Introduction
The immune system in higher vertebrates involves both innate and adaptive immune responses (Pancer and Cooper, Reference Pancer and Cooper2006). Innate immune mechanisms, which exist in all organisms from bacteria to humans, provide immediate frontline protection against infections (Beutler, Reference Beutler2004; Pancer and Cooper, Reference Pancer and Cooper2006). Evidence from both the host and virus indicates that invoking early and appropriate innate immune responses is critical for the outcome of most viral diseases, determining whether an infection is controlled or whether a persistent infection develops (Beutler, Reference Beutler2004; Pancer and Cooper, Reference Pancer and Cooper2006). The critical role of innate immune cells and their components not only dominates antiviral activity in the early phase of infection, but also potentiates the adaptive immune system for viral clearance (Hoebe et al., Reference Hoebe, Janssen and Beutler2004; Kabelitz and Medzhitov, Reference Kabelitz and Medzhitov2007). In this review, we provide a brief discussion of the major components of the innate antiviral immune system with particular emphasis on porcine-specific features. Our emphasis is on the response to and subversion of porcine innate antiviral immunity during infection caused by porcine reproductive and respiratory syndrome virus (PRRSV). For more general discussions about the challenges of PRRSV immunology and vaccinology, the reader is referred to several recent reviews (Kimman et al., Reference Kimman, Cornelissen, Moormann, Rebel and Stockhofe-Zurwieden2009; Darwich et al., Reference Darwich, Díaz and Mateu2010; Yoo et al., Reference Yoo, Song, Sun, Du, Kim and Liu2010).
Overview of mammalian innate antiviral immune system
Innate immune cells
A highly differentiated immune system appears to offer an evolutionary advantage to higher vertebrates. Nonetheless, all nucleated cells are readily capable of mounting innate immune responses upon exposure to a virus (Beutler, Reference Beutler2004). Mammalian innate immune cells, which are specialized for various functions such as pathogen recognition and killing, immune surveillance and antigen presentation, include granulocytes, natural killer (NK) cells, macrophages and dendritic cells (DCs), as well as epithelial and endothelial cells. Granulocytes, macrophages and NK cells are well documented for their role as effector cells in engulfing and digesting microorganisms or promoting active death of infected cells (Ludwig et al., Reference Ludwig, Geijtenbeek and van Kooyk2006; Appelberg, Reference Appelberg2007; Takeuchi and Akira, Reference Takeuchi and Akira2007; Vivier et al., Reference Vivier, Tomasello, Baratin, Walzer and Ugolini2008). Depending on their anatomical locations, epithelial and endothelial cells, as well as DCs and macrophages, are among the first groups of cells initially exposed to viruses (Barchet et al., Reference Barchet, Cella and Colonna2005; Opitz et al., Reference Opitz, Hippenstiel, Eitel and Suttorp2007; Wen et al., Reference Wen, Schaller, Dou, Hogaboam and Kunkel2008; Hammad and Lambrecht, Reference Hammad and Lambrecht2008). Their function in viral recognition and immune surveillance facilitates coordination of subsequent immune responses by linking to adaptive immunity.
Innate immune cells are dually functional as ‘sensors’ and ‘effectors’. Even cells that are primarily associated with killing, e.g., neutrophils, facilitate immune surveillance via Toll-like receptors (TLRs) (Haselmayer et al., Reference Haselmayer, Tenzer, Kwon, Jung, Schild and Radsak2006; Borregaard et al., Reference Borregaard, Sørensen and Theilgaard-Mönch2007). Furthermore, professional antigen presenting cells, such as conventional DCs, potently destroy engulfed pathogens through autophagy (Schmid et al., Reference Schmid, Dengjel, Schoor, Stevanovic and Münz2006; Lee and Iwasaki, Reference Lee and Iwasaki2008). This functional plasticity is best known in macrophages, which are composed of diverse subgroups of professional phagocytes as well as immunoregulatory cells (Figure 1; Hashimoto et al., Reference Hashimoto, Moki, Takizawa, Shiratsuchi and Nakanishi2007; Kumagai et al., Reference Kumagai, Takeuchi, Kato, Kumar, Matsui, Morii, Aozasa, Kawai and Akira2007; Randolph et al., Reference Randolph, Jakubzick and Qu2008). Because innate immune cells such as macrophages, epithelial cells and endothelial cells, often serve as the initial foothold for viral infection, these cells provide an excellent platform for examining virus–host interaction, viral recognition, signaling transduction and antiviral effector function (Opitz et al., Reference Opitz, Hippenstiel, Eitel and Suttorp2007; Hammad and Lambrecht, Reference Hammad and Lambrecht2008; Wen et al., Reference Wen, Schaller, Dou, Hogaboam and Kunkel2008).

Fig. 1. Innate immune homeostasis and interaction with viral infection. Innate immune cells exert antiviral activities of immune surveillance and direct inactivation using various virus-sensing and effector molecules. Whereas most viral attacks are controlled through innate immunity and synergistic induction of adaptive immunity (including vaccine-induced), virus isolates or species that have the capability to divert innate immunity, especially type I IFN responses, alter innate immune balance causing pandemic diseases (Katze et al., Reference Katze, Fornek, Palermo, Walters and Korth2008).
Accumulated evidence indicates that upon viral infection the overall immune response is dependent on the coordination of innate immune cells to exert immune surveillance and to produce immune effectors. The balance resulting from immune regulation or vaccine-induced innate-immune activation will elicit both innate and adaptive immunity to control virus replication. In contrast, if viruses divert the induction of innate immunity, especially a type I interferon (IFN) response, it is likely that disease will result from uncontrolled viral infections (Figure 1; Katze et al., Reference Katze, Fornek, Palermo, Walters and Korth2008). DCs and activated macrophages are antigen-presenting cells, which directly bridge innate and adaptive immunity. In addition, some newly defined groups of innate immune cells including innate lymphoid cells (ILCs), natural helper cells (NHCs) and innate type 2 helper cells (ih2), which are crucial for the development of lymphoid structures and secrete cytokines similar to those from T helper cells, are also important players that bridge innate and adaptive immunity. However, their role in antiviral responses remains obscure (Sawa et al., Reference Sawa, Cherrier, Lochner, Satoh-Takayama, Fehling, Langa, Di Santo and Eberl2010; Saenz et al., Reference Saenz, Noti and Artis2010; Veldhoen and Withers, Reference Veldhoen and Withers2010).
Monocytes represent 10–30% of all peripheral blood mononuclear cells (PBMC) and are progenitors of most tissue macrophages (Randolph et al., Reference Randolph, Jakubzick and Qu2008; Cassol et al., Reference Cassol, Cassetta, Alfano and Poli2010). These myeloid monocytes circulate for about 1–2 days before migrating into peripheral tissues and differentiating into resident tissue macrophages (Martinez et al., Reference Martinez, Helming and Gordon2009). Macrophages are anatomically and functionally heterogeneous. Tissue-specific macrophages are specific to anatomic locations including blood monocytes, peritoneal macrophages, pulmonary macrophages, Kupffer cells in the liver and microglia in the brain (Taylor et al., Reference Taylor, Martinez-Pomares, Stacey, Lin, Brown and Gordon2005; Naito, Reference Naito2008; Randolph et al., Reference Randolph, Jakubzick and Qu2008). Even within an organ, macrophages are further categorized based on their micro-anatomical location. The lung provides an example of this macrophage categorization. For example, pulmonary macrophages can be divided into three subgroups based on their microenvironment within the lung: pulmonary alveolar macrophages (PAMs), pulmonary intravascular macrophages (PIMs) and interstitial macrophages (ISMs) (Naito, Reference Naito2008; Randolph et al., Reference Randolph, Jakubzick and Qu2008).
PAMs are the most abundant pulmonary immune cells in the alveolus located at the interface between air and lung tissues. Substantial numbers of ISMs are detected within the lung stroma and PIMs are mature phagocytes adhered to capillary endothelial cells within the lung that cover approximately 16% of the lung capillary surface in species such as pigs and ruminants (Chitko-McKown and Blecha, Reference Chitko-McKown and Blecha1992). With respect to airway viral infections, PAMs are first to express early activation for scavenging and killing mucus-trapped viral particles through phagocytosis. To fulfill this task, activated PAMs are equipped with a variety of surface and internal receptors to detect antibody/complement-engaged viral particles (by surface Fc or complement receptors) and to recognize viral components by membrane-associated or cytosolic receptors (Fantuzzi et al., Reference Fantuzzi, Belardelli and Gessani2003; Beisswenger and Bals, Reference Beisswenger and Bals2005; Daffis et al., Reference Daffis, Samuel, Keller, Gale and Diamond2007; Kumagai et al., Reference Kumagai, Takeuchi, Kato, Kumar, Matsui, Morii, Aozasa, Kawai and Akira2007). PAMs are very active killer cells known to inactivate trapped viruses with both oxidative and non-oxidative mechanisms and to lower the chance of airborne viruses initializing infections on pneumocytes as well as pulmonary endothelial cells (Taylor et al., Reference Taylor, Martinez-Pomares, Stacey, Lin, Brown and Gordon2005; Naito, Reference Naito2008; Randolph et al., Reference Randolph, Jakubzick and Qu2008). PAMs are activated through phagocytosis or receptor recognition of viral components. They are active producers of type I IFNs and other pro-inflammatory cytokines, which lead to antiviral responses including the regulatory loop by type I IFNs and recruitment of other immune cells to infection sites (Kumagai et al., Reference Kumagai, Takeuchi, Kato, Kumar, Matsui, Morii, Aozasa, Kawai and Akira2007; Takeuchi and Akira, Reference Takeuchi and Akira2007). Normally, PAMs represent >90% of immune cells in bronchoalveolar lavage (BAL) fluid prior to the increase of granulocytes in BAL fluid after an infection (White et al., Reference White, Tecle, Crouch and Hartshorn2007). PAMs have been identified as the primary IFN-α producer in murine models upon respiratory viral infections (Kumagai et al., Reference Kumagai, Takeuchi, Kato, Kumar, Matsui, Morii, Aozasa, Kawai and Akira2007; Takeuchi and Akira, Reference Takeuchi and Akira2007).
Compared to PAMs, ISMs are less phagocytic and potentially more active in repairing tissue damage. PIMs are highly phagocytic and mainly exist in ruminants, pigs and horses (Longworth, Reference Longworth1997). Compared to PAMs, porcine PIMs are almost equally permissive to PRRSV infection and their bactericidal and phagocytic activities are significantly suppressed by PRRSV infection much like PAMs (Thanawongnuwech et al., Reference Thanawongnuwech, Halbur and Thacker2000).
The importance of macrophages in an antiviral immune response is also reflected by the virus’ ability to surpass this first line of surveillance allowing for a much higher chance of escaping innate immune defenses in turn causing persistent infections. Several viruses possess the ability to directly infect and undermine immune responses (such as production and signaling of type I IFNs) of macrophages (Table 1). For example, macrophages infected by human immunodeficiency virus-1 (HIV-1) and PRRSV are functionally compromised in many ways including cytokine production, receptor expression, phagocytosis and antigen presentation (Ieong et al., Reference Ieong, Reardon, Levitz and Kornfeld2000; Martinelli et al., Reference Martinelli, Cicala, Van Ryk, Goode, Macleod, Arthos and Fauci2007; Darwich et al., Reference Darwich, Díaz and Mateu2010; Thanawongnuwech and Suradhat, Reference Thanawongnuwech and Suradhat2010). In this regard, direct infection of macrophages may divert overall homeostasis of cell activation status causing exacerbating co-infections and complicated syndromes.
Table 1. Innate immune evasion mechanisms of various monocytotropic viruses

* Based on information from PAMs and other cells. ASFV, African swine fever virus; CSFV, classical swine fever virus; HIV1, human immune-deficiency virus-1; HRSV, human respiratory syncytial virus; IVA: human (swine) Influenza A; SAR, severe acute respiratory syndrome coronavirus; SeV, mouse Sendai virus; PCV2, porcine circovirus-2; PFMDV, porcine foot-and-mouth disease virus; PRRSV, porcine respiratory and reproductive syndrome virus; PrV, porcine pseudorabies virus. See figure legends for other abbreviations.
Macrophages have been classified into two major types according to their activation status and functional difference: the classical type 1 macrophages (M1) and alternative type 2 macrophages (M2). M1 cells are conventionally activated by Th1 cytokines such as IFN-γ and IL-12. Alternatively, M2 macrophages are more heterogeneous than M1 cells depending on the stimuli. M2a macrophages are induced by Th2 cytokines such as IL-4/IL-13, while M2b macrophages are activated by immune complexes, TLR stimulation, or by IL-1Ra. M2c macrophages are generated by stimulation with the immune suppressive cytokine IL-10 and glucocorticoids (Martinez et al., Reference Martinez, Helming and Gordon2009; Herbein and Varin, Reference Herbein and Varin2010; Odegaard and Chawla, Reference Odegaard and Chawla2011). In general, M1 macrophages are pro-inflammatory and induce cellular immunity with higher microbicidal as well as tissue-destructive activities. M2a/b cells are anti-inflammatory and induce humoral immunity with higher anti-parasite, tissue repairing as well as allergic activities. In contrast, deactivated M2c macrophages are primarily immunosuppressive along with secretion of anti-inflammatory cytokines (Martinez et al., Reference Martinez, Helming and Gordon2009; Herbein and Varin, Reference Herbein and Varin2010; Odegaard and Chawla, Reference Odegaard and Chawla2011). Accumulated evidence indicates that macrophages are functionally plastic cells with the potential to alter their activities progressively and reversibly in response to changes in the tissue environment (Stout et al., Reference Stout, Watkins and Suttles2009). For example, mouse peripheral monocytes or peritoneal macrophages shift from M2a to M2c or from M1 to M2 after sequential treatment with corresponding cytokines (Stout et al., Reference Stout, Jiang, Matta, Tietzel, Watkins and Suttles2005). Thus, hypothetically, macrophages at different activation statuses vary in their antimicrobial activity or capacity to resolve tissue damage from infections.
The link between macrophage polarization and virus infection has recently been studied in HIV-1 and respiratory syncytial virus (RSV)-infected human and murine cells (Cassol et al., Reference Cassol, Cassetta, Rizzi, Alfano and Poli2009; Shirey et al., Reference Shirey, Pletneva, Puche, Keegan, Prince, Blanco and Vogel2010). In vitro polarization of human blood monocytes into M1 cells prevents HIV-1 infection and M2a polarization inhibits viral replication at a post-integration level but facilitates macrophage-mediated transmission of HIV-1 to CD4+ T cells (Cassol et al., Reference Cassol, Cassetta, Rizzi, Alfano and Poli2009). In mice, RSV infection induces acute inflammatory responses at the early phase, but macrophages are skewed to the M2 phenotype progressively during the later phase of infection (Shirey et al., Reference Shirey, Pletneva, Puche, Keegan, Prince, Blanco and Vogel2010). This alternative transition of macrophages counteracts the early inflammatory responses for resolution of tissue damage, but decreases the higher antiviral ability associated with the M1 state, which may help prevent virus escape from innate immune surveillance. In addition, if the M2 phenotype persists, it may lead to a ‘Th2-skewed’ adaptive immune response resulting in hypersensitivity to allergies and autoimmune diseases (Shirey et al., Reference Shirey, Pletneva, Puche, Keegan, Prince, Blanco and Vogel2010). The increase of deactivated M2c macrophages in response to IL-10 and glucocorticoids at a later phase of viral infection decreases aggressive immune responses thus limiting pathological damage. However, some viruses, such as RSV and possibly PRRSV, may escape this mechanism facilitating the development of persistent/chronic infection. Therefore, the macrophage polarization scheme provides a valuable and straightforward framework for investigating the complexity of host–virus interaction in macrophages (Herbein and Varin, Reference Herbein and Varin2010).
There is broad divergence regarding permissiveness of porcine macrophages to PRRSV infection and although the activation status of macrophage subsets is critical in supporting virus infection and replication, this scheme has yet to be studied in pigs. In this respect, the differential expression of virus receptors [including heparin, sialoadhesin (CD169) and the scavenger receptor CD163] and immunomodulating cytokines should be scrutinized in different subsets of macrophages due to determinants correlating with macrophage activation and PRRSV permissiveness. Interestingly, in addition to membrane-bound CD163, a large amount of soluble CD163 is present in the circulation. It will be informative to determine how circulating soluble CD163 interacts with PRRSV (Van Gorp et al., Reference Van Gorp, Van Breedam, Delputte and Nauwynck2008; Patton et al., Reference Patton, Rowland, Yoo and Chang2009; Welch and Calvert, Reference Welch and Calvert2010). In addition, an increase of IL-10 and glucocorticoids as well as suppression of IFNs has been associated with PRRSV infection. It remains to be determined whether these changes contribute to deactivation of macrophages and thereby to PRRSV pathogenesis (Borghetti et al., Reference Borghetti, Saleri, Ferrari, Morganti, De Angelis, Franceschi, Bottarelli and Martelli2011).
Macrophages are abundant in mucosal membranes and, unlike DCs, do not migrate to distal tissues. PRRSV exists in tonsils and lymphoid nodes of infected piglets and a recent study of vertical transmission has shown that the targets of PRRSV replication are in the fetal thymus and presumably are monocytic (Rowland, Reference Rowland2010). Therefore, macrophages are likely contributors to PRRSV pathogenesis leading to infected DCs and blood monocytes, which may be more responsible for viral transmission in lymphatic tissues.
DCs represent a primary group of innate immune cells that bridge innate and adaptive immune responses (Iwasaki, Reference Iwasaki2007; Hammad and Lambrecht, Reference Hammad and Lambrecht2008; Wen et al., Reference Wen, Schaller, Dou, Hogaboam and Kunkel2008). The two major types of DCs are classified as conventional and plasmacytoid DCs (cDCs and pDCs, respectively). The cDCs are prominent antigen-presenting cells with high autophagy activity (a process to uptake an antigen). Although cDCs are very important in antigen presentation for T cells in secondary lymphoid tissues, no direct antiviral role of peripheral cDCs has been defined in a primary viral infection (Iwasaki, Reference Iwasaki2007; Hammad and Lambrecht, Reference Hammad and Lambrecht2008). The pDCs are known as natural IFN-producing cells for their high-level production of IFN-α after activation, which is essential for inducing a series of antiviral IFN-stimulated genes (ISGs) and establishing an antiviral state in surrounding cells (Barchet et al., Reference Barchet, Cella and Colonna2005; Sen and Sarkar, Reference Sen and Sarkar2007; Zuniga et al., Reference Zuniga, Hahm and Oldstone2007). To a lesser extent, pDC also produce TNF-α, IL-6 and IL-12, which are thought to influence T cells towards a Th1 response (Barchet et al., Reference Barchet, Cella and Colonna2005). When porcine pDCs are exposed to viral mimics (such as ligands for TLR7/8 and TLR9) and viruses [such as pseudorabies (PrV), swine influenza virus (SIV) and transmissible gastroenteritis coronavirus (TEGV)], the expression of IFN-α, TNF-α, IL-2, IL-6, and IFN-γ is significantly stimulated; however, production of IL-12 is only stimulated by the viral mimic and not viruses themselves. Significantly, the production of all of the aforementioned cytokines was not observed when pDCs were exposed to PRRSV, and IL-8 production was not responsive to both PRRSV and SIV (Calzada-Nova et al., Reference Calzada-Nova, Schnitzlein, Husmann and Zuckermann2010a). A small number of viruses, which include measles virus (MV), human RSV A2 strain and classical swine fever virus (CSFV), are able to infect DCs and subvert type I IFN synthesis and/or signaling to some extent (Table 1). In this regard, PRRSV is capable of infecting DCs, primarily monocyte-derived DCs (mDCs, representing mostly cDCs) but not lung DCs. Infection of mDCs by PRRSV significantly suppresses IFN-α but not IFN-β production (Loving et al., Reference Loving, Brockmeier and Sacco2007). PRRSV does not infect pDCs and the presence of either live or inactivated PRRSV did not induce IFN-α (Calzada-Nova et al., Reference Calzada-Nova, Schnitzlein, Husmann and Zuckermann2010b). In addition, PRRSV also inhibits the production of some pro-inflammatory cytokines including TNF-α and IL-6 but not IL-8 stimulated by a TLR9 agonist (Calzada-Nova et al., Reference Calzada-Nova, Schnitzlein, Husmann and Zuckermann2010a). However, CSFV-infected porcine pDCs had suppressed IFN-α production without inhibition of other cytokines (Carrasco et al., Reference Carrasco, Rigden, Vincent, Balmelli, Ceppi, Bauhofer, Tâche, Hjertner, McNeilly, van Gennip, McCullough and Summerfield2004). This suggests that the production and action of type I IFNs are primary innate antiviral responses targeted by multiple viruses for successful infection, whereas modulation of other innate immune responses are complementary for disease development (Table 1).
Cell-based viral recognition mechanisms
As illustrated in Figure 2, the innate immune system, similar to its adaptive counterpart, comprises both afferent and efferent arms to discriminate and kill pathogens (Beutler, Reference Beutler2004). In this context, animal cells use various receptors to perceive viral infections by recognizing pathogen-associated molecular patterns (PAMPs) culminating in the induction of antiviral responses (Pichlmair and Reis e Sousa, Reference Pichlmair and Reis e Sousa2007). Prominent among these receptors are TLRs, which are vertebrate homologues revealed and named after Drosophila Toll receptors (West et al., Reference West, Koblansky and Ghosh2006; Takeuchi and Akira, Reference Takeuchi and Akira2007). TLRs are critical for innate immune recognition and for inducing immune responses to most microbial infections (Beutler, Reference Beutler2004; West et al., Reference West, Koblansky and Ghosh2006). Mammalian genome projects reveal that each mammalian species has approximately 10 TLRs, which are functional for detection of a multitude of molecular ligands derived from various microorganisms (West et al., Reference West, Koblansky and Ghosh2006; Gay and Gangloff, Reference Gay and Gangloff2007). Six of these TLRs have been instrumental in the response to viral infection through sensing viral components (Akira et al., Reference Akira, Uematsu and Takeuchi2006; Pichlmair and Reis e Sousa, Reference Pichlmair and Reis e Sousa2007; Takeuchi and Akira, Reference Takeuchi and Akira2007). Among them, TLR2 and TLR4, hinged on the cell cytoplasmic membrane, recognize several viral proteins (Akira et al., Reference Akira, Uematsu and Takeuchi2006; Pichlmair and Reis e Sousa, Reference Pichlmair and Reis e Sousa2007; Takeuchi and Akira, Reference Takeuchi and Akira2007). The functional group of TLR3, TLR7, TLR8 and TLR9 sense viral nucleic acid, either virus-derived RNA or DNA molecules (Gay and Gangloff, Reference Gay and Gangloff2007; Forsbach et al., Reference Forsbach, Nemorin, Montino, Müller, Samulowitz, Vicari, Jurk, Mutwiri, Krieg, Lipford and Vollmer2008). Accordingly, these nucleic acid-sensing TLRs are responsive mainly in acidified intracellular compartments including late endosomes and lysosomes, where most viruses undergo a de-coating process in infection routes (Pichlmair and Reis e Sousa, Reference Pichlmair and Reis e Sousa2007). Besides TLR-mediated viral recognition, mainly in endosomal or lysomal compartments, animal cells also bear viral recognition and signaling mechanisms in the cytosol, where most viruses are obligated to carry out their entire or part of their infectious cycles (Pichlmair and Reis e Sousa, Reference Pichlmair and Reis e Sousa2007). Four cytosol pattern recognition receptors (PRRs), including retinotic acid inducible gene I protein (RIG-I), melanoma differentiation factor-5 (Mda5) and laboratory of genetics and physiology-2 (LGP2), recognize virus derived RNA, as well as a cytosol dsDNA sensor named DNA-dependent activator of IFN regulatory factors (IRFs) (DAI), have been collectively termed RIG-I-like receptors (RLRs) (Lee and Kim, Reference Lee and Kim2007; Pichlmair and Reis e Sousa, Reference Pichlmair and Reis e Sousa2007; Takaoka et al., Reference Takaoka, Wang, Choi, Yanai, Negishi, Ban, Lu, Miyagishi, Kodama, Honda, Ohba and Taniguchi2007). These cytosol receptors recognize distinct molecular patterns of virus-derived nucleic acids and signal the production of innate immune IFNs including types I and III IFNs (Takaoka and Yani, 2006, Reference Takaoka, Wang, Choi, Yanai, Negishi, Ban, Lu, Miyagishi, Kodama, Honda, Ohba and Taniguchi2007; Onoguchi et al., Reference Onoguchi, Yoneyama, Takemura, Akira, Taniguchi, Namiki and Fujita2007). Detailed information about the receptor ligand specificity and antiviral signaling transduction has been reviewed elsewhere (Akira et al., Reference Akira, Uematsu and Takeuchi2006; Onoguchi et al., Reference Onoguchi, Yoneyama, Takemura, Akira, Taniguchi, Namiki and Fujita2007; Pichlmair and Reis e Sousa, Reference Pichlmair and Reis e Sousa2007; Takeuchi and Akira, Reference Takeuchi and Akira2007; Takaoka et al., Reference Takaoka, Wang, Choi, Yanai, Negishi, Ban, Lu, Miyagishi, Kodama, Honda, Ohba and Taniguchi2007).
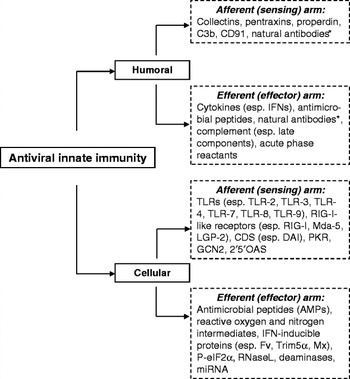
Fig. 2. Afferent and efferent arms of the innate antiviral immune response. Examples of humoral and cellular components of innate antiviral immune responses are indicated. *Natural antibodies belong to the ‘innate’ aspect of specific immunity. Abbreviations: CDS, cytosolic DNA sensor; DAI, DNA-dependent activator of IFN-regulatory factors; GCN2, general control nonderepressible-2; LGP-2, laboratory of genetics and physiology 2; Mda-5, melanoma differentiation-associated antigen 5; 2′5′OAS, 2′,5′-oligo-adenylate synthetase; PKR, protein kinase R; RIG-I, retinoic acid-inducible gene I; TLR, Toll-like receptor; TRIM5α, tripartite motif protein.
TLRs are conserved in pigs as indicated from gene sequences of porcine TLR1–11 (Shinkai et al., Reference Shinkai, Muneta, Suzuki, Eguchi-Ogawa, Awata and Uenishi2006a, Reference Shinkai, Tanaka, Morozumi, Eguchi-Ogawa, Okumura, Muneta, Awata and Uenishi2006b; Sang et al., Reference Sang, Yang, Ross, Rowland and Blecha2008a and unpublished data). In addition, porcine RIG-I and Mda5 of RLRs and the related adaptor protein of IFN-β promoter stimulator 1 (IPS-1) have been identified (Wang et al., Reference Wang, Fang, Li, Luo, Xie, Jiang, Chen and Xiao2008; Kojima-Shibata et al., Reference Kojima-Shibata, Shinkai, Morozumi, Jozaki, Toki, Matsumoto, Kadowaki, Suzuki and Uenishi2009). Pertaining to PRRSV infection, RNA helicase RIG-I has been shown to have differential expression in PRRSV infected tissues (Zhang et al., Reference Zhang, Wang, Schook, Hawken and Rutherford2000). We have shown that transcripts of TLR3, TLR7 and TLR9 are significantly stimulated in PRRSV infected lungs and macrophages by the North American type 2 PRRSV (Sang et al., Reference Sang, Yang, Ross, Rowland and Blecha2008a, Reference Sang, Ross, Rowland and Blechab and unpublished data). Using RNA library deep sequencing, Xiao et al. (Reference Xiao, Mo, Wang, Jia, Qin, Yu, Niu, Zhao, Liu and Chen2010a, Reference Xiao, Jia, Mo, Wang, Qin, He, Zhao, Huang, Li, Yu, Niu, Liu and Chenb) recently showed that multiple TLRs, RIG-I and Mda5 are stimulated by an emerged sub-strain of highly virulent Chinese-type PRRSV, but no stimulation of TLR3 was reported. It is not clear whether the highly virulent Chinese-type virus has a mechanism to suppress TLR3 gene transcription. However, we know that suppression of TLR3 expression increases PRRSV replication and infection in porcine cells (Sang et al., Reference Sang, Ross, Rowland and Blecha2008b). The antagonism of PRRSV to inactivate the RIG-I-adaptor protein (i.e. IPS-1) and to reduce the activation of TLR3-adaptor protein (TRIF) has been demonstrated in a PRRSV-permissive monkey kidney cell line (MARC-145) (Luo et al., Reference Luo, Xiao, Jiang, Jin, Wang, Liu, Chen and Fang2008). Thus, accumulated evidence shows that PRRSV has evolved to deviate porcine cells from perceiving and transmitting antiviral signaling before the production of antiviral effectors (Figure 3) (Luo et al., Reference Luo, Xiao, Jiang, Jin, Wang, Liu, Chen and Fang2008; Sang et al., Reference Sang, Ross, Rowland and Blecha2008b; Xiao et al., Reference Xiao, Mo, Wang, Jia, Qin, Yu, Niu, Zhao, Liu and Chen2010a, Reference Xiao, Jia, Mo, Wang, Qin, He, Zhao, Huang, Li, Yu, Niu, Liu and Chenb).

Fig. 3. Interactions between PRRSV and porcine innate immunity. PRRSV, via non-structural proteins (Nsp) or nucleocapsid (N) protein, alter antiviral signaling through suppression of upstream virus perception of type I IFN production and action. Major virus-targeting factors include transcription factors that mediate the production/action of type I IFN and other innate immune antiviral effectors such as inflammatory cytokines and antimicrobials. Lines with blunt heads indicate suppression and lines with ball heads indicate suppression or activation. Abbreviations: AMP, antimicrobial peptide; AP-1, activator protein 1; Cytk, cytokine; IFN, interferon; IFNAR, IFN-α receptor; IRF, IFN-regulatory factor; ISG, IFN-stimulated gene; ISRE, IFN-stimulated response element; JAK/Tyk2, Janus kinase/tyrosine-protein kinase 2; NF-κB, nuclear factor κB.
Type I IFNs and other antiviral effectors in innate immunity
Major classes of innate immune effectors are listed in Figure 2 and extensively reviewed elsewhere (Beutler, Reference Beutler2004; Klotman and Chang, Reference Klotman and Chang2006; Takaoka and Yanai, Reference Takaoka and Yanai2006; Lehrer, Reference Lehrer2007; Umbach and Cullen, Reference Umbach and Cullen2009; Hartshorn, Reference Hartshorn2010). Two major groups of innate immune effectors, type I IFNs and antimicrobial peptides (AMPs), and their role in antiviral responses will be discussed here. Type I IFNs are prominent in eliciting antiviral responses, and comprise several subtypes in mammals: IFN-α, IFN-β, IFN-ε, IFN-ω and IFN-κ (Pestka, Reference Pestka2007). Humans have multiple IFN-αs, and single members of IFN-β, IFN-ε, IFN-κ and IFN-ω (Takaoka and Yanai, Reference Takaoka and Yanai2006). Additional type I IFNs include IFN-δ, -τ and -ζ (limitin), which are only detected in pigs and cattle (IFN-δ), ruminants (IFN-τ) and mice (IFN-ζ) (Takaoka and Yanai, Reference Takaoka and Yanai2006).
In pigs, type I IFNs consist of multiple IFN-α, IFN-δ and IFN-ω like molecules, such as porcine IFN-α, which are encoded by as many as 17 functional genes (Sang et al., Reference Sang, Rowland, Hesse and Blecha2010a) (Table 2). In addition, pigs have single gene loci encoding each of IFN-β, IFN-ε and IFN-κ (Artursson et al., Reference Artursson, Gobl, Lindersson, Johansson and Alm1992; Sang et al., Reference Sang, Rowland, Hesse and Blecha2010a). In most mammalian species, ubiquitously expressed IFN-α/β are among the most studied subtypes in antiviral responses. Although less extensively studied, the tissue-/cell-specific expressed subtypes, such as IFN-ω in various leukocytes, IFN-δ/ε in female reproductive tissues and IFN-κ in epidermal keratinocytes, are potently induced by viral infection in these cell types and confer antiviral states on uninfected cells (Takaoka and Yanai, Reference Takaoka and Yanai2006; Pestka, Reference Pestka2007). Using a real-time RT-PCR array, we have detected significant expression of multiple-type IFN genes in porcine skin, intestine, lymph nodes, spleen and testis. For example, in skin, 15 IFN genes belonging to all subclasses are highly expressed, likely contributing to the skin's antiviral role as protection from repetitive exposure to viral attacks (Table 2). IFNs, except for subclass IFN-α, are highly expressed in intestine (10 genes) and lymph nodes (9 genes). In contrast, bone marrow cells and liver showed a relatively weaker expression pattern of type I IFNs. Relative to subtype differences, IFN-α1/8/12 and IFN-β are detected in all tested tissues; and porcine IFN-δ5/6/8, IFN-ω1/2/3 and single-subtype subclasses IFN-ε and IFN-κ are detectable in most tested tissues. IFN-αω, a unique subtype only found in pigs and cattle, is highly expressed in porcine skin, but detectable in the intestine, lymph nodes and spleen, implying an intensification of IFN response (Sang et al., Reference Sang, Rowland, Hesse and Blecha2010a) (Table 2).
Table 2. Porcine IFN family members and gene candidates, receptors, number of amino acids, and major expression pattern

Modified from Chang et al. (Reference Chang, Pang, Chen, Chia, Tsai and Jeng2006), Takaoka and Yanai (Reference Takaoka and Yanai2006), and Sang et al. (Reference Sang, Rowland, Hesse and Blecha2010a, Reference Sang, Rowland and Blechab). Found only in
a pigs and cattle, bruminants, or cmice. dψ, pseudo genes; MLN, mesenteric lymph nodes; NA, not applicable.
Type I IFNs are central cytokines in antiviral innate immunity. The local production of type I IFNs around infection sites comprises a major antiviral barrier to inactivate viruses and limit virus spreading. Natural or modified IFN peptides have been well documented for various IFN-based antiviral therapies, which are effective against many viral diseases including viral hepatitis, HIV and SARS infections (Haagmans and Osterhaus, Reference Haagmans and Osterhaus2006; Deutsch and Hadziyannis, Reference Deutsch and Hadziyannis2008; Sulkowski and Benhamou, Reference Sulkowski and Benhamou2007). Type I IFNs, produced during the early phase of virus-cell interaction, not only activate antiviral responses via autocrine mechanisms, but also diffuse or transmit systemically to induce an antiviral state in surrounding and distal cells. The induction of an antiviral state, which involves the suppression of cellular metabolic levels of protein synthesis and the profound expression of genes encoding antiviral products (Haller and Weber, Reference Haller and Weber2007; Zuniga et al., Reference Zuniga, Hahm and Oldstone2007), is critical for developing effective immune protection against viral infections. Type I IFNs collectively induce antiviral responses through a common receptor composed of two subunits, IFN-α/β receptor (IFNAR)-1 and IFNAR-2 (Table 2). However, the efficacy for induction of antiviral responses is different among subtypes and even between members belonging to the same subtype. For example, human IFN-αs vary in their ability to activate human NK cells, IFN-β shows more potency than IFN-α2 in inhibition of monocyte proliferation (García-Sastre and Biron, Reference García-Sastre and Biron2006; Takaoka and Yanai, Reference Takaoka and Yanai2006). Functional differences among type I IFNs are related to their diverse affinities and kinetics in interaction with IFNAR subunits. In addition, differential expression of each type I IFN and receptor subunits with regard to tissue/cell types also contributes to distinct regulation of antiviral responses (Uzé et al., Reference Uzé, Schreiber, Piehler and Pellegrini2007).
Porcine IFNs have varying levels of activity against PRRSV and other viruses in cells. In general, most subtypes of the IFN-α subclass are highly active against PRRSV; however, other subclasses including IFN-β, IFN-δ, IFN-ε and IFN-κ, are not (Sang et al., Reference Sang, Rowland, Hesse and Blecha2010a). Studies indicate that IFN-α subtypes are mainly down-regulated in response to PRRSV infection (Loving et al., Reference Loving, Brockmeier and Sacco2007; Jung et al., Reference Jung, Renukaradhya, Alekseev, Fang, Tang and Saif2009; Sang et al., Reference Sang, Rowland, Hesse and Blecha2010a). This suggests that IFN-α subtypes, not IFN-β, should receive emphasis in the modulation of porcine anti-PRRSV innate immunity. This viewpoint is supported by findings showing that PRRSV isolates differ in their sensitivity to IFN-α suppression (Lee et al., Reference Lee, Schommer and Kleiboeker2004) and by the recent identification of PRRSV-diminishing IFN-α production through intervening signal transducers and activators of transcription (STAT)1–IRF7 signaling in pDCs (Calzada-Nova et al., Reference Calzada-Nova, Schnitzlein, Husmann and Zuckermann2010b).
The interaction of type I IFNs with their receptors leads to the activation of transcription factors of STATs by two IFNAR-associated kinases. The activated STAT1, STAT2 and IRF9 form an activator complex of IFN-activated trimeric transcription factor, ISGF3, which interacts with the IFN-stimulated response element (ISRE) in promoters of ISGs to prompt transcription. Hundreds of ISGs have direct virus targeting functions (e.g. MxA, RNase L and RNA deaminases), amplifying antiviral resistance (e.g. PKR, 2′5′OAS, and type I IFN themselves), and sequestration of cellular metabolic processes to repress virus replication (e.g. PKR-mediated arrest of protein synthesis) (Sen and Sarkar, Reference Sen and Sarkar2007). It is notable that most notorious viruses have improved their capability to evade or subvert the IFN system for their own benefit. Extensive reviews (Iannello et al., Reference Iannello, Debbeche, Martin, Attalah, Samarani and Ahmad2006; Haller and Weber, Reference Haller and Weber2007; Loo and Gale, Reference Loo and Gale2007) on this topic indicate that a collection of virus-derived factors may interfere with IFN production and/or IFN-action pathways (Table 1).
In addition to type I IFNs, three type III IFNs (IFN-λ1–λ3, also known as IL-29, IL-28A and IL-28B, respectively), have been identified. Accumulated evidence indicates that type III IFNs are induced through similar signal transduction pathways as type I IFNs (Ank and Paludan, Reference Ank and Paludan2009). For example, activation of signaling pathways mediated by TLR3 and RIG-I, which has been well characterized to induce IFN-α/β production, also induces type III IFNs in murine and human cells (Ank and Paludan, Reference Ank and Paludan2009). The antiviral activity of human type III IFNs has been associated with multiple viral infections including those caused by hepatitis C virus, influenza A virus and cytomegalovirus (Ank et al., Reference Ank, West and Paludan2006; Ank and Paludan, Reference Ank and Paludan2009). However, type III IFNs are distinct from type I IFNs in their gene and protein structures as well as their receptors and expression patterns. For example, genes of all known mammalian type III IFNs have multiple exons (usually five) in contrast to the single-exon genes for type I IFNs (Fox et al., Reference Fox, Sheppard and O'Hara2009). The protein structure of human IFN-λ3 more closely resembles IL-22 of the IL-10 cytokine family rather than other IFNs (Gad et al., Reference Gad, Dellgren, Hamming, Vends, Paludan and Hartmann2009). Critically, type III IFNs act through cell receptors of the IL-28RA/IL-10R2 complex rather than the IFNAR1/IFNAR2 receptors of the type I IFNs (Kotenko et al., Reference Kotenko, Gallagher, Baurin, Lewis-Antes, Shen, Shah, Langer, Sheikh, Dickensheets and Donnelly2003; Sheppard et al., Reference Sheppard, Kindsvogel, Xu, Henderson, Schlutsmeyer, Whitmore, Kuestner, Garrigues, Birks, Roraback, Ostrander, Dong, Shin, Presnell, Fox, Haldeman, Cooper, Taft, Gilbert, Grant, Tackett, Krivan, McKnight, Clegg, Foster and Klucher2003). In addition, type III IFNs and their receptors are prominent in epithelial tissues, suggesting their involvement in epithelial antiviral immunity (Ank and Paludan, Reference Ank and Paludan2009). Thus, type III IFNs comprise a group of newly identified antiviral cytokines that are functionally similar to type I IFNs and elicit first-line antiviral responses, especially in epithelial cells. We have shown that pigs have at least two type III IFNs, IFN-λ1 and IFN-λ3, and that both exert similar but lower activity than IFN-α/β against PRRSV in cells (Sang et al., Reference Sang, Rowland and Blecha2010b). Non-IFN cytokines, chemokines and AMPs are other groups of important innate immune effectors (Figure 2) (Beutler, Reference Beutler2004; Klotman and Chang, Reference Klotman and Chang2006; Pancer and Cooper, Reference Pancer and Cooper2006; Takaoka and Yanai, Reference Takaoka and Yanai2006; Lehrer, Reference Lehrer2007; Sang and Blecha, Reference Sang and Blecha2008; Umbach and Cullen, Reference Umbach and Cullen2009; Hartshorn, Reference Hartshorn2010). The innate immune roles of cytokines and chemokines have been emphasized in aspects of pro-/anti-inflammation and attraction/activation of immune cells, which are important for overall immune responses. Although the antiviral activity of AMPs has been known for some time (Daher et al., Reference Daher, Selsted and Lehrer1986), research in this area has recently intensified (reviewed in Klotman and Chang, Reference Klotman and Chang2006; Lehrer, Reference Lehrer2007). We, along with others, have demonstrated that PRRSV infection suppresses the expression of AMPs and general antimicrobial activity especially in the lungs of young pigs (Sang and Blecha, Reference Sang and Blecha2009; Sang et al., Reference Sang, Ruchala, Lehrer, Ross, Rowland and Blecha2009; Jung et al., Reference Jung, Gurnani, Renukaradhya and Saif2010). Direct inactivation of PRRSV by porcine β-defensin (pBD) 3 and protegrin 4 has also been demonstrated in vitro (Sang et al., Reference Sang, Ruchala, Lehrer, Ross, Rowland and Blecha2009). Animal AMPs exert antiviral activity by distortion of virion glycoproteins and lipid membranes in enveloped viruses, and by impeding viral entrance into host cells. Other mechanisms of AMP antiviral activity have also been proposed, including down regulation of viral receptors (e.g. hBD3 for CXCR4 of receptor to HIV-1) (Feng et al., Reference Feng, Dubyak, Lederman and Weinberg2006), modulation of cellular antiviral signaling (e.g. HNP-1 for PKC signaling) (Salvatore et al., Reference Salvatore, Garcia-Sastre, Ruchala, Lehrer, Chang and Klotman2007) and potentiating adaptive immunity (Klotman and Chang, Reference Klotman and Chang2006). In addition, other carbohydrate binding proteins such as collectins, including Mannan-binding lectin (MBL), surfactant protein A (SP-A), surfactant protein D (SP-D) and ficolin, have been shown to participate in innate immune responses exerting antiviral activity. Recently, a porcine plasma ficolin was reported to have inhibitory activity against PRRSV replication in a N-acetylated glycan-dependent manner (Keirstead et al., Reference Keirstead, Lee, Yoo, Brooks and Hayes2008). These findings indicate that intervening in the interaction between sugar moieties of the viral envelope and host cells is a target for innate immune molecules to inhibit PRRSV infection (Klotman and Chang, Reference Klotman and Chang2006; Lehrer, Reference Lehrer2007).
The interaction of PRRSV and porcine innate immunity
Aberration of porcine innate immunity by PRRSV
Two decades ago, initial reports of PRRSV occurred almost simultaneously in the U.S. and central Europe. These viruses were defined as type 1 European-PRRSV and type 2 North American-PRRSV. PRRS continues to be the most significant worldwide swine disease and a persistent challenge in both immunology and vaccinology (Kimman et al., Reference Kimman, Cornelissen, Moormann, Rebel and Stockhofe-Zurwieden2009; Darwich et al., Reference Darwich, Díaz and Mateu2010; Huang and Meng, Reference Huang and Meng2010; Murtaugh et al., Reference Murtaugh, Stadejek, Abrahante, Lam and Leung2010). PRRSV evolves at a high mutation rate compared with other RNA viruses and has the potential to subvert host innate immune responses by various means. These include intervening in cell recognition of the virus, diverting antiviral cytokine (especially type I IFNs, IL-1, IL-10 and TNF-α) production and action, directing cytolysis, reducing antigen presentation activity and suppressing phagocytic and microbicidal activity (Darwich et al., Reference Darwich, Díaz and Mateu2010; Thanawongnuwech and Suradhat, Reference Thanawongnuwech and Suradhat2010; Yoo et al., Reference Yoo, Song, Sun, Du, Kim and Liu2010). The innate immune aberration could further contribute to inefficiently bridging adaptive immunity, which in cooperation with other diverting mechanisms on adaptive immunity, causes overall immune inefficacy to PRRSV and other co-infections (Darwich et al., Reference Darwich, Díaz and Mateu2010).
Collectively, data show that PRRSV infection leads to an increase of most if not all viral sensing TLRs in the lungs or lymphoid organs, including TLR2, TLR3, TLR4, TLR7, TLR8 and TLR9 (Sang et al., Reference Sang, Ross, Rowland and Blecha2008b; Liu et al., Reference Liu, Chaung, Chang, Peng and Chung2009; Xiao et al., Reference Xiao, Mo, Wang, Jia, Qin, Yu, Niu, Zhao, Liu and Chen2010a, Reference Xiao, Jia, Mo, Wang, Qin, He, Zhao, Huang, Li, Yu, Niu, Liu and Chenb). The increased TLR transcripts of TLR7 and TLR9 may last for at least 1-week post infection (Xiao et al., Reference Xiao, Mo, Wang, Jia, Qin, Yu, Niu, Zhao, Liu and Chen2010a, Reference Xiao, Jia, Mo, Wang, Qin, He, Zhao, Huang, Li, Yu, Niu, Liu and Chenb) with TLR2 and TLR4 returning toward the basal levels after 3-days post infection. It appears that upregulation of TLR3 is condition-dependent. The in vitro infection of PAMs and mDCs (Sang et al., Reference Sang, Ross, Rowland and Blecha2008b; Chaung et al., Reference Chaung, Chen, Hsieh and Chung2010) or in vivo infection of fetal lungs (Sang et al., Reference Sang, Ross, Rowland and Blecha2008b) fails to increase or even transiently decrease TLR3 expression. Similarly, no significant increase of TLR3 and TLR8 expression was found in lungs of pigs infected with the highly virulent Chinese-type PRRSV (Xiao et al., Reference Xiao, Mo, Wang, Jia, Qin, Yu, Niu, Zhao, Liu and Chen2010a, Reference Xiao, Jia, Mo, Wang, Qin, He, Zhao, Huang, Li, Yu, Niu, Liu and Chenb). Expression of cytosol virus sensing receptors of RIG-I and Mda5 is also significantly stimulated in lungs infected by type 2 PRRSV (Xiao et al., Reference Xiao, Mo, Wang, Jia, Qin, Yu, Niu, Zhao, Liu and Chen2010a, Reference Xiao, Jia, Mo, Wang, Qin, He, Zhao, Huang, Li, Yu, Niu, Liu and Chenb). The increase in these virus-sensing receptors may potentially lead to antiviral responses. Overexpression of TLR3 resulted in enhancement and reduction of TLR3 suppressed anti-PRRSV activity in virus-infected cells (Sang et al., Reference Sang, Ross, Rowland and Blecha2008b). Although it is unknown whether PRRSV has a mechanism to reduce the expression of these receptors, the activation of TLR7 and TLR9 signaling in pDCs appears inhibited by the presence of PRRSV (Calzada-Nova et al., Reference Calzada-Nova, Schnitzlein, Husmann and Zuckermann2010a). The implication of data using MARC-145 cells is that PRRSV may interfere with adaptor proteins to suppress both RLR- and TLR3-mediated stimulation of IFN production (Luo et al., Reference Luo, Xiao, Jiang, Jin, Wang, Liu, Chen and Fang2008; Miller et al., Reference Miller, Lager and Kehrli2009). Notably, the acute activation of these receptor-mediated signaling pathways also leads to increased production of pro-inflammatory cytokines and chemokines as well as activation of the complement systems; this process is thought to be the cause of pneumonia in PRRS cases (Xiao et al., Reference Xiao, Mo, Wang, Jia, Qin, Yu, Niu, Zhao, Liu and Chen2010a). In addition, the inflammatory response could be amplified in the presence of bacterial endotoxin (Qiao et al., Reference Qiao, Feng, Bao, Guo, Wan, Xiao, Yang and Zhang2011) or low-avidity immune complex (Monsalvo et al., Reference Monsalvo, Batalle, Lopez, Krause, Klemenc, Hernandez, Maskin, Bugna, Rubinstein, Aguilar, Dalurzo, Libster, Savy, Baumeister, Aguilar, Cabral, Font, Solari, Weller, Johnson, Echavarria, Edwards, Chappell, Crowe, Williams, Melendi and Polack2011) to promote macrophages skewing to the M2 status (Stout et al., Reference Stout, Watkins and Suttles2009). Human M2 macrophages show higher expression of CD163, heparin sulfate and IL-10 (Cassol et al., Reference Cassol, Cassetta, Alfano and Poli2010); similar responses in pigs could potentially exacerbate PRRSV infection (Patton et al., Reference Patton, Rowland, Yoo and Chang2009; Welch and Calvert, Reference Welch and Calvert2010).
The production and action of the type I IFN system are hallmarks of innate antiviral immunity. Earlier reports have shown that pigs infected with PRRSV produce very low levels of type I IFNs. Exogenous application of IFN-α could control the virus infection in porcine PAMs and MARC-145 cells (Albina et al., Reference Albina, Carrat and Charley1998; Buddaert et al., Reference Buddaert, Van Reeth and Pensaert1998). Studies by Lee et al. (Reference Lee, Schommer and Kleiboeker2004) showed that different field isolates and even virus quasi-species rescued from individual plaque clones of the same isolate vary in their ability to induce IFN-α and susceptibility to IFN-α treatment. PRRSV also infects mDCs and significantly suppresses type I IFN production of the IFN-α subtype but not the IFN-β subtype (Loving et al. Reference Loving, Brockmeier and Sacco2007). Extensive analysis of porcine type I IFN profiles indicates that pigs have as many as 39 functional genes including 17 IFN-α, 11 IFN-δ, 7 IFN-ω, as well as a single member of each of the IFN-αω, IFN-β, IFN-ε and IFN-κ subtypes (Sang et al., Reference Sang, Rowland, Hesse and Blecha2010a). Comparative antiviral analyses in both porcine PAMs and MARC-145 cells indicate that most IFN-α and IFN-αω have higher activity against PRRSV infection than do other subtype members (Sang et al., Reference Sang, Rowland, Hesse and Blecha2010a).
Determined by the stability of the ternary IFN-receptor complex, type I IFNs biological activities proceed in two directions, antiviral activity and immunomodulatory activity (Uzé et al., Reference Uzé, Schreiber, Piehler and Pellegrini2007; Kalie et al., Reference Kalie, Jaitin, Podoplelova, Piehler and Schreiber2008). Whereas IFN-α subtypes generally have more antiviral potency, IFN-β displays more immunomodulatory activity such as promotion of cell proliferation (Kalie et al., Reference Kalie, Jaitin, Podoplelova, Piehler and Schreiber2008). Recent studies have shown that IFN-β has anti-inflammatory properties inducing IL-10 production in human mDCs (Wang et al., Reference Wang, Brown, Garcia, Tang, Benakanakere, Greenway, Alard, Kinane and Martin2011) and regulating alternative activation of macrophages through induction of IL-4, IL-13 and IL-10 in RSV infected murine lungs (Shirey et al., Reference Shirey, Pletneva, Puche, Keegan, Prince, Blanco and Vogel2010). Interestingly, PRRSV suppression of IFN-β production through targeting IRF3 has been mostly observed in MARC-145 cells or experimental human cell lines (Miller et al., Reference Miller, Laegreid, Bono, Chitko-McKown and Fox2004; Luo et al., Reference Luo, Xiao, Jiang, Jin, Wang, Liu, Chen and Fang2008; Shi et al., Reference Shi, Wang, Zhi, Xing, Zhao, Deng and Zhang2010; Chen et al., Reference Chen, Lawson, Sun, Zhou, Guan, Christopher-Hennings, Nelson and Fang2010a; Kim et al., Reference Kim, Sun, Lai, Song and Yoo2010; Li et al., Reference Li, Zheng, Zhou, Zhang, Shi, Hu and Wang2010; Beura et al., Reference Beura, Sarkar, Kwon, Subramaniam, Jones, Pattnaik and Osorio2010; Song et al., Reference Song, Krell and Yoo2010). These observations are not always consistent with the data from infected pigs or porcine cells (Loving et al., Reference Loving, Brockmeier and Sacco2007; Genini et al., Reference Genini, Delputte, Malinverni, Cecere, Stella, Nauwynck and Giuffra2008). In contrast, in vivo or ex vivo tests in porcine lungs strongly support PRRSV-mediated IFN-α suppression in porcine lungs (Jung et al., Reference Jung, Renukaradhya, Alekseev, Fang, Tang and Saif2009), PAMs (Albina et al., Reference Albina, Carrat and Charley1998; Genini et al., Reference Genini, Delputte, Malinverni, Cecere, Stella, Nauwynck and Giuffra2008; Patel et al., Reference Patel, Nan, Shen, Ritthipichai, Zhu and Zhang2010), mDC (Loving et al., Reference Loving, Brockmeier and Sacco2007) and pDCs (Calzada-Nova et al., Reference Calzada-Nova, Schnitzlein, Husmann and Zuckermann2010a, Reference Calzada-Nova, Schnitzlein, Husmann and Zuckermannb).
Cell type difference in respect to response to PRRSV mediated IFN-suppression has recently been noted (Loving et al., Reference Loving, Brockmeier, Vincent, Lager and Sacco2008; Sang et al., Reference Sang, Rowland, Hesse and Blecha2010a; He et al., Reference He, Overend, Ambrogio, Maganti, Grubman and Garmendia2011). In addition, the suppression of IFN-δ or IFN-ω (termed SPI IFN in the reference) has also been observed (Xiao et al., Reference Xiao, Jia, Mo, Wang, Qin, He, Zhao, Huang, Li, Yu, Niu, Liu and Chen2010b; Sang, unpublished data); therefore, whether all type I IFNs are generally suppressed or differentially regulated by PRRSV in vivo to facilitate virus infection should be determined. PRRSV suppresses type I IFN signaling primarily in infected cells, as indicated by suppression of ISG15 and ISG56 in PRRSV infected MARC-145 and PAMs (Patel et al., Reference Patel, Nan, Shen, Ritthipichai, Zhu and Zhang2010). However, exogenous application of IFNs does prevent PRRSV-suppression of IFN signaling due to the treatment of PAMs with either porcine type I, II or III IFNs, and especially IFN-α, which induce significant anti-PRRSV activity (Rowland et al., Reference Rowland, Robinson, Stefanick, Kim, Guanghua, Lawson and Benfield2001; Sang et al., Reference Sang, Rowland, Hesse and Blecha2010a, Reference Sang, Rowland and Blechab).
PRRSV infection also diverts the production of other cytokines and antimicrobial molecules, such as AMPs and nitric oxide (NO). However, reports regarding PRRSV regulation of cytokines are quite controversial. Both increases and decreases of pro-inflammatory cytokines such as TNF-α, IL-1 and IL-6, have been shown in PRRSV infected pigs and PAMs (Darwich et al., Reference Darwich, Díaz and Mateu2010). PRRSV does prevent the production of IL-8 (Calzada-Nova et al., Reference Calzada-Nova, Schnitzlein, Husmann and Zuckermann2010a) in pDCs but other studies have found that PRRSV induces IL-8 (Darwich et al., Reference Darwich, Díaz and Mateu2010). More extensive cytokine analyses have linked IL-1β, IL-8 and IFN-γ but not IL-12 with PRRSV clearance (Lawson et al., Reference Lawson, Lunney, Zuckermann, Osorio, Nelson, Welbon, Clement, Fang, Wong, Kulas and Christopher-Hennings2010; Lunney et al., Reference Lunney, Fritz, Reecy, Kuhar, Prucnal, Molina, Christopher-Hennings, Zimmerman and Rowland2010). IL-10 is an immunosuppressive cytokine, which is up-regulated by PRRSV infection (Thanawongnuwech and Suradhat, Reference Thanawongnuwech and Suradhat2010) and induces PAM permissiveness to PRRSV (Patton et al., Reference Patton, Rowland, Yoo and Chang2009). Similar to the regulation of other cytokines, PRRSV infected pigs or cells have been shown to have either increased or decreased IL-10 production (Klinge et al., Reference Klinge, Vaughn, Roof, Bautista and Murtaugh2009; Darwich et al., Reference Darwich, Díaz and Mateu2010; Subramaniam et al., Reference Subramaniam, Sur, Kwon, Pattnaik and Osorio2011). Therefore, as suggested by studies of Díaz et al. (Reference Díaz, Darwich, Pappaterra, Pujols and Mateu2006) and Silva-Camp et al. (Reference Silva-Campa, Cordoba, Fraile, Flores-Mendoza, Montoya and Hernández2010), PRRSV-regulation of IL-10 may be both pig- and virus-strain dependent. Studies evaluating PRRSV-suppression of antimicrobial and phagocytic activities are consistent in virus-infected lungs and PAMs, showing that, in general, PRRSV decreases the production of AMPs and NO, and suppresses the microbicidal activity of both PAMs and NK cells (Thanawongnuwech et al., Reference Thanawongnuwech, Halbur and Thacker2000; Jung et al., Reference Jung, Renukaradhya, Alekseev, Fang, Tang and Saif2009; Sang et al., Reference Sang, Ruchala, Lehrer, Ross, Rowland and Blecha2009). PRRSV also directs cytolysis in infected PAMs and mDCs and cell death is prominent in activated M2 macrophages (unpublished data). The suppression of microbicidal activity and direction of cell death of innate immune cells at the cellular and molecular levels may be linked to co-infections with PRRSV.
Viral mechanisms responsible for innate immune aberration
PRRSV is an enveloped virus, which has a ~15 kb (+)ssRNA genome containing nine open reading frames (ORF). The 5′ end ORF1a and ORF1b encode two replicase polyproteins, pp1a and pp1b. The proteolytic pp1a products self cleave into nine non-structural proteins (Nsp) (Nsp1α, Nsp1β and Nsp2–8), and cleave pp1b into four Nsp (Nsp9–12). The 3′ end seven ORFs encode four minor (GP2a, GP3, GP4 and E proteins) and three major (GP5, M and N proteins) structural proteins. Besides their essential role in viral replication, recent studies highlight the roles of the Nsp in immune modulation of innate immune effectors (Fang and Snijder, Reference Fang and Snijder2010; Yoo et al., Reference Yoo, Song, Sun, Du, Kim and Liu2010). Four viral Nsp have strong to moderate inhibitory effects (Nsp1>Nsp2>Nsp11>Nsp4) on IFN-β production through inactivating IRF3, which is a key transcription factor responsible for activation of the IFN-β promoter (Beura et al., Reference Beura, Sarkar, Kwon, Subramaniam, Jones, Pattnaik and Osorio2010).
Nsp1 and its two autocleaved products, Nsp1α and Nsp1β, have been shown to have the highest activity inhibiting IRF3 activation (Beura et al., Reference Beura, Sarkar, Kwon, Subramaniam, Jones, Pattnaik and Osorio2010; Yoo et al., Reference Yoo, Song, Sun, Du, Kim and Liu2010). Beura et al. (Reference Beura, Sarkar, Kwon, Subramaniam, Jones, Pattnaik and Osorio2010) indicated that Nsp1α and Nsp1β block IRF3 nuclear translocation; however, Yoo et al. (Reference Yoo, Song, Sun, Du, Kim and Liu2010) observed no blocking of Nsp1α and Nsp1β in IRF3 nuclear translocation, and proposed a mechanism based on Nsp1 promoting degradation of the CREB (cyclic AMP response element binding)-binding protein (CBP). CBP has histone acetyltransferase activity functioning in dissociation of histones from the DNA promoter region, and the CBP/p300 co-activators function in concert with a variety of transcription factors including STATs, NF-κB, PIAS1 and the IRF family (Yoo et al., Reference Yoo, Song, Sun, Du, Kim and Liu2010). Nsp1-mediated CBP-degradation may provide a general explanation of PRRSV immune suppression via interaction with IRF3 as well as STAT2, because the decrease in STAT2 at the protein level has been reported in PRRSV-infected cells (Patel et al., Reference Patel, Nan, Shen, Ritthipichai, Zhu and Zhang2010). In another report, both Nsp1α and Nsp1β were shown to dramatically inhibit IFN-β expression and Nsp1β also suppressed IFN signaling via inhibiting STAT1 nuclear translocation (Chen et al., Reference Chen, Lawson, Sun, Zhou, Guan, Christopher-Hennings, Nelson and Fang2010a). Patel et al. (Reference Patel, Nan, Shen, Ritthipichai, Zhu and Zhang2010) further demonstrated that STAT1 is not the only factor inhibited as nuclear translocation of ISGF3 (ISG factor 3, composed by STAT1/STAT2/IRF9) was also inhibited by Nsp1β to suppress IFN-α signaling in the virus-infected cells. PRRSV does not infect pDCs, but the presence of some uncharacterized viral component blocks STAT1 nuclear localization thereby reducing the availability of IRF7, which has been thought to be a mechanism for PRRSV inhibition of IFN-α production in pDCs (Calzada-Nova et al., Reference Calzada-Nova, Schnitzlein, Husmann and Zuckermann2010b). Nsp1α and Nsp1β also block NF-κB activation (Song et al., Reference Song, Krell and Yoo2010), a critical transcription factor in innate immune signaling not only for antiviral responses; therefore, Nsp1 proteins may also be the viral components responsible for suppressing other cytokines and AMPs in addition to IFNs (Figure 3).
PRRSV Nsp2 represents another major immunomodulatory protein (Fang and Snijder, Reference Fang and Snijder2010). The region of 691–722 residues in Nsp2 has been shown to be important for virus mediation of production of pro-inflammatory cytokines including IL-1β and TNF-α (Chen et al., Reference Chen, Zhou, Lunney, Lawson, Sun, Brown, Christopher-Hennings, Knudsen, Nelson and Fang2010b). Biochemically, Nsp2 belongs to the deubiquitinase superfamily; through this activity, it can interfere with the polyubiquitination process of ISG15 and IKKα (inhibitor of nuclear factor kappa-B kinase (NF-κB) subunit α), thereby targeting the IFN response and NF-κB signaling pathways respectively (Sun et al., Reference Sun, Chen, Lawson and Fang2010).
PRRSV Nsp11 also has dual roles in suppression of IFN responses (Yoo et al., Reference Yoo, Song, Sun, Du, Kim and Liu2010) and reduction of NF-κB activation (Beura et al., Reference Beura, Sarkar, Kwon, Subramaniam, Jones, Pattnaik and Osorio2010). Nsp11 has endo-RNase activity; its suppression of IFN response may partially be due to cleavage of viral RNA patterns to dampen the binding by multiple cellular antiviral receptors including TLR3/7/8/9, RIG-I, and Mda5, thus reducing the upstream viral sensing in antiviral signaling (Yoo et al., Reference Yoo, Song, Sun, Du, Kim and Liu2010). In addition, Nsp11 has been shown to block IRF3 phosphorylation and nuclear translocation (Yoo et al., Reference Yoo, Song, Sun, Du, Kim and Liu2010), but it is unclear whether this resulted from weakened upstream signaling (i.e., diminishing dsRNA binding) or Nsp11 targeting IRF3 directly through other mechanisms.
As previously mentioned, PRRSV has been reported to strongly induce IL-10 production during the early phase of infection in pigs (Thanawongnuwech and Suradhat, Reference Thanawongnuwech and Suradhat2010) and in cultured porcine PBMCs and PAMs (Yoo et al., Reference Yoo, Song, Sun, Du, Kim and Liu2010). The viral mechanism to induce IL-10 is reported to be the nucleocapsid (N) protein (Yoo et al., Reference Yoo, Song, Sun, Du, Kim and Liu2010). The N protein is a small basic protein of 123 (or 128 of type I PRRSV) residues and it is the most abundant virion component and the most immunogenic protein in virus-infected pigs and cells (Music and Gagnon, Reference Music and Gagnon2010). The mechanism of N protein induction of IL-10 is unknown. Given that nucleolus-localized N protein interacts with several cell transfactors (Yoo et al., Reference Yoo, Song, Sun, Du, Kim and Liu2010), there is potential of the N protein directly targeting the IL-10 gene promoter. In addition, recent evidence shows that IFN-β, produced from LPS activation of macrophages or DCs, induces IL-10 in these cells (Chang et al., Reference Chang, Guo, Doyle and Cheng2007; Wang et al., Reference Wang, Brown, Garcia, Tang, Benakanakere, Greenway, Alard, Kinane and Martin2011). The N protein may activate the NF-κB pathway to induce IFN-β production through TLR4 activation of NF-κB pathways, therefore, inducing IL-10 stimulation and NF-κB activation with LPS-TLR4 signaling (Chang et al., Reference Chang, Guo, Doyle and Cheng2007; Wang et al., Reference Wang, Brown, Garcia, Tang, Benakanakere, Greenway, Alard, Kinane and Martin2011). This assumption is supported by the understanding that N protein does activate NF-κB in MARC-145 cells and the region between residue 30–73 of N protein is essential for this function (Luo et al., Reference Luo, Fang, Jiang, Jin, Wang, Wang, Chen and Xiao2011), and TLR4 is induced in PRRSV infected pigs (Xiao et al., Reference Xiao, Mo, Wang, Jia, Qin, Yu, Niu, Zhao, Liu and Chen2010a). Integrating the points of N protein activation of NF-κB, LPS-TLR4 mediated IFN-β production and IL-10 induction by IFN-β explains several controversial observations such as the induction/no-induction of IFN-β and IL-10 as well as the activation/suppression of NF-κB in PRRSV infected pigs or cells. Therefore, the consequent levels of IFN-β and IL-10 (and probably other innate immune effectors too) are not only dependent on PRRSV infection but also the activation of LPS-TLR4 signaling by some bacterial endotoxins; and the suppression or activation of NF-κB signaling should be variable according to the intensity and tempo between the N protein's positive and Nsp's negative effects. In summary, innate immune deficiency caused by PRRSV infection complicates the viral disease creating a complex syndrome. The challenge faced is not only from the small virus per se, but mostly, if not always, from the interaction of other opportunistic infections in a host with a disruption in homeostasis.
Concluding remarks
In Figure 4, we briefly summarize our understanding of PRRSV diversion of innate immunity at the levels of the animal, cell and molecule. We propose that through viral parasitization and interaction to deviate the expression and/or activation states of innate immune molecules, PRRSV alters the environment for development/activation of innate immune cells, and particularly dominates the functional status of monocytic cells including tissue macrophages and circulating DCs by direct infection or standby suppression in pDCs. The viral aberration of innate immunity has been shown at the cellular level in two aspects. Firstly, the skewing or adapting of innate immune cells to the status of anti-inflammation and immunosuppression at the early phase of infection, such as the M2 status of macrophages, which are observed to be more permissive to PRRSV infection than M1 cells (Patton et al., Reference Patton, Rowland, Yoo and Chang2009; Sang, unpublished data). Secondly, there is suppression of immune surveillance including dampened microbicidal and antigen processing activity in DCs and macrophages (Sang et al., Reference Sang, Ruchala, Lehrer, Ross, Rowland and Blecha2009; Jung et al., Reference Jung, Renukaradhya, Alekseev, Fang, Tang and Saif2009; Thanawongnuwech and Suradhat, Reference Thanawongnuwech and Suradhat2010).

Fig. 4. Tactics to counteract PRRSV deviation of porcine innate immunity at the molecular, cellular and animal levels. Viral aberration is indicated in the top portion of the figure. Vector-based expression or suppression of the virus-aberrant antiviral effectors (or signaling molecules) may work alone or in concert with a subunit vaccine to stimulate effective anti-PRRSV protection. Innate immune cells, especially PRRSV-infected monocytic cells, are platforms to manipulate virus-aberrant cell activation status and innate immune response. In pigs, drug-based approaches to modulate inflammatory and lipid metabolic status may be feasible to alter host response to vaccination or infection.
Pertaining to the characteristics of tissue- or cell-tropism, the primary sites of PRRSV infection, including reproductive and pulmonary tissues, contain monocytic cells that are naturally inclined to a M2 or immunosuppressive status, especially those in the reproductive tissues with immune privilege. This viewpoint suggests that PRRSV has evolved to adapt rather than skew innate immune cells to establish an infection at the animal level, and the high-virulence PRRSV strains, which cause significant host mortality because of the dramatic inflammatory response (Xiao et al., Reference Xiao, Mo, Wang, Jia, Qin, Yu, Niu, Zhao, Liu and Chen2010a), are unique cases of unsuccessful parasitization. Therefore, the suppression of M1 activation especially antiviral status induced by type I IFNs in innate immune cells during the early phase of infection might be a key for designing counteractions to target PRRSV infection at both cell and animal levels. However, unlike in ex vivo cells, time and intensity are more critical for the success of inflammatory and immune regulation in vivo (Figure 4). In this line, some herbal therapies, which have been shown effective in treatment of inflammatory symptoms in SARS (Leung, Reference Leung2007) and pandemic influenza (Ge et al., Reference Ge, Wang, Xu, Gu, Liu, Xiao, Zhou, Liu, Yang and Su2010), are worth evaluating in PRRSV pandemics. In addition, lipid metabolites such as sphingosine-1-phosphate (S1P) have been known for critical regulation of inflammation and immune cell recruitment/activation (Spiegel and Milstien, Reference Spiegel and Milstien2011). Several recent studies elegantly linked lipid metabolism to immune status of macrophages (Im et al., Reference Im, Yousef, Blaschitz, Liu, Edwards, Young, Raffatellu and Osborne2011), T cells and DCs (Herber et al., Reference Herber, Cao, Nefedova, Novitskiy, Nagaraj, Tyurin, Corzo, Cho, Celis, Lennox, Knight, Padhya, McCaffrey, McCaffrey, Antonia, Fishman, Ferris, Kagan and Gabrilovich2010), as well as cytokine storms in influenza-infected lungs (Teijaro et al., Reference Teijaro, Walsh, Cahalan, Fremgen, Roberts, Scott, Martinborough, Peach, Oldstone and Rosen2011). It will be informative to examine the role of modulators/metabolites of lipid metabolism in regulation of inflammatory and immune status during PRRSV infection and vaccine development.
Finally, the development of vaccines to induce effective protection against heterologous PRRSV isolates should be focused at the cellular and molecular levels. To this end, the adjuvant mechanisms underlying innate immunity (Coffman et al., Reference Coffman, Sher and Seder2010) should be considered in respect to PRRSV-diverted innate immune components as reviewed above. Positive effects have been obtained in studies with expression of IFN-α alone (Brockmeier et al., Reference Brockmeier, Lager, Grubman, Brough, Ettyreddy, Sacco, Gauger, Loving, Vorwald, Kehrli and Lehmkuhl2009) or conjugated expression of innate immune effectors including CD40L (Cao et al., Reference Cao, Wang, Du, Li, Wang and Jiang2010), GM-CSF (Wang et al., Reference Wang, Li, Jiang, Li, Zeshan, Cao and Wang2009) and HSP70 (Li et al., Reference Li, Jiang, Li, Wang, Cao, Wang and Zeshan2009b) with the viral epitopes using adenovirus vectors. In addition, several studies using RNA interference techniques have shown promise in suppression of PRRSV infection in both cells and pigs (Lu et al., Reference Lu, Ho and Kwang2006; Li G et al., Reference Li, Jiang, Li, Wang, Huang, Bai, Cao, Wu, Chen and Zeshan2009a), implying that miRNA-mediated antiviral mechanisms, which have been found in regulation of HCV and HIV infection (Skalsky and Cullen, Reference Skalsky and Cullen2010), could be also functional in PRRSV-host interaction. Viruses are dependent on cell metabolism and genome-wide screening of host factors critical for host–viral interaction has revealed a large number of candidates belonging to metabolic pathways besides immune genes (Karlas et al., Reference Karlas, Machuy, Shin, Pleissner, Artarini, Heuer, Becker, Khalil, Ogilvie, Hess, Mäurer, Müller, Wolff, Rudel and Meyer2010; König et al., Reference König, Stertz, Zhou, Inoue, Hoffmann, Bhattacharyya, Alamares, Tscherne, Ortigoza, Liang, Gao, Andrews, Bandyopadhyay, De Jesus, Tu, Pache, Shih, Orth, Bonamy, Miraglia, Ideker, García-Sastre, Young, Palese, Shaw and Chanda2010). To this end, it is likely that aberration of lipid metabolism is a major consequence in PRRSV-host interaction (Xiao et al., Reference Xiao, Jia, Mo, Wang, Qin, He, Zhao, Huang, Li, Yu, Niu, Liu and Chen2010b) and the regulation of lipid signaling has potential to prime activation status and immune surveillance of innate immune cells. In summary, to induce ideal anti-PRRSV protection, strategies should be considered for targeting innate immune components to counteract viral replication/spreading and subversion of immunity as well as to mitigate immune pathology from excessive/persistent activated innate immune responses (Figure 4).
Acknowledgments
We thank Kansas Agricultural Experiment Station for their contribution under number 12-112-J.








