INTRODUCTION
The cultivation of watermelon (Citrullus lanatus) in Brazil covers an area of approximately 91 thousand ha (IBGE [Instituto Brasileiro de Geografia e Estatística], 2010), predominantly in sandy soils with conventional tillage for land preparation, using ploughs and harrows (Castellane and Cortez, Reference Castellane and Cortez1995; Villa et al., Reference Villa, Groppo, Tessarioli Neto and Gelmini2001). Intensive tillage, characteristic of conventional agriculture, exposes the soil to erosion by the action of rain and wind, and also leads to the formation of a compacted subsurface layer, resulting from the pressure exerted by the use of harrow and plough (Lal et al., Reference Lal, Reicosky and Hanson2007). The preservation of soil and water for food production is the basis of conservational agriculture, with basic principles of minimum soil tillage or no tillage, permanent organic soil protection with cover crops or commercial crops and crop diversification with species rotation or association (FAO [Food and Agriculture Organization], 2011). Several studies have highlighted the importance of no tillage due to their effects on soil properties. Straw permanence on the surface and no-tillage soil foster soil property improvements compared with conventional tillage (Hoyt et al., Reference Hoyt, Monks and Monaco1994; Morse, Reference Morse1999).
Crop rotation, a key component for quality production and sustainability in soils under no tillage, conditions the improvement of their structure and makes possible the formation of biopores with wide variation in size, allowing better root growth (Williams and Weil, Reference Williams and Weil2004). It is also an alternative for the control of subsurface compaction and soil structural and physical quality in the no-tillage system (Derpsch et al., Reference Derpsch, Sidiras and Roth1986).
The relation is quite clear between no tillage and crop rotation with cover crops such as sunn hemp, velvet bean, bristle oat and millet, since one of the principles of the system is the maintenance of straw on the soil surface, which these species do very effectively. Besides the protection of soil surface due to maintenance of straw and root growth (Zuazo and Pleguezuelo, Reference Zuazo and Pleguezuelo2008), cover crops offer several traits that improve crop environment, such as incorporation of atmospheric nitrogen (N) while using legume species, nutrient cycling, improvement of physical and chemical attributes of the soil, among other benefits that help to increase crop productivity (Rosolen et al., Reference Rosolen, Foloni and Tiritan2002; Yau et al., Reference Yau, Sidahmed and Haidar2010).
Due to these significant advantages, no-tillage practices are progressively increasing around the world, being practised in an area of approximately 72 million hectares, mainly in the United States, Brazil, Argentina, Canada and Australia (Bolliger, Reference Bolliger, Magid, Amado, Skóra Neto, Ribeiro, Calegari, Ralisch and Neergaard2006). In Brazil, the acreage under no tillage is about 25.5 million hectares, mostly under cereal crops (Febrapdp [Federação Brasileira de plantio Direto na Palha], 2012), while for vegetables, it is still practised little, perhaps due to the scarcity of scientific information about this technology. However, the few scientific papers related to the topic confirm the good performance of vegetable crops in no tillage in rotation with cover crops (Castro et al., Reference Castro, Alves, Almeida and Ribeiro2004; Perin et al., Reference Perin, Santos, Urquiaga, Guerra and Cecon2004; Wang et al., Reference Wang, Klassen, Li and Codallo2009).
Thus, the aim of this work was to study the physical and chemical properties of soil and agronomic performance of watermelon grown in different methods of tillage and after cultivation of cover crops.
MATERIAL AND METHODS
Description of experimental site
The experiment was carried out in a rural area of Oriente-SP with geographical coordinates of 22°13′ S, 50°06′ W, average altitude of 480 m, a sandy loam Alfisol with the following chemical characteristics: pH (H2O) = 4.8; phosphorus (P) = 0.193 mmolc dm−3; potassium (K) = 1.0 mmolc dm−3; calcium (Ca) = 4.0 mmolc dm−3; magnesium (Mg) = 3.0 mmolc dm−3; aluminium (Al) = 1.0 mmolc dm−3; H+Al = 18.0 mmolc dm−3; cation exchange capacity (CEC) = 26.0 mmolc dm−3; base saturation (V%) = 30.8% and organic matter (OM) = 0.8 g dm−3; and 56.0, 9.0 and 935.0 g kg−1 of clay, silt and sand respectively. This area was under degraded pasture conditions.
While growing cover crops, the total rainfall was 124.6 mm and the average maximum temperature was 26.9 °C and average minimum temperature 15.0 °C. During the watermelon cultivation, the total rainfall was 317.4 mm and the average maximum temperature was 28.9 °C and the minimum temperature was 18.8 °C. Such conditions were adequate for a good growth and development of these crops.
Treatments and experimental design
The experimental treatments comprised minimum tillage (subsoiling), no tillage and cultivation of white lupine and bristle oat as cover crops. Conventional soil tillage was used as the control treatment, simulating the usual technology of the grower.
The experiment was designed as randomised blocks in factorial arrangement plus control treatment with four replications, totalling 20 experimental units. The experimental unit was 7 × 10 m, a total area of 70 m2, and the total area used for the experiment was 1400 m2.
Weed management, lime and fertiliser application
Initially, the Brachiaria pasture in the degradation stage was desiccated with glyphosate (N-(fosfonometil) glicina, C3H8NO5P) herbicide. Fifteen days after desiccation, liming, phosphate and gypsum were applied to the soil. Lime was applied in the amount of 2.0 Mg ha−1 with 60% of reactive power of total neutralization (RPTN) to raise the base saturation to 80% based on the results of the chemical analysis of the soil. Lime was not incorporated into any treatments but was eventually incorporated only in conventional treatment at the time of tillage for watermelon cultivation. According to Trani et al. (Reference Trani, Melo, Passos, Tavares, Nagai, Scivittaro, van Raij, Cantarella, Quaggio and Furlani1996), the fertilisation was performed with thermophosphate with micronutrients, containing 16% P2O5, 18% Ca, 7% Mg, 10% silicon (Si), 0.1% boron (B), 0.05% copper (Cu), 0.3% manganese (Mn) and 0.55% zinc (Zn), in the amount of 1.0 Mg ha−1. Also, it was done by the application of 1.0 Mg ha−1 of gypsum, with the objective of providing calcium in deeper soil profiles. This fertilisation was done in the total area of the experiment.
Minimum tillage and cover crop cultivation
Next, subsoiling was conducted at 60-cm depth with a three-rod subsoiler in minimum tillage experimental units. In areas destined for no tillage, the soil was left intact. Afterwards, cover crops were seeded, except in the experimental units reserved for conventional tillage.
Bristle oats were seeded at 0.3 m inter-row and a density of 60 seeds per metre, and white lupine were seeded at 0.4 m inter-row and a density of 10 seeds per metre, using a no-tillage cereal seeder containing a straw cutting disc of 20 inches. After seeding, the area was irrigated with 5 mm of water to promote germination, emergence and establishment of cover crops. Irrigation was discontinued after the establishment of cover crops, leaving the plants to grow in natural conditions. At 90 days after sowing, the cover crops were cut with a manual mower, leaving the residue of the plant shoot to rest on soil surface.
Conventional tillage and watermelon cultivation establishment
To establish watermelon, tillage was carried out in conventional tillage experimental units, proceeding in the farmer's usual way of tilling the soil to approximately 0.4-m depth with a mouldboard plough. After ploughing, the experimental units were harrowed. Next, grooves were opened for watermelon cultivation at approximately 0.2-m depth and the fertiliser was manually applied in the bottom of the planting furrow. Later, the grooves were closed with specific equipment, leaving the ground ready for planting. Sowing lines for the watermelon cultivation were opened in minimum tillage (subsoiling) and no-tillage treatments with the aid of straw cutting coulters fitted with fertiliser dispensers, similar to no-tillage cereal seeder. At planting, fertilisation was applied in all treatments: 1.0 Mg ha−1 with 4% N, 14% P2O5 and 8% K2O formulation (Trani et al. Reference Trani, Melo, Passos, Tavares, Nagai, Scivittaro, van Raij, Cantarella, Quaggio and Furlani1996). The watermelon seed used was hybrid Top Gun, at a spacing of 3 × 0.8 m, depositing the seeds at up to 3-inches depth with two seeds per hole with the aid of a specific manual seeder for watermelon. Thinning was done 15 days after emergence, leaving one plant per hole.
The control of pests, diseases and weeds was carried out according to Villa et al. (Reference Villa, Groppo, Tessarioli Neto and Gelmini2001), and the topdressing was done by drip fertigation with 50 kg ha−1 of N and 50 kg ha−1 of K2O split applications at 15, 30 and 50 days after emergence of seedlings (Trani et al., Reference Trani, Melo, Passos, Tavares, Nagai, Scivittaro, van Raij, Cantarella, Quaggio and Furlani1996). Irrigation was monitored by tensiometers installed at 0.3-m depth, and the amount of water added was a function of soil tensiometry results and crop phenological stage (Marouelli et al., Reference Marouelli, Silva and Silva1994).
Evaluated characteristics
Cover crop productivity and nutrient cycling
At the end of the cultivation cycle of cover crops, samples were taken from the shoots of bristle oat and white lupine, from 1.0 m2 at two points of each experimental unit for the analysis of dry mass productivity and nutrient content of cover crop shoots. After harvest, the samples were washed with distilled water and neutral detergent, dried in a forced air circulation oven at 65 °C until reaching constant weight, and weighed on a digital scale to determine the dry mass productivity of each species. Later, samples were prepared for nutrient determination; samples were crushed and sent to laboratory for the analysis of macronutrients: N, P, K, Ca and Mg, and micronutrients: B, Cu, iron (Fe), Mn and Zn.
Nutrient content of watermelon
For the analysis of nutritional status of watermelon, the fifth mature leaf from the tip of the vegetative bud (apical tuft) was picked from three plants out of each experimental unit at 50 days after sowing (Trani et al., Reference Trani, Melo, Passos, Tavares, Nagai, Scivittaro, van Raij, Cantarella, Quaggio and Furlani1996). The samples were washed with distilled water and neutral detergent, dried in a forced air circulation oven at 65 °C until reaching constant weight, then crushed and sent to laboratory for the analysis of macronutrient content: nitrogen, phosphorus, potassium, calcium and magnesium, and macronutrients: B, Cu, Fe, Mn and Zn.
Penetration resistance and soil fertility
Measurements of soil penetration resistance (PR) were carried out with a penetrometre model PLG1020 in a 60-cm profile at three points of each experimental unit at the beginning and end of the watermelon cultivation cycle. Soil samples for chemical analysis were collected from 0 to 5-, 5 to 10- and 10 to 20-cm depths at three points of each experimental unit and on two occasions, i.e. at the beginning and end of the watermelon cultivation. The samples were sent to laboratory for the analysis of nutrients, pH, organic matter, base saturation and CEC.
Watermelon root growth
For the root growth study, the minirizhotron method was used with CI-600 root scanner equipment. First, acrylic transparent tubes of 65-mm diameter were inserted vertically in the soil profile to a depth of 40 and 10 cm of watermelon stem at one point of each experimental unit. Later, the root image generation was done with CI-600 root scanner, generating an image of 360° at the depths of 0–20 and 20–40 cm in soil profile at 80 days after watermelon seeding. With the images, and with the aid of WinRhizotron software, it was possible to quantify the length, explored surface area, diameter, volume and the number of roots.
Watermelon productivity
Watermelon productivity was evaluated at 83 days after sowing, picking ripe fruit of 10 central plants from each experimental unit, measuring the number of commercial and non-commercial fruits, fresh fruit mass for commercial and non-commercial on a digital scale and the sugar content (°Brix) of commercial fruit with an optical handheld refractometer (Optech model RCZ) with a scale of 0 to 32% and precision of 0.2%. To measure soluble solids (°Brix), the fruit were parted in two and measured at the three points in each part: central portion and median above and below the centre of the fruit pulp.
Statistical analysis
The effects of tillage type (minimum tillage or no tillage) of cover crops (bristle oat or white lupine), and the effects of interaction between these two factors were evaluated by the analysis of variance using PROC GLM in SAS (Littell et al., Reference Littell, Stroup and Freund2002). The partition of treatment effects was performed by orthogonal contrasts with 1 degree-of-freedom (DOF). In addition, the Dunnett test was used for comparison between the control and the other treatments. For soil fertility and penetration resistance, the analysis was performed by comparison of means (t-test) at each depth only where the analysis of variance indicated significance.
RESULTS
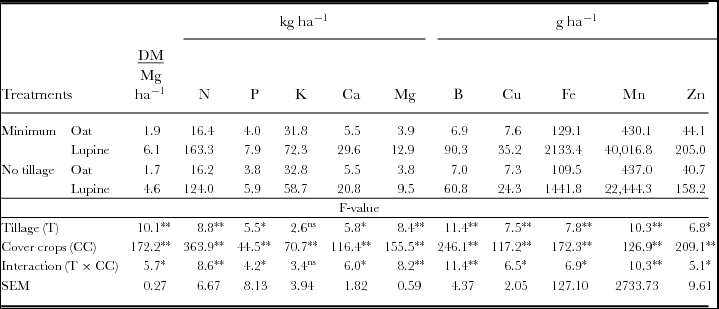
There was significant interaction between the treatments of tillage and cover crops and the shoot dry mass production of cover crop where the minimum tillage increased white lupine productivity compared with no tillage (Table 1). Regarding the accumulation of nutrient by cover crops, there was significant interaction between the experimental treatments for N, P, Ca and Mg. The minimum tillage provided greater accumulation of N, P, Ca and Mg in white lupine shoot than in no tillage. Regarding K, accumulation had a significant effect only for cover crops, and white lupine had the highest amount, with no interaction between treatments. There was a significant interaction between the treatments for the accumulation of micronutrients: B, Cu, Fe, Mn and Zn. In minimum tillage, white lupine had greater accumulation of these micronutrients than in no tillage, and in bristle oats, accumulation was the same in both methods of tillage.
Table 1. Shoot dry biomass of cover crops (DM) and nutrient-accumulated N, P, K, Ca, Mg, B, Cu, Fe, Mn and Zn.

*Significant difference (ANOVA, p < 0.05); **significant difference (ANOVA, p < 0.01); ns: no significant difference.
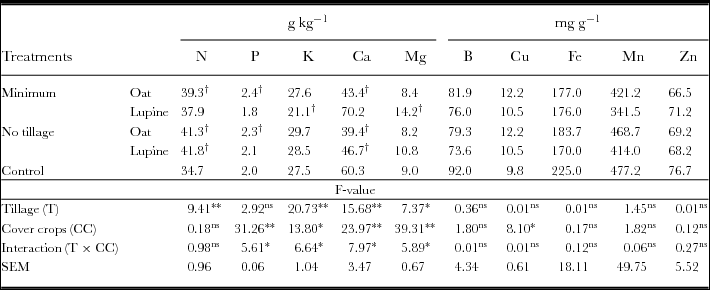
Regarding the watermelon nutritional status, the tillage method had significant effect on the foliar concentration of N, where in no tillage its concentration was higher than in minimum tillage (Table 2). The cover crops were not effective in increasing the concentration of N in watermelon leaves. In minimum tillage with bristle oats and no tillage with both cover crops, the N concentration was higher than in conventional tillage.
Table 2. Concentration of nutrients, N, P, K, Ca, Mg, B, Cu, Fe, Mn and Zn in leaves of watermelon.

*Significant difference (ANOVA, p < 0.05); **significant difference (ANOVA, p < 0.01); ns: no significant difference.
†Significant difference (Dunnets, p < 0.05). This test was applied to confront main treatments with control.
There was an interaction between tillage methods and cover crops for P and K concentration in watermelon leaves. When cultivated on bristle oats, leaf P and K concentration was similar in both the tillage methods, while cultivation on white lupine straw had a leaf concentration of P and K higher in no tillage compared with minimum tillage. Watermelon cultivation on bristle oat straw had higher leaf P concentration than in conventional tillage in both the methods of conservation tillage. The leaf K concentration of watermelon was lower in minimum tillage with white lupine compared with conventional tillage.
There was also interaction between tillage method and cover crops for watermelon leaf concentrations of Ca and Mg. Minimum tillage on white lupine straw provided higher concentration of both Ca and Mg, while on bristle oats straw, concentrations of Ca and Mg were not affected by the tillage method. In conventional tillage, leaf Ca concentrations of watermelon were higher than in minimum tillage with bristle oats and that in no tillage with both cover crops. The watermelon leaf concentration of Mg was higher in minimum tillage with white lupine than in conventional tillage. With the exception of Cu, which had higher leaf concentration in watermelon when grown in bristle oat straw, concentrations of other micronutrients, i.e. B, Fe, Mn and Zn, did not change with experimental treatments.
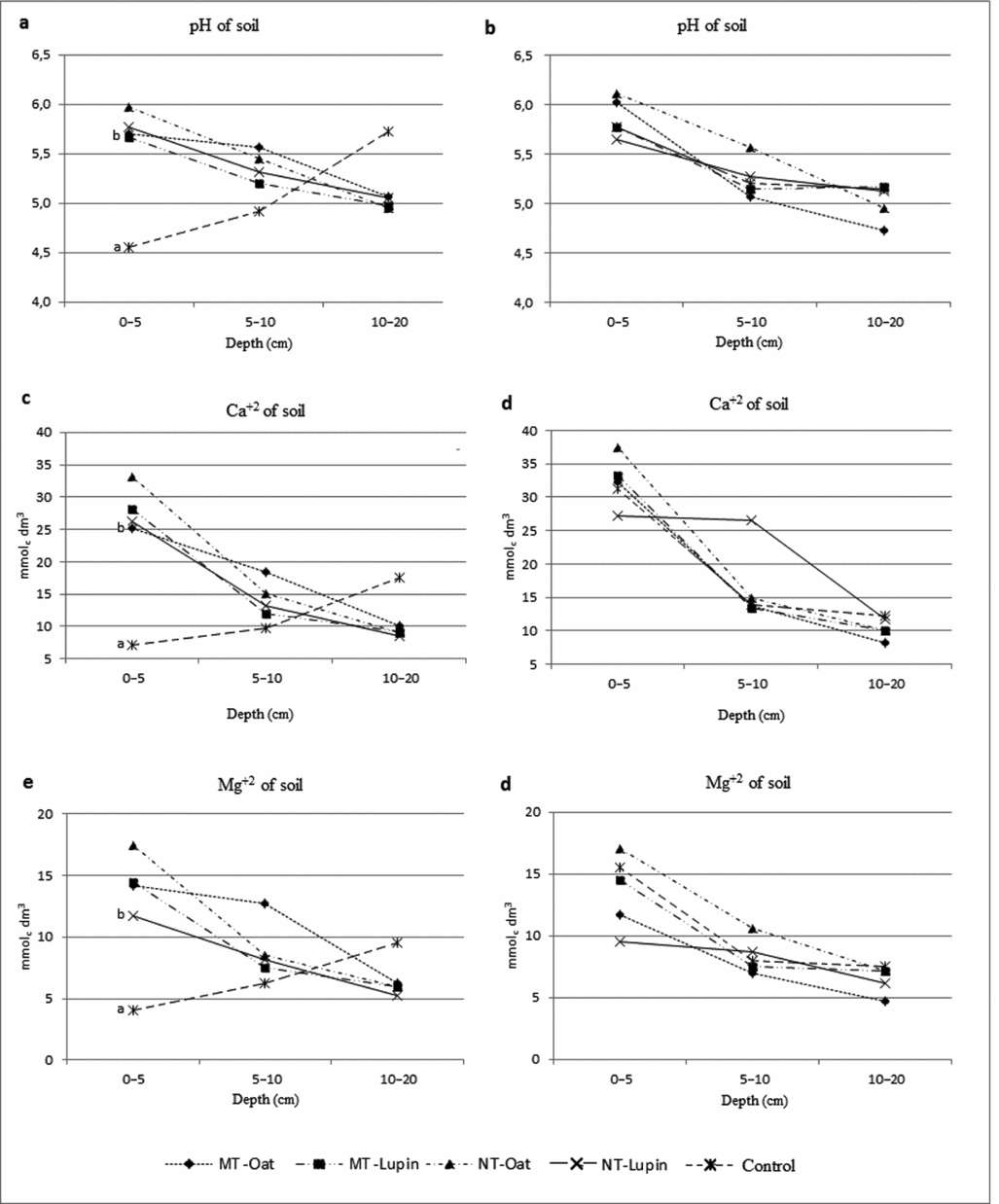
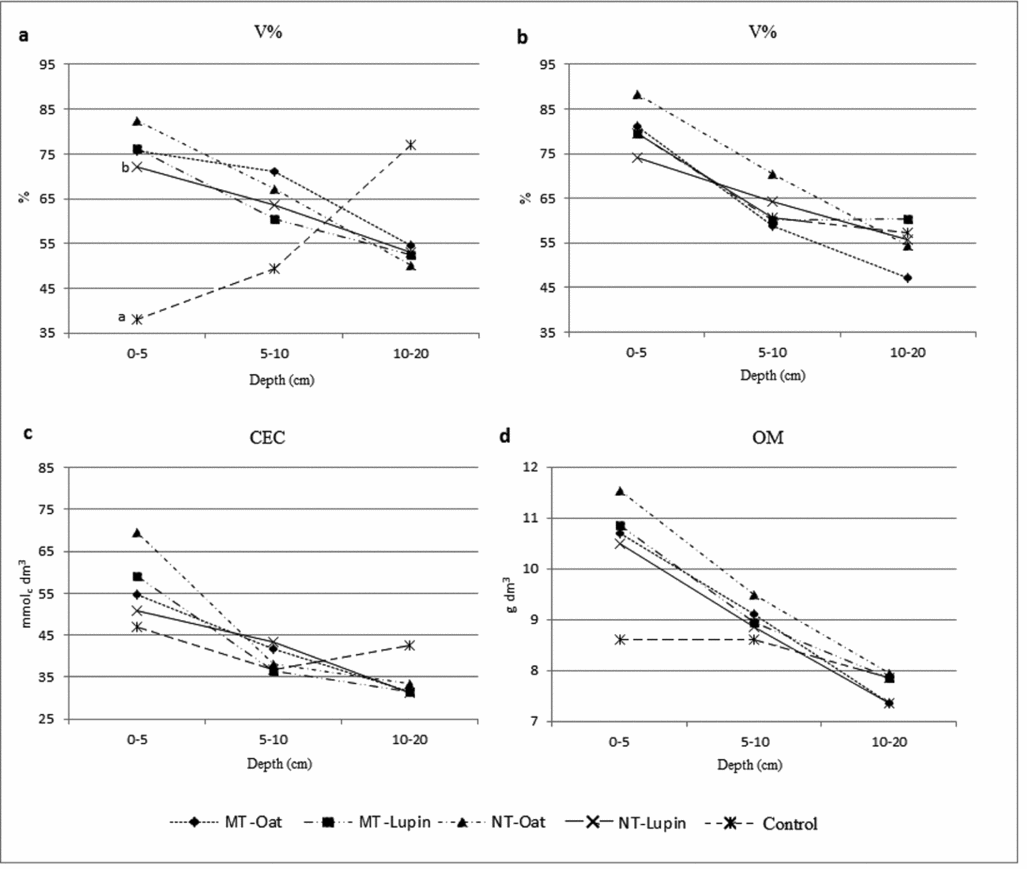
Regarding soil fertility, there was interaction between treatments, depths (0–5, 5–10 and 10–20 cm) and sampling times, early and late cultivation cycle of watermelon, for pH, Ca and Mg (Figure 1). At the first sampling time, pH, Ca and Mg had higher values at 0–5-cm depths in treatments with no tillage and minimum tillage for both cover crops compared with conventional tillage, and at depths of 5–10 and 10–20 cm, treatments did not alter the values of pH, Ca and Mg. At the second sampling time, the soil tillage treatments, including conventional tillage, and both cover crops had the same pH, Ca and Mg values at all depths (Figures 1b, d and f). As for the base saturation, there was no interaction between the treatments and the sampling depth. In the first sampling, conventional tillage had lower base saturation than other treatments, only at a depth of 0–5 cm (Figure 2c), and in the second sampling there was no effect of treatments in the studied depths (Figure 2d). For CEC and soil organic matter, there was no interaction between the factors studied, with only depth effect where the values were decreasing in profile regardless of the treatments (Figures 2e and f).

Figure 1. pH value, calcium (Ca) and magnesium (Mg) in three depths (0–5; 5–10 and 10–20 cm) of soil profile and two instances: 0 day (1a, c and e) and 90 days (1b, d and f) after watermelon sowing. MT = minimum tillage; NT = no tillage. Different letters in each depth indicate significant difference (t-test, p < 0.05).

Figure 2. (a, b) Basis saturation (V%), (c) cationic exchange capacity (CEC) and (d) organic matter (OM) in three depths (0–5; 5–10 and 10–20 cm) of soil profile, and at two instances: 0 and 90 days after watermelon sowing. MT = minimum tillage and NT = no tillage. Different letters in each depth indicate significant difference (t-test, p < 0.05).
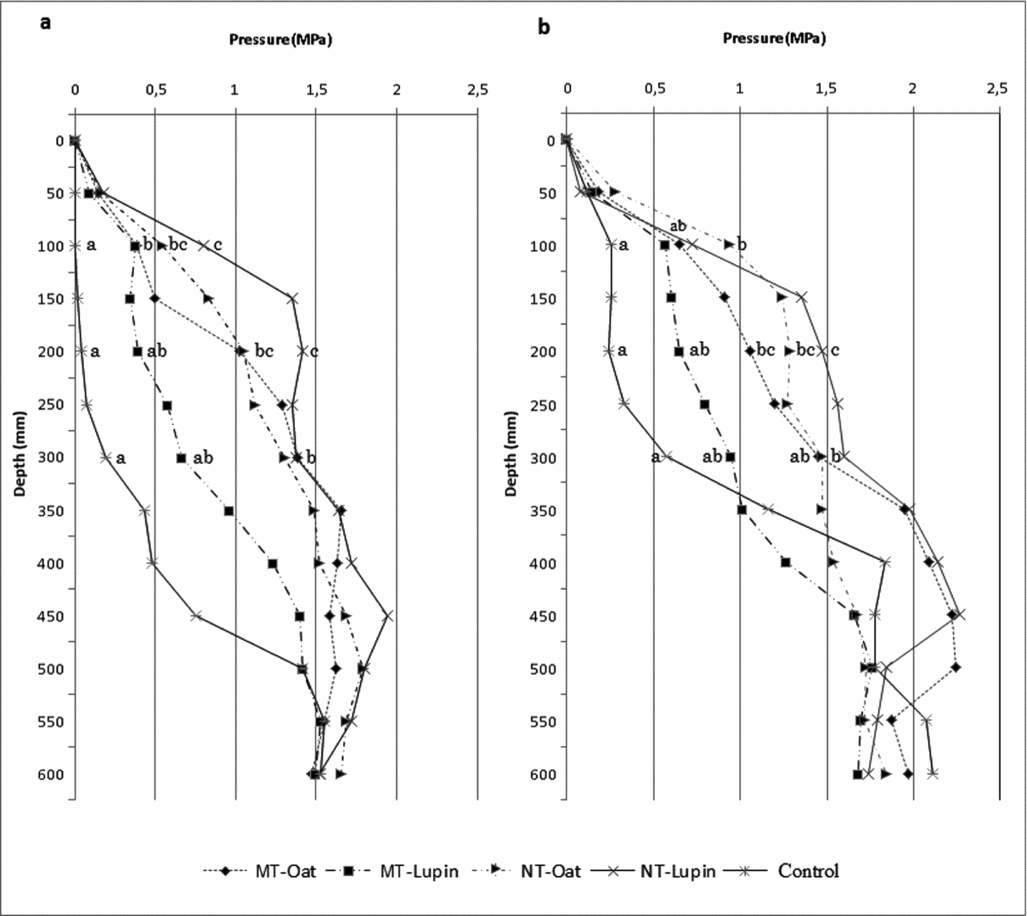
There was significant interaction between treatments and sampling depths for soil penetration resistance. At the first sampling, at the early stage of watermelon cultivation, penetration resistance difference between treatments at a depth of 30 cm and below did not differ (Figure 3). At 10-cm depth, conventional tillage had lower penetration resistance than other tillage methods, probably due to the harrowing disruptive soil action. The minimum tillage with both cover crops resulted in lower penetration resistance than no tillage with white lupine at 10-cm depth. At 20- and 30-cm depth of profile, conventional tillage had the lowest penetration resistance, with no difference from minimum tillage with white lupine. At the second sampling, at the end of watermelon cultivation cycle, conventional tillage penetration resistance was significantly lower than no tillage with bristle oat at a depth of 10 cm. At 20 cm, conventional tillage had a penetration resistance similar to minimum tillage with white lupine, however lower than the others, and at a depth of 30 cm, conventional tillage had lower penetration resistance than no tillage with lupine.

Figure 3. Penetration resistence (MPa) in soil depth at two instances, at the beginning and the end of the watermelon grown, in the tretaments of tillage. MT = minimum tillage and NT = no tillage. Different letters within lines indicate significant difference (t-test, p < 0.05).
For root growth characteristics of watermelon, there was no difference between the soil profile studied depths. However, the tillage methods had significant effect on total root length, explored surface area and root's total volume, where in minimum tillage watermelon roots had higher values for these characteristics in relation to no tillage (Table 3). There was no effect of tillage methods and cover crop on the diameter and number of roots. It was found that compared with conventional tillage, no tillage with both cover crops was inferior in root length, and tillage with bristle oat was not lower in explored surface area and number of roots of watermelon.
Table 3. Parameters of root growth of watermelon: total length (TL), surface area explored (SAE), diameter medium of roots in mm (DR), total volume of root system (VT) and number of root tips (RT), at the 0–40 cm of soil depth in 440 cm2.
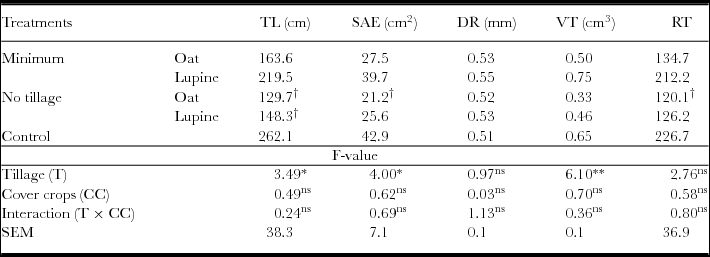
*Significant difference (ANOVA, p < 0.05); **significant difference (ANOVA, p < 0.01); ns: no significant difference.
†Significant difference (Dunnets, p < 0.05). This test was applied to confront main treatments with control.
Regarding watermelon fruit production, there was significant interaction between the studied factors to commercial fresh fruit mass (CFFM), number of commercial (NCF) and non-commercial fruits (NNCF). When cultivated on bristle oat straw, the minimum tillage provided greater CFFM and NCF than no tillage (Table 4). In cultivation on white lupine straw, no tillage reduced the amount of discarded fruits in relation to minimum tillage. Only no tillage on bristle oat straw had lower CFFM and NCF values compared with conventional tillage. There was no difference between experimental treatments for degrees °Brix of commercial fruit pulp, the sensorial quality being similar between treatments.
Table 4. Commercial fresh fruit mass (CFFM), non-commercial fresh fruit mass (NCFFM), number of commercial fruits (NCF), number of non-commercial fruits (NNCF) and °Brix of commercial fruits.
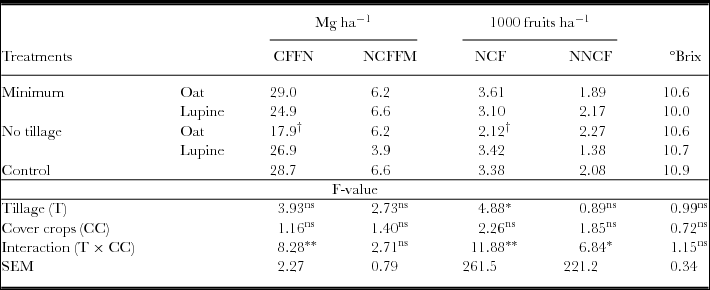
*Significant difference (ANOVA, p < 0.05); **significant difference (ANOVA, p < 0.01); ns: no significant difference.
†Significant difference (Dunnets, p < 0.05). This test was applied to confront main treatments with control.
DISCUSSION
Cover crops biomass productivity and nutrient cycling
Water restriction due to lack of rain and the absence of nitrogen fertilisation on crop cycle affect the yield of cover crops, mainly bristle oat. The better dry mass productivity of white lupine shoot compared with oats provided greater nutrient uptake, and consequently higher amount of cycled nutrients, but the amount of dry mass produced was not reflected in an increase of organic matter in the soil. Gonçalves and Carreta (Reference Gonçalves and Carreta1999) also reported higher dry mass production of white lupine relative to bristle oat with the same values of this work. Being a leguminous species, the amount of N in white lupine shoot was much higher than that of bristle oat, a fact related to biological nitrogen fixation (Vance, Reference Vance2001). However, the amount of N incorporated into the system by white lupine was not enough to raise the concentration in the leaves of watermelon. An increase of N in watermelon leaves due to the no-tillage method regardless of cover crops was observed, and it may be that this fact is related to the maintenance of straw on soil surface, thus improving the nitrogen foliar content of watermelon due the gradual mineralization of organic matter (Torres et al., Reference Torres, Pereira and Fabian2008). No tillage may be recommended as a viable alternative to increasing organic carbon in soil surface and consequently the availability of N to plants (López et al., Reference López, Blanco-Moure, Limón and Garcia2012). Increasing the concentration of watermelon leaf calcium and magnesium was favoured by cultivation on white lupine straw, mainly on minimum tillage, demonstrating the ability of this cover crop in cycling these nutrients. The dry mass production of bristle oat, in spite of being inferior to white lupine, accumulated a great amount of P and consequently increased the leaf P concentration in watermelon, thereby being an excellent alternative of P cycling in the system. One hypothesis that we idealized in the study was that the lupin would be more efficient in P absorption and accumulation in shoot due to the genetic characteristics of the root system (O’Rourke et al., Reference O’Rourke, Yang, Miller, Bucciarelli, Liu, Rydeen, Bozsoki, Uhde-Stone, Tu, Allan, Gronwald and Vance2013). However, we noted that bristle oat had better performance, the fact that we assigned to our fasciculate root architecture. This characteristic provides better soil volume exploitation, and, in fact, can be contributed to P absorption by roots and accumulation in the shoot (Shen et al., Reference Shen, Yuan, Zhang, Li, Bai, Chen, Zhang and Zhang2011).
Soil fertility and resistance to root penetration
Changes detected in soil fertility due to tillage methods were evident by the values of pH, Ca, Mg, CTC and V%, but only at the first sampling time. Compared with conventional tillage, minimum tillage and no tillage, the higher content of Ca and Mg was evident and consequently higher values of pH, CTC and V% in shallower soil profile of 0.0 to 5.0 cm, but at greater depths there were no changes due to tillage methods. This demonstrates that lime incorporation by harrowing reduces pH, Ca, Mg, CTC and V% in shallower soil profile (Adekiya et al., Reference Adekiya, Ojeniyi and Agbede2011). The results marked the low mobility of lime in soil profile over the conservation methods of tillage, with limited effect on the surface layer (Caires et al., Reference Caires, Alleoni, Cambri and Barth2005; Godsey et al., Reference Godsey, Pierzysnki, Mengel and Lamond2007).
Conventional tillage favoured the concentration of watermelon leaf Ca, possibly due to greater root growth provided by the tillage method, increasing the surface contact with the soil for nutrient absorption.
The minimum tillage with white lupine as a cover crop favoured the reduction of penetration resistance up to 30-cm depth compared with other conservation tillage with values similar to those of conventional tillage. The action of harrow in conventional tillage causes soil surface disruption and thus reduces penetration resistance and exposes the soil to the erosion process (Lal et al., Reference Lal, Reicosky and Hanson2007). Being a sandy soil, the natural penetration resistance does not extrapolate values considered critical for restricting root growth that is 2.0 MPa (Handerson, Reference Handerson1991). However, higher penetration resistance in no tillage with bristle oats restricted root growth and yield performance of watermelon.
Cover crops are known as production system components to assist reducing soil compaction and enhancing soil structure due to vigorous root growth (Rosolen et al., Reference Rosolen, Foloni and Tiritan2002; Williams and Weil, Reference Williams and Weil2004). In this work, the white lupine positive effect was found in reducing resistance to penetration of roots, mainly with minimum tillage.
Watermelon root growth and production
Conventional tillage favoured root growth of watermelon in relation to tillage because of disruption in the plough layer soil by the action of harrow to reduce resistance to root penetration. Greater resistance to penetration of roots has a negative effect on root growth in most of the cultivated species (Wolf et al., Reference Wolf, Topoleski, Gundersheim and Ingall1995). However, Drost and Wilcox-Lee (Reference Drost and Wilcox-Lee2000) reported the favourable effect of asparagus root growth in no tillage.
The root system of cover crops works as biological subsoilers, reducing compaction of deeper profiles, which promotes root growth of next cultivation (Calonego and Rosolen, Reference Calonego and Rosolen2010; Chen and Wiel, Reference Chen and Weil2010). In this sense, white lupine was quite efficient, favouring watermelon root growth. We cannot relate the increase in root growth of watermelon with soil fertility because change in acidity and base saturation was restricted to the surface soil layer of 0–5 cm, and below that depth there was no change in the chemical characteristics of soil given to the treatments.
The lower productive performance of watermelon in no tillage with bristle oat straw seems related to restriction in root growth due to high resistance to root penetration of this treatment, and also to lower leaf concentrations of Ca and Mg. Keshavarzpour and Rashidi (Reference Keshavarzpour and Rashidi2008) reported reduction in productivity of watermelon in no tillage compared with conventional tillage due to lower root growth and high resistance to root penetration in no tillage.
CONCLUSIONS
The minimum tillage with lupine alleviated the penetration resistance of soil, conferring a great root growth of watermelon.
Conservationist methods of tillage maintained Ca and Mg concentration in the soil surface layer, thus increasing pH and base saturations at a depth of 5 cm from the surface, thereby ensuring adequate nutritional status and yield of watermelon.
White lupine is the best option of cover crop because of its good performance in dry mass production and nutrient cycling in comparison to bristle oat.
Acknowledgements
To Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) for the financial support to project 2010/02312-1.