Introduction
Necrotising otitis externa is a severe inflammatory process, occurring mostly among diabetic patients, which affects both soft tissue and bone. The association between necrotising otitis externa and diabetes is thought to be due to local micro-angiopathies and neuropathies, secondary to long-standing diabetes and ageing. This subject has been extensively described in the medical literature, mostly in conjunction with diabetic foot osteomyelitis. Furthermore, when considering the clinical approach to these two entities, it appears that necrotising otitis externa and diabetic foot osteomyelitis share many similarities regarding the diagnostic and treatment algorithms.
In contrast to necrotising otitis externa, in which most of the reported data come from small case series (because of the rarity of the disease), diabetic foot osteomyelitis is a common condition, and as a consequence substantially more information has been reported based on larger case series. This article aimed to compare the approaches to necrotising otitis externa and diabetic foot osteomyelitis, and to emphasise specific issues that should be considered in the treatment algorithm for necrotising otitis externa patients. A PubMed search was conducted using the key words ‘otitis externa’, ‘necrotising otitis externa’, ‘malignant otitis externa’, ‘osteomyelitis’ and ‘diabetic foot’. The similarities and differences of these entities are described below.
Aetiology and pathophysiology
Necrotising otitis externa (also known as malignant otitis externa) was first described in 1968 by Chandler.Reference Chandler1 The inflammatory process begins as pathogens penetrate the overlying skin of the external ear canal, mostly due to local trauma. As the inflammation proceeds, it penetrates the nodes of Santorini and the osseocartilaginous junction, disseminating into the temporal cortical bone. This leads to osteomyelitis of the temporal bone and skull base. The inflammation occurs most commonly among diabetic patients, although immunocompromised and human immunodeficiency virus patients are also at high risk of developing necrotising otitis externa.
Diabetic foot osteomyelitis also originates as a result of contiguous spread from local soft tissue infection. Classical locations for the development of diabetic foot osteomyelitis are the phalanges, and metatarsal and calcaneus bones. The inciting event, most commonly local trauma or pressure, occurs at locations affected by microvascular insufficiency and peripheral neuropathy secondary to diabetes. As the inflammation continues, local soft tissue necrosis occurs, leading to the formation of an ulcer. Independent risk factors for the development of diabetic foot ulcer include a past history of ulcers, peripheral neuropathy (neuropathy disability score above 6 out of 10), reduced local pulse and foot deformity.Reference Abbott, Carrington, Ashe, Bath, Every and Griffiths2 Bone involvement occurs via direct spread of the offending pathogen from the ulcer. Independent risk factors for the development of diabetic foot osteomyelitis include recurrent deep ulcers and multiple ulcers.Reference Lavery, Peters, Armstrong, Wendel, Murdoch and Lipsky3
Bacteriology
Although not a member of normal flora of the external ear,Reference Stroman, Roland, Dohar and Burt4 Pseudomonas aeruginosa was originally reported as the offending pathogen in as many as 99.2 per cent of necrotising otitis externa cases.Reference Rubin and Yu5 Although P aeruginosa is still the most common pathogen, rates of P aeruginosa necrotising otitis externa have been declining in recent years, while rates of sterile necrotising otitis externa have been increasing.Reference Mahdyoun, Pulcini, Gahide, Raffaelli, Savoldelli and Castillo6 This raises the concern of bacterial resistance. In recent studies, P aeruginosa was reported as the offending pathogen in 76 per cent of necrotising otitis externa patients.Reference Mahdyoun, Pulcini, Gahide, Raffaelli, Savoldelli and Castillo6 Other reported pathogens include Staphylococcus aureus,Reference Bayardelle, Jolivet-Granger and Larochelle7 klebsiella species,Reference Garcia Rodriguez, Montes Martinez, Gómez González, Ramos Macias and Lopez Alburquerque8 Streptococcus epidermidis,Reference Barrow and Levenson9 and fungal pathogens such as aspergillus speciesReference Hollis and Evans10 and candida species.Reference Bae, Lee, Park, Bae, Ma and Kim11
A superficial swab of the external ear canal is currently the accepted approach for isolating the offending pathogen in necrotising otitis externa. When isolated, the antibiotic regimen is tailored according to the susceptibility profile of the agent. Deep soft tissue cultures in the setting of necrotising otitis externa have been used in refractory cases and in suspected fungal infections.Reference Gruber, Roitman, Doweck, Uri, Shaked-Mishan and Kolop-Feldman12 The use of bone cultures has not been reported in the setting of necrotising otitis externa.
In contrast to necrotising otitis externa, diabetic foot osteomyelitis infections are essentially polymicrobial, with aerobic Gram-positive cocci being the most common pathogen and Gram-negative bacilli being common co-pathogens in long-standing chronic infections.Reference Lipsky, Berendt, Cornia, Pile, Peters and Armstrong13 Among the pathogens causing diabetic foot infection, S aureus is the most common, followed by P aeruginosa. The prevalence of P aeruginosa diabetic foot osteomyelitis is reported to be higher in warm climates. Fungal diabetic foot osteomyelitis is rare, and is caused by haematogenous dissemination or extension from an adjacent focus of infection.Reference Yener, Topcu, Manisali, Comlekci and Yesil14
The recommended location for obtaining culture in diabetic foot osteomyelitis is bone.Reference Abbott, Carrington, Ashe, Bath, Every and Griffiths2, Reference Lew and Waldvogel15 Superficial wound cultures and cultures taken from draining sinuses are not used for isolation of the offending pathogen.Reference Abbott, Carrington, Ashe, Bath, Every and Griffiths2, Reference Lew and Waldvogel15 When comparing superficial swab cultures from bone biopsy in the setting of diabetic foot osteomyelitis, less than 50 per cent of the superficial swab cultures will show concordance with bone biopsy.Reference Game16
Imaging
Imaging modalities for the diagnosis of necrotising otitis externa and diabetic foot osteomyelitis include plain radiography, computed tomography (CT) imaging, magnetic resonance imaging (MRI) and nuclear imaging.
High-resolution CT scanning of the temporal bone is commonly used in the initial evaluation of suspected necrotising otitis externa. Bony changes seen on high-resolution CT include external ear canal, mastoid, temporomandibular joint and skull base involvement. Soft tissue involvement, such as middle-ear and mastoid fullness, and nasopharynx involvement, may also be seen on initial high-resolution CT scan, and these findings have been shown to have prognostic value.Reference Peleg, Perez, Raveh, Berelowitz and Cohen17 However, these findings are not specific to necrotising otitis externa, and may be due to other conditions such as malignancy, cholesteatoma and keratosis obturans. Another major disadvantage of high-resolution CT is that bony changes are evident on CT only after 30 per cent of demineralisation has occurred, thus limiting its sensitivity. Nevertheless, its widespread availability means that high-resolution CT is useful for assessing disease extent in the initial evaluation of necrotising otitis externa, and for excluding other ear pathologies.
Nuclear imaging is used widely for the diagnosis of necrotising otitis externa. Combined technetium-99 and gallium-67 imaging provide information regarding bone involvement, which thus helps the physician distinguish between severe otitis externa and necrotising otitis externa. Gallium-67 clears comparatively fast once the inflammatory process has resolved, and hence it may be used for evaluating treatment response (see the Follow up section below). The main disadvantages of nuclear imaging are high cost, lack of availability and poor anatomical localisation, with the latter necessitating further imaging if surgery is considered.
Magnetic resonance imaging offers a better resolution than high-resolution CT in the evaluation of soft tissue, especially the parotid gland, meninges and cranial nerves.Reference Okpala, Siraj, Nilssen and Pringle18 Although it has been recommended in the setting of skull base osteomyelitis and cranial nerve palsy,Reference Peleg, Perez, Raveh, Berelowitz and Cohen17 MRI is not used regularly, and its use is largely based on its availability and the treating physician's preferences.
The imaging algorithm for diagnosing diabetic foot osteomyelitis differs substantially from that for necrotising otitis externa. Plain radiography is used routinely in the initial evaluation of diabetic foot osteomyelitis, but has no role in the diagnosis of necrotising otitis externa. Early changes seen on plain radiography include osteopenia, lytic lesions and periosteal thickening. The overall sensitivity and specificity of plain radiography, however, are reported to be quite low, especially at early stages of the disease.Reference Game and Jeffcoate19
Magnetic resonance imaging provides excellent anatomical localisation and soft tissue involvement in the setting of suspected osteomyelitis. Bone changes may be detected as early as 3–5 days from disease onset.Reference Pineda, Espinosa and Pena20 The sensitivity of MRI in the detection of diabetic osteomyelitis has been reported to be 90 per cent, with a specificity of 79 per cent.Reference Dinh, Abad and Safdar21 Given its high sensitivity, MRI is considered the imaging modality of choice for the diagnosis of diabetic foot osteomyelitis.Reference Lipsky, Berendt, Cornia, Pile, Peters and Armstrong13
In nuclear imaging, technetium-99 and indium-111 are also used for the diagnosis of diabetic foot osteomyelitis, but to a lesser degree. The reported sensitivity of nuclear imaging for detecting diabetic foot osteomyelitis is 74 per cent, with a specificity of 68 per cent.Reference Tomas, Patel, Marwin and Palestro22 Currently, nuclear imaging is recommended for the diagnosis of diabetic foot osteomyelitis only in cases where MRI is contraindicated.Reference Lipsky, Berendt, Cornia, Pile, Peters and Armstrong13
Treatment
Antibiotics
The current accepted duration for intravenous antibiotic treatment for diabetic foot osteomyelitis is four to six weeks.Reference Lew and Waldvogel15 Necrotising otitis externa may have a similar treatment duration, although large variation exists in the reported literature.
Initial empirical antibiotic treatment in the setting of suspected necrotising otitis externa includes antipseudomonal antibiotics, such as quinolone or third-generation cephalosporin. Once the offending pathogen has been isolated, treatment is tailored, based on the pathogen's antibiotic profile.
Although reports have shown no difference in outcome between culture-positive and culture-negative necrotising otitis externa patients who were treated with a dual antibiotic course,Reference Rubin and Yu5, Reference Loh and Loh23 one should always consider fungal necrotising otitis externa, and close monitoring is of vital importance. In cases where no improvement is observed, obtaining deep tissue culture may assist in the isolation of offending pathogens. The use of polymerase chain reaction in deep tissue cultures for the detection of fungal necrotising otitis externa has been reported previouslyReference Gruber, Roitman, Doweck, Uri, Shaked-Mishan and Kolop-Feldman12 and should be carried out whenever possible.
Common antibiotic regimens for the treatment of diabetic foot osteomyelitis include fluoroquinolone, clindamycin and rifampin.Reference Peters and Lipsky24 Similar to necrotising otitis externa, further treatment is based on the pathogen's antibiotic profile.
Surgery
The indications for surgical interventions in necrotising otitis externa have changed dramatically since the introduction of anti-pseudomonal antibiotics. While clear guidelines are lacking, surgery has been reported in the following circumstances: (1) for debridement of necrotic tissue;Reference Mahdyoun, Pulcini, Gahide, Raffaelli, Savoldelli and Castillo6, Reference Hollis and Evans10 (2) when obtaining deep tissue biopsies, especially in culture-negative patients;Reference Hollis and Evans10, Reference Gruber, Roitman, Doweck, Uri, Shaked-Mishan and Kolop-Feldman12 (3) for surgical exploration in refractory cases;Reference Mahdyoun, Pulcini, Gahide, Raffaelli, Savoldelli and Castillo6, Reference Gruber, Roitman, Doweck, Uri, Shaked-Mishan and Kolop-Feldman12 and (4) for facial nerve decompression in the presence of facial palsy.Reference Karaman, Yilmaz, Ibrahimov, Haciyev and Enver25 To date, there is no consensus regarding the timing and extent of surgery in the setting of necrotising otitis externa.
Although the role of surgery in the treatment of diabetic foot osteomyelitis has been a matter of debate in recent years, surgery is performed much more commonly and earlier in the course of the disease as compared to necrotising otitis externa. Surgery in the treatment of diabetic foot osteomyelitis is considered when clinical resolution is not achieved, following a week of antibiotic therapy or in cases where bone stability is endangered.Reference Lew and Waldvogel15 Surgical intervention aims to remove all necrotic bone and soft tissue, and enables bone biopsy for definitive culture testing. External fixation and amputation is considered, based on the location and extent of disease.Reference Lew and Waldvogel15, Reference Marais, Ferreira, Aldous, Sartorius and Le Roux26
Glycaemic control
Although diabetes is the most important risk factor for the development of necrotising otitis externa, there are limited data regarding the correlation between diabetes severity and disease extent. Stevens et al.,Reference Stevens, Lambert, Baker and Mayer27 and Loh and Loh,Reference Loh and Loh23 reported that poor diabetic control was not an indicator of poor prognosis for necrotising otitis externa. Carfrea and Kesser,Reference Carfrea and Kesser28 and Hollis and Evans,Reference Hollis and Evans10 on the other hand, recommended strict glycaemic control as a treatment for necrotising otitis externa.
The effect of diabetes severity and prognosis in the setting of diabetic foot osteomyelitis has been addressed in the literature. Elevated haemoglobin A1C levels have been reported as a risk factor for foot amputation and ulcer formation.Reference Davis, Norman, Bruce and Davis29 Moreover, the 2012 diabetic foot infection guidelines recommend that glycaemic control should be achieved among diabetic foot osteomyelitis patients prior to discharge from hospital.Reference Lipsky, Berendt, Cornia, Pile, Peters and Armstrong13
Follow up
Periodic nuclear imaging has been recommended for evaluating treatment response in the setting of necrotising otitis externa. By using repeated technetium-99 and gallium-67 scanning during and at the end of the treatment course, the physician gains objective information regarding ongoing bone disease. Despite the high sensitivity and specificity of nuclear imaging, there have been reports of false negative results in necrotising otitis externa patients.Reference Grandis, Branstetter and Yu30, Reference Chen and Hsieh31 Inflammatory markers such as leukocyte count, sedimentation rate and C-reactive protein, combined with periodic physical examinations, may also be used for assessing disease progression. Loh and Loh reported a reduction of 21.7 per cent in mean erythrocyte sedimentation rates among patients with resolved necrotising otitis externa, compared to patients with persistent disease.Reference Loh and Loh23 The same trend was also observed in C-reactive protein rates.
In contrast to necrotising otitis externa, follow-up evaluation in diabetic foot osteomyelitis is based mostly on close physical evaluation. Although no systemic parameters have been shown to correlate with long-term resolution of diabetic foot osteomyelitis, current recommendations indicate that any changes in systemic inflammatory markers should raise the suspicion for diabetic foot osteomyelitis relapse.Reference Lipsky, Berendt, Cornia, Pile, Peters and Armstrong13 Re-imaging at the end of treatment is not recommended in the setting of diabetic foot osteomyelitis.
Discussion
Despite the obvious differences between diabetic foot osteomyelitis and necrotising otitis externa, one cannot overlook the similarities in aetiology, predisposing factors, pathology and, as a consequence, the similarity in the treatment algorithm for these conditions. Based on the present comparison, it is difficult to recommend major changes in the treatment algorithm for necrotising otitis externa. However, certain aspects of the current algorithm for diabetic foot osteomyelitis might be useful also for necrotising otitis externa patients. A summary of the differences and similarities of the two conditions is presented in Table 1.
Table 1. Comparison between necrotising otitis externa and diabetic foot osteomyelitis
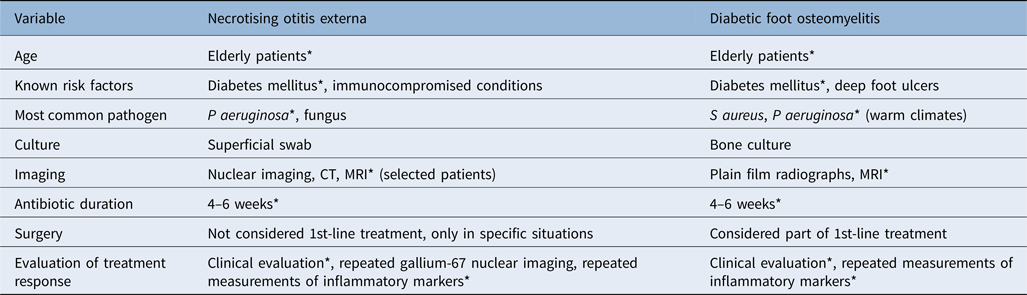
*Indicates similarities. CT = computed tomography; MRI = magnetic resonance imaging
The similarities regarding patient population raises basic questions concerning the pathophysiological effects of long-standing diabetes on the external ear. Further histological data are required to establish these effects, and these may change our approach to necrotising otitis externa. Moreover, if these effects exist, the role of primary prevention and periodic evaluation among high-risk patients should be strongly considered, as in the case of diabetic foot osteomyelitis prevention.
As mentioned earlier, isolated pathogens from diabetic foot osteomyelitis culture correlate with superficial swab cultures in less than 50 per cent of cases. There are no data to indicate whether this is similar in necrotising otitis externa; however, if this holds true, the role of early surgical debridement, and deep tissue and bone cultures, should be re-evaluated. This correlation is especially important in the setting of suspected fungal necrotising otitis externa, as local steroid and antibiotic treatment may lead to changes in the normal flora of the external ear, causing secondary fungal infection.
Although nuclear imaging has traditionally been recommended for the diagnosis of necrotising otitis externa over MRI, we could not find studies comparing the two. On the one hand, it is possible that resolution restrictions in the detection of microscopic bony changes, and the lack of medulla in the aerated mastoid bone, reduce the sensitivity and specificity of MRI in the detection of necrotising otitis externa compared to reported data in diabetic foot osteomyelitis. On the other hand, MRI has been found to be useful in the setting of advanced disease and suspected skull base osteomyelitis.Reference Peleg, Perez, Raveh, Berelowitz and Cohen17 Stevens et al. presented a case series of necrotising otitis externa patients and divided them into two groups based on severity.Reference Stevens, Lambert, Baker and Mayer27 They concluded there might be a subgroup of necrotising otitis externa patients who do not respond to parenteral antibiotics and local debridement. It is probable that this subgroup of patients would benefit from MRI as the initial imaging of choice.
Follow up in necrotising otitis externa patients has traditionally advocated the use of repeated nuclear imaging in order to assess treatment response. This is not a common practice in the evaluation of diabetic foot osteomyelitis, and if repeated imaging is required, MRI is the imaging modality of choice. Although white blood cell count and inflammatory markers are not consistently elevated in necrotising otitis externa, similar to diabetic foot osteomyelitis, any elevation in these parameters during treatment raises suspicion of disease relapse and should be addressed promptly.
Conclusion
Necrotising otitis externa and diabetic foot osteomyelitis share common features of local pathology secondary to diabetes. Primary prevention has not been defined in necrotising otitis externa, unlike in diabetic foot osteomyelitis, and should be considered. Magnetic resonance imaging and early surgical debridement may be employed earlier in advanced necrotising otitis externa patients. It is important to examine the correlation between swab findings from the external ear canal and deep tissue cultures, in order to conclude on proper antimicrobial coverage. Similar to diabetic foot osteomyelitis, treatment response in necrotising otitis externa can be evaluated by physical evaluation and measurement of inflammatory markers, rather than repeated imaging.
Competing interests
None declared



