Introduction
The proboscis provides some of the most important morphological characters used in acanthocephalan taxonomy (Van Cleave, Reference Van Cleave1953). Many acanthocephalan species are discriminated from congeners on the basis of the size and form of the proboscis, and especially the number, arrangement, size and shape of the proboscis hooks (Petrochenko, Reference Petrochenko1956; Yamaguti, Reference Yamaguti1963; Golvan, Reference Golvan1969). Furthermore, patterns of serial variation in hook morphometrics have been used to detect cryptic species (Huffman and Bullock, Reference Huffman and Bullock1975; Wayland, Reference Wayland2010).
During a helminthological survey of Chinese marine fishes, many specimens of Neorhadinorhynchus Yamaguti, Reference Yamaguti1939 were collected from the frigate tuna Auxis thazard Lacépède (Perciformes: Scombridae) in the South China Sea. Examination of this material using light and scanning electron microscopy revealed three distinct morphotypes, characterized by the length of the proboscis, and the number of hooks per longitudinal row. One of these morphotypes conformed to the diagnosis of Neorhadinorhynchus nudus (Harada, Reference Harada1938), a common parasite of scombrid and carangid fishes in the Pacific Ocean and Red Sea (Harada, Reference Harada1938; Yamaguti, Reference Yamaguti1939, Reference Yamaguti1963; Amin and Nahhas, Reference Amin and Nahhas1994; Hassanine, Reference Hassanine2006), and was used in a redescription of the taxon (Li et al., Reference Li, Chen and Yang2018). The other two morphotypes were suspected to represent a different, as yet undescribed species. In the present study, we tested the hypothesis that these three morphotypes correspond to three distinct lineages, by analysing nuclear and mitochondrial DNA sequences from a few individuals of each morphotype. The cytochrome c oxidase subunit 1 (cox1) gene and internal transcribed spacer (ITS) region were selected with the expectation that they would reveal population structure and discriminate congeneric species (Král'ová-Hromadová et al., Reference Král'ová-Hromadová, Tietz, Shinn and Spakulová2003; Steinauer et al., Reference Steinauer, Nickol and Ortí2007). The more slowly evolving 18S rRNA was chosen to provide resolution at higher taxonomic ranks (García-Varela and Nadler, Reference García-Varela and Nadler2005).
Material and methods
Morphological observation
Specimens of N. nudus collected from the intestine of A. thazard in the South China Sea (off Shanwei, Guangdong Province, China) were kept in tap water for several hours until the proboscis everted, and then fixed and stored in 80% ethanol until studied. Acanthocephalans were identified to the specific level based on the following morphological characters: the morphology and size of the trunk, proboscis, proboscis receptacle and uterine bell, the number of longitudinal rows of proboscis hooks and the hooks per longitudinal row, the number and size of the cement glands and testes, and the length of the neck and uterus. For light microscopy, the worms were cleared in lactophenol and examined as wet mounts. Drawings of the proboscis were made with the aid of a Nikon microscope drawing attachment. Voucher specimens (accession numbers: HBNU–F-A-2017005L, HBNU–F-A-2018002L, HBNU–F-A-2018003L) are deposited in College of Life Sciences, Hebei Normal University, Hebei Province, P. R. China.
Molecular procedures
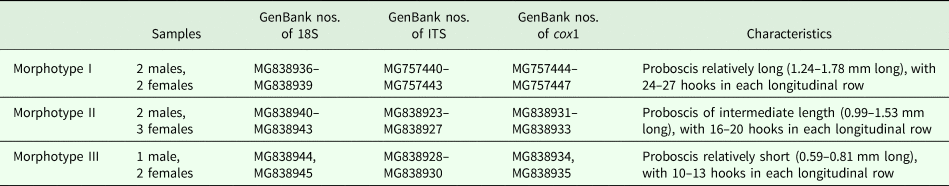
A total of 12 specimens representing the three morphotypes were selected for molecular analysis (see Table 1 for details). Genomic DNA from each individual was extracted using a Column Genomic DNA Isolation Kit (Shanghai Sangon, China) according to the manufacturer's instructions. DNA was resuspended in elution buffer and kept at −20 °C until use.
Table 1. Specimens of Neorhadinorhynchus nudus collected from Auxis thazard (Lacépède) (Perciformes: Scombridae) in the South China Sea selected for molecular analysis

Part of the gene encoding the small subunit 18S rRNA was amplified by polymerase chain reaction (PCR) using the forward primer (5′-AGATTAAGCCATGCATGCGT-3′) and the reverse primer (5′-GCAGGTTCACCTACGGAAA-3′) (Garey et al., Reference Garey, Near, Nonnemacher and Nadler1996). A segment of the cox1 gene was amplified by PCR using the forward primer (5′-GGTCAACAAATCATAAAGATATTGG-3′) and the reverse primer (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) (Goméz et al., Reference Goméz, Serra, Carvalho and Lunt2002). A region of the ITS was amplified by PCR using the forward primer (5′-GTCGTAACAAGGTTTCCGTA-3′) and the reverse primer (5′-TATGCTTAAATTCAGCGGGT-3′) (Král'ová-Hromadová et al., Reference Král'ová-Hromadová, Tietz, Shinn and Spakulová2003). The cycling conditions were as described previously (Li et al., Reference Li, Chen, Amin and Yang2017). PCR products were checked on GoldView-stained 1.5% agarose gels and purified with a Column PCR Product Purification Kit (Shanghai Sangon). Sequencing was carried out using a DyeDeoxyTerminator Cycle Sequencing Kit (v.2, Applied Biosystems, California, USA) and an automated sequencer (ABI-PRISM 377, South Carolina, USA). Sequencing of each sample was carried out for both strands.
Sequences were aligned using ClustalW2 (Larkin et al., Reference Larkin, Blackshields, Brown, Chenna, McGettigan, McWilliam, Valentin, Wallace, Wilm, Lopez, Thompson, Gibson and Higgins2007) with some manual adjustment. The DNA sequences obtained herein were compared (using the algorithm BLASTn) with those available in the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov). The functional consequences of nucleotide polymorphisms in the cox1 sequences were assessed by translating them into amino acid sequences using the EMBOSS program Transeq (Rice et al., Reference Rice, Longden and Bleasby2000; Li et al., Reference Li, Cowley, Uludag, Gur, McWilliam, Squizzato, Park, Buso and Lopez2015) with the invertebrate mitochondrial codon table.
Phylogenetic analyses A haplotype network was constructed from the cox1 DNA sequence data using the method of statistical parsimony (Templeton et al., Reference Templeton, Crandall and Sing1992) and an infinite sites model, as implemented in the R package pegas (Paradis, Reference Paradis2010). Phylogenetic trees for the cox1 haplotypes were inferred using both maximum parsimony (MP) and maximum likelihood (ML), as implemented in MEGA7 (Kumar et al., Reference Kumar, Stecher and Tamura2016). Filisoma bucerium Van Cleave, 1940 (DQ089722) was selected as the outgroup, because it is the only other species of the family Cavisomidae for which a DNA barcode is available. The N. nudus and F. bucerium cox1 sequences were aligned using the MUSCLE algorithm (Edgar, Reference Edgar2004) implemented in MEGA7, with the default alignment parameters, and then refined manually. The optimal nucleotide substitution model for the cox1 dataset was determined to be the Hasegawa–Kishino–Yano with invariant sites (Hasegawa et al., Reference Hasegawa, Kishino and Yano1985). Bootstrap resampling (n = 1000) was used to quantify clade support.
Results
Morphological analysis
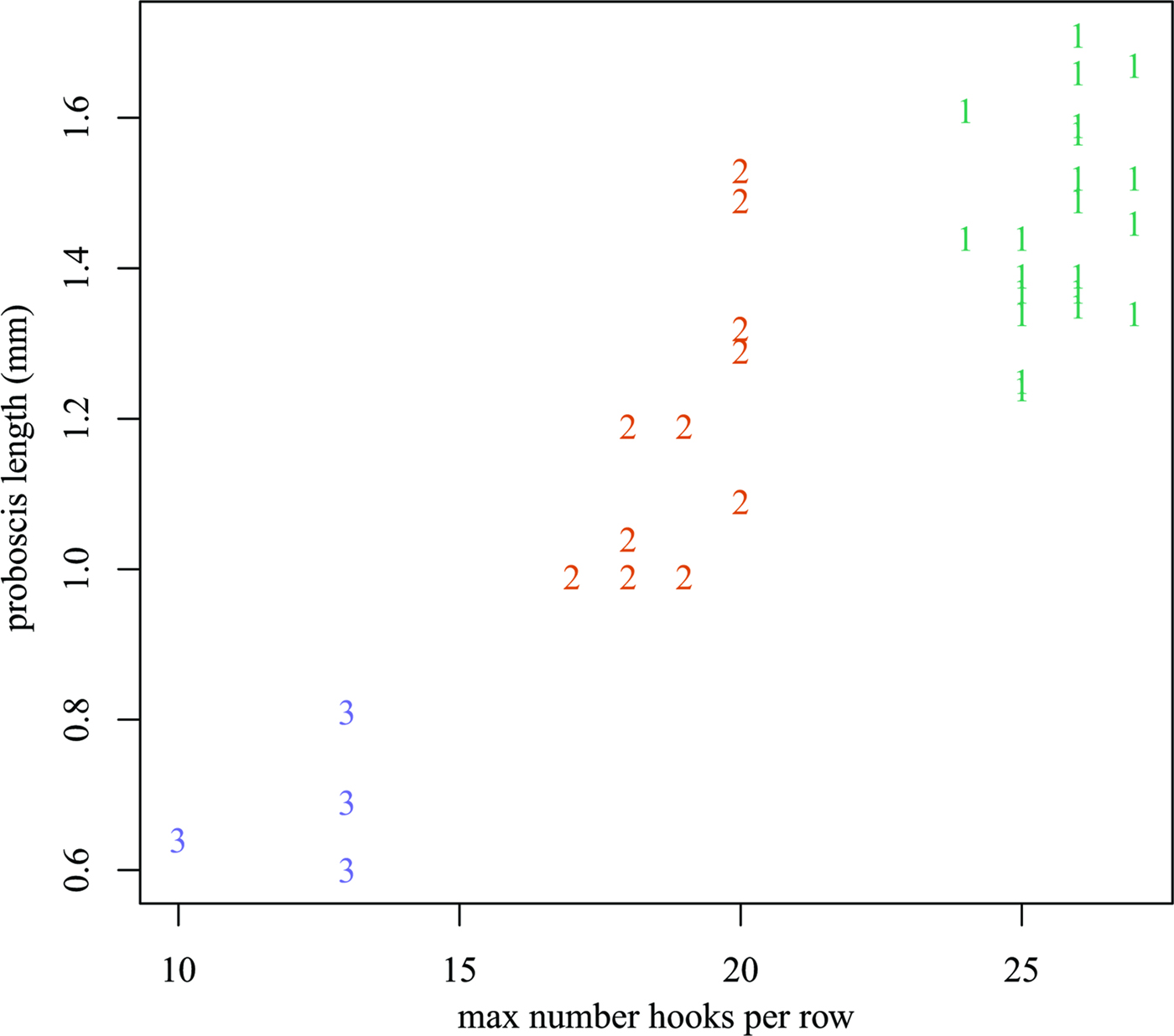
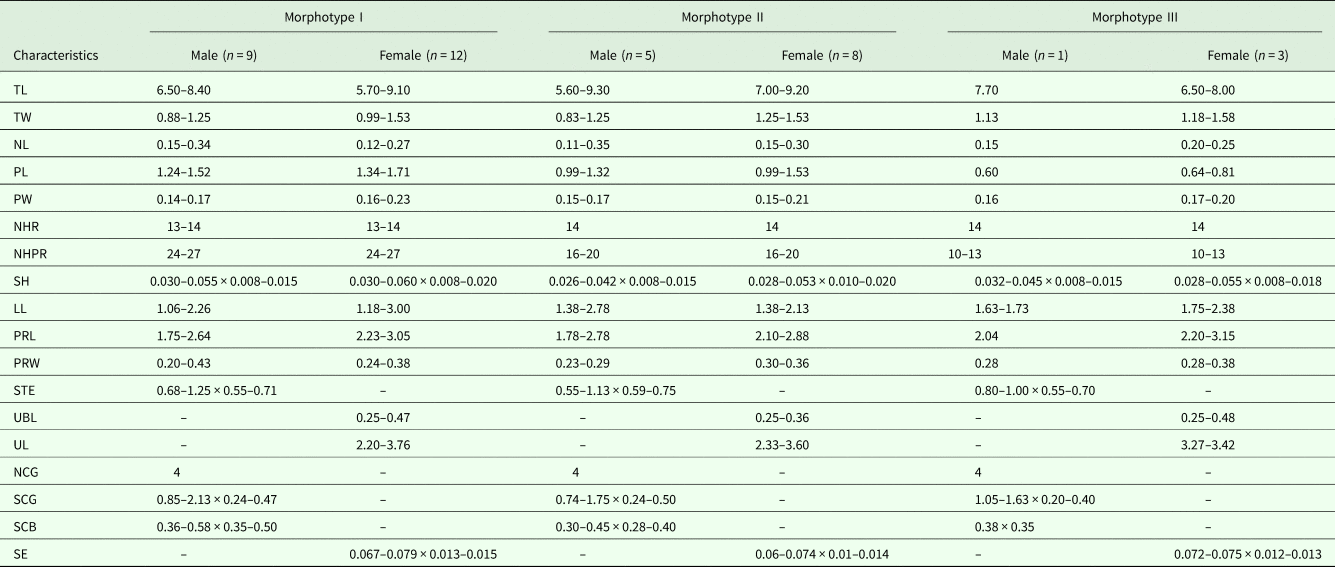
A total of 38 individuals of N. nudus were collected from three specimens of A. thazard. Based on the number of hooks per longitudinal row and the length of proboscis (Fig 1 and Table 1), these acanthocephalans were readily classified into three morphotypes, designated I, II and III. All three morphotypes were present in each of the host fish examined. Morphotype I had the longest proboscis (1.24–1.78 mm) and the most hooks per longitudinal row (24–27). Morphotype III had the shortest proboscis (0.59–0.81 mm) and the smallest number of hooks per longitudinal row (10–13). Morphotype II had a proboscis that was intermediate in form between those of the other two morphotypes, with a length of 0.99–1.53 mm and 16–20 hooks per longitudinal row. Morphometric and meristic data for the three morphotypes are provided in Table 2. Anatomical differences between the three morphotypes appear to be restricted to the number of hooks per longitudinal row and the length of the proboscis. Pairwise scatter plots and principal component analysis failed to find additional combinations of morphometric and/or meristic variables which might discriminate the three morphotypes (Fig 2).

Fig. 1. Scanning electron micrographs of the different number of hooks per longitudinal row and the length of the proboscis of Neorhadinorhynchus nudus (Harada, Reference Harada1938) from Auxis thazard (Lacépède) (Perciformes: Scombridae) in the South China Sea. (A) Morphotype I with the longest proboscis and 24–27 hooks per longitudinal row; (B) morphotype III with the shortest proboscis and 10–13 hooks per longitudinal row; (C) morphotype II with a proboscis of intermediate length and 16–20 hooks per longitudinal row.
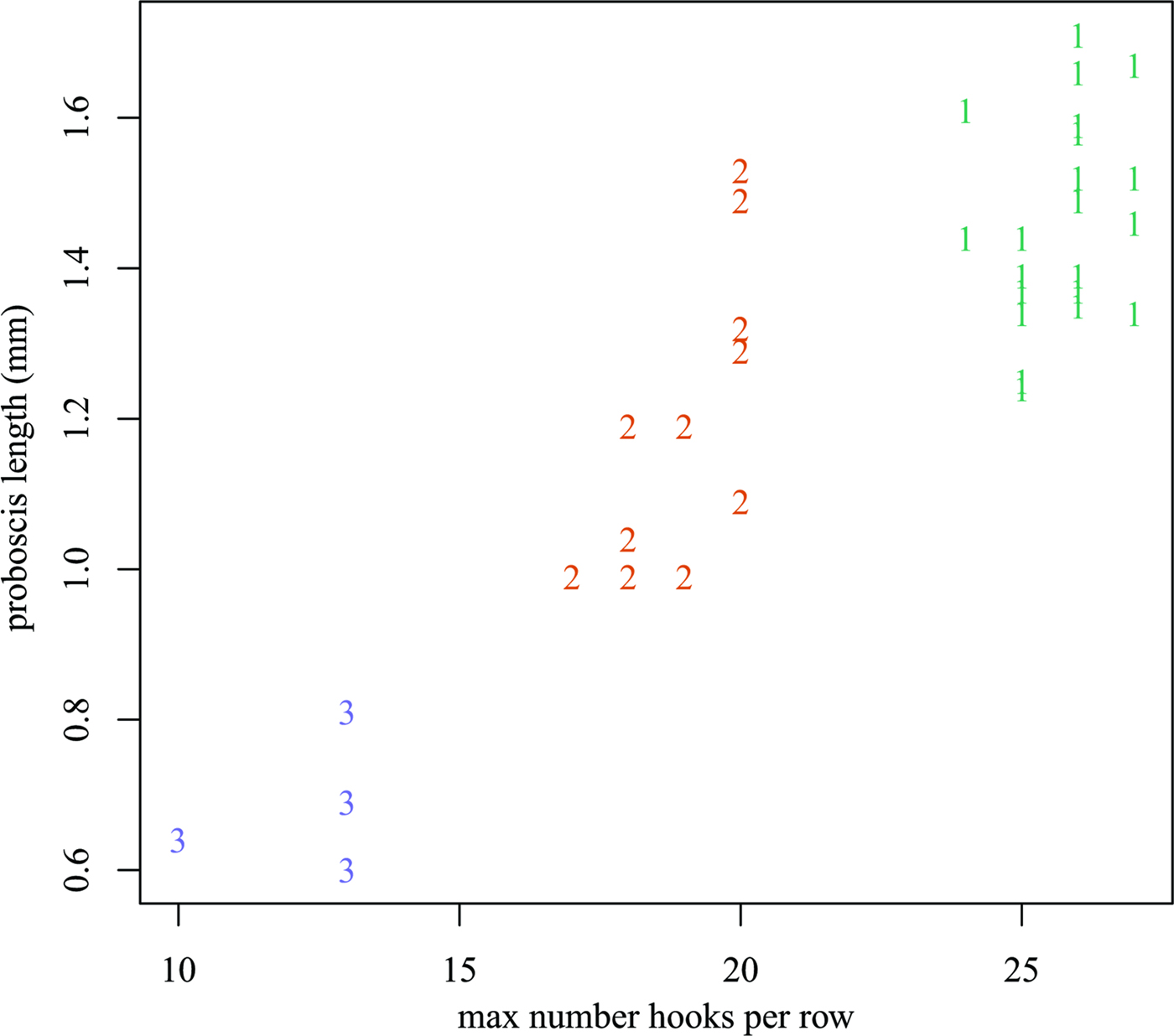
Fig. 2. Scatterplot of proboscis length (mm) vs the maximum number of hooks per longitudinal row. Plotting symbols indicate the morphotype.
Table 2. Morphometric comparisons of the different morphotypes of Neorhadinorhynchus nudus from Auxis thazard (Lacépède) (Perciformes: Scombridae) in the South China Sea (mm)

TL, trunk length; TW, trunk width; NL, neck length; PL, proboscis length; PW, proboscis width; NHR, number of longitudinal rows of proboscis hooks; NHPR, number of hooks per longitudinal row; SH, size of hooks; LL, lemnisci length; PRL, proboscis receptacle length; PRW, proboscis receptacle width; STE, size of testes; UBL, uterine bell length; UL, uterus length; NCG, number of cement glands; SCG, size of cement glands; SCB, size of copulatory bursa; SE, size of eggs. Morphological data of morphotype I is from Li et al. (Reference Li, Chen and Yang2018).
Molecular characterization
Partial 18S rDNA region
Partial 18S rDNA sequences, 1201 bp in length, were obtained from ten specimens of N. nudus (four from morphotype I, four from morphotype II and two from morphotype III). All ten sequences are identical. They have been deposited in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) under accession numbers MG838936–MG838945. There are only two other cavisomid species F. bucerium Van Cleave, 1940 and F. rizalinum Tubangui & Masilungan, 1946 with 18S rDNA data registered in GenBank (https://www.ncbi.nlm.nih.gov/genbank/). Nucleotide divergence between N. nudus and F bucerium (AF064814) was 14.5%, whereas divergence between N. nudus and F. rizalinum (JX014229) was 21.7%.
Partial ITS region
Partial ITS sequences, 559 bp in length, were obtained from 12 specimens of N. nudus (four from morphotype I, five from morphotype II and three from morphotype III). All sequences are identical. They have been deposited in GenBank under accession numbers MG757440–MG757443 and MG838923–MG838930. GenBank contains ITS sequence data for only one other cavisomid species, F. bucerium (AF286305). The ITS sequences of F. bucerium and N. nudus display over 40% nucleotide divergence.
Partial cox1 region
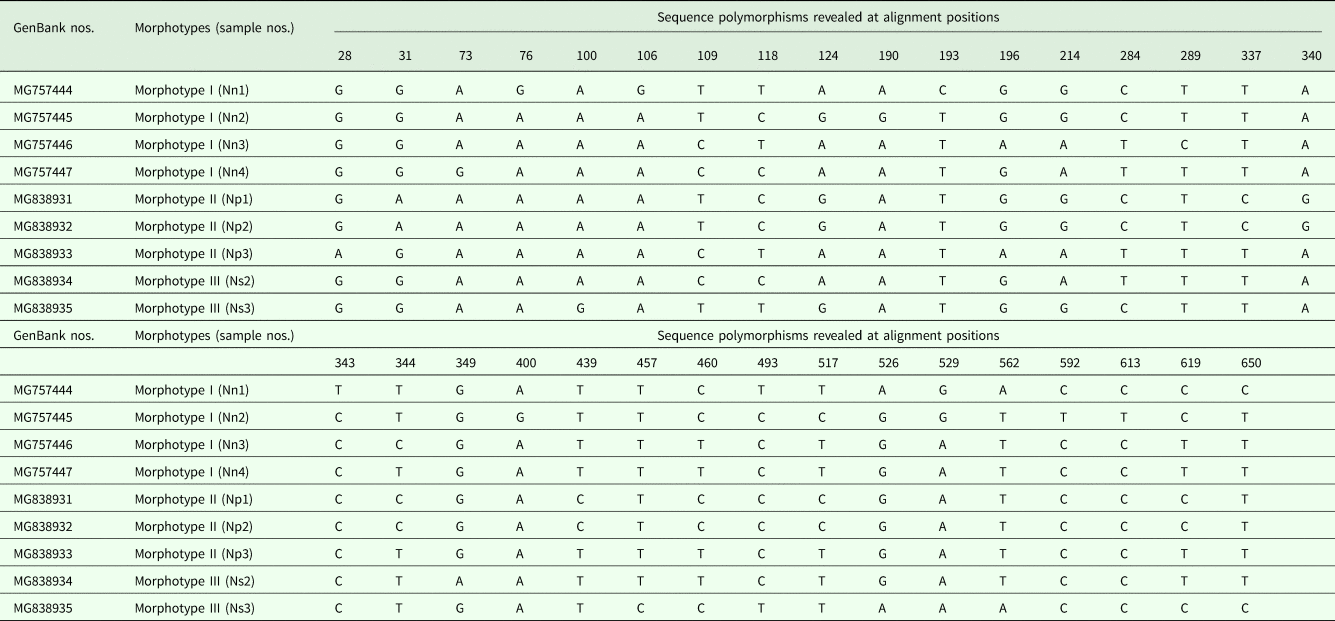
Partial cox1 sequences, 669 bp in length, were obtained from nine specimens of N. nudus (four from morphotype I, three from morphotype II and two from morphotype III) and represented eight unique haplotypes. The two specimens sharing the same haplotype belonged to morphotype II. Nucleotide polymorphisms were found at 33 (4.9%) of the sites; 30 (4.5%) at a third codon position and three (0.4%) at a first codon position (sequences were read in frame 2). Translation of the partial cox1 nucleotide sequences demonstrated that they all encode the same sequence of amino acids. Nucleotide divergence between pairs of haplotypes ranged from 0.30 to 2.54% (see Table 3 for details). The cox1 sequences of N. nudus are deposited in GenBank under accession numbers MG757444–MG757447 and MG838931–MG838935. There is only one other cavisomid species F. bucerium with cox1 data (DQ089722) registered in GenBank, and pairwise comparison between N. nudus and F. bucerium showed over 30.0% nucleotide differences in the cox1 region.
Table 3. Sequence polymorphisms revealed at alignment positions among the cox1 sequences of Neorhadinorhynchus nudus obtained in the present study

Phylogenetic analyses
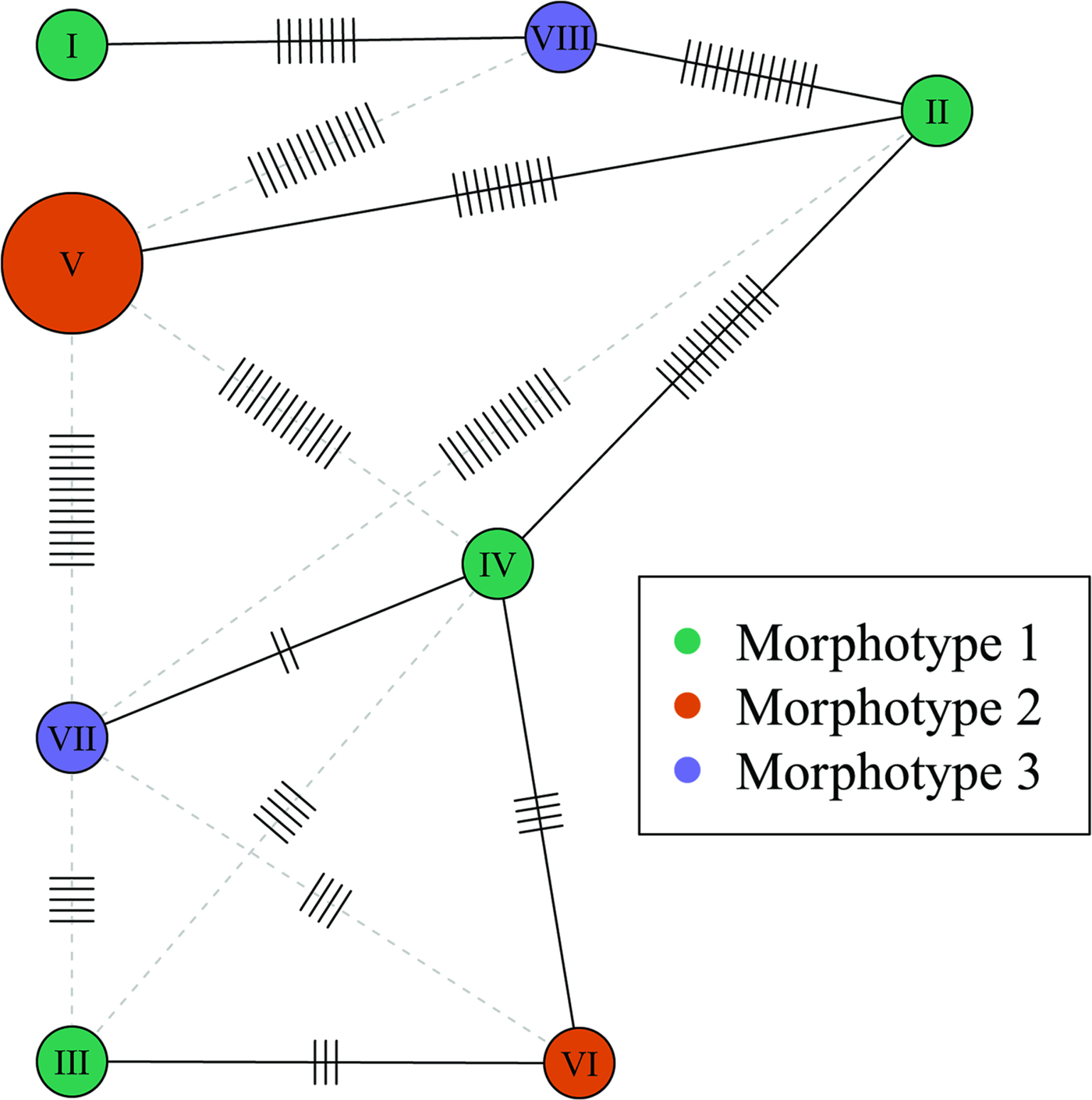
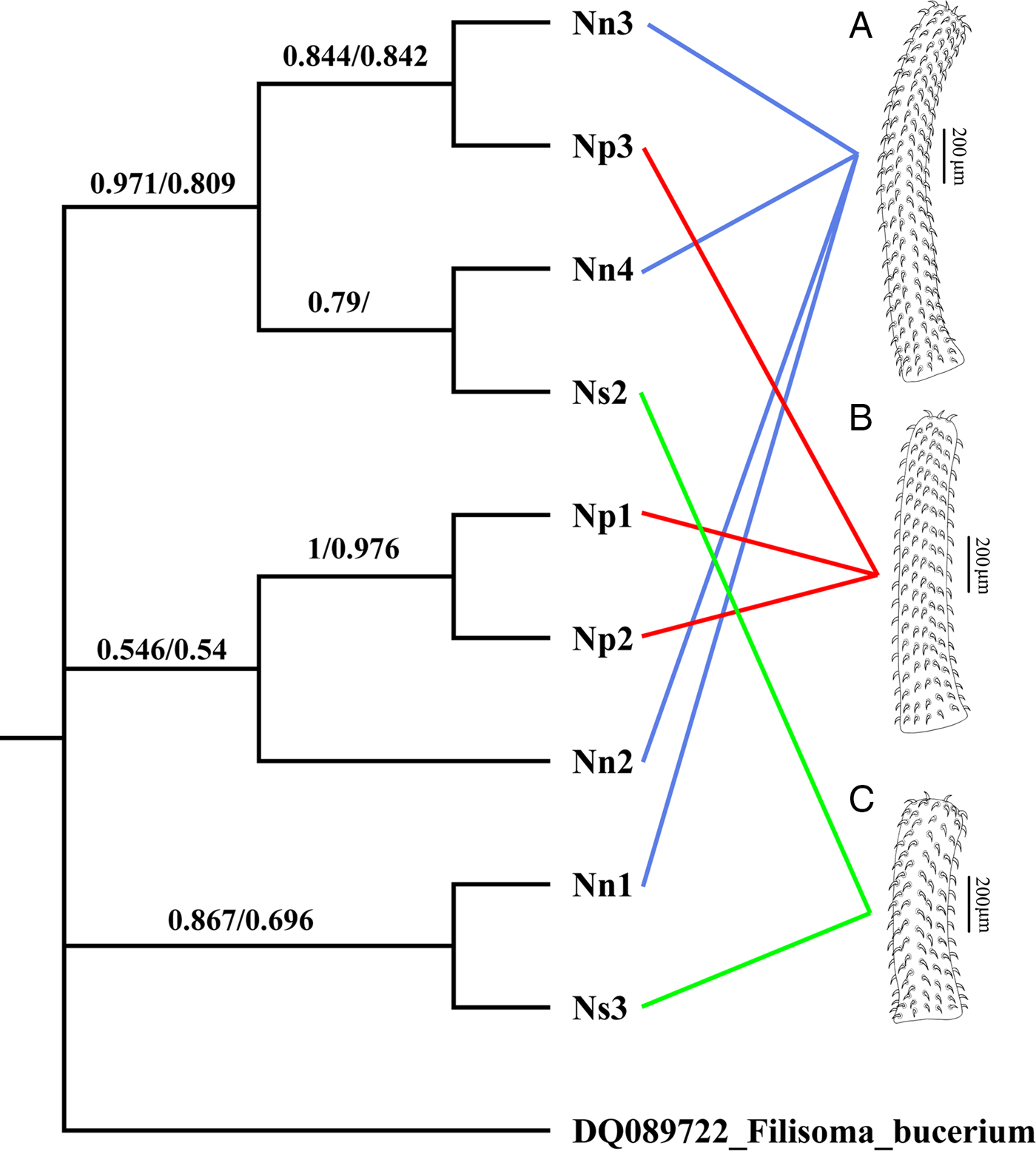
The cox1 haplotype network (Fig. 3) shows no evidence that the three morphotypes represent distinct genetic lineages. For example, haplotypes IV and VII, exhibiting the most divergent phenotypes (morphotypes I and III, respectively) differ by only two nucleotide substitutions. Moreover, one of the two joint longest links in the minimum spanning tree (13 nucleotide substitutions) connects haplotypes II and IV, both of which exhibit morphotype I. Phylogenetic trees constructed from the cox1 dataset using ML and MP had almost an identical topology, but there were small differences in clade support (Fig. 4). None of the three morphotypes formed a monophyletic group in these trees. Moreover, there was strong bootstrap support (97% in the ML tree) for the clade comprising samples Nn3, Np3, Nn4 and Ns2, which includes representatives of all three morphotypes.
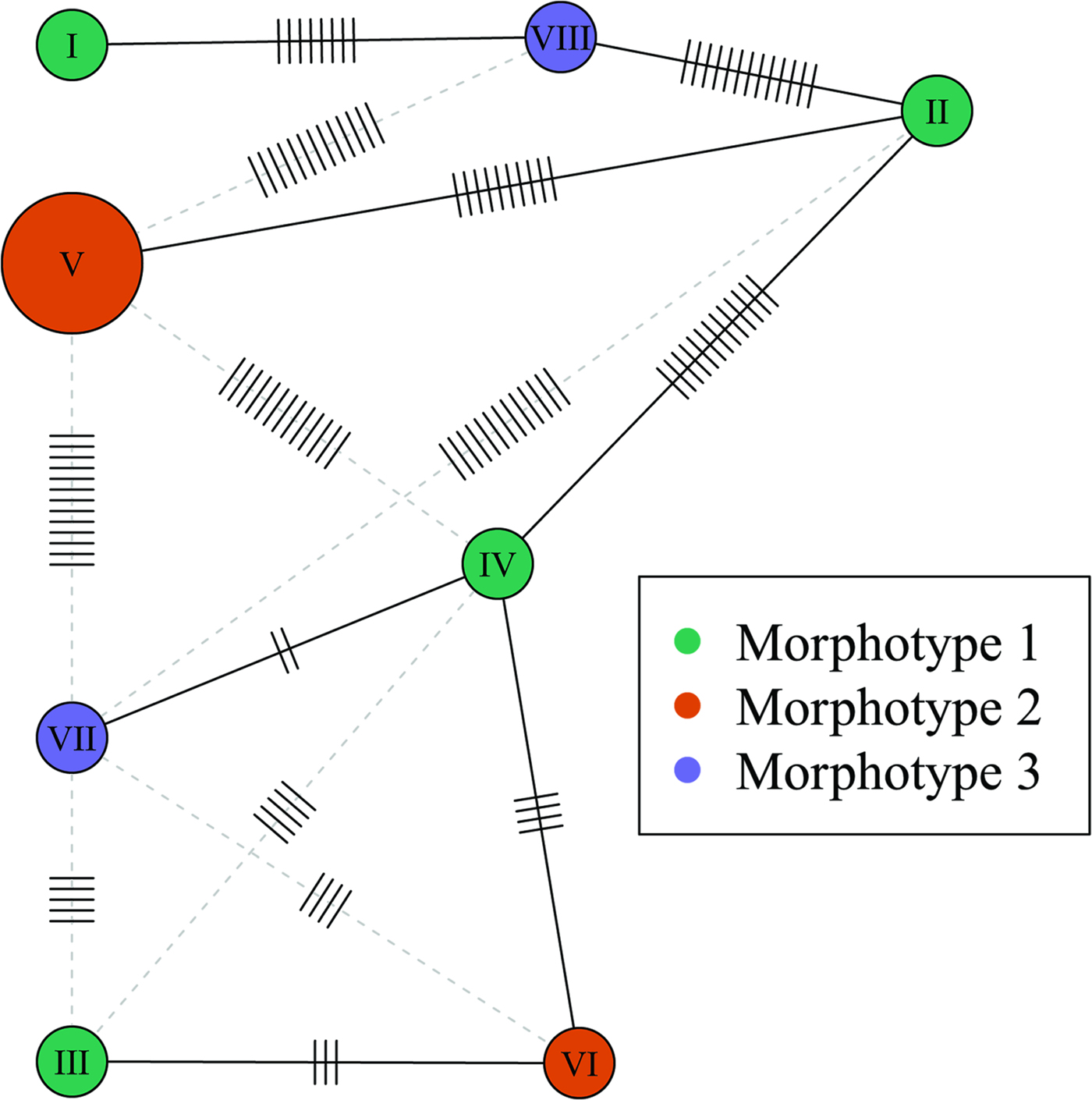
Fig. 3. Haplotype network based on the partial cox1 sequences. Node size is proportional to haplotype frequency. Node colour indicates the morphotype. The minimum spanning tree is represented by solid black links (edges). Alternate links are shown as grey-dashed lines. Mutations are indicated by small segments on the links. Haplotypes correspond to the following samples: I, Nn1; II, Nn2; III, Nn3; IV, Nn4; V, Np1 and Np2; VI, Np3; VII, Ns2; VIII, Ns3.
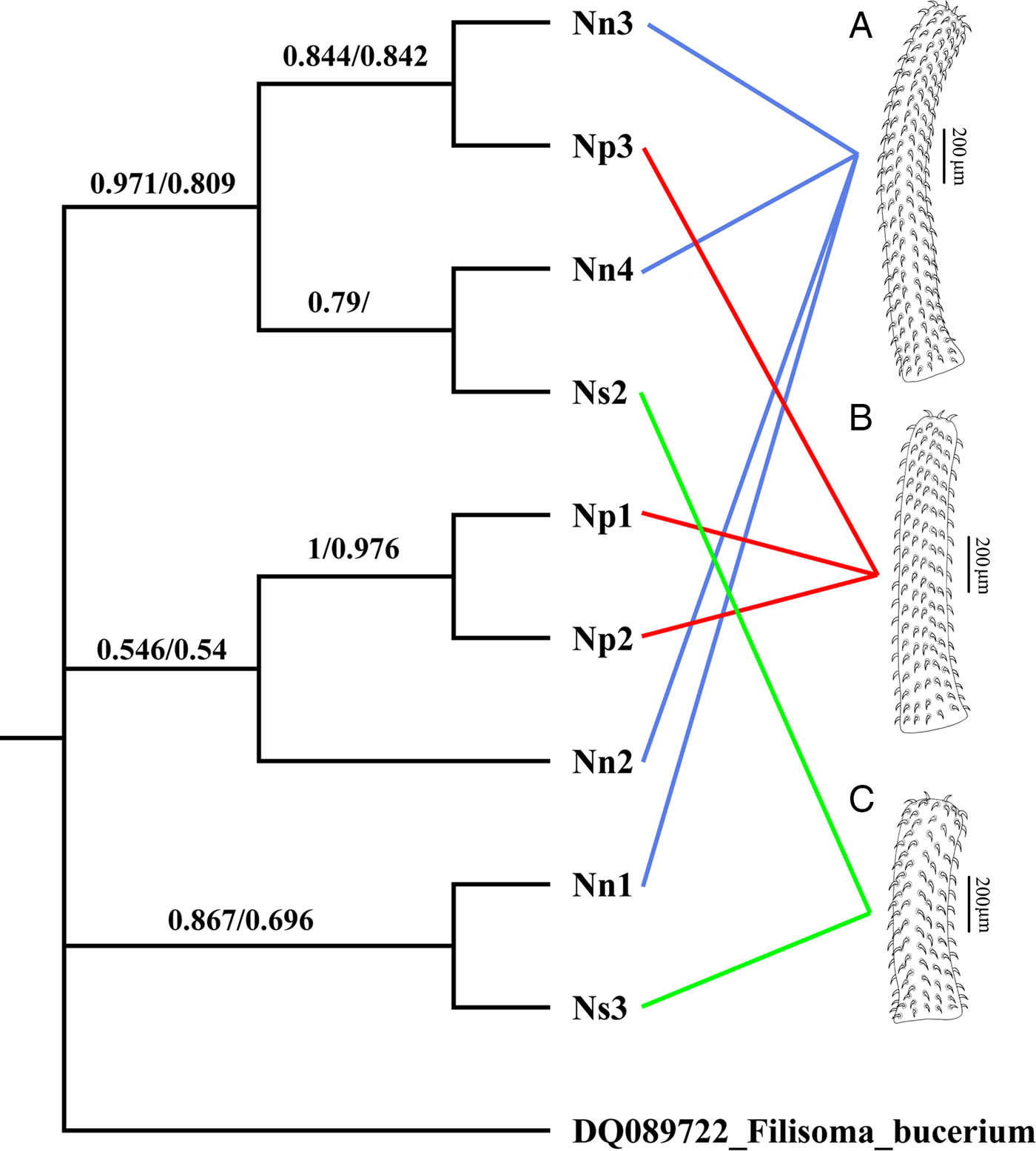
Fig. 4. ML tree showing the phylogenetic relationships of nine specimens of Neorhadinorhynchus nudus (Harada, Reference Harada1938) from Auxis thazard (Lacépède) (Perciformes: Scombridae) in the South China Sea based on the mitochondrial cox1 sequences. Numbers at the nodes are the clade credibility values (%) for each method of phylogeny reconstruction (ML/MP). The tree is rooted on the outgroup Filisoma bucerium Van Cleave, 1940 (DQ089722). (A) Morphotype I with 24–27 hooks per longitudinal row; (B) morphotype II with 16–20 hooks per longitudinal row; (C) morphotype III with 10–13 hooks per longitudinal row.
Discussion
In recent years, acanthocephalan taxonomists have started to augment their morphological descriptions of new species with DNA sequence data (e.g. Amin et al., Reference Amin, Evans, Heckmann and El-Naggar2013; Tkach et al., Reference Tkach, Lisitsyna, Crossley, Binh and Bush2013; Braicovich et al., Reference Braicovich, Lanfranchi, Farber, Marvaldi, Luque and Timi2014; Li et al., Reference Li, Chen, Amin and Yang2017). Nevertheless, the vast majority of the c. 1300 currently recognized species (Amin, Reference Amin2013) were defined under the traditional morphospecies concept (Cain, Reference Cain1953; Ruse, Reference Ruse1969). Within Neorhadinorhynchus, none of the eight currently recognized species were characterized using molecular markers when they were originally described (Fukui and Morisita, Reference Fukui and Morisita1937; Harada, Reference Harada1938; Golvan, Reference Golvan1969; Mordvinova, Reference Mordvinova1988; Amin and Nahhas, Reference Amin and Nahhas1994; Amin and Ha, Reference Amin and Ha2011; Smales et al., Reference Smales, Al-Hasson, Al-Niaeem and Al-Azizz2015). However, Li et al. (Reference Li, Chen and Yang2018) used both morphological and molecular characters in their redescription of N. nudus.
The acanthocephalans collected in the present study exhibit far greater variation in proboscis characters than has previously been reported among conspecifics. The three morphotypes are clearly delimited by disjunct ranges for the number of hooks per longitudinal row, and so under the morphospecies concept they would be considered distinct taxa. However, analysis of the cox1 sequence data provides convincing evidence that these morphotypes do not represent monophyletic groups. Moreover, the level of genetic variation within this collection of worms is a strong indication that they are systematically homogeneous.
Across the animal kingdom, uncorrected nucleotide divergence in the cox1 gene between populations, sibling species and morphologically distinct congeneric species is on average 0.89, 3.78 and 11.06%, respectively (Kartavtsev, Reference Kartavtsev2011). More specifically, within the acanthocephalan order Echinorhynchida, Steinauer et al. (Reference Steinauer, Nickol and Ortí2007) found that for pairwise comparisons of six closely related species of Leptorhynchoides Kostylev, 1924, the proportion of nucleotide substitutions in the cox1 gene ranged from 6.3 to 11.6%. Divergence within these six taxa ranged from 0.4 to 2.8%. Therefore, the 0.3–2.54% divergence observed in the present study is strongly indicative of an intraspecific comparison. Identical ITS sequences for all sequenced individuals of N. nudus are further evidence for the systematic homogeneity of this collection of acanthocephalans. In an interspecific or even an interpopulation comparison, nucleotide variation in the ITS region would be expected (Král'ová-Hromadová et al., Reference Král'ová-Hromadová, Tietz, Shinn and Spakulová2003). Nucleotide polymorphisms in the slowly evolving 18S rRNA gene were neither anticipated nor found, but this DNA sequence will be a valuable reference for future studies examining the phylogenetic relationships of higher taxa within the Acanthocephala (García-Varela and Nadler, Reference García-Varela and Nadler2005).
Molecular genetic studies on other morphologically variable acanthocephalan taxa have shown that the morphospecies concept often conflates cryptic species (e.g. Buron et al., Reference Buron, Renaud and Euzet1986; Väinölä et al., Reference Väinölä, Valtonen and Gibson1994; Steinauer et al., Reference Steinauer, Nickol and Ortí2007; Martínez-Aquino et al., Reference Martínez-Aquino, Reyna-Fabián, Rosas-Valdez, Razo-Mendivil, Ponce de León and García-Varela2009). This study highlights the opposite, less commonly reported problem, where the use of the morphospecies concept would result in the splitting of a biological species into genetically indistinct taxa. The present study is not the first record of clearly defined morphotypes within an acanthocephalan species. Li et al. (Reference Li, Chen, Amin and Yang2017) observed two phenotypes in Pomphorhynchus zhoushanensis Li et al. (Reference Li, Chen, Amin and Yang2017). In one the neck bulb was symmetrical and in the other asymmetrical. Analysis of molecular markers confirmed that the two forms were conspecific (Li et al., Reference Li, Chen, Amin and Yang2017).
The most recent key to the species of Neorhadinorhynchus Yamaguti, Reference Yamaguti1939 relied almost exclusively on proboscis characters (Amin and Nahhas, Reference Amin and Nahhas1994), which the current study has shown may lack the stability required for delimiting taxa in this genus. When the key was devised, N. nudus was thought to exhibit 24–25 hooks per longitudinal row (Amin and Nahhas, Reference Amin and Nahhas1994). The number of hooks per row observed in the present study (10–27) encompasses almost the full range of variation found across the entire genus. N eorhadinorhynchus basrahiensis Smales et al., Reference Smales, Al-Hasson, Al-Niaeem and Al-Azizz2015 has the fewest hooks per longitudinal row, with a reported range of 9–10 (Smales et al., Reference Smales, Al-Hasson, Al-Niaeem and Al-Azizz2015). N eorhadinorhynchus atypicalis Amin and Ha, Reference Amin and Ha2011 also displays 27 hooks per longitudinal row (Amin and Ha, Reference Amin and Ha2011), which is the maximum number reported for a species of this genus. Tandem molecular phylogenetic and morphological analyses will be required to determine the true species diversity in Neorhadinorhynchus and to identify the best anatomical characters for differential diagnosis of these taxa.
The cause of the broad anatomical variation in the proboscis of N. nudus can only be speculated. It may be the result of phenotypic plasticity, i.e. ‘the property of individual genotypes to produce different phenotypes when exposed to different environmental conditions’, such as temperature or nutrient availability (Pigliucci et al., Reference Pigliucci, Murren and Schlichting2006). Many of the studies that have attempted to identify sources of phenotypic variation in acanthocephalans (e.g. Grabda-Kazubska and Ejsymont, Reference Grabda-Kazubska and Ejsymont1969; Amin, Reference Amin1975; Buckner and Nickol, Reference Buckner and Nickol1975; Amin and Redlin, Reference Amin and Redlin1980; Amin, Reference Amin1984; Shostak et al., Reference Shostak, Dick, Szalai and Bernier1986; Brown, Reference Brown1987) were conducted at a time when molecular systematics was in its infancy, and so did not make use of genetic data to differentiate intraspecific from interspecific variation. Recently, Sobecka et al. (Reference Sobecka, Szostakowska, MacKenzie, Hemmingsen, Prajsnar and Eydal2012) investigated intraspecific morphological variation in a species of the Echinorhynchus gadi Zoega in Müller, 1776 group, after first using molecular markers to confirm that their collection of acanthocephalans was systematically homogenous. Morphological variation was subtle, compared with that found in the present study, and showed an association with geographical locality, definitive host subspecies and size of the acanthocephalan infrapopulation.
Morphogenesis of the acanthocephalan proboscis, including hooks, occurs in the acanthella larva, within the intermediate host (Schmidt, Reference Schmidt, Crompton and Nickol1985). Therefore, to identify factors that might influence this process, both the immediate environment of the acanthella and the habitat of the intermediate host must be considered. For example, different species of intermediate hosts might present different environments for the acanthella in terms of nutrient availability, physical space and, if they occupy different habitats, temperature regime.
The development of Echinorhynchus truttae Schrank, 1788 (another member of the Echinorhynchida) in its intermediate host Gammarus pulex (L.) is influenced by infection intensity, which is presumably a proxy for the magnitude of competition for resources, such as nutrients and space. Awachie (Reference Awachie1966) found that in heavily infected intermediate hosts, cystacanths of this species were smaller and more slender than those developing under less crowded conditions, and in some cases were malformed or irregularly shaped. If the intermediate host(s) of N. nudus can be identified, and maintained in the laboratory, phenotypic plasticity could be investigated via experimentation.
In summary, the present study has shown that N. nudus displays far greater intraspecific variation in proboscis characters than has previously been reported for an acanthocephalan species. This variation is partitioned into three morphotypes, each of which might erroneously be recognized as a distinct taxon in the absence of genetic data. We recommend that when species of Acanthocephala are described or redescribed, appropriate molecular markers (e.g. cox1 or ITS sequences) should be used to distinguish intraspecific from interspecific morphological variation, and to provide a reference for diagnostic purposes.
Acknowledgements
We are grateful to Associate Professor Rui-Yong An (Hebei Normal University, Shijiazhuang, P. R. China) for identifying the fish host.
Financial support
This study was supported by the National Natural Science Foundation of China (NSFC-31872197) and Youth Top Talent Support Program of Hebei Province to Dr Liang Li.
Conflict of interest
None.
Ethics approval
This study was conducted according to the Hebei Normal University Experiments in Animals Policy and was approved by the Animal Ethics Committee of Hebei Normal University as complying with the Animal Protection Law of the People's Republic of China.