Introduction
Didymozoids (Platyhelminthes, Trematoda) are poorly known parasites, often highly prevalent and abundant in several wild and reared marine fish (Munday et al., Reference Munday, Sawada, Cribb and Hayward2003; Mladineo and Tudor, Reference Mladineo and Tudor2004). Until 1985, Didymozoidae included 212 species placed in 81 genera, infecting mainly tropical and subtropical host species, with 23 species occurring in Mediterranean fish (Nikolaeva, Reference Nikolaeva and Hargis1985). However, in recent years the growing interest for fishery, protection and aquaculture of several fish from the Mediterranean Sea has increased the number of didymozoid species recorded in this region (40 taxa, Mele et al., Reference Mele, Pennino, Piras, Macías, Gómez-Vives, Alemany, Montero, Garippa and Merella2016; Pérez-del-Olmo et al., Reference Pérez-del-Olmo, Kostadinova and Gibson2016). Didymozoid parasites have been reported on the gills of the wild dusky groupers (Epinephelus marginatus, Lowe, 1834) (Osteichthyes, Serranidae), one of the largest top predators in the Atlantic and Mediterranean littoral ecosystems. In a first account, white capsules have been reported attached to the gills, pseudobranchs and orobranchial cavity of dusky grouper in the eastern Atlantic Ocean and ascribed them to Didymodiclinus branchialis (Yamaguti, Reference Yamaguti1970) (Gijón-Botella and López-Román, Reference Gijón-Botella and López-Román1987). Also, Canestri-Trotti et al. (Reference Canestri-Trotti, Fioravanti, Patarnello and Restani1994) reported similar findings on dusky grouper from the Adriatic Sea, with the uncertain attribution to the genera between Gonapodasmius (Ishii, 1935) and Indoglomeritrema (Madhavi and Hanumantha, 1983). Thus, the same parasitological lesions were observed on E. marginatus from several localities of the western Mediterranean Sea (Panebianco et al., Reference Panebianco, Giannetto and Conte1995; Azzurro et al., Reference Azzurro, Berrilli, De Liberato, Di Cave and Orecchia2002; Polinas et al., Reference Polinas, Mele, Padrós, Merella, Antuofermo, Gouraguine and Reñones2018). Recently, De Benedetto et al. (Reference De Benedetto, Arfuso, Ferrara, Brianti and Gaglio2021) found the infection also in fish from central Mediterranean Sea and ascribed it to Didymodiclinus sp.
The present study provides the morphological and molecular description of a didymozoid gill parasite of E. marginatus from the Mediterranean Sea (Balearic Islands and Sicily), with the aim of clarifying the taxonomy of this organism. Moreover, a key of identification for the genus Didymodiclinus is proposed.
Materials and methods
Fish sampling and parasitological analysis
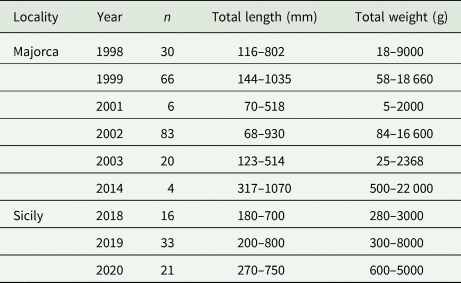
A total of 279 dusky grouper specimens were examined for didymozoid gill parasites (Table 1). Two hundred and nine fish were caught off Majorca (western Mediterranean Sea) between 1998 and 2014; 70 were collected from different fish markets or seized during official controls by veterinarians in the east coast of Sicily (central Mediterranean Sea) between 2018 and 2020. Fish were measured (Total length) to the next millimetre and weighed (Total weight) to the next gram (Table 1) and then gills and opercula were examined for didymozoid capsules. Samples of infected gills and pseudobranchs were excised, placed in Petri dishes and dissected. Didymozoid capsules were opened to release the worms, which were recorded as alive, dead or degraded. Specimens were stored in 70% ethanol for morphological analysis or at −80°C for molecular analyses.
Table 1. Data of examined Epinephelus marginatus from the Mediterranean sea
DNA isolation and polymerase chain reaction
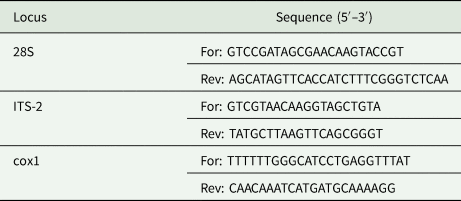
Genomic DNA was extracted and purified using the NucleoSpin Plant II (Macherey-Nagel, Düren, North Rhine-Westphalia, Germany), according to the manufacturer's protocol. The obtained genomic DNA was used to evaluate 3 different markers, namely 2 ribosomal DNA markers, the 28S ribosomal RNA (28S) and partial internal transcribed spacer 2 (ITS-2) regions, and the mitochondrial cytochrome oxidase 1 (cox1) gene, by polymerase chain reaction (PCR). The loci of interest were amplified using the primer sets listed in Table 2 and the recombinant Taq DNA polymerase (Invitrogen, Carlsbad, California, United States) according to the manufacturer's instructions. PCR reactions (50 μL total volume) were performed in an Ep-Gradient Mastercycler (Eppendorf, Hamburg, Germany) using the following cycling parameters for 28S rDNA: 94°C for 30 s, 35 cycles of 94°C for 30 s, 58°C for 30 s and 72°C for 1 min, with a final step of 72°C for 10 min. For ITS-2 and Cox1 the following amplification profile was set: 94°C for 30 s, 35 cycles of 94°C for 30 s, 56°C for 90 s and 72°C for 90 s, with a final extension of 72°C for 10 min. PCR products were analysed by 1.5% agarose gel electrophoresis and samples that were successfully amplified were then purified using the E.Z.N.A. gel extraction kit (Omega Bio-tek, Norcross, Georgia, United States).
Table 2. List of targeted loci, sequence of the oligonucleotide forward (For) and reverse (Rev) primers used for PCR (Mladineo et al., Reference Mladineo, Tomaš and Stanić2015)
DNA sequencing and alignment
Extracted samples were sequenced in both the forward and reverse directions on an Applied Biosystems 3730 DNA analyser (Thermo Fisher Scientific, Waltham, Massachusetts, United States) and the obtained DNA sequences were analysed by BLASTN similarity search against the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The 28S, ITS-2 and cox1 sequences obtained from the isolates were aligned with the available nucleotide sequences of Didymozoidae (Table 3) using the MUSCLE algorithm and further used for phylogenetic analyses. Neighbour-joining (NJ) and maximum likelihood (ML) trees were constructed selecting the GTR + G + I nucleotide substitution model for all datasets with the bootstrap method (1000 replications) to evaluate the reliability of internal branches (Lefort et al., Reference Lefort, Longueville and Gascuel2017). NJ and ML phylogenetic analyses were performed using MEGA X and PhyML 3.0 (Guindon et al., Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010; Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018), respectively. The Bayesian inference (BI) analysis was carried out using MrBayes 3.2.6 (Ronquist and Huelsenbeck, Reference Ronquist and Huelsenbeck2003) with the GTR model (1 000 000 generations, sampling every 500 generations and burn-in fraction 0.25). The trees were rooted with Prosogonotrema bilabiatum (28S), Hemiuridae sp. (ITS-2) and Genarchopsis goppo (cox1) chosen as outgroups.
Table 3. Nucleotide sequences of the 28S rRNA, ITS and cox1 markers used to evaluate the phylogenetic relations among our isolates and other Didymozoidae
na, data not available.
Results
Gills and pseudobranchs of 92 fish from Majorca and 13 from Sicily (prevalence 44.9 and 18.6%, respectively) were infected with didymozoids. The main morphological traits of these parasites did not correspond to those of any of the previously described species, indicating they belonging to a new one.
Didymodiclinus marginati n. sp.
Type host: Epinephelus marginatus (Lowe, 1834) (Osteichthyes: Serranidae), dusky grouper.
Type locality: Majorca, Spain, western Mediterranean Sea.
Other localities: Sicily, Italy, Ionian Sea, Central Mediterranean Sea.
Type specimens: The holotype (NHM UK 2022.4.8.1), paratype (NHM UK 2022.4.8.2) and vouchers (NHM UK 2022.4.8.3-6) were deposited in the Invertebrates Collection at the Natural History Museum, London, UK.
Site in host: Gills and pseudobranchs.
Infection parameters: Prevalence: 38% of host, 45% in Majorcan fish and 19% of Sicilian fish; mean intensity: 10.0 worms per infected host, 9.8 in Majorcan fish and 11.8 in Sicilian fish.
Representative DNA sequences: GenBank accession numbers: MW489519 (ITS-2); MW489507 (28S rRNA gene) and MW540490 (COX1).
ZooBank registration: To comply with the regulations set out in Article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for D. marginati n. sp. is urn:lsid:zoobank.org:act:3B39D377-55DC-46BE-BE9B-F5821E39A7C0.
Etymology: Specific name refers to the name of the host species, E. marginatus.
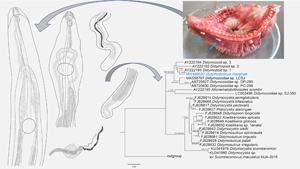
Description (Fig. 1)
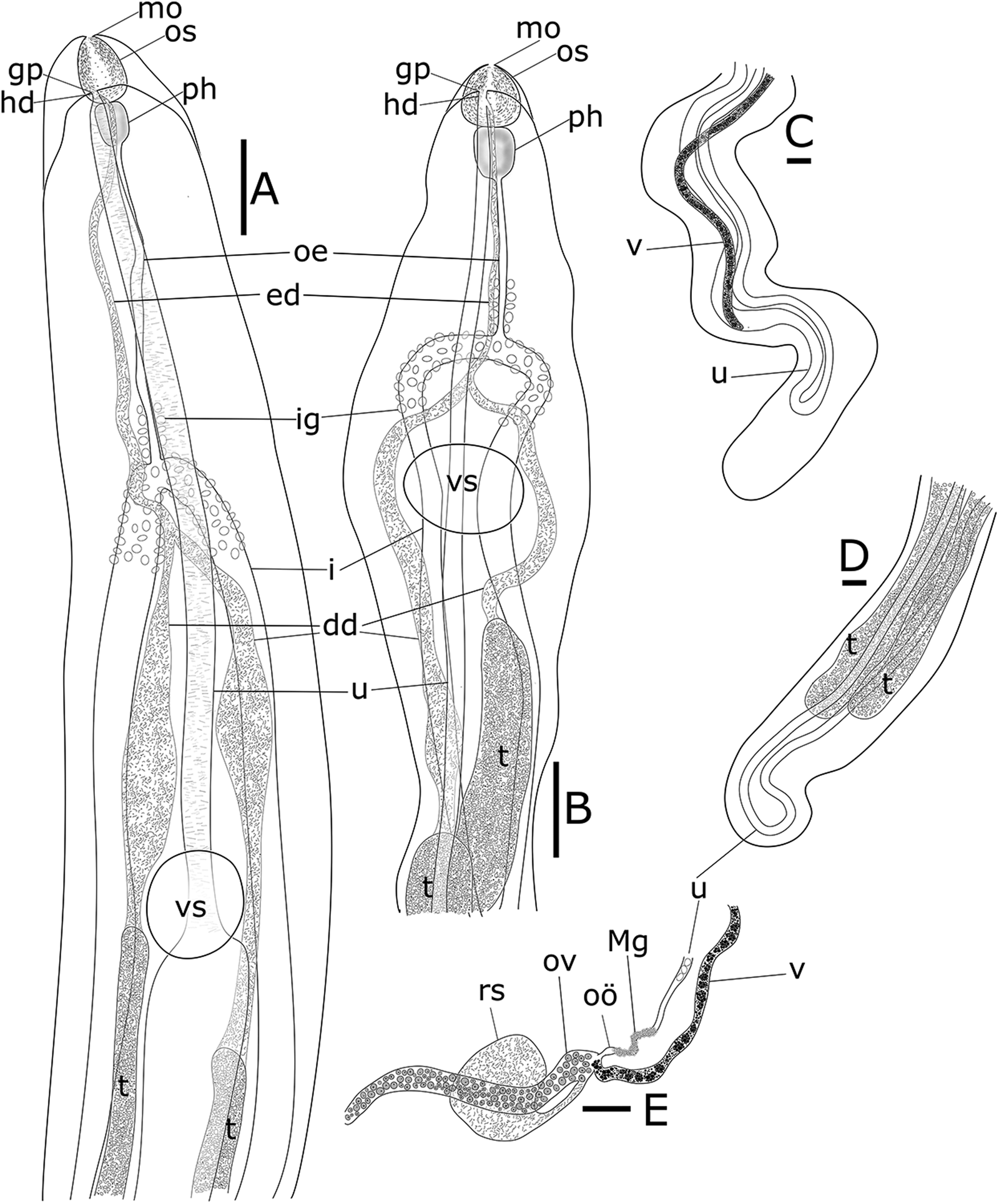
Fig. 1. Didymodiclinus marginati n. sp. ex the pseudobranch filaments of Epinephelus marginatus. (A) Functional female, holotype, anterior end of the body, ventral view. (B) Functional male, paratype, anterior end of the body, ventral view. (C) Functional female, holotype, posterior end of the body, ventral view. (D) Functional male, paratype, posterior end of the body, ventral view. (E) Functional female, holotype, genital junction. dd, deferent duct; ed, ejaculatory duct; gp, genital pore; hd, hermaphroditic duct; i, intestine; ig, intestinal glands; Mg, Mehlis gland; mo; mouth opening; oe, oesophagus; oö, oötype; os, oral sucker; ov, ovarium; ph, pharynx; rs, receptaculum seminis; vs, ventral sucker; t, testis; u, uterus; v, vitellarium. Scale bar: 100 μm.
[Based on 15 adult specimens, 6 functional females and 9 functional males from dusky groupers from Mallorca, western Mediterranean Sea. The values of the holotype NHM UK 2022.4.8.1 (functional female) and paratype NHM UK 2022.4.8.2 (functional male) are expressed in the text, whereas the range of the measurements is reported in Table 4.] Capsule containing a couple of parasites with convoluted posterior ends. Gonochoristic, with weak sexual dimorphism i.e. underdeveloped male and female organs in both functional female and male specimens, respectively. All measurements are given in micrometres.
Table 4. Morphometric data of species of the genus Didymodiclinus
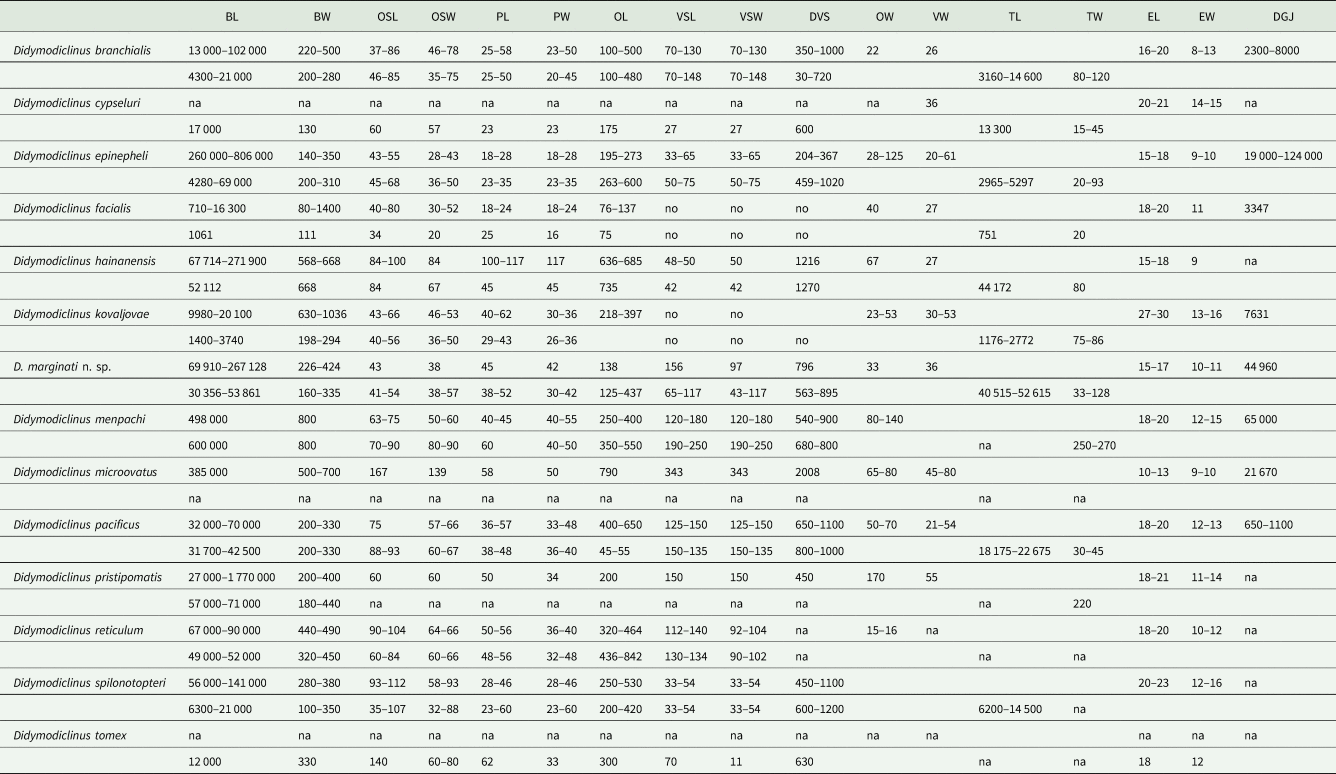
BL, body length; BW, body width; OSL, sucker length; OSW, sucker width; PL, pharynx length; PW, pharynx width; OL, oesophagus length; VSL, ventral sucker length; VSW, ventral sucker width; DVS, distance between anterior tip of body and VS; OW, ovary width; VW, vitellarium width; TL, testis length; TW, testis width; EL, egg length; EW, egg width; DGJ, distance between the anterior tip and the genital junction; na, data not available.
Functional male specimen filiform, not divided in longitudinal regions, smaller than functional female, with anterior end dorso-ventrally flattened and somewhat lanceolate, 53 861 long, 260 wide (Fig. 1A). Oral sucker terminal, spheroidal, 54 × 57. Pharynx spheroidal, 52 × 38: oesophagus bifurcating into 2 caeca, 437 wide. Ventral sucker 65 × 117, at 907 from the anterior end. Pharynx, oesophagus and proximal part of caeca covered with gland cells. Testes 2, parallel, 52 136−52 615 × 111–128, starting at 50 from the posterior end. Vasa deferentia sinuous and spermatic duct filled with spermatozoa. Vasa deferentia joint at 571 from the anterior end. Rudiments of ovary and vitellarium barely visible. Uterus with 1 loop first descending. Metraterm 60 wide, joined with spermatic duct in a very short hermaphroditic duct, 17 long, reaching genital pore ventrally to the oral sucker. In alive specimens the genital pore is located on an extensible temporary papilla. Descending uterus filled with few (2–100) apparently immature eggs, 15 × 10.
Functional female filiform, not divided into regions, with anterior end dorso-ventrally flattened, 77 680 long, 226 wide. Oral sucker terminal, spheroidal, 62 × 47 (Fig. 1B). Pharynx ellipsoidal, 45 × 34. Oesophagus bifurcating into 2 caeca, 156 long, reaching the posterior end of body. Ventral sucker diameter 103, distant 796 from anterior end. Pharynx, oesophagus and proximal part of caeca covered with gland cells. Testes 2, parallel, 6304–7307 × 44–54, starting at 55 805 from the posterior end (Fig. 1D). Vasa deferentia straight and spermatic duct filled with spermatozoa. Vasa deferentia joint at 442 from the anterior end. Ovary long, simple, tubular, sinuous, 33 wide, starting 852 from anterior end of body and reaching with a short oviduct the genital junction at 44 960 (3/5 of the body). Seminal receptacle cystic, 191 × 171, joining the oviduct. Vitellarium long, simple, tubular, posterior to genital junction, 36 wide, starting at 355 from posterior end and joining the oviduct (Fig. 1C). Mehlis gland present around the first part of the uterus. Uterus with 1 loop, first descending, filled with eggs. Metraterm well developed, 90 wide. Genital pore ventral to the oral sucker. In alive specimens the genital pore is located on an extensible temporary papilla. Mature eggs, 15–17 × 10–11 (Fig. 1E).
Remarks
This species morphologically agrees with the diagnosis of the genus Didymodiclinus (Pozdnyakov, Reference Pozdnyakov1993) by the number of characters: the encapsulation in gills and pseudobranchs, a not clearly marked sexual dimorphism, specimens of both sexes with long filiform not regionalized body, a simple short oesophagus, intestinal caeca reaching the posterior extremity, ventral sucker present. Moreover, glandular cells are present around oesophagus and proximal region of the intestinal caeca. Functional males are smaller than functional females, with 2 parallel testes reaching the posterior end, and similarly to Didymodiclinus menpachi (Yamaguti, Reference Yamaguti1970) with rudiments of the female reproductive system. Functional females have long and narrow ovaries located in the anterior part of body and vitellarium with similar form, located in the posterior part. The central portion of the female reproductive system is situated approximately in the middle of the body.
Didymodiclinus marginati n. sp. can be distinguished from D. branchialis because of the larger body length and shorter oesophagus, and also the presence of the seminal receptacle, not observed in the latter species (Table 4). The presence of Mehlis gland, larger pharynx and ventral sucker and shorter oesophagus distinguishes the new species from Didymodiclinus epinepheli (Abdul-Salam et al., Reference Abdul-Salam, Sreelatha and Farah1990). Didymodiclinus marginati n. sp. has accessory gland cells around the oesophagus, a character not observed in Didymodiclinus pacificus (Yamaguti, Reference Yamaguti1938) and Didymodiclinus pristipomatis (Yamaguti, Reference Yamaguti1934). In addition, D. marginati n. sp. has smaller oral sucker and eggs and shorter oesophagus than these 2 species. Concerning the differences with the rest of the congeneric species, D. marginati n. sp. has larger body than Didymodiclinus cypseluri (Yamaguti, Reference Yamaguti1940) and Didymodiclinus hainanensis (Gu and Shen, Reference Gu and Shen1983), but shorter than D. menpachi and Didymodiclinus microovatus (Reimer, Reference Reimer1980). It also has smaller eggs than D. cypseluri, D. menpachi, Didymodiclinus reticulum (Madhavi and Murugesh, Reference Madhavi and Murugesh1994) and Didymodiclinus spilonotopteri (Yamaguti, Reference Yamaguti1970), but larger than D. microovatus.
All previously described species are also characterized by their different hosts and location within the host tissues, being from other geographical localities. Among the most similar species: D. branchialis was recorded in the nostril of Epinephelus quernus Seale, 1901 from Hawaii; D. epinepheli on the gills of Epinephelus tauvina (Forsskål, 1775) from the Arabian Gulf and Epinephelus coioides (Hamilton, 1822) from the Philippines; D. pacificus on the gills of an epinephelid from the Pacific Ocean; D. pristipomatis in the mouth of Epinephelus akaara (Temminck and Schlegel, 1842) from the Japan Sea (Yamaguti, Reference Yamaguti1971; Abdul-Salam et al., Reference Abdul-Salam, Sreelatha and Farah1990; Cruz-Lacierda et al., Reference Cruz-Lacierda, Lester, Eusebio, Marcial and Pedrajas2001).
Molecular results
All the isolates showed positive amplification for 28S, ITS-2 and cox1 genes. Partial sequences of 28S (746 nt, 15 replicates), ITS-2 (640 nt, 15 replicates) and cox1 (530 nt, 15 replicates) were obtained for D. marginati n. sp. The nucleotide sequences of the amplified products of each gene were identical among the isolates from specimens collected in Majorca and Sicily. The representative DNA sequences for 28S, ITS-2 and cox1 were submitted to GenBank (accession numbers MW489507, MW489519 and MW540490, respectively). The representative sequences of 28S had 97.98 and 97.2% of similarity to that of Didymozoidae sp. (AY222193) and Didymozoidae sp. (AY222194) from GenBank, with 13 and 18 nt of differences, respectively. The ITS-2 sequences had 90.3% of similarity to that of Didymozoidae (KP452505) from GenBank with 74 nt of difference. The obtained sequences of cox1 had 75.2% of similarity to Didymosulcus katsuwonicola (KF379726) from GenBank, with 71 nt of difference.
The alignments of 28S sequences used 625 informative positions for NJ, ML and BI analyses. The dataset included sequences of all the species deposited in GenBank. The phylogenetic trees (Fig. 2) resulted in very similar topology with reasonably high posterior probabilities and bootstrap values in most of the nodes. Didymodiclinus marginati n. sp. (sub-family Didymodiclininae) grouped with unidentified didymozoids from Epinephelus cyanopodus (AY222193). The genetic distance between D. marginati n. sp. and the relatives within this clade ranged between 2 (12 nt, AY222193) and 5% (46 nt, AB725627 and AB725630). The alignments of ITS-2 and cox1 sequences used 151 and 244 informative positions, respectively. The results depicted a distribution of taxa similar to that of the 28S, however several basal nodes were not resolved (Figs 3 and 4). In fact, the sequences of D. marginati n. sp. clustered as the sister taxon of didymozoids from other coral reef fish (i.e. Didymozoidae sp. DC-280 and Didymozoidae sp. PC-298).
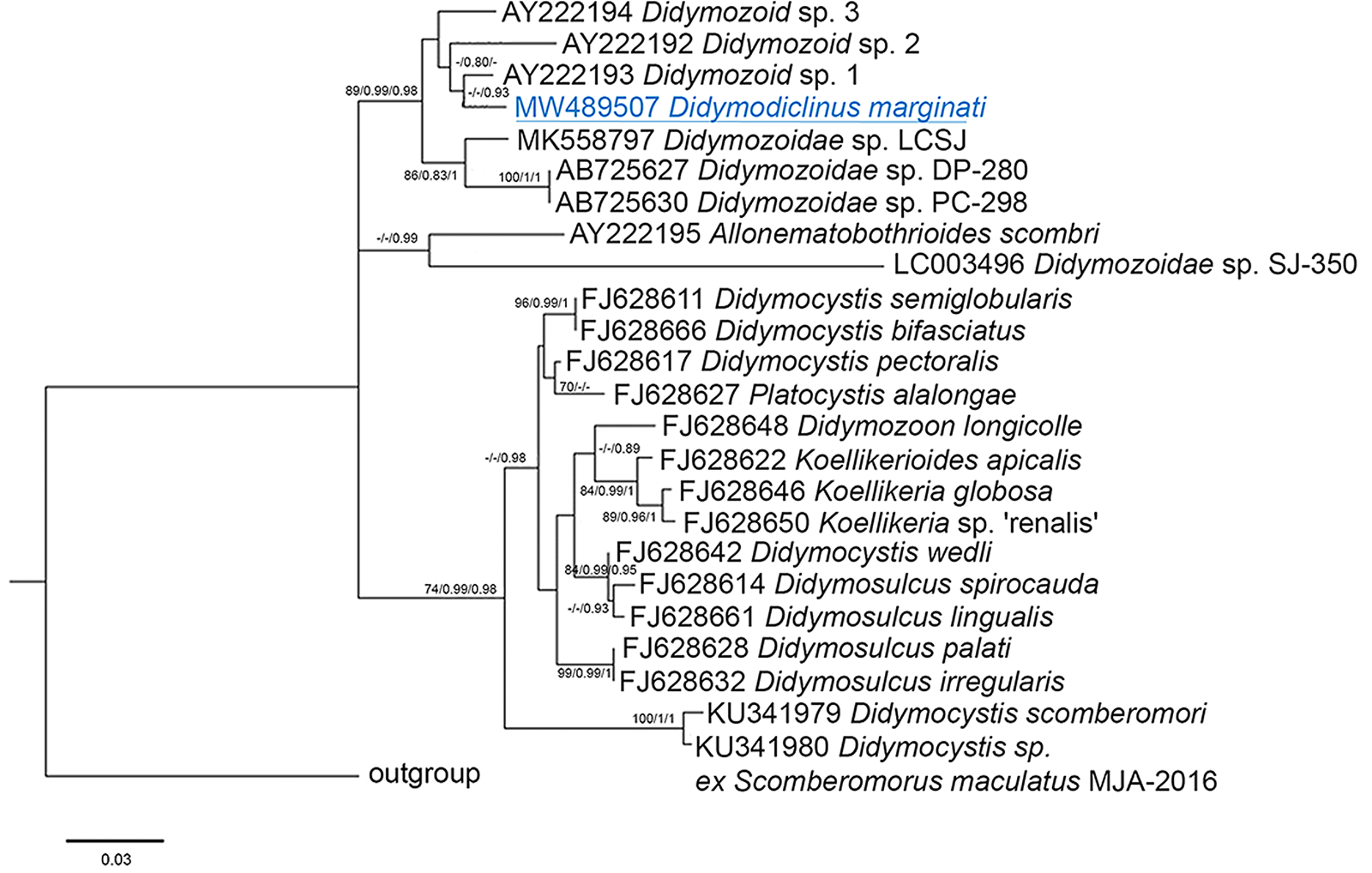
Fig. 2. Phylogenetic relationships between the isolates of the present study and other Didymozoidae as inferred from sequences of 28S rDNA analysed by NJ, ML and BI methods (ML tree is represented). Numbers at the nodes refer to NJ/ML/BI analysis; only bootstrap values above 70% (for NJ) or 0.7 (for ML and Bayesian posterior probabilities) are shown. GenBank accession numbers are indicated before species names. The species newly analysed in this study is underlined. Outgroup: Prosogonotrema bilabiatum.
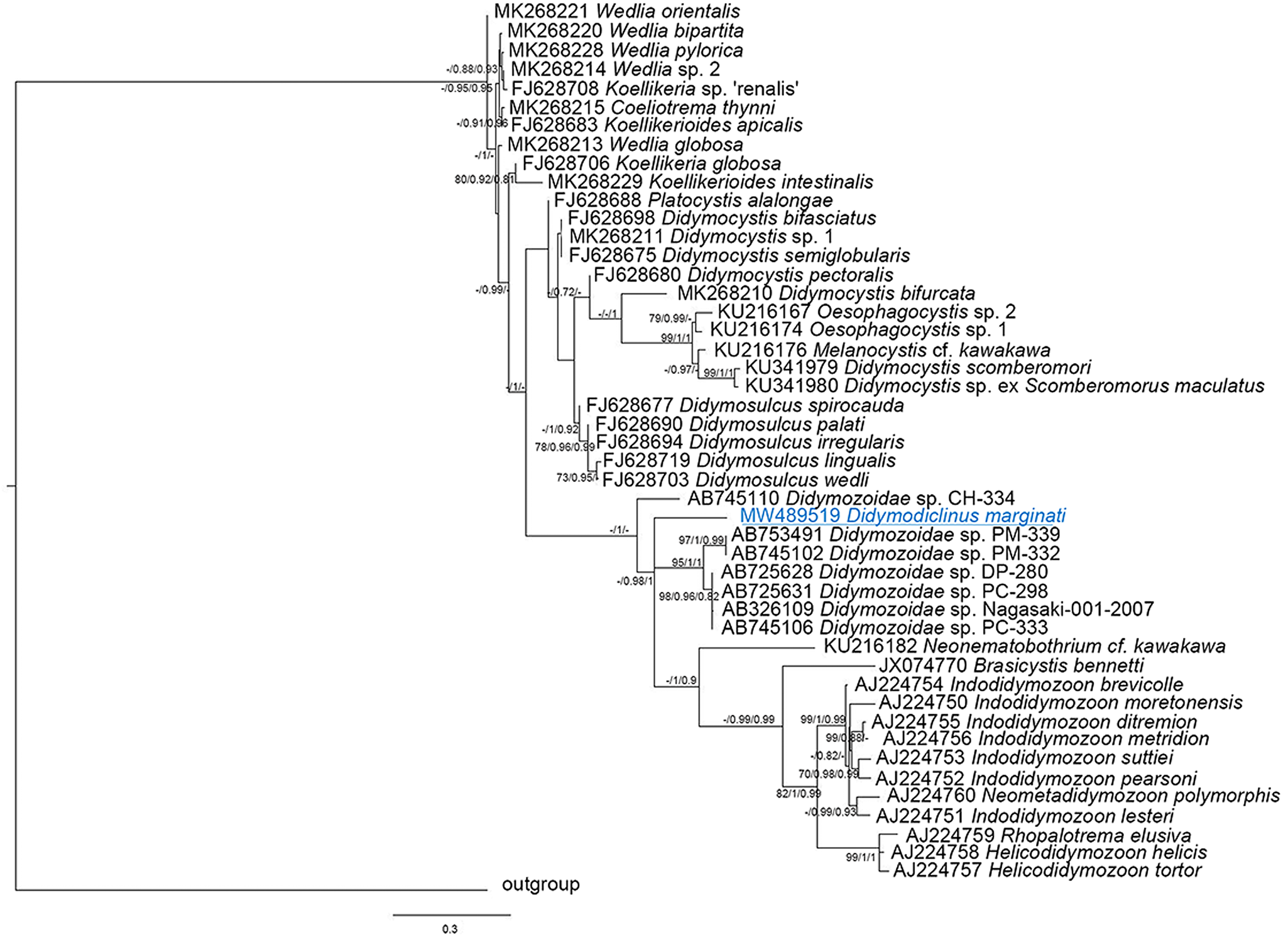
Fig. 3. Phylogenetic relationships between the isolates of the present study and other Didymozoidae as inferred from sequences of ITS-2 rDNA analysed by NJ, ML and BI methods (ML tree is represented). Numbers at the nodes refer to NJ/ML/BI analysis; only bootstrap values above 70% (for NJ) or 0.7 (for ML and Bayesian posterior probabilities) are shown. GenBank accession numbers are indicated before species names. The species newly analysed in this study is underlined. Outgroup: Hemiuridae sp.
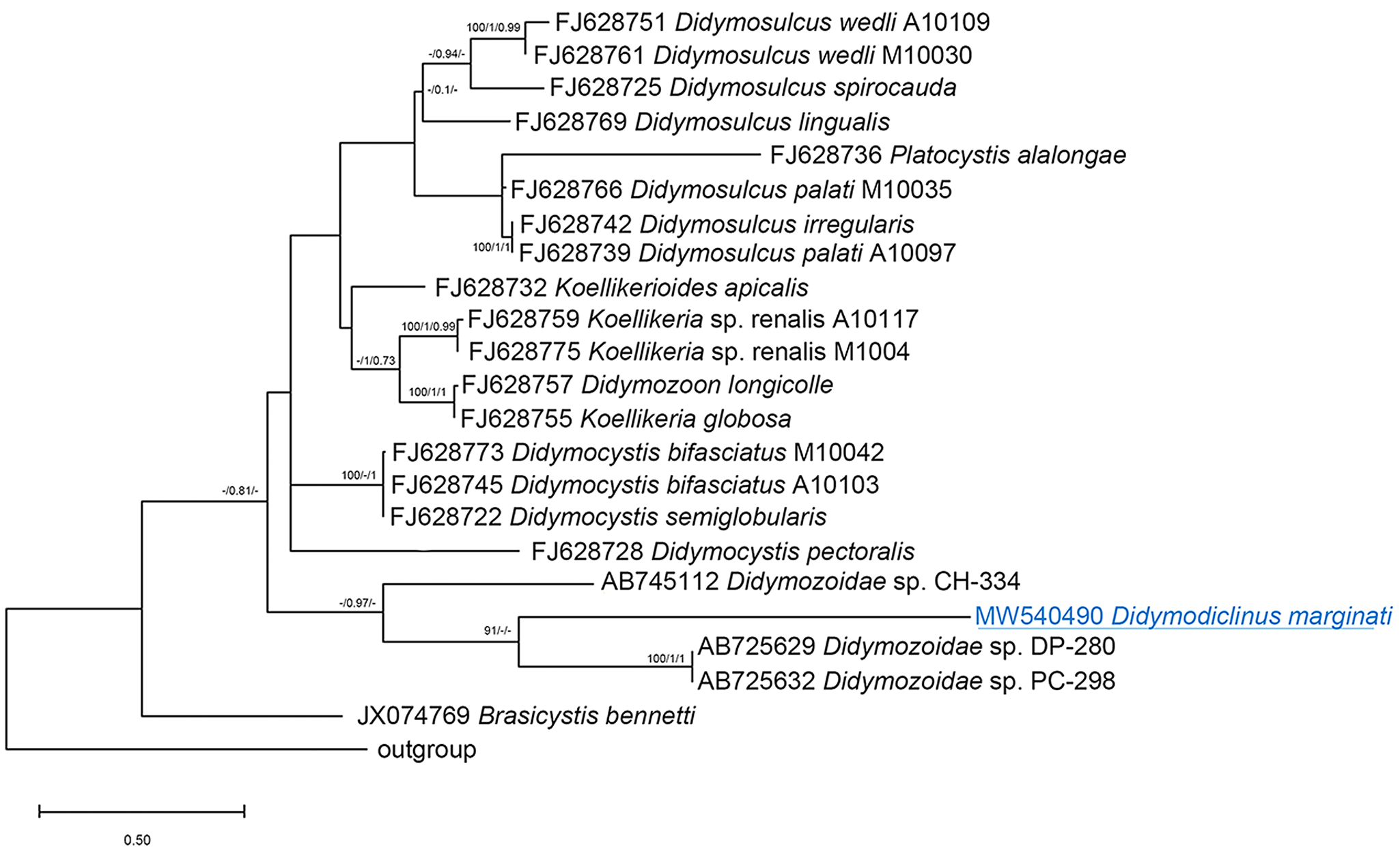
Fig. 4. Phylogenetic relationships between the isolates of the present study and other Didymozoidae as inferred from sequences of cox1 DNA analysed by NJ, ML and BI methods (ML tree is represented). Numbers at the nodes refer to NJ/ML/BI analysis; only bootstrap values above 70% (for NJ) or 0.7 (for ML and Bayesian posterior probabilities) are shown. GenBank accession numbers are indicated before species names. The species newly analysed in this study is underlined. Outgroup: Genarchopsis goppo.
Discussion
Didymozoidae is one of the most taxonomically complex and rich in synonyms of digenean families; the main reliable morphological character used for the identification of the species of this family is the genital complex (Pozdnyakov and Gibson, Reference Pozdnyakov, Gibson, Bray, Gibson and Jones2008), which often represents a challenge due to the variety of body sizes and subjective/partial descriptions reported in literature (Pozdnyakov and Gibson, Reference Pozdnyakov, Gibson, Bray, Gibson and Jones2008; Mladineo et al., Reference Mladineo, Bott, Nowak and Block2010). Pozdnyakov (Reference Pozdnyakov1993) erected the family Didymodiclinidae to include gonochoristic didymozoids, arranging them into 3 subfamilies: Didymodiclininae, with filiform body without marked sexual dimorphism; and the other 2 with globular bodies, Nephrodidymotrematinae with strongly marked sexual dimorphism and body of partners fused, Koellikeriinae without these characteristics. The subfamily Didymodiclininae included only 2 genera separated by the presence (Didymodiclinus) or absence (Paragonapodasmius) of the ventral sucker. Pozdnyakov and Gibson (Reference Pozdnyakov, Gibson, Bray, Gibson and Jones2008) rejected the family Didymodiclinidae, including the subfamilies Didymodiclininae within the Didymozoidae. Apart from the general description of Didymodiclinus (Pozdnyakov, Reference Pozdnyakov1993), the species herein described has some characters that can be useful to distinguish it from the other species representative of the genus: anterior end dorso-ventrally flattened between suckers; functional males with rudiments of the female reproductive system; functional females with Mehlis gland around the first part of descending uterus. Furthermore, the genital papilla was observed in live specimens, but it is a difficult discriminating character because it is visible only in alive specimens (hardly available). This structure seems to work as a muscular organ protruded for mating, but it is also evident in stressful situations (e.g. during the dissection of capsules in live specimens; Salvatore Mele personal observation).
Concerning molecular analysis, the comparisons of the results of 3 loci indicated that the sequences of the new species are related to those of unknown didymozoids of other serranid and haemulid hosts, especially to one found in E. cyanopodus from Australia (AY222193). However, the phylogenetic tree generated with the ITS-2 loci did not have enough significant power to resolve most of the nodes, because the region amplified was too short and included a large number of invariant sites (71% of pair of bases analysed). Conversely, the 28S alignment showed that D. marginati n. sp. clusters apart from the didymozoids of other coral reef fish from the Indian and Pacific Oceans and Yucatan (larval stage) and from the didymozoids of scombrid fish.
Key to identification of Didymodiclinus species
The following dichotomous keys are provided to facilitate the identification of the trematodes belonging to the genus Didymodiclinus based on the comparison between the species of the genus.

Data
The data presented in this study are available on request from the corresponding author.
Acknowledgements
The authors thank F. E. Montero for his critical comments and suggestions on an earlier draft of the manuscript.
Author contributions
S. M., G. D. B. and G. G. conceived and designed the study. S. M., G. D. B. and G. G. performed the veterinary examinations and sampling. A. G., S. O. and K. R. performed the molecular analysis. S. M., G. D. B. and A. G. wrote the article. G. G., P. M., O. R. and GI. G. critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Our study was planned on internal organs sampled from fish markets. For this reason, according to national decree-law 26/2014 (2010-63-EU directive), no institutional review board statement was required.