jung, Ross and Hill1999b), which inhibits a broad spectrum of Gram-positive bacteria including mastitis-causing bacteria such as streptococci and staphylococci (Ryan et al. Reference Ryan, Meaney, Ross and Hill1998). Indeed, it was previously shown that the bacteriocin itself can reduce the incidence of mastitis after experimental challenge in non-lactating dairy cows when incorporated in a Teat Seal preparation (Ryan et al. Reference Ryan, Flynn, Hill, Ross and Meaney1999a). More recently, a whey containing lacticin 3147 formulation in a bismuth-based teat seal was effective in reducing bacterial recoveries from teats deliberately infected with Staphylococcus aureus in lactating dairy cows (Crispie et al. Reference Crispie, Twomey, Flynn, Hill, Ross and Meaney2005).
In the present study, a live culture of Lc. lactis was assessed for efficacy in the treatment of bovine mastitis. Naturally infected animals were used in two trials, limiting case numbers and study size to small, preliminary trials of a size comparable to that reported previously (Greene et al. Reference Greene, Gano, Smith, Hogan and Todhunter1991). In Trial 1, the live culture treatment was used in subclinical cases caused mostly by Staph. aureus, whereas in Trial 2, the efficacy of infusing a live culture of Lc. lactis DPC3147 for the treatment of acute clinical mastitis in lactating dairy cows was assessed.
Materials and Methods
Selection of animals and clinical assessment
Cows for both trials were selected from four adjacent herds mainly consisting of Holstein-Friesian cows as well as small numbers of New Zealand Friesians, Norwegian Reds, Normandes and Montbelliards. Following selection of suitable quarters, Trial 1 was undertaken over a 2-week period in July 2003. Detection of naturally occurring clinical cases of mastitis and enrolment of quarters in Trial 2 was undertaken over a period of 8 months beginning in January 2005. All cows enrolled in the study were routinely milked twice a day where pre-milking udder preparation consisted of washing with water, forestripping and drying teats with service paper towels. Following milking, teats were sprayed with Deosan Summer Teatcare Plus® (RTU, Diversey Lever) a ready-to-use teat skin disinfectant containing chlorhexidine. At the end of their lactation period, the teats of all cows were infused with long-acting antibiotics (dry cow treatment).
In Trial 1, chronic subclinical mastitis cases were used to evaluate the treatment. Selection of animals was made using a combination of SCC and bacteriological data from quarter milk samples. Composite milk of individual cows was also sampled at monthly intervals throughout the lactation. SCC in the milk samples was determined using a Somacount 300® (Bentley Instruments Incorporated, USA). Similarly to previous studies (Berry & Meaney, Reference Berry and Meaney2006; Kivaria et al. Reference Kivaria, Noordhuizen and Nielen2007; Sandgren et al. Reference Sandgren, Waller and Emanuelson2008) cases were defined as ‘chronic subclinical’ when the SCC was elevated (>3×105 cells/ml) in at least two samples and a pathogen was present or when the SCC was elevated (>3×105 cells/ml) in at least three samples and no pathogen was present. There was no swelling of the udder or visible abnormalities in the milk of chronic subclinically infected quarters. Twenty-two chronic subclinical infections were selected in 12 cows in Trial 1. Eleven cases were infused with culture and 11 cases were infused with an intramammary antibiotic.
In Trial 2, newly acquired clinical cases of mastitis were detected by trained dairy personnel during the process of forestripping. Quarters were classified as having clinical mastitis when an udder quarter was inflamed and visible abnormalities such as clots were present in the milk. Clinical cases were subsequently assessed by a member of the study team as to the severity of the individual case and classified as either mild or severe based on milk and udder appearance and rectal temperature. If the clots in the foremilk were small to medium in size and the milk and udder showed slight abnormalities, such as ‘watery’ consistency and slight swelling and tenderness of the udder quarter, the case was enrolled as mild. Mild cases had rectal temperatures ranging from 38·5°C to 39·5°C. In contrast, if clots were large and the foremilk appeared abnormal in character, the disease was classified as severe. In severe cases the udder quarter was swollen, hot and painful to the touch and rectal body temperatures were 39·5°C or higher. Overall in Trial 2, 50 cases of clinical mastitis were identified in 48 cows and randomly assigned at the time of detection to either culture or antibiotic treatment respectively. Two cows showing clinical mastitis in two different quarters at different time points were assigned once to culture treatment and once to antibiotic treatment. Udder condition, rectal temperatures and SCC were assessed throughout the trial by trained farm staff and veterinary personnel.
Preparation of live culture treatments
The live culture was prepared as follows: Lc. lactis DPC3147 (1%, v/v) was grown at 30°C for 16 h in M17 broth (DIFCO, Becton, Dickinson and Company, Maryland, USA), containing 0·5% lactose (w/v) (LM17). In Trial 1, 2 ml of this overnight culture was mixed with 3 ml of sterile Water for Injection B.P.® (Antigen Pharmaceuticals Ltd., Roscrea, Ireland) and the 5 ml diluted culture/water mix was used as the treatment.
In Trial 2, in an effort to develop a commercially viable live culture product, a washed cell suspension was infused. Treatments were prepared in this trial by incubating Lc. lactis DPC3147 for 16 h at 30°C in LM17. The cells were subsequently harvested by centrifugation at 3500 g for 15 min and the supernatant discarded. The cell pellet was then washed with sterile water, centrifuged again and resuspended in sterile water. This suspension was divided into 5-ml volumes and frozen at −80°C. The frozen culture was then freeze dried in a Modulyo 4K Freeze drier (Edwards, Sussex, UK). Before treatment, the freeze-dried cell pellet was resuspended in 5 ml of sterile Water for Injection B.P.® (Antigen Pharmaceuticals Ltd., Roscrea, Ireland). The resuspended live culture was then used as a single treatment. This suspension contained approximately 9·1±0·5 log10 cfu/ml (colony forming units, average of 12 different counts) of live Lc. lactis DPC3147.
Treatment protocol
To compare the efficacy of treatment with lactococci v. antibiotics, two trials were conducted, involving cows with chronic subclinical or newly acquired clinical mastitis. Infected quarters in each trial were treated with live culture or with antibiotics. As it is unethical to withhold treatment from animals that are known to be infected, untreated controls could not be used in the experiments.
In both trials, treatments were infused in an aseptic manner after milking. For the infusion of the cultures, a syringe with a blunted smoothed tip was used to prevent injury to the teat. In Trial 1, the culture and water mixture was infused after the morning milking on day 1 and day 2 of the trial, with a 24-h interval between treatments. The antibiotic control treatment was administered at 12-h intervals as per the manufacturer's instructions, with administration following the morning and evening milkings on day 1 and following the morning milking on day 2. As Staph. aureus is one of the major causes of subclinical mastitis in Ireland and >80% of Staph. aureus isolates from Irish dairy herds exhibit a significant level of resistance towards penicillin G (Barrett, Reference Barrett2004), the control treatment was a commercial intramammary antibiotic formulation containing amoxycillin (200 mg), clavulanic acid (50 mg) and prednisolone (10 mg) (Synulox®, Pfizer Animal Health, New York, USA). Milk for human consumption was withheld for five milkings after the last treatment as instructed by the manufacturer.
In Trial 2, the resuspended culture was infused on day 1, day 2 and day 3 with 24-h intervals between treatments. To standardize treatment intervals for the two treatments and to provide a broad-spectrum treatment for naturally occurring clinical cases, the antibiotic treatment in this trial was a commercial formulation containing 150 mg penethamate hydriodide, 150 mg dihydrostreptomycin (as sulphate), 50 mg framycetin sulphate and 5 mg prednisolone (Leo Yellow®, Leo Laboratories Limited, Ireland). This product was infused at 24-h intervals on day 1, day 2 and day 3 as recommended by the manufacturer. Milk from cows treated with the antibiotic was withheld from the bulk supply for seven milkings after the last treatment.
Milk sampling protocol
Milk samples in both trials were taken prior to treatment and on day 12 and day 14 after treatment in Trial 1 and Trial 2, respectively, to monitor the progress of the infused quarter. Before sample collection, teats were cleaned by washing with water and then dried with service-paper towels. Immediately before sampling, teats were disinfected with cotton wool swabs containing methylated spirits (Blackhall Pharmaceutical Distributors Ltd., Swords, Co. Dublin, Ireland). Samples (20–25 ml) were collected in single-use sterile plastic containers. In Trial 1, as the treated quarters had chronic subclinical infections, SCC was measured and used as an indication of disease. In Trial 2, the California Mastitis Test (CMT) and the presence of clots were used as subjective measures of SCC as described previously (Crispie et al. Reference Crispie, Twomey, Flynn, Hill, Ross and Meaney2005). A clinical response in Trial 2 was defined as a day 14 post-treatment sample that showed no visible clots or flakes, whose udder condition appeared normal and that had SCC <9×106 cells/ml. The presence or absence of pathogens was not taken into account when classifying clinical response.
Microbiological assessment and bacterial enumeration
Milks obtained from animals in the studies were analysed microbiologically following recommendations of the International Dairy Federation (1981). In brief, 10 μl of each milk sample was surface plated on aesculin blood agar (ABA). ABA was prepared from Blood Agar Base No. 2 (Merck®, Darmstadt, Germany), containing 0·1% aesculin (w/v) (Sigma-Aldrich® Ireland Ltd., Dublin, Ireland) and 5–8% (v/v) whole calf-blood. Plates were incubated overnight at 37°C and bacteria were identified on colony appearance, growth characteristics, morphology and on type of haemolysis. For analysis of the bacterial counts in Trial 1, arbitrary values were assigned to staphylococcal counts in a manner similar to that outlined by Zadoks et al. Reference Zadoks, Gillespie, Barkema, Sampimon, Oliver and Schukken2003.
Bacteriological eliminations in Trials 1 and 2 were defined as the absence of the infecting bacteria in the last sample post-infusion (day 12 and day 14 respectively). In Trial 2, a bacteriological response was defined as a day 14 post-treatment sample with SCC <1×106 cells/ml and a pathogen count <500 cfu/ml. These values were in a similar range to those used by Corlett et al. (Reference Corlett, Peters, Paape and Schultze1984) as a threshold to define infected from non-infected quarters in a study examining the effect of an intramammary device in the control of mastitis.
In cases where the same bacterial species isolated on day 1 persisted throughout the experimental period in Trial 2, bacteria were purified after isolation and cultures were stored in 80% glycerol at −20°C. These isolates were later subjected to 16s rRNA sequencing which was carried out essentially as described by Simpson et al. (Reference Simpson, Stanton, Fitzgerald and Ross2002).
Statistical analysis and data processing
The overall number of cases showing a clinical or a bacteriological response in Trial 2 was analysed using Chi-Square analysis, allowing a comparison of cases treated with the culture with cases in the control group (intramammary antibiotic treatment). Fisher's Exact Probability Test was used for a comparison of clinical responses and bacteriological responses in the subgroups.
SCC was available in precise counts up to 9·99×106 cells/ml milk. Beyond this value, the somatic cells in a sample were estimated using CMT score and clinical appearance.
Results
Given that we had previously demonstrated that the bacteriocin lacticin 3147 was effective in preventing both staphylococcal and streptococcal mastitis (Ryan et al. Reference Ryan, Meaney, Ross and Hill1998, Reference Ryan, Flynn, Hill, Ross and Meaney1999a; Twomey et al. Reference Twomey, Wheelock, Flynn, Meaney, Hill and Ross2000; Crispie et al. Reference Crispie, Twomey, Flynn, Hill, Ross and Meaney2005) we decided to test the efficacy of the producing culture for treatment of the disease. It is important to emphasize that in both these trials high numbers of lactococci could be found in most quarters at the first milking post infusion but the culture did not survive for longer than 14 d in any of the treated udder quarters (data not shown).
Trial 1
In Trial 1, 22 chronic subclinical infections in 12 cows were treated with live culture or an intramammary antibiotic and the effects on pathogen survival and on SCC were observed. Most cows had two infected quarters and in these animals, one infected quarter was treated with Lc. lactis and the other with antibiotic. All cows were in late lactation, 6 cows were in 1st or 2nd lactation and 6 were in greater than 3rd lactation. Overall, pathogens were eliminated from four quarters treated with Lc. lactis and from three Staph. aureus infected quarters treated with amoxycillin/clavulanic acid (Table 1). SCC levels remained relatively unchanged in both groups.
Table 1. Bacteriological and SCC response to intramammary infusion of Lc. lactis DPC3147 or an intramammary antibiotic containing amoxycillin/clavulanic acid in chronic subclinical infections in Trial 1

† Cases 1–11 were treated with Lc. lactis and cases 12–22 were treated with the antibiotic control treatment
‡ Bacteria were enumerated and scored according to the following: 0=Absence of pathogens +=<40 cfu/10 μl, ++=40–400 cfu/μl and +++=>400 cfu/μl
§ Str. uberis infection
Bacteriology
Of the 22 chronic subclinical infections, most were caused by Staph. aureus. The level of pathogen found and the SCC of milk samples on days 0 and 12 are shown (Table 1). Overall, on day 12, 7 of 11 quarters treated with Lc. lactis and 5 of 11 treated with antibiotics were pathogen-free. On day 12, 3 of the 6 quarters treated with Lc. lactis DPC3147 that had originally been infected with Staph. aureus were no longer shedding staphylococci or any other pathogen. One quarter in the culture treated group that had been chronically infected with Streptococcus uberis also showed no bacterial growth on day 12. In the group treated with antibiotics, 3 of the 8 quarters originally infected with Staph. aureus were pathogen-free on day 12. In each treatment group, culture and amoxycillin/clavulanic acid, one case that had no pathogen isolation on day 0 showed staphylococcal growth at the last sampling (Table 1).
SCC response
Overall, in Trial 1, SCC remained relatively unchanged regardless of treatment. On the final day of sampling (day 12), one quarter in each treatment group had an SCC<3×105 cells/ml (Table 1). Average log SCC pre- and post-treatment in the lactococci-treated group were 6·33±0·41 (day 0) and 6·27±0·43 cells/ml (day 12) and average log SCC pre- and post-treatment in the antibiotic treated group were 6·34±0·37 (day 0) and 6·22±0·46 cells/ml (day 12).
Trial 2
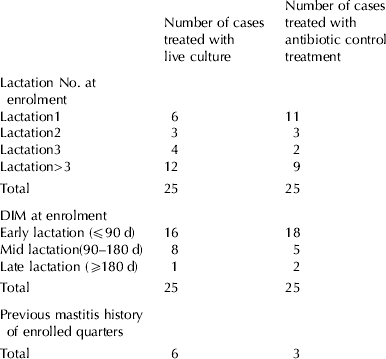
Fifty cases of clinical mastitis in 48 cows were selected for Trial 2, with two cows presenting clinical mastitis in two different quarters at different time points. The parity, lactation status and previous mastitis history of the cows enrolled in Trial 2 are shown in Table 2. To mimic normal farming conditions, where farmers treat clinical cases ‘blindly’ as they appear throughout the lactation period, cases were enrolled and randomized at the time of detection, immediately prior to treatment. Using this technique, the treatment groups could not be stratified either on parity, lactation status or mastitis history. The effect of the treatments on bacteriology and disease symptoms was assessed in the 50 cases as described in Materials and Methods.
Table 2. Cow parity, days in milk (DIM) and previous history of mastitis at the time of enrolment in Trial 2
Bacteriology
Table 3 shows the pathogen types recovered from milk samples taken on day 0 and day 14 for both culture and antibiotic treated groups, respectively. Pathogens were present in 35 of the day 0 samples, culture (n=17) and antibiotic (n=18). Overall bacteriological elimination rates were 61% (11/18) and 47% (8/17) for antibiotic and live culture treatments respectively (Table 3).
Table 3. Bacteriological elimination and bacteriological responses in clinical quarters treated with live culture (Top) or antibiotics (Bottom) in Trial 2

† Cases treated with live culture
‡ quarter dried off before day 14, secretions plated
§ one quarter showed elimination of the pathogen isolated on day 0 and showed a different pathogen on day 14, indicating a new infection with a different organism had occurred
¶ mixed infection with Str. uberis and yeasts
†† Cases treated with antibiotic
Eighteen of the 35 pathogen positive cases on day 0 still showed growth of pathogens in the live culture (n=11) and antibiotic treated groups (n=7) on day 14. Based on colony morphology and haemolysis as well as 16s rRNA sequencing (data not shown), it was concluded that in all but two cases, the pathogen isolated from the final sample was the same as the pathogen isolated on day 0, indicating a persisting infection or re-infection with the same pathogen. The two cases where a new infection occurred were both in quarters treated with the culture. In one of these cases, Streptococcus dysgalactiae was isolated on day 0 and a non-haemolytic staphylococcus was isolated on day 14. In the second case, a mixture of Streptococcus uberis and yeasts was isolated on day 0 and Staph. aureus was isolated on day 14. These two cases were therefore classified as showing bacteriological elimination, as the original infecting organism was no longer present (Table 3).
The treatment groups were also divided on the basis of severity of mastitis in addition to showing pathogen growth on day 0, i.e. culture mild (n=7), culture severe (n=10), antibiotic mild (n=8) and antibiotic severe (n=10) subgroups. In the group treated with the culture, two of the mild (2/7) and four of the severe cases (4/10) had no detectable pathogen on day 14. In the antibiotic-treated group, 4 of the 8 mild pathogen-positive cases and 7 of the 10 severe cases were negative on day 14.
Overall, 16 of the 50 treated quarters (32%) showed a bacteriological response, i.e. had a day 14 post-treatment sample with an SCC <1×106 cells/ml and a pathogen count of <500 cfu/ml. In the group treated with Lc. lactis, this was achieved in 7 of 25 quarters (28%), whereas 9 cases responded to the antibiotic treatment (38%) (Table 3). The difference in overall bacteriological response rates between the Lc. lactis treated group and the antibiotic treated group was not statistically significant (P=0·5443). Treatment groups were subdivided according to the severity of mastitis on day 0 (mild and severe). In the mild clinical group, 4 of 13 cases treated with Lc. lactis and 4 of 12 cases treated with the antibiotic showed a bacteriological response. In the severe cases, 3 of 12 quarters treated with the culture and 5 of 13 quarters treated with the antibiotic showed a bacteriological response (Table 3).
In 15 of the day 0 samples, no pathogen growth was observed following overnight incubation at 37°C. Of these, 10 remained negative on day 14: 5 had been treated with antibiotic (3 in the mild group, 2 in the severe group) and a further 5 had been treated with Lc. lactis (4 in the mild group and 1 in the severe group). Interestingly, 5 of the 15 day 0 pathogen negative samples showed bacterial growth on day 14. Str. uberis (n=2) and Str. dysgalactiae (n=1) were isolated from three of these cases treated with the culture. Both Str. uberis infected quarters were associated with mild clinical symptoms, whereas the Str. dysgalactiae infection was classified as severe. Staph. aureus was isolated from one mild and one severe mastitis case in the antibiotic treated group on day 14.
Clinical response
Fifty quarters were diagnosed with mastitis and selected for the trial. There were 13 and 12 out of the 25 mild cases in culture or antibiotic treated groups, respectively. Furthermore, 12 cases treated with the culture and 13 cases treated with the antibiotic were diagnosed as severe on day 0. An illustration of udder secretions from mild and severe cases on day 0 is shown in Fig. 1. Overall, 15 of the 25 cases treated with the culture and 18 of the 25 cases treated with the antibiotic showed a clinical response at day 14. This difference was not significant (P=0·5443). Nine of the 13 mild clinical quarters treated with Lc. lactis and 8 of 12 mild cases treated with the antibiotic showed a clinical response at the final sampling. In the severe cases, 6 of 12 cases treated with the culture responded clinically on day 14, compared with 10 of 13 cases treated with the antibiotic (Fig. 1).

Fig. 1. Trial 2: Clinical responses on day 14 in culture and antibiotic treated quarters with photographs of a milk sample on day 0 from one mild clinical and one severe clinical case.
Discussion
The aim of the two studies reported here was to evaluate the effectiveness and safety of a live culture of Lc. lactis DPC3147 for the treatment of clinical and chronic subclinical cases of naturally occurring mastitis.
In Trial 1, the efficiacy of the live culture treatment was examined in cows with chronic subclinical Staph. aureus mastitis. Although some studies consider treating chronic subclinical cases during lactation to be beneficial (Swinkels et al. Reference Swinkels, Hogeveen and Zadoks2005) it is generally regarded as controversial and most farmers do not attempt it. Usually, best treatment results for chronic subclinical mastitis are achieved when affected quarters are treated during the dry period. If dry cow therapy fails, it has been suggested that the best option is to cull the animals (Sandgren et al. Reference Sandgren, Waller and Emanuelson2008). The mode of action of the culture treatment is believed to be very different from conventional antibiotic treatments (Crispie et al. Reference Crispie, Alonso-Gomez, O'Loughlin, Klostermann, Flynn, Arkins, Meaney, Ross and Hill2008), however, and for this reason, it was decided initially to examine the effect of the treatment on subclinical mastitis cases in lactating cows from both safety and efficacy perspectives. Of the cases initially shedding Staph. aureus, no staphylococci could be isolated at the last sampling in 4 of 7 infected quarters (57%) treated with the culture and in 3 of 8 infected quarters (38%) treated with antibiotics. Previously, bacteriological cure rates for Staph. aureus-induced subclinical mastitis was found to be 43% for amoxycillin and cephapirin (Wilson et al. Reference Wilson, Gonzalez, Case, Garrison and Groehn1999) and thus the bacteriological elimination rate appeared to be similar to cure rates of some antibiotics, in this small preliminary trial. Cured quarters, however, are usually defined as not only being pathogen free, but also having an SCC <2×105 cfu/ml. As SCC was not considered in our definition of bacterial elimination, a direct comparison cannot be made, but our results imply similar trends for both treatments. If the similar results found for live culture and antibiotic treatment were confirmed in a larger study, the treatment of subclinical mastitis with the culture during lactation could be considered beneficial, as no cost through milk withdrawal would arise. In cases of treatment success, the major financial loss due to culling would also be prevented.
The results of Trial 2 showed that the difference between the overall bacteriological response rate and the clinical response rate for the culture treatment v. the antibiotic treatment was not statistically significant (P=0·5443 and P=0·5443 for bacteriological responses and clinical responses, respectively). In terms of bacterial elimination, 11 of 18 (61%) and 6 of 17 (35%) cases treated with antibiotic and culture treatment, respectively, were pathogen free at the end of Trial 2. At first glance, this would suggest that the antibiotic treatment seemed to compare favourably with the culture treatment. However, a further two cases treated with the live culture, although not pathogen free on day 14 had in fact eliminated the original infecting organism, bringing the elimination rate for this treatment to 47% (8/17). Although the numbers are relatively small and again SCC was not taken into consideration, indications are that the live culture may therefore be as effective as the antibiotic treatment.
Bacteriological elimination of Staph. aureus infections in Trial 2 was achieved for 3 of 7 and 4 of 8 cases in the culture and antibiotic group, respectively. Owens et al. (Reference Owens, Ray, Watts and Yancey1997) previously found that using a combination of penicillin and novobiocin, bacteriological cure rates for clinical mastitis caused by Staph. aureus could be as high as 70% if treatment was initiated at the early stage of infection (<28 d) and could be as low as 35% for animals with chronic infections. These figures compare favourably with the bacteriological elimination rates achieved with Lc. lactis and the antibiotic control used in Trial 2, especially if we consider that some of the Staph. aureus treated cases may have been chronic. It is well known that Staph. aureus causes microabscesses inside the mammary gland and therefore the pathogens may be protected from antibiotic access and shed in a cyclical manner. Consecutive sampling is sometimes recommended for a more accurate diagnosis of the mastitis event (Sears et al. Reference Sears, Smith, English, Herer and Gonzalez1990). Since this level of sampling was not carried out in Trial 2, it is possible that some of the 15 day-0 pathogen-negative samples may well have been associated with chronic Staph. aureus infections and the intermittent shedding pattern of these pathogens.
Other pathogens isolated in the trials were Str. uberis (1 and 4 cases in Trials 1 and 2, respectively) and Str. dysgalactiae (10 in Trial 2). Bacteriological cure rates for both streptococcal strains when treated with penicillin alone were reported to be 60–90% (Sandholm et al. Reference Sandholm, Honkanen-Buzalski, Kaartinen and Pyörälä1995) and when treated with a combination of penicillin and novobiocin were 90% and 91%, respectively (Owens et al. Reference Owens, Ray, Watts and Yancey1997). Three out of five Str. dysgalactiae related cases persisted in each treatment group in Trial 2, therefore elimination rates for both treatments were lower than the published cure rates. The three cases of Str. uberis mastitis treated with the antibiotic in Trial 2 showed no pathogen growth on day 14. The elimination rate for this pathogen by antibiotics in Trial 2 is therefore similar to the published cure rates. In contrast, the only Str. uberis case in Trial 2 treated with the culture persisted on day 14. Str. uberis is a mastitis pathogen that can internalize into epithelial cells for up to 120 h without affecting the viability of the host cell (Almeida et al. Reference Almeida, Batcha and Oliver2005). Trials by Crispie et al. (accompanying paper) have shown that Lc. lactis DPC3147 causes an immediate, short-lived effect on the immune system and therefore a longer interval between doses might be more effective in treating slow progressing Str. uberis type infections.
As it is unethical to withhold treatment from animals that are known to be infected, untreated controls could not be used in the experiments and thus the results of the experiments must be considered in conjunction with spontaneous recovery. Most spontaneous recoveries occur in quarters with mild or recent infections and only rarely in the case of well-established or chronic infections such as those encountered in Trial 1. However, some cows recover from clinical mastitis spontaneously: the rate of spontaneous recovery of untreated mastitis caused by Staph. aureus is approximately 15–20% and in untreated cases of streptococcal mastitis is approximately 20–25% (Sandholm et al. Reference Sandholm, Honkanen-Buzalski, Kaartinen and Pyörälä1995; Nickerson et al. Reference Nickerson, Owens, Tomita and Widel1999). The small numbers of cases in these studies make comparison difficult but it appears that the rate of elimination of pathogens observed is higher than that expected from spontaneous recovery. Moreover, the rates were comparable across treatment, i.e the live culture appeared to be as effective as the antibiotic treatments. Factors such as age, and previous history of mastitis can also effect recoveries as it has been shown that mastitis in older cows, or in cows with a previous history of mastitis, is more difficult to treat than in younger animals (Sandgren et al. Reference Sandgren, Waller and Emanuelson2008). In Trial 2, cases of clinical mastitis were randomly assigned to culture or antibiotic treatment as they arose, without knowledge of factors such as parity, days in milk at the time of enrolment and previous mastitis history. When these data were examined retrospectively, it was observed that overall, cows in the culture-treated group were older than in the antibiotic group. Additionally, twice as many cases in the culture group had a previous history of mastitis or high SCC, compared with the antibiotic group. In a larger trial involving more cases, the culture treatment might therefore show better results than in the present study.
Like many other intramammary antibiotics, the formulations used in this study contained a mixture of antibiotics and the anti-inflammatory component prednisolone. Prednisolone belongs to a group of substances called steroidal anti-inflammatories and mainly aids the reduction of swelling and related pain in intramammary treatments (Lees, Reference Lees1991). Indeed, the better accessibility of the antibiotics to the pathogens due to lower inflammation possibly led to a quicker recovery. This may be an explanation for the numerical differences encountered in response rates of the severe cases for both treatments in Trial 2. In contrast, treatment with Lc. lactis DPC3147 seems to be associated with transient elevated SCC. This effect on the immune system might aid in the recovery of an inflamed quarter, especially as the mammary gland immune response in Staph. aureus related mastitis is evidently suppressed and down-regulated (Alluwaimi, Reference Alluwaimi2004). In support of this, recent results from our laboratory have confirmed that the infusion of live cultures into quarters of low-cell-count animals is associated with a rapid influx of neutrophils in the first 2 d post infusion (Crispie et al. Reference Crispie, Alonso-Gomez, O'Loughlin, Klostermann, Flynn, Arkins, Meaney, Ross and Hill2008). It is believed that this influx of PMN may enhance the intramammary immune response. The influx of PMN was also associated with a short-lived rise in SCC; however SCC rapidly decreased to pre-treatment levels, probably reflecting the inability of Lc. lactis to establish in the treated quarters, and no lasting adverse effects from the treatment were observed in any of the animals in any of the trials, demonstrating the safety of the treatment.
Alternative treatments such as the live culture treatment may have an application in the growing organic sector where the use of antibiotic treatments is limited or even prohibited. The live culture treatment also has potential to reduce the costs associated with the disease, as is estimated that approximately 5·7% of the cost of mastitis is due to discarding of milk containing antibiotic residues (Costello, Reference Costello2004). Lc. lactis DPC3147 is used in food production (Ryan et al. Reference Ryan, Meaney, Ross and Hill1998) and it is likely that as a result, milk from quarters treated with this organism would only have to be withheld until normal milk character is restored. Additionally, the ongoing discovery of more multiple resistant strains of pathogens, which is well documented through the risk posed by methicillin and vancomycin resistant Staph. aureus (MRSA and VRSA), will require a reduction in the use of antibiotics in every sector in the future. It would now be interesting to compare antibiotic therapies with this more natural approach in the larger field trials that would be required for regulatory approval.






