People divide roughly, it seems to me, into two kinds, or rather a continuum is stretched between two extremes. There are people people and things people.
—W. D. Hamilton (Reference Hamilton and Ridley2005, p. 205)1. Introduction
We describe a new hypothesis that seeks to conceptually unify the analyses of psychosis and autism, two disorders of the human social brain (Burns Reference Burns2004; Reference Burns2006a; McAlonan et al. Reference McAlonan, Cheung, Cheung, Suckling, Lam, Tai, Yip, Murphy and Chua2005). The core of this hypothesis is that psychosis and autism represent two extremes on a cognitive spectrum with normality at its center. Social cognition is thus underdeveloped in autism, but hyper-developed to dysfunction in psychosis. We also suggest that these forms of deviation from normal social brain development in either direction are mediated in part by alterations in developmental and metabolic systems affected by genomic imprinting, notably via effects of genes that are imprinted in the brain and in the placenta (Davies et al. Reference Davies, Isles, Smith, Karunadasa, Burrmann, Humby, Ojarikre, Biggin, Skuse, Burgoyne and Wilkinson2005; Tycko & Morison Reference Tycko and Morison2002). Genomic imprinting involves a developmental and physiological tug-of-war, in the growing fetus and child, between the effects of paternally expressed (maternally “imprinted,” that is, maternally silenced) genes, which favor enhanced growth as well as selfishness in interactions with the mother, and the effects of maternally expressed (paternally silenced) genes, which favor relatively constrained growth and other traits that tend to benefit mothers (Haig Reference Haig2000b; Reference Haig2004b). Relatively small genetic or epigenetic disruptions of this tug-of-war may increase the fitness of the child or mother, respectively, as in some disorders of placentation mediated by dysregulated imprinting (Haig Reference Haig and Stearns1999b; Oudejans et al. Reference Oudejans, Mulders, Lachmeijer, van Dijk, Könst, Westerman, van Wijk, Leegwater, Kato, Matsuda, Wake, Dekker, Pals, ten Kate and Blankenstein2004); but larger alterations are pathological, and we hypothesize that they contribute to the development of either autistic-spectrum disorders (due to a paternal-gene bias) or psychotic-spectrum disorders (due to a maternal-gene bias) via their effects on growth, neurodevelopment, cognition, and behavior.
We unpack our hypothesis by first providing a brief background on the social brain, and how its development is altered in autism and psychosis. Second, we provide an overview of genomic imprinting and explain Haig's (2000b; 2004b) “conflict theory” for how imprinting has evolved. Third, we describe our nosological framework for conceptualizing autistic-spectrum conditions and what we call psychotic-spectrum conditions, and we explain how the conflict theory of imprinting provides an evolutionary basis for elucidating their genetic, epigenetic, and neurodevelopmental causes. Fourth, we describe how Prader-Willi syndrome and Angelman syndrome, which are caused by alterations of a region of chromosome 15 harboring imprinted genes, provide useful tests of the role of imprinted genes in autism and psychosis. Fifth, we contrast autistic-spectrum and psychotic-spectrum conditions for a wide range of anatomical, neurological, developmental, cognitive, behavioral, and epidemiological data. Our hypothesis predicts that to the degree that they represent opposite, generalized disorders, autistic- and psychotic-spectrum conditions should exhibit diametric phenotypes for traits related to growth, development, and the social brain. Finally, we develop a conceptual model for how sex differences interact with genomic-imprinting effects, which can help to explain some key features of autistic- and psychotic-spectrum epidemiology and symptoms.
We appreciate that autistic-spectrum and psychotic-spectrum conditions are each highly heterogeneous, with myriad causes (Ronald et al. Reference Ronald, Happé, Bolton, Butcher, Price, Wheelwright, Baron-Cohen and Plomin2006; Ross et al. Reference Ross, Margolis, Reading, Pletnikov and Coyle2006a), and we are thus not proposing that these conditions are caused in any exclusive sense by alterations to genomic imprinting. We also stress that describing an evolutionary framework for understanding autism and psychosis does not in any way imply that these conditions should be considered as adaptive, even though the autistic and psychotic spectrums each involve a pattern of specialized cognitive strengths and impairments in relatively high-functioning individuals (Claridge Reference Claridge1997; Mottron et al. Reference Mottron, Dawson, Soulières, Hubert and Burack2006). Our main goal instead is to integrate predictions from evolutionary theory and genetics with psychology, neuroscience, and psychiatry, to further our understanding of the major disorders of human cognition, affect, and behavior.
2. The social brain
A key initial insight into human evolution was the idea that the primary selective pressures shaping human cognitive development may be social rather than ecological (Emery Reference Emery2000). This idea can be traced to Chance and Mead (Reference Chance and Mead1953), Jolly (Reference Jolly1966), Humphrey (Reference Humphrey, Bateson and Hinde1976; Reference Humphrey1983), Alexander (Reference Alexander, Mellars and Stringer1989), and Brothers (Reference Brothers1990), who have suggested that living in large, complex groups, under strong within-group and between-group social competition for resources and mates, has selected for a “social brain,” functionally designed by evolution mainly for solving social problems.
Recent studies have described how early development of components of the social brain is impaired in autism, which may lead to a cascade of social deficits, and how many of the core features of schizophrenia can also be understood in terms of dysregulation in multiple aspects of uniquely human social cognition (Arbib & Mundhenk Reference Arbib and Mundhenk2005; Baron-Cohen & Belmonte Reference Baron-Cohen and Belmonte2005; Benes & Berretta Reference Benes and Berretta2001; Burns Reference Burns2004; Reference Burns2006a). These advances have suggested that autism and schizophrenia are related to one another in some conceptual and etiological ways, because they both involve alterations in recently evolved human social behavior as central features (Burns Reference Burns2006a). Although both disorders can be conceived as dimensional, grading more or less finely into normality (e.g., Frith & Happé Reference Frith and Happé2005; see also, Hill & Frith Reference Hill and Frith2003; Linney et al. Reference Linney, Murray, Peters, MacDonald, Rijsdijk and Sham2003; Schürhoff et al. Reference Schürhoff, Laguerre, Szöke, Méary and Leboyer2005), the relationship of autistic-spectrum conditions with schizophrenia, and other conditions on the psychotic spectrum, has yet to be explicitly investigated in any detail. We do so here, in the context of evolutionary theory and genetics, with a focus on effects of genomic imprinting.
3. Genomic imprinting
Analysis of the social brain in contemporary neuroscience and psychology is yielding stunning insights into human cognition and psychiatric disorders. However, the conceptual social brain is not yet an evolutionary brain, because it has yet to fully incorporate central features of evolutionary biology, such as inclusive fitness theory (Foster et al. Reference Foster, Wenseleers, Ratnieks and Queller2006; Hamilton Reference Hamilton1964), intragenomic conflict (Burt & Trivers Reference Burt and Trivers2006), social-behavioral ecology (e.g., Krebs & Davies Reference Krebs and Davies1991), sex differences attributable to sex-differential selective pressures (e.g., Andersson Reference Andersson1994), and the genomic basis of recent human evolution (e.g., Crespi Reference Crespi2006; Voight et al. Reference Voight, Kudaravalli, Wen and Pritchard2006). Each of these bodies of theory and data has important implications for understanding the evolution of the human social brain, its developmental-genetic underpinnings, and its dysregulation.
Inclusive fitness theory forms a cornerstone of biology, in explaining how social interactions between genetically related individuals, such as mothers and offspring, have evolved (Alexander Reference Alexander1987; Hamilton Reference Hamilton1964; Hrdy Reference Hrdy1999). This theory predicts that under any degree of multiple paternity, genes subject to imprinting whose phenotypic effects lead to offspring extracting relatively high levels of limiting resources from mothers and other maternal kin are expected to be silenced in the maternal germ line (and thus paternally expressed in offspring) (Burt & Trivers Reference Burt and Trivers2006; Haig Reference Haig2000b; Reference Haig2004b). In turn, these effects should be countered by selection for paternal silencing (and thus expression from only the maternally inherited chromosome) of genes whose phenotypic effects restrain the transfer of mother's resources, bringing maternal investment towards her own optimum level. This conflict theory for the evolution and patterns of genomic imprinting has been supported by a large body of evidence on the functions and expression patterns of imprinted genes (Cattanach et al. Reference Cattanach, Beechey and Peters2004; Haig Reference Haig1996; Reference Haig and Stearns1999b; Reference Haig2004a; Reference Haig2004b; McMinn et al. Reference McMinn, Wei, Schupf, Cusmai, Johnson, Smith, Weksberg, Thaker and Tycko2006; Plagge et al. Reference Plagge, Gordon, Dean, Boiani, Cinti, Peters and Kelsey2004; Smith et al. Reference Smith, Garfield and Ward2006), and it provides a robust theoretical framework for analyzing the roles of imprinted genes in human development and evolution. Although imprinted genes comprise only about 1% of the genome, they are disproportionately involved in growth, especially with regard to placental and brain development and function (Tycko & Morison Reference Tycko and Morison2002); they are highly pleiotropic in their effects; and they can be dysregulated in more ways than non-imprinted genes. Thus, imprinted-gene expression can be affected by alterations in nucleotide sequence, by epigenetic variation (such as methylation and histone modification), by “imprinter” genes that regulate application, maintenance, and removal of imprints (Wilkins Reference Wilkins2005), and by environmentally induced effects on imprinted gene expression (Dolinoy et al. Reference Dolinoy, Weidman and Jirtle2006).
Most studies of genomic imprinting have focused on genes expressed during prenatal and neonatal development, where conflict is manifested in aspects of maternal–fetal interactions during placentation and neonatal growth (Angiolini et al. Reference Angiolini, Fowden, Coan, Sandovici, Smith, Dean, Burton, Tycko, Reik, Sibley and Constância2006; Crespi & Semeniuk Reference Crespi and Semeniuk2004; Haig Reference Haig1993; Reference Haig1996; Reference Haig2004a; Reference Haig2004b). The placenta has evolved as a focal point for genomic conflict due to its function in the transfer of resources between mutually dependent individuals that bear genes with partially divergent inclusive fitness interests (Coan et al. Reference Coan, Burton and Ferguson-Smith2005; Haig Reference Haig1993; Reference Haig1996). Many of the common disorders of pregnancy, including gestational diabetes, pre-eclampsia, and fetal growth restriction, arise in part from breakdowns in the dynamically balanced, “tug-of-war” nature of physiological systems subject to maternal–fetal conflict and imprinting effects (Cattanach et al. Reference Cattanach, Beechey and Peters2004; Haig Reference Haig1993; Reference Haig1996; Reference Haig and Stearns1999b; McMinn et al. Reference McMinn, Wei, Schupf, Cusmai, Johnson, Smith, Weksberg, Thaker and Tycko2006; Oudejans et al. Reference Oudejans, Mulders, Lachmeijer, van Dijk, Könst, Westerman, van Wijk, Leegwater, Kato, Matsuda, Wake, Dekker, Pals, ten Kate and Blankenstein2004; Reik et al. Reference Reik, Constancia, Fowden, Anderson, Dean, Ferguson-Smith, Tycko and Sibley2003).
A considerable proportion of known imprinted genes are expressed exclusively or predominantly in the brain, where they influence aspects of behavior (Curley et al. Reference Curley, Barton, Surani and Keverne2004; Davies et al. Reference Davies, Isles and Wilkinson2001; Reference Davies, Isles, Smith, Karunadasa, Burrmann, Humby, Ojarikre, Biggin, Skuse, Burgoyne and Wilkinson2005; Reference Davies, Isles, Burgoyne and Wilkinson2006; Isles et al. Reference Isles, Davies and Wilkinson2006; Keverne Reference Keverne2001a; Reference Keverne2001b). The brain can be conceived as analogous to the placenta in that both organs mediate the transfer of fitness-limiting resources in networks of kin (Badcock & Crespi Reference Badcock and Crespi2006). As in the case of placentation, disruption in systems involving brain-expressed imprinted genes can lead to major neurological and physiological disorders (Badcock Reference Badcock2000; Davies et al. Reference Davies, Isles and Wilkinson2001; Haig & Wharton Reference Haig and Wharton2003; Isles et al. Reference Isles, Davies and Wilkinson2006). Developmental systems regulated by imprinting effects are unusual in that they can be disrupted in two diametrically opposed ways, towards either paternal-gene or maternal-gene bias. Disorders affected by imprinting should thus exhibit diametric phenotypes, as seen clearly, for example, in Beckwith-Wiedemann syndrome involving overgrowth versus the Silver-Russell undergrowth syndrome (Cerrato et al. Reference Cerrato, Sparago, Di Matteo, Zou, Dean, Sasaki, Smith, Genesio, Bruggemann, Reik and Riccio2005; Eggermann et al. Reference Eggermann, Meyer, Obermann, Heil, Schüler, Ranke, Eggermann and Wollmann2005; Reference Eggermann, Schönherr, Meyer, Obermann, Mavany, Eggermann, Ranke and Wollmann2006). We propose that such diametric effects extend to brain and behavior, and these effects help to account for some of the major features of human cognitive architecture.
4. The imprinted brain
In 2002, Badcock proposed that insights from autism research suggest that we have evolved two parallel cognitive systems, which he termed mentalistic and mechanistic cognition (see Badcock Reference Badcock, Crawford and Salmon2004). Mentalistic cognition (or simply mentalism, otherwise know as theory of mind, folk psychology, or mentalizing) evolved for interaction with other people in a psychological environment, whereas mechanistic cognition (folk physics) evolved in parallel for interaction with the physical environment (for a comparable view, see Kuhlmeier et al. Reference Kuhlmeier, Bloom and Wynn2004). Badcock also proposed that if, as is generally accepted, many symptoms of autism can be seen in terms of deficits in functions such as gaze-monitoring, intentionality, shared attention, and theory of mind in general (Baron-Cohen Reference Baron-Cohen1995), then some common symptoms of paranoid schizophrenia, such as delusions of being watched or spied on, erotomania or delusions of persecution, conspiratorial delusions, and religious and magical delusions, could be seen as pathologically hypertrophied equivalents, or in general terms as hyper-mentalism (Badcock Reference Badcock, Crawford and Salmon2004; see also Abu-Akel Reference Abu-Akel1999; Abu-Akel & Bailey Reference Abu-Akel and Bailey2000 for supporting views). Badcock and Crespi (Reference Badcock and Crespi2006) suggested that evolutionary and genetic foundations of autism might be found in some combination of enhanced expression of paternally active genes and reduced expression of maternally active ones in brain development and behavior (Badcock & Crespi Reference Badcock and Crespi2006). Here, we extend these basic insights by showing how the two extremes of the mechanistic-mentalistic continuum – what we call autistic- and psychotic-spectrum conditions – can be represented as diametric opposites for a large suite of phenotypic traits, with the diametric nature understood in terms of the two possible directions, paternal and maternal, for imbalances in imprinted gene effects. The cognitive and behavioral effects of such imbalances are most clear for known syndromes mediated by imprinting effects, but can, we contend, be generalized and extended to major disorders of the social brain.
4.1. Autistic-spectrum conditions
Autism is a spectrum of conditions, all of which involve some combination of impairments in social interaction; language and communication; and repetitive, restricted behaviors or interests (Happé et al. Reference Happé, Ronald and Plomin2006). This spectrum includes Kanner (infantile) autism, Asperger syndrome, and a set of other conditions including Rett syndrome (LaSalle et al. Reference LaSalle, Hogart and Thatcher2005), Fragile X syndrome (Belmonte & Bourgeron Reference Belmonte and Bourgeron2006), and Turner syndrome (Skuse Reference Skuse2005), all of which involve autistic features in a substantial proportion of affected individuals. Conti-Ramsden et al. (Reference Conti-Ramsden, Simkin and Botting2006), Herbert and Kenet (Reference Herbert and Kenet2007), and Smith (Reference Smith2007) also describe close links between autism and Specific Language Impairment, and obsessive-compulsive disorder (OCD) may exhibit a closer association with the autistic spectrum than with the psychotic spectrum (Abramson et al. Reference Abramson, Ravan, Wright, Wieduwilt, Wolpert, Donnelly, Pericak-Vance and Cuccaro2005; Bejerot Reference Bejerot2007; Bürgy Reference Bürgy2007; Fineberg et al. Reference Fineberg, Saxena, Zohar and Craig2007). We have therefore conceptualized the autistic spectrum in terms of its three main criteria and their main component phenotypes (Fig. 1), showing that these criteria partially overlap in their phenotypic expression, and by implication in their genetic underpinnings (Happé et al. Reference Happé, Ronald and Plomin2006). By our hypothesis for the phenotypic structure of autistic conditions, at the core of these features we find a reduction in mentalistic cognition, affect, and behavior – a relatively underdeveloped social brain.

Figure 1. The autistic spectrum can be visualized in terms of three suites of traits that partially overlap in their phenotypic expression and genetic underpinnings, with each suite of traits grading more or less smoothly into each other and into normality. At the core of the autistic spectrum we find a reduction in mentalistic cognition, affect, and behavior, which can be mediated by effects on the development of social reciprocity, language and communication, and restrictive interests and activities, or by some combination of effects from these three domains. Recent studies suggest that the degree of genetic and phenotypic overlap between these three domains of the autistic spectrum appears similar in magnitude to the overlap between the three main conditions characterizing the psychotic spectrum, which are shown in Figure 2.
Previous theory for understanding the evolutionary and developmental bases of autism has focused on sex differences and how they relate to autistic phenotypes. Asperger (Reference Asperger and Frith1991) thus suggested that “the autistic personality is an extreme variant of male intelligence,” and Baron-Cohen (Reference Baron-Cohen2002; Reference Baron-Cohen2003) has developed this idea into an “extreme male brain” theory of autism, which posits that the primary differences between autistic and normal cognition parallel the differences between the sexes. By this theory, autism can be “explained psychologically by an impaired capacity for empathizing, or modeling the mental states governing the behavior of people, along with a superior capacity for systemizing, or inferring the rules governing the behavior of objects” (Baron-Cohen & Belmonte Reference Baron-Cohen and Belmonte2005, p. 109; see also Baron-Cohen Reference Baron-Cohen2002; Baron-Cohen et al. Reference Cohen, Pichard, Tordjman, Baumann, Burglen, Excoffier, Lazar, Mazet, Pinquier, Verloes and Héron2005). This hypothesis is consistent with a large body of evidence, including (1) a male-biased sex ratio in autism; (2) enhanced empathy, better ability to detect emotions, and faster language development in girls, whereas boys show increased ability and interests in activities related to systemizing (Baron-Cohen Reference Baron-Cohen2002; Baron-Cohen et al. Reference Cohen, Pichard, Tordjman, Baumann, Burglen, Excoffier, Lazar, Mazet, Pinquier, Verloes and Héron2005; McClure et al. Reference McClure, Monk, Nelson, Zarahn, Leibenluft, Bilder, Charney, Ernst and Pine2004); (3) links between higher prenatal exposure to testosterone and autistic traits (Knickmeyer et al. Reference Knickmeyer, Baron-Cohen, Raggatt and Taylor2005; Lutchmaya et al. Reference Lutchmaya, Baron-Cohen and Raggatt2002a; Reference Lutchmaya, Baron-Cohen and Raggatt2002b); and (4) higher scores for males on a test characterizing individuals along an autistic spectrum (Baron-Cohen et al. Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley2001; Reference Baron-Cohen, Knickmeyer and Belmonte2005).
Badcock and Crespi (Reference Badcock and Crespi2006) have described genetic, neurological, and behavioral evidence relevant to the hypothesis that important aspects of autism may represent not the extreme male brain per se, but rather the extreme paternally biased imprinted brain. Thus, autism disproportionately involves imbalances in development that lead to increased effects of paternally expressed genes at loci subject to imprinting, relative to maternally expressed ones. Such paternally expressed genes are expected to drive development and cognition towards a more resource-demanding phenotype, similar to a phenotype generally more characteristic of males than females (Badcock & Crespi Reference Badcock and Crespi2006). The imprinted brain theory for autism is consistent with Baron-Cohen's body of evidence, but it can also help explain other key features of autism, such as the much more male-biased sex ratio in Asperger syndrome and high-functioning autism than in severe, Kanner autism (Folstein & Rosen-Sheidley Reference Folstein and Rosen-Sheidley2001), and the observation that many factors other than sex and fetal testosterone are involved. Evidence for epigenetic dysregulation of imprinted genes in autism is also reviewed by Schanen (Reference Schanen2006).
Badcock and Crespi (Reference Badcock and Crespi2006) also describe how some central aspects of the autistic spectrum may be explained by their hypothesis. Thus, extreme deficits in the so-called maternal brain (mainly the highly developed neocortex) (Keverne Reference Keverne2001a) but more or less normal function of the paternal brain (mainly the limbic system), may lead to the loss of language, mental retardation, and repetitive behavior typical of infantile (Kanner) autism, whereas increased paternal-brain effects, but relatively spared maternal-brain function, may lead to high-functioning autism or Asperger syndrome, which involves specific deficits in social cooperation and reciprocity (Badcock & Crespi Reference Badcock and Crespi2006; Constantino & Todd Reference Constantino and Todd2005; Fitzgerald Reference Fitzgerald2004, pp. 30–41; Rinehart et al. Reference Rinehart, Bradshaw, Brereton and Tonge2002a). In both cases, autism results in part from disrupted tension between neurodevelopmental and physiological agents of intragenomic conflict. As for imprinted gene effects in placental disorders and carcinogenesis (e.g., Angiolini et al. Reference Angiolini, Fowden, Coan, Sandovici, Smith, Dean, Burton, Tycko, Reik, Sibley and Constância2006; Lee Reference Lee2003; McMinn et al. Reference McMinn, Wei, Schupf, Cusmai, Johnson, Smith, Weksberg, Thaker and Tycko2006), the resulting phenotype is more or less pathological, but the nature of the deviation from normality provides insight into its underlying genomic, physiological, and evolutionary causes. The main phenotypic feature of autism that may reflect the conflict theory of genomic imprinting is that autism involves increased “self-oriented” and indeed “selfish” behavior, expressed most clearly as deficits of cooperative social behavior and augmentation of mechanistic cognition (Badcock Reference Badcock, Crawford and Salmon2004; Badcock & Crespi Reference Badcock and Crespi2006). We use the term mechanistic (rather than systemizing) cognition because mechanistic refers more generally to the physical world, including aspects of sensation; cause–effect inference; mechanistic relationships of child with mother (Kanner Reference Kanner1949); and bottom-up, non-abstract, less centrally coherent processing of information (Vermeulen Reference Vermeulen2001, p. 28).
4.2. Psychotic-spectrum conditions
Psychosis is literally a disordering of the psyche, the Greek “soul.” In schizophrenia, such disordering commonly involves delusions and auditory hallucinations, loss of coherence and logic in thought and discourse, and emotionality (affect) externally reduced or inappropriate to social context (Tamminga & Holcomb Reference Tamminga and Holcomb2005). Auditory hallucinations, a primary symptom found in over 60% of persons diagnosed with schizophrenia, are also common in persons with bipolar disorder or major depression (Baethge et al. Reference Baethge, Baldessarini, Freudenthal, Streeruwitz, Bauer and Bschor2005; Kempf et al. Reference Kempf, Hussain and Potash2005; 2007; Tsuang et al. Reference Tsuang, Taylor and Faraone2004), as well as in non-clinical settings (Bentall Reference Bentall2003a). Bipolar disorder and major depression often involve other psychotic symptoms such as delusions, as well as symptoms related to dysregulated emotionality (Boks et al. Reference Boks, Leask, Vermunt and Kahn2007b). Schizophrenia, bipolar disorder, and major depression thus exhibit broad phenotypic overlap, as shown in Figure 2; and they also partially overlap in their polygenic underpinnings (Blackwood et al. Reference Blackwood, Pickard, Thomson, Evans, Porteous and Muir2007; Craddock & Forty Reference Craddock and Forty2006; Potash Reference Potash2006; Van Den Bogaert et al. Reference Van Den Bogaert, Del-Favero and Van Broeckhoven2006). These so-called psychotic-spectrum conditions also include schizotypy (Claridge Reference Claridge1997), Klinefelter syndrome (Boks et al. Reference Boks, de Vette, Sommer, van Rijn, Giltay, Swaab and Kahn2007a), velocardiofacial syndrome (Feinstein et al. Reference Feinstein, Eliez, Blasey and Reiss2002), and dyslexia (Condray Reference Condray2005), all of which exhibit a notably elevated incidence of schizophrenia or affective psychosis, or a suite of physiological and neurological phenotypes characteristic of these conditions. We have conceptualized schizophrenia, bipolar disorder, and major depression as exhibiting partial overlap in their phenotypic features, with psychosis and hyper-mentalistic cognition, affect, and behavior at their core (Fig. 2).

Figure 2. The psychotic spectrum can be visualized in terms of three main conditions – schizophrenia, bipolar disorder, and major depression – that grade into one another and exhibit partial overlap in their phenotypic expression and genetic underpinnings. These three conditions have historically been considered as largely separate, but recent genetic studies, and consideration of intermediate conditions, have demonstrated that they share a broad range of features and risk factors. At the core of the three conditions we find hyper-development in aspects of mentalistic cognition, affect and behavior, especially psychotic symptoms such as hallucinations and delusions.
Like autism, schizophrenia, bipolar disorder, and major depression each grades more or less smoothly from disorder into normality (Claridge Reference Claridge1997; Happé et al. Reference Happé, Ronald and Plomin2006). Each of these conditions also exhibits a strong genetic component to its expression, but with many genes involved and different combinations of these genes underlying the phenotypes involved (Rapoport et al. Reference Rapoport, Addington, Frangou and Psych2005; Tamminga & Holcomb Reference Tamminga and Holcomb2005). Psychosis in schizophrenia, bipolar disorder, major depression, and schizotypy involves so-called positive, first-rank symptoms, which mainly include magical ideation, delusions, hallucinations, paranoia, thought disorder, and referential thinking. Such positive symptoms comprise a much higher proportion of the genetic liability to schizophrenia and schizotypy than do negative symptoms (Kremen et al. Reference Kremen, Faraone, Toomey, Seidman and Tsuang1998; Vollema et al. Reference Vollema, Sitskoorn, Appels and Kahn2002; Yaralian et al. Reference Yaralian, Raine, Lencz, Hooley, Bihrle, Mills and Ventura2000), and positive and negative symptoms appear to be independently heritable to a considerable degree (Fanous et al. Reference Fanous, Gardner, Walsh and Kendler2001; Linney et al. Reference Linney, Murray, Peters, MacDonald, Rijsdijk and Sham2003).
A logical consequence of the imprinted-brain hypothesis for the etiology of autism is that the converse disruption, towards stronger relative effects of maternally expressed imprinted genes, should also involve altered growth, development, and cognition. We describe evidence here that this direction of disrupted imprinting represents a contributing cause in the development of psychotic-spectrum conditions. By contrast with autism, imbalances towards increased effects of maternally expressed imprinted genes, or reduced effects from paternally expressed imprinted genes, should engender changes in physiology, morphology, and behavior that can be construed as more or less pathological manifestations of effects that are normally beneficial to mothers and other maternal relatives (Haig Reference Haig, LeCroy and Moller2000a; Reference Haig2000b; Reference Haig2003; Reference Haig2004b).
Our hypothesis is focused primarily on explaining phenotypes involved in psychosis, as these represent central traits exhibited in schizophrenia, schizotypy, bipolar disorder, and major depression (Fig. 2) (Crow Reference Crow2004a; Reference Crow2004b; Reference Crow2004c; Keverne Reference Keverne1999). Negative symptoms such as social withdrawal, perseveration, apathy, and flat affect – as seen mainly in “deficit” schizophrenia – apparently involve a relatively large element of major neurophysiological pathology (such as grey matter loss) as well as altered function (e.g., Chua et al. Reference Chua, Wright, Poline, Liddle, Murray, Frackowiak, Friston and McGuire1997; Frith Reference Frith1992). Such symptoms have been used as evidence for “autism” or “autistic traits” in schizophrenia, velocardiofacial syndrome, and Prader-Willi syndrome (Frith & Frith Reference Frith, Frith and Bebbington1991; Nylander & Gillberg Reference Nylander and Gillberg2001; Sheitman et al. Reference Sheitman, Kraus, Bodfish and Carmel2004; Veltman et al. Reference Veltman, Thompson, Roberts, Thomas, Whittington and Bolton2004; Reference Veltman, Craig and Bolton2005; Vorstman et al. Reference Vorstman, Morcus, Duijff, Klaassen, Heineman-de Boer, Beemer, Swaab, Kahn and van Engeland2006), but in each case these inferences of similarity have been based entirely on observation or data from questionnaires, interviews, scales, or checklists. By contrast, biological criteria, including neuroanatomy, neurophysiology, and genetics, demonstrate notable similarities of velocardiofacial syndrome, Klinefelter syndrome and Prader-Willi syndrome with disorders on the psychotic spectrum, especially schizophrenia (e.g., DeLisi et al. Reference DeLisi, Maurizio, Svetina, Ardekani, Szulc, Nierenberg, Leonard and Harvey2005; Eliez Reference Eliez2007; Eliez & van Amelsvoort Reference Eliez, van Amelsvoort, Murphy and Scambler2005; Holsen & Thompson Reference Holsen and Thompson2004; Lee et al. Reference Lee, Walker, Karten, Kuny, Tennese, O'Neill and Wevrick2005).
The most useful information for evaluating our hypothesis comes from the relatively non-pathological points on the salient cognitive spectra: For autism, this is Asperger syndrome, high-functioning autism, and non-clinical individuals with autistic traits; and for psychosis, this is manifested most clearly in “healthy schizotypy” (Claridge Reference Claridge1997). However, we will consider all traits and conditions on the psychotic spectrum as potentially amenable to some degree of falsifiable explication by our hypothesis. Thus, by analogy with hypothesized Kevernian maternal-brain and paternal-brain effects in autistic conditions (Badcock & Crespi Reference Badcock and Crespi2006), negative symptoms of schizophrenia and depression such as anhedonia, loss of will, flat affect, and psychomotor retardation may be associated with relatively decreased paternal-brain influences and a maternal brain that is either relatively unaffected, or that sends hyper-mentalistic outputs to the limbic system (e.g., paranoia eliciting fear, or feelings of guilt imposing anhedonia). In comparison, positive symptoms appear to be more a consequence of increased maternal-brain influences on cognition and behavior, with the paternal brain relatively unaltered.
As autism involves traits characteristic of an “extreme male brain” (Baron-Cohen et al. Reference Cohen, Pichard, Tordjman, Baumann, Burglen, Excoffier, Lazar, Mazet, Pinquier, Verloes and Héron2005), we predict that, in comparison, psychotic-spectrum disorders should reflect neuroanatomy, cognition, and behavior that are relatively more characteristic of females. We stress that the male–female axis, and the phenotypic axis defined by effects of paternally versus maternally expressed imprinted genes, are not the same: Both sexes exhibit effects from brain-expressed imprinted genes, and sex differences are driven by selection in diverse contexts. But the axes overlap; they may share mechanisms of development, and, as described later, the way that these axes interact may help to explain sex biases in the incidence and some major features of autistic- and psychotic-spectrum conditions.
5. Prader-Willi and Angelman syndromes
Prader-Willi and Angelman syndromes result from opposite disruptions (usually deletions or duplications) of a suite of imprinted genes on chromosome 15. Prader-Willi syndrome is caused by the downstream developmental effects of imbalance towards increased relative expression of maternal genes in this region, and Angelman syndrome is due to imbalance towards less maternal gene expression (Bittel & Butler Reference Bittel and Butler2005; Dan & Boyd Reference Dan and Boyd2003; Whittington et al. Reference Whittington, Holland, Webb, Butler, Clarke and Boer2004; Yamasaki et al. Reference Yamasaki, Joh, Ohta, Masuzaki, Ishimaru, Mukai, Niikawa, Ogawa, Wagstaff and Kishino2003). Both syndromes have major impacts on cognition, behavior, and psychopathology, and as a result, they provide useful tests of our hypothesis. If our hypothesis is correct, then Prader-Willi syndrome should involve increased rates of psychosis, and Angelman syndrome should involve a high incidence of autism. The power of such predictions is tempered primarily by the large magnitude of the perturbations that cause these syndromes: Reducing levels of imprinted gene expression to zero or doubling them (Bittel & Butler Reference Bittel and Butler2005) probably leads to any number of purely pathological effects that may not be clearly indicative of the nature of the disrupted adaptive systems.
The phenotype of Prader-Willi syndrome can be divided into two main life-history stages. Prior to the usual age of weaning, this syndrome involves lack of appetite, poor suckling ability, a weak cry, inactivity, and sleepiness; by contrast, after this age, it involves extreme and unselective overeating (Dykens et al. Reference Dykens, Hodapp and Finucane2000; Holland et al. Reference Holland, Whittington and Hinton2003; Whittington & Holland Reference Whittington and Holland2004). Haig and Wharton (Reference Haig and Wharton2003) have suggested that these features of Prader-Willi syndrome reflect an extreme manifestations of traits that benefit the mother by making the baby less demanding on her resources, both before weaning (when food intake and energetic demands are reduced) and after weaning (when ingestion of any solid food available may ease provisioning). Prader-Willi syndrome also involves low birth weight and growth hormone deficiency (Gillessen- Kaesbach et al. 1995; Goldstone Reference Goldstone2004), which are consistent with increased relative developmental effects from maternally expressed imprinted genes.
Prader-Willi syndrome engenders a very high incidence of psychosis in adulthood (Verhoeven et al. Reference Verhoeven, Tuinier and Curfs2003; Vogels et al. Reference Vogels, Matthijs, Legius, Devriendt and Fryns2003; Reference Vogels, De Hert, Descheemaeker, Govers, Devriendt, Legius, Prinzie and Fryns2004). Such psychosis is found predominantly in cases of maternal uniparental disomy (UPD) (with 61% of individuals exhibiting symptoms) compared to deletion (17%) (Soni et al. Reference Soni, Whittington, Holland, Webb, Maina, Boer and Clarke2007). The genetic differences between disomy and deletion include: (a) higher expression levels of maternally expressed genes in disomy, for genes in the PWS region; (b) haploinsufficiency of non-imprinted genes in this region, in deletion cases; and (c) loss of expression, in disomy, of any paternally expressed genes on chromosome 15 outside the Prader-Willi region (Bittel et al. Reference Bittel, Kibiryeva, Talebizadeh and Butler2003; Whittington et al. Reference Whittington, Holland, Webb, Butler, Clarke and Boer2004). Thus, the UPD genotype exhibits a greater deviation towards increased relative expression of maternal genes. Biological similarities between Prader-Willi syndrome and psychotic-spectrum conditions include enlarged ventricles (Miller et al. Reference Miller, Couch, Schmalfuss, He, Liu and Driscoll2007), altered serotoninergic and dopaminergic neurotransmission patterns (Akefeldt et al. Reference Akefeldt, Ekman, Gillberg and Mansson1998; Holsen & Thompson Reference Holsen and Thompson2004), impaired stereopsis (Chen et al. Reference Chen, Bidwell and Holzman2005; Fox et al. Reference Fox, Sinatra, Mooney, Feurer and Butler1999), and a high pain threshold (Kuwako et al. Reference Kuwako, Hosokawa, Nishimura, Uetsuki, Yamada, Nada, Okada and Yoshikawa2005; Singh et al. Reference Singh, Giles and Nasrallah2006). Lee et al. (Reference Lee, Walker, Karten, Kuny, Tennese, O'Neill and Wevrick2005) postulated that “Prader-Willi syndrome is one of an emerging class of neurodevelopmental disorders that includes BBS [Bardet-Biedl syndrome], schizophrenia, and lissencephaly, which are in part caused by defects in centrosome function in cytoskeletal rearrangement during neurite extension” (p. 628). Neuroanatomically, Prader-Willi syndrome is apparently mediated by impaired development of the hypothalamus (Goldstone Reference Goldstone2004), the neurological nexus of the paternal brain.
Veltman et al. (Reference Veltman, Thompson, Roberts, Thomas, Whittington and Bolton2004; 2005) discuss the presence of autistic symptoms in Prader-Willi syndrome, which primarily involves obsessive behaviors and deficits in social interaction (e.g., social withdrawal), with language abilities largely intact. Such symptoms are about twice as common in uniparental disomy than deletion cases (Veltman et al. Reference Veltman, Craig and Bolton2005), which is consistent with an alternative interpretation of these patterns as indicating expected aspects of a personality “premorbid” for schizophrenia, a condition which involves notable deficits in social and language development (Cannon et al. Reference Cannon, Jones, Gilvarry, Rifkin, McKenzie, Foerster and Murray1997; Sporn et al. Reference Sporn, Addington, Gogtay, Ordoñez, Gornick, Clasen, Greenstein, Tossell, Gochman, Lenane, Sharp, Straub and Rapoport2004a; Vourdas et al. Reference Vourdas, Pipe, Corrigall and Frangou2003). More generally, childhood diagnoses of autism in individuals with neurogenetic syndromes showing greatly increased rates of psychotic-spectrum disorders in adulthood, such as Klinefelter syndrome (Boks et al. Reference Boks, de Vette, Sommer, van Rijn, Giltay, Swaab and Kahn2007a; DeLisi et al. Reference DeLisi, Maurizio, Svetina, Ardekani, Szulc, Nierenberg, Leonard and Harvey2005; Jha et al. Reference Jha, Sheth and Ghaziuddin2007) and velocardiofacial syndrome (Antshel et al. Reference Antshel, Aneja, Strunge, Peebles, Fremont, Stallone, Abdulsabur, Higgins, Shprintzen and Kates2007; Gothelf Reference Gothelf2007; Vorstman et al. Reference Vorstman, Morcus, Duijff, Klaassen, Heineman-de Boer, Beemer, Swaab, Kahn and van Engeland2006) may represent “false positives” (Feinsten & Singh 2007), motivated by superficial childhood similarities between autism and “premorbid” psychotic-spectrum conditions (Eliez Reference Eliez2007) that are not underlain by genetic, neurological, or other biological criteria. Such considerations also apply to diagnoses of atypical autism in childhood, which Mouridsen et al. (Reference Mouridsen, Rich and Isager2008) found to be followed in adulthood by diagnoses of “schizophrenia spectrum disorders” in 31 (35%) of 89 cases.
Symptoms of Angelman syndrome in childhood include prolonged suckling, frequent laughter, hyperactivity, and frequent waking (Badcock Reference Badcock2000; Cohen et al. Reference Cohen, Pichard, Tordjman, Baumann, Burglen, Excoffier, Lazar, Mazet, Pinquier, Verloes and Héron2005; Williams et al. Reference Williams, Beaudet, Clayton-Smith, Knoll, Kyllerman, Laan, Magenis, Moncla, Schinzel, Summers and Wagstaff2006a). As in severe cases of autism, speech is often absent (Holm et al. Reference Holm, Cassidy, Butler, Hanchett, Greenswag, Whitman and Greenberg1993). Angelman syndrome also exhibits a disproportionately high rate of autistic traits that include deficits in reciprocal social behavior, poor eye contact, intolerance to change, and repetitive and stereotyped behaviors (Cohen et al. Reference Cohen, Pichard, Tordjman, Baumann, Burglen, Excoffier, Lazar, Mazet, Pinquier, Verloes and Héron2005; Peters et al. Reference Peters, Beaudet, Madduri and Bacino2004; Schroer et al. Reference Schroer, Phelan, Michaelis, Crawford, Skinner, Cuccaro, Simensen, Bishop, Skinner, Fender and Stevenson1998; Trillingsgaard & Østergaard Reference Trillingsgaard and Østergaard2004). Peters et al. (Reference Peters, Beaudet, Madduri and Bacino2004) found that 42% of Angelman children in a long-term study met DSM-IV criteria for autism, and Sahoo et al. (Reference Sahoo, Peters, Madduri, Glaze, German, Bird, Barbieri-Welge, Bichell, Beaudet and Bacino2006) diagnosed 48% as autistic, with a higher frequency (80%) in cases of the larger, “Type 1” deletion at 15q11-q13. Angelman syndrome also involves mildly increased body weight in early childhood in three of the classes of genetic alteration that cause it (paternal UPD15, imprinting center alteration, and UBE3A mutation), as well as in some mouse models (Johnstone et al. Reference Johnstone, DuBose, Futtner, Elmore, Brannan and Resnick2006; Lossie et al. Reference Lossie, Whitney, Amidon, Dong, Chen, Theriaque, Hutson, Nicholls, Zori, Williams and Driscoll2001). Further biological evidence for similarities between Angelman syndrome and autism includes high rates of seizures, an epileptiform EEG (electroencephalogram), and ataxia in both conditions (Williams et al. Reference Williams, Beaudet, Clayton-Smith, Knoll, Kyllerman, Laan, Magenis, Moncla, Schinzel, Summers and Wagstaff2006a); genetic associations of UBE3A alleles with autism (Nurmi et al. Reference Nurmi, Bradford, Chen, Hall, Arnone, Gardiner, Hutcheson, Gilbert, Pericak-Vance, Copeland-Yates, Michaelis, Wassink, Santangelo, Sheffield, Piven, Folstein, Haines and Sutcliffe2001); and genetic models that posit a strong role for UBE3A dysregulation in autism (Jiang et al. Reference Jiang, Sahoo, Michaelis, Bercovich, Bressler, Kashork, Liu, Shaffer, Schroer, Stockton, Spielman, Stevenson and Beaudet2004). An important contrast is macrocephaly in autism (Lainhart et al. Reference Lainhart, Bigler, Bocian, Coon, Dinh, Dawson, Deutsch, Dunn, Estes, Tager-Flusberg, Folstein, Hepburn, Hyman, McMahon, Minshew, Munson, Osann, Ozonoff, Rodier, Rogers, Sigman, Spence, Stodgell and Volkmar2006; Stanfield et al., in press), but acquired microcephaly in Angelman syndrome.
Taken together, the high rates of autistic-spectrum traits in Angelman syndrome, and psychotic-spectrum traits in Prader-Willi syndrome, suggest that diametric dysregulation of imprinted genes – towards increased paternal and maternal expression, respectively – mediates the expression of diametric behavioral and psychiatric phenotypes. By our hypothesis, individuals with Beckwith-Wiedemann syndrome should also show autistic features, and Silver-Russell syndrome should involve traits relatively characteristic of the psychotic spectrum.
6. Diametric phenotypes of psychosis and autism
The term autism was originally coined by Bleuler in the context of negative symptoms of schizophrenia, and Kanner (Reference Kanner1965) struggled to establish autism as a disorder separate from childhood-onset schizophrenia until Kolvin's (1971) classic study showing bimodality in timing of onset for “childhood psychosis.” The comorbidity of autism and schizophrenia is apparently low (Goussé et al. Reference Goussé, Plumet, Chabane, Mouren-Siméoni, Ferradian and Leboyer2002). Leyfer et al. (Reference Leyfer, Folstein, Bacalman, Davis, Dinh, Morgan, Tager-Flusberg and Lainhart2006) found no comorbid cases in a sample of 109 autistics, but they were ages 5–17, so few cases would be expected. (By contrast, depression, attention-deficit/hyperactivity disorder [ADHD], and obsessive-compulsive disorder [OCD] were markedly elevated.) Volkmar and Cohen (Reference Volkmar and Cohen1991) reported one case of schizophrenia in 163 adolescent and adult autistic individuals, which is at or below the general prevalence of about 1% in the overall population. Stahlberg et al. (Reference Stahlberg, Soderstrom, Rastam and Gillberg2004) analyzed 129 adults (mean age 32) with autistic-spectrum disorders, and found no schizophrenia in 13 cases of autism, 1 case of schizophrenia, and 5 cases of “other psychotic disorder” in 49 Asperger syndrome cases, and 3 cases of schizophrenia and 1 case of “other psychotic disorder” in 67 cases of PDD-NOS (pervasive developmental disorder not otherwise specified). This latter association is of questionable salience to the hypothesis, given that Sporn et al. (Reference Sporn, Addington, Gogtay, Ordoñez, Gornick, Clasen, Greenstein, Tossell, Gochman, Lenane, Sharp, Straub and Rapoport2004a) have described a high incidence of PDD-NOS associated with later-onset schizophrenia.
The main complications of interpreting comorbidity studies are that apparent Asperger syndrome cases may involve “autistic” features expressed in some negative symptoms of schizophrenia and schizotypy (Konstantareas & Hewitt Reference Konstantareas and Hewitt2001; Goldstein et al. Reference Goldstein, Minshew, Allen and Seaton2002); the lack of communication skills in Kanner autism may make diagnosis of psychosis problematic; the presence of psychotic symptoms formally excludes an autism diagnosis by DSM criteria; and childhood “autism” may represent premorbidity for schizophrenia, as described earlier.
One of the strongest predictions of our hypothesis follows from the diametric nature of disruptions to systems affected by imprinted genes. Thus, the suite of phenotypic traits that characterize autistic- and psychotic-spectrum conditions should exhibit patterns of symmetrical and opposite phenotypes for traits related to growth and development, as well as aspects of social cognition and behavior. In this section, we describe evidence from studies of growth, development, neuroanatomy, cognition, behavior, and epidemiology for diametric phenotypes in autism and psychosis (Table 1). We focus on the most recent studies and comprehensive reviews, and we encourage neuroscientists, psychiatrists, and psychologists to consider the evidence as a convergent whole, constructively engage the core arguments and predictions, and suggest alternative possible explanations for the patterns that we describe.
Table 1. Diametrically opposed phenotypes of autistic- and psychotic-spectrum conditions [Note: Recent, salient references are indicated by number after each entry and collated at the bottom of the table. Full references are in the Consolidated References list, and discussion is provided in the target article main text.]

Key references: (1) Anderson et al. Reference Anderson, Jacobs-Stannard, Chawarska, Volkmar and Kliman2007; (2) Wahlbeck et al. Reference Wahlbeck, Forsén, Osmond, Barker and Eriksson2001a; (3) Rees & Inder (Reference Rees and Inder2005); (4) Sugie et al. Reference Sugie, Sugie, Fukuda and Ito2005; (5) Mraz et al. Reference Mraz, Green, Dumont-Mathieu, Makin and Fein2007; (6) Dissanayake et al. Reference Dissanayake, Bui, Huggins and Loesch2006; (7) Nilsson et al. Reference Nilsson, Stålberg, Lichtenstein, Cnattingius, Olausson and Hultman2005; (8) Niemi et al. Reference Niemi, Suvisaari, Haukka and Lönnqvist2005; (9) Cannon et al. Reference Cannon, Jones and Murray2002; (10) Sacco et al. Reference Sacco, Militerni, Frolli, Bravaccio, Gritti, Elia, Curatolo, Manzi, Trillo, Lenti, Saccani, Schneider, Melmed, Reichelt, Pascucci, Puglisi-Allegra and Persico2007; (11) Fukumoto et al., in press; (12) Gunnell et al. Reference Gunnell, Rasmussen, Fouskakis, Tynelius and Harrison2003; (13) Haukka et al., in press; (14) Manning et al. Reference Manning, Baron-Cohen, Wheelwright and Sanders2001; (15) Milne et al. Reference Milne, White, Campbell, Swettenham, Hansen and Ramus2006; (16) Arató et al. Reference Arató, Frecska, Beck, An and Kiss2004; (17) Walder et al. Reference Walder, Andersson, McMillan, Breedlove and Walker2006a; (18) Mills et al. Reference Mills, Hediger, Molloy, Chrousos, Manning-Courtney, Yu, Brasington and England2007; (19) Connolly et al. Reference Connolly, Chez, Streif, Keeling, Golumbek, Kwon, Riviello, Robinson, Neuman and Deuel2006; (20) Moises et al. Reference Moises, Zoega and Gottesman2002; (21) Weickert et al. Reference Weickert, Ligons, Romanczyk, Ungaro, Hyde, Herman, Weinberger and Kleinman2005; (22) Palomino et al. Reference Palomino, Vallejo-Illarramendi, González-Pinto, Aldama, González-Gómez, Mosquera, González-García and Matute2006; (23) Herbert et al. Reference Herbert, Ziegler, Makris, Filipek, Kemper, Normandin, Sanders, Kennedy and Caviness2004; (24) Lainhart et al. Reference Lainhart, Bigler, Bocian, Coon, Dinh, Dawson, Deutsch, Dunn, Estes, Tager-Flusberg, Folstein, Hepburn, Hyman, McMahon, Minshew, Munson, Osann, Ozonoff, Rodier, Rogers, Sigman, Spence, Stodgell and Volkmar2006; (25) Hardan et al. Reference Hardan, Muddasani, Vemulapalli, Keshavan and Minshew2006; (26) Kieseppä et al. Reference Kieseppä, van Erp, Haukka, Partonen, Cannon, Poutanen, Kaprio and Lönnqvist2003; (27) McDonald et al. Reference McDonald, Bullmore, Sham, Chitnis, Suckling, MacCabe, Walshe and Murray2005; (28) Tamminga & Holcomb Reference Tamminga and Holcomb2005; (29) McIntosh et al. Reference McIntosh, Job, Moorhead, Harrison, Whalley, Johnstone and Lawrie2006; (30) Goghari et al. Reference Goghari, Rehm, Carter and Macdonald2007; (31) McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happé, Howlin and Murphy2002; (32) Rapoport et al. Reference Rapoport, Addington, Frangou and Psych2005; (33) Schumann et al. Reference Schumann, Hamstra, Goodlin-Jones, Lotspeich, Kwon, Buonocore, Lammers, Reiss and Amaral2004; (34) Stanfield et al., in press; (35) Aleman & Kahn Reference Aleman and Kahn2005; (36) Alexander et al. Reference Alexander, Lee, Lazar, Boudos, Dubray, Oakes, Miller, Lu, Jeong, McMahon, Bigler and Lainhart2007; (37) Brambilla et al. Reference Brambilla, Cerini, Gasparini, Versace, Andreone, Vittorini, Barbui, Pelizza, Nosè, Barlocco, Marrella, Gregis, Tournikioti, David, Keshavan and Tansella2005; (38) Tuncer et al. Reference Tuncer, Hatipoglu and Ozates2005; (39) Herbert et al. Reference Herbert, Harris, Adrien, Ziegler, Makris, Kennedy, Lange, Chabris, Bakardjiev, Hodgson, Takeoka, Tager-Flusberg and Caviness2002; (40) Herbert et al. Reference Herbert, Ziegler, Deutsch, O'Brien, Kennedy, Filipek, Bakardjiev, Hodgson, Takeoka, Makris and Caviness2005; (41) Leask & Crow Reference Leask and Crow2005; (42) Weiss et al. Reference Weiss, Hofer, Golaszewski, Siedentopf, Felber and Fleischhacker2006; (43) Gunter et al. Reference Gunter, Ghaziuddin and Ellis2002; (44) Mohr et al. Reference Mohr, Röhrenbach, Laska and Brugger2001; (45) Hulshoff Pol et al. Reference Hulshoff Pol, Schnack, Mandl, Brans, van Haren, Neeltje, Baaré, van Oel, Collins, Evans and Kahn2006; (46) Williams et al. Reference Williams, Whiten, Suddendorf and Perrett2001; (47) Hadjikhani et al. Reference Hadjikhani, Joseph, Snyder and Tager-Flusberg2007; (48) Arbib & Mundhenk Reference Arbib and Mundhenk2005; (49) Ristic et al. Reference Ristic, Mottron, Friesen, Iarocci, Burack and Kingstone2005; (50) McKay et al. Reference McKay, Langdon and Coltheart2005; (51) Langdon et al. Reference Langdon, Corner, McLaren, Coltheart and Ward2006b; (52) Gallese Reference Gallese2006; (53) Kimhy et al. Reference Kimhy, Goetz, Yale, Corcoran and Malaspina2005; (54) Kennedy et al. Reference Kennedy, McDonough, Kelly and Berrios2002; (55) Tomasello et al. Reference Tomasello, Carpenter, Call, Behne and Moll2005; (56) Toichi et al. Reference Toichi, Kamio, Okada, Sakihama, Youngstrom, Findling and Yamamoto2002; (57) Grandin Reference Grandin2004; (58) Baron-Cohen & Belmonte Reference Baron-Cohen and Belmonte2005; (59) Frith Reference Frith2003; (60) Harrington et al. Reference Harrington, Langdon, Siegert and McClure2005a; Reference Harrington, Siegert and McClure2005b; (61) Rim Reference Rim1994; (62) Pilowsky et al. Reference Pilowsky, Yirmiya, Arbelle and Mozes2000; (63) Dinn et al. Reference Dinn, Harris, Aycicegi, Greene and Andover2002; (64) Happé et al. Reference Happé, Ehlers, Fletcher, Frith, Johansson, Gillberg, Dolan, Frackowiak and Frith1996; (65) Luna et al. Reference Luna, Minshew, Garver, Lazar, Thulborn, Eddy and Sweeney2002; (66) Dapretto et al. Reference Dapretto, Davies, Pfeifer, Scott, Sigman, Bookheimer and Iacoboni2006; (67) Silk et al. Reference Silk, Rinehart, Bradshaw, Tonge, Egan, O'Boyle and Cunnington2006; (68) Quintana et al. Reference Quintana, Davidson, Kovalik, Marder and Mazziotta2001; (69) Whalley et al. Reference Whalley, Simonotto, Flett, Marshall, Ebmeier, Owens, Goddard, Johnstone and Lawrie2004; (70) Seidman et al. Reference Seidman, Thermenos, Poldrack, Peace, Koch, Faraone and Tsuang2006; (71) Kennedy et al. Reference Kennedy, Redcay and Courchesne2006; (72) Garrity et al. Reference Garrity, Pearlson, McKiernan, Lloyd, Kiehl and Calhoun2007; (73) Harrison et al. Reference Harrison, Yücel, Pujol and Pantelis2007; (74) U. Frith Reference Frith2004; (75) Camisa et al. Reference Camisa, Bockbrader, Lysaker, Rae, Brenner and O'Donnell2005; (76) Losh & Capps Reference Losh and Capps2003; (77) Blanc et al. Reference Blanc, Adrien, Roux and Barthélémy2005; (78) Honey et al. Reference Honey, Leekam, Turner and McConachie2006; (79) Claridge et al. Reference Claridge, Pryor and Watkins1990; (80) Nettle Reference Nettle2001; (81) Happé Reference Happé1994; (82) Landry & Bryson Reference Landry and Bryson2004; (83) Brugger & Graves Reference Brugger and Graves1997a; (84) Brugger & Graves Reference Brugger and Graves1997b; (85) Mathes et al. Reference Mathes, Wood, Proffitt, Stuart, Buchanan, Velakoulis, Brewer, McGorry and Pantelis2005; (86) Whitehouse et al. Reference Whitehouse, Maybery and Durkin2006; (87) Jones & Fernyhough Reference Jones and Fernyhough2007; (88) Baron-Cohen et al. Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley2001; (89) Baron-Cohen et al. Reference Cohen, Pichard, Tordjman, Baumann, Burglen, Excoffier, Lazar, Mazet, Pinquier, Verloes and Héron2005; (90) Toulopoulou et al. Reference Toulopoulou, Mapua-Filbey, Quraishi, Kravariti, Morris, McDonald, Walshe, Bramon and Murray2005; (91) Kravariti et al. Reference Kravariti, Toulopoulou, Mapua-Filbey, Schulze, Walshe, Sham, Murray and McDonald2006; (92) Happé & Frith Reference Happé and Frith2006; (93) Bellgrove et al. Reference Bellgrove, Vance and Bradshaw2003; (94) Sumich et al. Reference Sumich, Chitnis, Fannon, O'Ceallaigh, Doku, Faldrowicz and Sharma2005; (95) Just et al. Reference Just, Cherkassky, Keller and Minshew2004; (96) Turkeltaub et al. Reference Turkeltaub, Flowers, Verbalis, Miranda, Gareau and Eden2004; (97) Bersani et al. Reference Bersani, Maneschi, Tarolla and Pancheri2006; (98) Edgar et al. Reference Edgar, Yeo, Gangestad, Blake, Davis, Lewine and Cañive2006.
6.1. Growth and neuroanatomy
6.1.1. Brain size, birth weight, growth, and placentation
Whole brain size is reduced in schizophrenia from birth onwards (Cannon et al. Reference Cannon, Jones and Murray2002; Gur et al. Reference Gur, Keshavan and Lawrie2007; McIntosh et al. Reference McIntosh, Job, Moorhead, Harrison, Whalley, Johnstone and Lawrie2006; Tamminga & Holcomb Reference Tamminga and Holcomb2005), due to reductions in grey matter (neuronal tissue) (e.g., Narr et al. Reference Narr, Bilder, Toga, Woods, Rex, Szeszko, Robinson, Sevy, Gunduz-Bruce, Wang, DeLuca and Thompson2005; Woods et al. Reference Woods, Ward and Johnson2005), reduced and altered white matter (mainly brain fatty acids) (e.g., Kieseppä et al. Reference Kieseppä, van Erp, Haukka, Partonen, Cannon, Poutanen, Kaprio and Lönnqvist2003; McDonald et al. Reference McDonald, Bullmore, Sham, Chitnis, Wickham, Bramon and Murray2004; Reference McDonald, Bullmore, Sham, Chitnis, Suckling, MacCabe, Walshe and Murray2005), and lower cortical thickness (Goghari et al. Reference Goghari, Rehm, Carter and Macdonald2007; Kuperberg et al. Reference Kuperberg, Broome, McGuire, David, Eddy, Ozawa, Goff, West, Williams, van der Kouwe, Salat, Dale and Fischl2003). Moises et al. (Reference Moises, Zoega and Gottesman2002) also noted that a considerable range of growth deficiencies, including low birth weight, late maturation, and small brain size, are found in schizophrenia. Reduced brain size may be due in part to slow brain maturation in individuals who develop psychosis (Crow Reference Crow1995; Crow et al. Reference Crow, Done and Sacker1996; James et al. Reference James, Crow, Renowden, Wardell, Smith and Anslow1999; Saugstad Reference Saugstad1998; Reference Saugstad1999).
In autism, cortical thickness is increased (Hardan et al. Reference Hardan, Muddasani, Vemulapalli, Keshavan and Minshew2006), and increased head and brain size is one of the most consistent anatomical findings across studies (DiCicco-Bloom et al. Reference DiCicco-Bloom, Lord, Zwaigenbaum, Courchesne, Dager, Schmitz, Schultz, Crawley and Young2006; Dissanayake et al. Reference Dissanayake, Bui, Huggins and Loesch2006; Lainhart et al. Reference Lainhart, Bigler, Bocian, Coon, Dinh, Dawson, Deutsch, Dunn, Estes, Tager-Flusberg, Folstein, Hepburn, Hyman, McMahon, Minshew, Munson, Osann, Ozonoff, Rodier, Rogers, Sigman, Spence, Stodgell and Volkmar2006; Stanfield et al., in press). In autism, brain size undergoes a striking growth spurt between birth and age four (Cody et al. Reference Cody, Pelphrey and Piven2002; Courchesne & Pierce Reference Courchesne and Pierce2005a; Reference Courchesne and Pierce2005b; Courchesne et al. Reference Courchesne, Redcay and Kennedy2004; Herbert Reference Herbert2005; Lainhart et al. Reference Lainhart, Bigler, Bocian, Coon, Dinh, Dawson, Deutsch, Dunn, Estes, Tager-Flusberg, Folstein, Hepburn, Hyman, McMahon, Minshew, Munson, Osann, Ozonoff, Rodier, Rogers, Sigman, Spence, Stodgell and Volkmar2006; Penn Reference Penn2006; Redcay & Courchesne Reference Redcay and Courchesne2005), an acceleration driven differentially by increases in (metabolically expensive) white matter volume (Herbert et al. Reference Herbert, Ziegler, Makris, Filipek, Kemper, Normandin, Sanders, Kennedy and Caviness2004; Lainhart Reference Lainhart2006; see also McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happé, Howlin and Murphy2002). Remarkably, a recent study of Asperger syndrome showed that grey matter volume did not decrease with age (from 15 to 50), as it does substantially in normal individuals (McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happé, Howlin and Murphy2002; see also Ge et al. Reference Ge, Grossman, Babb, Rabin, Mannon and Kolson2002; Woods et al. Reference Woods, Ward and Johnson2005); these data suggest that autism and schizophrenia exhibit divergent patterns of grey matter loss, with little to no loss in autism, moderate loss in normal development, and high rates of loss in schizophrenia.
These differences in brain size and development between autistic and schizophrenic individuals are broadly paralleled by differences in birth weight and growth. Thus, autism can involve higher birth weight compared to controls (Mraz et al. Reference Mraz, Green, Dumont-Mathieu, Makin and Fein2007; Sacco et al. Reference Sacco, Militerni, Frolli, Bravaccio, Gritti, Elia, Curatolo, Manzi, Trillo, Lenti, Saccani, Schneider, Melmed, Reichelt, Pascucci, Puglisi-Allegra and Persico2007; Sugie et al. Reference Sugie, Sugie, Fukuda and Ito2005) and faster body growth (Dissanayake et al. Reference Dissanayake, Bui, Huggins and Loesch2006; Fukumoto et al. Reference Fukumoto, Hashimoto, Ito, Nishimura, Tsuda, Miyazaki, Mori, Arisawa and Kagami2008; Mraz et al. Reference Mraz, Green, Dumont-Mathieu, Makin and Fein2007), although some studies report a lack of birth weight difference (Cederlund & Gillberg Reference Cederlund and Gillberg2004; Juul-Dam et al. Reference Juul-Dam, Townsend and Courchesne2001; Larsson et al. Reference Larsson, Eaton, Madsen, Vestergaard, Olesen, Agerbo, Schendel, Thorsen and Mortensen2005) or lower birth weight in autism (Kolevzon et al. Reference Kolevzon, Gross and Reichenberg2007). By contrast, schizophrenia and major depression entail lower weight at birth with considerable consistency across studies (Cannon et al. Reference Cannon, Jones and Murray2002; Costello et al. Reference Costello, Worthman, Erkanli and Angold2007; Gale & Martyn Reference Gale and Martyn2004; Gunnell & Holly Reference Gunnell and Holly2004; Niemi et al. Reference Niemi, Suvisaari, Haukka and Lönnqvist2005; Nilsson et al. Reference Nilsson, Stålberg, Lichtenstein, Cnattingius, Olausson and Hultman2005; Wahlbeck et al. Reference Wahlbeck, Forsén, Osmond, Barker and Eriksson2001a). Imprinted genes are known to exert strong effects on birth weight and childhood weight in humans (Gorlova et al. Reference Gorlova, Amos, Wang, Shete, Turner and Boerwinkle2003; Lindsay et al. Reference Lindsay, Kobes, Knowler and Hanson2002), and Svensson et al. (Reference Svensson, Pawitan, Cnattingius, Reilly and Lichtenstein2006) have demonstrated familial aggregation of low birth weight, with effects from maternal, paternal, and fetal genes. The clearest evidence for enhanced growth in autism comes from Mills et al. (Reference Mills, Hediger, Molloy, Chrousos, Manning-Courtney, Yu, Brasington and England2007), who reported significantly increased head size, body weight, body mass index, and levels of growth hormones (including the key, paternally expressed growth factor IGF2) in autistic children compared to normal controls. Diametric patterns of growth can also help to explain the higher incidence of schizophrenia than autism, given that there should be many more genetic, epigenetic, and environmental ways to disrupt and reduce growth than to increase it. We also note that genomic conflicts and strong selection may contribute to the high heritabilities of autism and schizophrenia via such processes as antagonistic pleiotropy, evolutionary disequilibrium, and increased scope for mutation-selection balance via disruption of developmental tugs-of-war (Crespi Reference Crespi2006; Crespi et al. Reference Crespi, Summers and Dorus2007).
Fetal development is critically dependent upon placentation, which in humans is highly invasive and has evolved in the context of constrained maternal–fetal conflict (Haig Reference Haig1993). Anderson et al. (Reference Anderson, Jacobs-Stannard, Chawarska, Volkmar and Kliman2007) recently described a highly significant, three-fold increase in placental inclusions in autism. Such inclusions are caused by increased proliferative growth of cytotrophoblast, the stem-cell-like component of the placenta. Placental inclusions are also found disproportionately in Beckwith-Wiedemann syndrome and hydatiform moles (molar pregnancies involving placental overgrowth), both of which represent disorders of genomic imprinting that involve excessive effects from paternal gene expression (Devriendt Reference Devriendt2005; Fisher et al. Reference Fisher, Hodges, Rees, Sebire, Seckl, Newlands, Genest and Castrillon2002; Ohama et al. Reference Ohama, Ueda, Okamoto, Takenaka and Fujiwara1986; Saxena et al. Reference Saxena, Frank, Panichkul, Van den Veyver, Tycko and Thaker2003). Placental development in general is strongly regulated by imprinted genes, with paternally expressed genes favoring increased placental growth (Dunger et al. Reference Dunger, Petry and Ong2006; Fowden et al. Reference Fowden, Sibley, Reik and Constancia2006; McMinn et al. Reference McMinn, Wei, Schupf, Cusmai, Johnson, Smith, Weksberg, Thaker and Tycko2006; Reik et al. Reference Reik, Constancia, Fowden, Anderson, Dean, Ferguson-Smith, Tycko and Sibley2003).
Risk of schizophrenia decreases with increased placental weight (Wahlbeck et al. Reference Wahlbeck, Forsén, Osmond, Barker and Eriksson2001a). Intrauterine growth restriction, which is caused predominantly by placental underdevelopment and engenders increased fetal hypoxia (Gagnon Reference Gagnon2003), is also a strong risk factor for schizophrenia (Abel & Allin Reference Abel, Allin, Baker and Sibley2006; Cannon et al. Reference Cannon, Rosso, Hollister, Bearden, Sanchez and Hadley2000; Rees & Inder Reference Rees and Inder2005). Effects of fetal hypoxia on brain development are also indicated by experiments in rats that link hypoxia with altered lateralization of the dopaminergic system (Brake et al. Reference Brake, Sullivan and Gratton2000). Finally, in humans, monozygotic twin concordance for schizophrenia is much higher when the twins share a placenta (60%), than when they do not (11%) (Davis et al. Reference Davis, Phelps and Bracha1995). Abel (Reference Abel2004) describes additional evidence that fetal growth restriction, mediated by imprinting effects, contributes to the development of schizophrenia.
Placentation is crucial to brain development, and it represents a key arena for imprinted-gene conflict, because fetal brain growth, especially deposition of fatty acids, is one of the most metabolically costly processes during pregnancy, as well as exerting severe energetic costs in early postnatal development (Foley & Lee Reference Foley and Lee1991; Herrera Reference Herrera2002; Kuzawa Reference Kuzawa1998). Mothers bear virtually all of these costs, and indeed, during the late stages of pregnancy mothers metabolize their own brain fat for transfer to the fetus. We hypothesize that the contrasting patterns of brain size, growth, composition, and birth weight in psychosis and autism are mediated by effects of maternal versus paternal genes, with paternal genes driving the acquisition of increased brain fatty acids in particular. Directly parallel arguments have been made concerning intragenomic conflict over body fat in human babies (Haig Reference Haig and Stearns1999b): Human neonates exhibit by far the highest average body fat content of any mammal, which may represent an adaptation to sequester resources to fuel sustained brain growth in early childhood (Badcock Reference Badcock2000, pp. 208–212; Cunnane & Crawford Reference Cunnane and Crawford2003; Kuzawa Reference Kuzawa1998). A role for imprinted genes in human fat metabolism is suggested by Silver-Russell syndrome, which is caused by a bias towards relative maternal gene expression and involves a striking lack of subcutaneous fat (Monk & Moore Reference Monk and Moore2004).
Patterns and predictions regarding early brain growth in autism and schizophrenia are critically important to the theory proposed here, because altered early brain growth rates are expected to strongly influence patterns of brain connectivity and cerebral lateralization, as described in detail in the next sections.
6.1.2. Hippocampus and amygdala size
The hippocampus is centrally involved in learning and the consolidation of memory, with the right side more involved in spatial cognition, and the left side dedicated more to aspects of memory (Burgess et al. Reference Burgess, Maguire and O'Keefe2002; Piefke & Fink Reference Piefke and Fink2005). By contrast, the amygdala provides social and emotional valence to sensory perceptions, such as the recognition of fearful stimuli, providing input on emotional content to brain structures such as the hippocampus and neocortex (Adolphs et al. Reference Adolphs, Baron-Cohen and Tranel2002; Sander et al. Reference Sander, Grafman and Zalla2003; Skuse et al. Reference Skuse, Morris and Lawrence2003). Integrated activity of the amygdala, hippocampus, and social-brain components of the neocortex has been hypothesized as a core aspect of the social brain system, that processes the rapid, complex information flow involved in human interaction, especially interactions involving emotion, language, and facial expression.
Autism and schizophrenia both involve alterations in structure and function of the interacting amygdala, hippocampus, and prefrontal cortex (Baron-Cohen et al. Reference Cohen, Pichard, Tordjman, Baumann, Burglen, Excoffier, Lazar, Mazet, Pinquier, Verloes and Héron2005; Berretta et al. Reference Berretta, Munno and Benes2001; Burns Reference Burns2004; Reference Burns2006a; Gisabella et al. Reference Gisabella, Bolshakov and Benes2005; J. D. Johnson Reference Johnson2005). The available evidence indicates that relative to brain size, the hippocampus and amygdala are larger in autism than in controls (at least during early development) (Schumann et al. Reference Schumann, Hamstra, Goodlin-Jones, Lotspeich, Kwon, Buonocore, Lammers, Reiss and Amaral2004; Stanfield et al., in press), and (in most studies, and in adults) smaller in schizophrenia and schizotypy (Aleman & Kahn Reference Aleman and Kahn2005; Geuze et al. Reference Geuze, Vermetten and Bremner2005; Gur et al. Reference Gur, Kohler, Turetsky, Siegel, Kanes, Bilker, Brennan and Gur2004; Reference Gur, Keshavan and Lawrie2007; Kuroki et al. Reference Kuroki, Kubicki, Nestor, Salisbury, Park, Levitt, Woolston, Frumin, Niznikiewicz, Westin, Maier, McCarley and Shenton2006; Lawrie et al. Reference Lawrie, Whalley, Job and Johnstone2003; Narr et al. Reference Narr, van Erp, Cannon, Woods, Thompson, Jang, Blanton, Poutanen, Huttunen, Lönnqvist, Standerksjold-Nordenstam, Kaprio, Mazziotta and Toga2002; Reference Narr, Thompson, Szeszko, Robinson, Jang, Woods, Kim, Hayashi, Asunction, Toga and Bilder2004; Suzuki et al. Reference Suzuki, Zhou, Takahashi, Hagino, Kawasaki, Niu, Matsui, Seto and Kurachi2005; Tamminga & Holcomb Reference Tamminga and Holcomb2005; van Elst & Trimble Reference van Elst and Trimble2003). In schizophrenia, smaller size and altered shape of the hippocampus may be functionally related to positive symptoms such as paranoia and delusions, in that the hippocampus mediates the creation, maintenance, and updating of contextual social and spatial “worldviews” and beliefs, via interactions with the neocortex and amygdala (e.g., Gray Reference Gray1998; J. D. Johnson Reference Johnson2005). In autism, increased hippocampus size may be related to enhanced visual-spatial, mathematical, and mechanistic aspects of cognition (Baron-Cohen et al. Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley2001; Minshew et al. Reference Minshew, Goldstein and Siegel1997), as best seen in Asperger syndrome mechanistic skills, and the abilities of autistic savants at calculation and memory (Heaton & Wallace Reference Heaton and Wallace2004; Pring Reference Pring2005; see also Young et al. Reference Young, Ridding and Morrell2004). Individuals with schizophrenia, and schizotypal individuals, exhibit cognitive profiles of impaired visual-spatial and arithmetic abilities, relative to verbal abilities, as described in more detail in the section on sex difference further on.
6.1.3. Cerebral lateralization
Schizophrenia involves reduced structural and functional brain asymmetry, as indicated by an increased incidence of mixed or inconsistent handedness, imaging studies of neuroanatomy with a focus on language-related regions such as the planum temporale, asymmetries in neurotransmitter activity, and higher impairments in verbal ability for individuals less lateralized for handedness (Chance et al. Reference Chance, Esiri and Crow2005; Collinson et al. Reference Collinson, Mackay, James, Quested, Phillips, Roberts and Crow2003; Crow Reference Crow1997; Reference Crow1998; Reference Crow2000; Crow et al. Reference Crow, Crow, Done and Leask1998; DeLisi et al. Reference DeLisi, Svetina, Razi, Shields, Wellman and Crow2002; Honea et al. Reference Honea, Crow, Passingham and Mackay2005; Leask & Crow Reference Leask and Crow2005; Mitchell & Crow Reference Mitchell and Crow2005; Schiffman et al. Reference Schiffman, Pestle, Mednick, Ekstrom, Sorensen and Mednick2005; Shirakawa et al. Reference Shirakawa, Kitamura, Lin, Hashimoto and Maeda2001; Sommer et al. Reference Sommer, Ramsey and Kahn2001; Weiss et al. Reference Weiss, Hofer, Golaszewski, Siedentopf, Felber and Fleischhacker2006). This reduced brain lateralization and lower degree of torque in schizophrenia is apparently associated with slower brain development (Crow et al. Reference Crow, Done and Sacker1996; Reference Crow, Crow, Done and Leask1998; Saugstad Reference Saugstad1998; Reference Saugstad1999), relatively increased dysfunction of components of the left hemisphere compared to the right (e.g., Honea et al. Reference Honea, Crow, Passingham and Mackay2005; Kasai et al. Reference Kasai, Shenton, Salisbury, Hirayasu, Lee, Ciszewski, Yurgelun-Todd, Kikinis, Jolesz and McCarley2003a; Reference Kasai, Shenton, Salisbury, Hirayasu, Onitsuka, Spencer, Yurgelun-Todd, Kikinis, Jolesz and McCarley2003b; Mucci et al. Reference Mucci, Galderisi, Bucci, Tresca, Forte, Koenig and Maj2005) with diminished left-hemisphere specialization for language (Dollfus et al. Reference Dollfus, Razafimandimby, Delamillieure, Brazo, Joliot, Mazoyer and Tzourio-Mazoyer2005; Mitchell & Crow Reference Mitchell and Crow2005), and an increase in the extent of positive symptoms such as delusions (Verdoux et al. Reference Verdoux, Liraud, Droulout, Theillay, Parrot and Franck2004). Similar patterns have been detected in healthy individuals, in whom the degree of schizotypal cognition is positively associated with mixed handedness and other evidence of reduced cerebral lateralization (Barnett & Corballis Reference Barnett and Corballis2002; Jaspers-Fayer & Peters Reference Jaspers-Fayer and Peters2005; Preti et al. Reference Preti, Sardu and Piga2007; Shaw et al. Reference Shaw, Claridge and Clark2001).
Impaired or reduced left-hemisphere language function in schizophrenia and schizotypy may result in greater reliance on right-hemisphere processing of some components of thought and language (Fisher et al. Reference Fisher, Mohanty, Herrington, Koven, Miller and Heller2004; Mohr et al. Reference Mohr, Krummenacher, Landis, Sandor, Fathi and Brugger2005; Taylor et al. Reference Taylor, Zäch and Brugger2002). A crucial result of such a shift may be more “coarse” semantic processing; generation of “loose,” more-distant associations between events and thoughts (Pizzagalli et al. Reference Pizzagalli, Lehmann, Gianotti, Koenig, Tanaka, Wackermann and Brugger2000); overestimation of meaningfulness of naturally occurring coincidences; increased paranormal ideation; and at the extreme, delusion, paranoia, and other positive symptoms of schizophrenia (Brugger Reference Brugger, Houran and Lange2001; Brugger & Graves Reference Brugger and Graves1997a; Reference Brugger and Graves1997b; Leonhard & Brugger Reference Leonhard and Brugger1998). This hypothesis is supported by a diverse range of additional evidence, from modelling of neural networks (Hoffman et al. Reference Hoffman, Hampson, Varanko and McGlashan2004), to neurocognitive and psychological analyses of schizotypy (Claridge Reference Claridge1997), and the use of dopamine agonists to restore left-hemisphere language dominance (Mohr et al. Reference Mohr, Krummenacher, Landis, Sandor, Fathi and Brugger2005). The hypothesis also provides a relatively simple neuroanatomical and neurophysiological explanation for the links between creativity and psychosis as a cognitive style that involves more-distant and more-novel associations between components of thought (Barrantes-Vidal Reference Barrantes-Vidal2004; Brugger Reference Brugger, Houran and Lange2001; Gianotti et al. Reference Gianotti, Mohr, Pizzagalli, Lehmann and Brugger2001). The general links of imagination and creativity with psychosis (Claridge et al. Reference Claridge, Pryor and Watkins1990; Nettle Reference Nettle2001; Sack et al. Reference Sack, van de Ven Vincent, Etschenberg, Schatz and Linden2005) strongly contrast with the lower levels of pretend play and symbolic creativity, as well as increased repetitive and compulsive behavior, in autism (Atlas & Lapidus Reference Atlas and Lapidus1987; Blanc et al. Reference Blanc, Adrien, Roux and Barthélémy2005; Boucher Reference Boucher2007; U. Frith Reference Frith2004; Honey et al. Reference Honey, Leekam, Turner and McConachie2006; Turner Reference Turner1999).
Whereas schizophrenia is associated with reduced asymmetry, autism tends to involve increased size of some right-hemisphere cortical structures (i.e., reversed asymmetry compared to normal), and reversed lateralization of language (Bigler et al. Reference Bigler, Mortensen, Neeley, Ozonoff, Krasny, Johnson, Lu, Provencal, McMahon and Lainhart2007; De Fossé et al. Reference De Fossé, Hodge, Makris, Kennedy, Caviness, McGrath, Steele, Ziegler, Herbert, Frazier, Tager-Flusberg and Harris2004; Escalante-Mead et al. Reference Escalante-Mead, Minshew and Sweeney2003; Flagg et al. Reference Flagg, Cardy, Roberts and Roberts2005; Herbert et al. Reference Herbert, Harris, Adrien, Ziegler, Makris, Kennedy, Lange, Chabris, Bakardjiev, Hodgson, Takeoka, Tager-Flusberg and Caviness2002; Reference Herbert, Ziegler, Deutsch, O'Brien, Kennedy, Filipek, Bakardjiev, Hodgson, Takeoka, Makris and Caviness2005; see also Rinehart et al. Reference Rinehart, Bradshaw, Brereton and Tonge2002b; Sutton et al. Reference Sutton, Burnette, Mundy, Meyer, Vaughan, Sanders and Yale2005). As the right hemisphere develops earlier than the left hemisphere in the fetal and neonatal stages (Chiron et al. Reference Chiron, Leboyer, Leon, Jambaqué, Nuttin and Syrota1995; see also Rinehart et al. Reference Rinehart, Bradshaw, Brereton and Tonge2002b), a pattern of rightward asymmetry in autism may be due simply to a faster, earlier pattern of brain development – a heterochronic shift opposite to a slower developmental profile in schizophrenia (Saugstad Reference Saugstad1999). Accelerated early brain development in autism, and relatively slow brain development in schizophrenia, may thus both lead to anomalous lateralization of cognitive functions.
6.1.4. Corpus callosum size and brain connectivity
In autism, the corpus callosum is relatively small (for brain size) compared to control individuals (Cody et al. Reference Cody, Pelphrey and Piven2002; Egaas et al. Reference Egaas, Courchesne and Saitoh1995; Nydén et al. Reference Nydén, Carlsson, Carlsson and Gillberg2004; Piven et al. Reference Piven, Bailey, Ranson and Arndt1997; Sherr et al. Reference Sherr, Owen, Albertson, Pinkel, Cotter, Slavotinek, Hetts, Jeremy, Schilmoeller, Schilmoeller, Wakahiro and Barkovich2005; Stanfield et al., in press; Waiter et al. Reference Waiter, Williams, Murray, Gilchrist, Perrett and Whiten2005). A pattern of reduced interhemispheric brain connectivity in autistic individuals (Belmonte et al. Reference Belmonte, Allen, Beckel-Mitchener, Boulanger, Carper and Webb2004a; Reference Belmonte, Cook, Anderson, Rubenstein, Greenough, Beckel-Mitchener, Courchesne, Boulanger, Powell, Levitt, Perry, Jiang, DeLorey and Tierney2004b; Nydén et al. Reference Nydén, Carlsson, Carlsson and Gillberg2004) fits with one of the central hypotheses for the cognitive architecture of autism: that it involves increased local and decreased global information processing, reduced “central coherence,” increased “bottom-up” relative to “top-down” processing, and decreased overall brain connectivity (Baron-Cohen & Belmonte Reference Baron-Cohen and Belmonte2005; Belmonte et al. Reference Belmonte, Allen, Beckel-Mitchener, Boulanger, Carper and Webb2004a; Reference Belmonte, Cook, Anderson, Rubenstein, Greenough, Beckel-Mitchener, Courchesne, Boulanger, Powell, Levitt, Perry, Jiang, DeLorey and Tierney2004b; Happé & Frith Reference Happé and Frith2006; Just et al. Reference Just, Cherkassky, Keller and Minshew2004). Courchesne and Pierce (Reference Courchesne and Pierce2005b) have referred to autistic neurocognition as the frontal cortex unconsciously “talking to itself,” concomitant with loss of language and other elements of social cognition.
Findings regarding corpus callosum size in schizophrenia are mixed and inconsistent, apparently due to marked variation with age, sex, handedness, and clinical profile (Bachmann et al. Reference Bachmann, Pantel, Flender, Bottmer, Essig and Schroder2003; Brambilla et al. Reference Brambilla, Cerini, Gasparini, Versace, Andreone, Vittorini, Barbui, Pelizza, Nosè, Barlocco, Marrella, Gregis, Tournikioti, David, Keshavan and Tansella2005; Downhill et al. Reference Downhill, Buchsbaum, Wei, Spiegel-Cohen, Hazlett, Haznedar, Silverman and Siever2000; Highley et al. Reference Highley, DeLisi, Roberts, Webb, Relja, Razi and Crow2003; Luders et al. Reference Luders, Rex, Narr, Woods, Jancke, Thompson, Mazziotta and Toga2003; Tuncer et al. Reference Tuncer, Hatipoglu and Ozates2005). The issue of brain connectivity in schizophrenia has yet to reach a consensus among researchers, as it has for autism, perhaps due to the high clinical heterogeneity in schizophrenia. A considerable body of evidence suggests that schizophrenia involves altered intrahemispheric and interhemispheric connectivity, but such changes take particular forms, such as altered transfer of verbal information between hemispheres (Barnett & Kirk Reference Barnett and Kirk2005; Barnett et al. Reference Barnett, Corballis and Kirk2005; Endrass et al. Reference Endrass, Mohr and Rockstroh2002), that are difficult to interpret without a cognitive model for their significance. One possibility suggested by neural network models, and consistent with increased relative corpus callosum size, is that schizophrenia involves reduced local (relative to global) connections (Siekmeier & Hoffman Reference Siekmeier and Hoffman2002), which would provide a contrast with the enhanced local connectivity and processing found in autism (Courchesne & Pierce Reference Courchesne and Pierce2005a; Reference Courchesne and Pierce2005b; Happé & Frith Reference Happé and Frith2006). This hypothesis is supported by several findings, including: (1) the results of Whalley et al. (Reference Whalley, Simonotto, Marshall, Owens, Goddard, Johnstone and Lawrie2005), who recorded increased connectivity of left parietal with left prefrontal regions in individuals at high risk of schizophrenia; (2) hyperconnectivity across brain regions for some (though not other) EEG frequencies in schizophrenia (Sritharan et al. Reference Sritharan, Line, Sergejew, Silberstein, Egan and Copolov2005; Strelets et al. Reference Strelets, Novototsky-Vlasov and Golikova2002; see also McCreery & Claridge Reference McCreery and Claridge1996); and (3) coactivation of inner speech and language regions normally activated in sequence (Hubl et al. Reference Hubl, Koenig, Strik, Federspiel, Kreis, Boesch, Maier, Schroth, Lovblad and Dierks2004) and inferred wider-spreading activation of brain regions in schizophrenia (Chance et al. Reference Chance, Esiri and Crow2005) and schizotypy (Pizzagalli et al. Reference Pizzagalli, Lehmann and Brugger2001; see also Niebauer Reference Niebauer2004). Sumich et al. (Reference Sumich, Chitnis, Fannon, O'Ceallaigh, Doku, Faldrowicz and Sharma2005) provide MRI evidence that unreality symptoms and hallucinations in schizophrenia involve dysfunctions in Heschl's gyrus that impair bottom-up processing, “giving greater perceptual control to ‘top-down’ mechanisms” (p. 947); Seal et al. (Reference Seal, Aleman and McGuire2004) provide evidence for “heightened influence of top-down processing on perception” in auditory hallucinations of schizophrenics; and Carter et al. (Reference Carter, Robertson, Nordahl, Chaderjian and Oshora-Celaya1996), Granholm et al. (Reference Granholm, Perry, Filoteo and Braff1999), and Bellgrove et al. (Reference Bellgrove, Vance and Bradshaw2003) have shown that schizophrenia involves greater impairments in local as opposed to global processing of stimuli, and exaggerated global-processing advantages for some tasks.
Finally, the neuroanatomical and cognitive correlates of dyslexia are notably similar to those found in schizophrenia and schizotypy (Bersani et al. Reference Bersani, Maneschi, Tarolla and Pancheri2006; Bradshaw & Nettleton Reference Bradshaw and Nettleton1983, pp. 242–54; Edgar et al. Reference Edgar, Yeo, Gangestad, Blake, Davis, Lewine and Cañive2006; Heim et al. Reference Heim, Kissler, Elbert and Rockstroh2004; Richardson Reference Richardson1994), and dyslexia has also been linked with “enhanced ability to process visual-spatial information globally (holistically) rather than locally (part by part)” (von Károlyi et al. Reference von Károlyi, Winner, Gray and Sherman2003). By contrast, hyperlexia, the spontaneous and highly precocious mastery of reading in children (Grigorenko et al. Reference Grigorenko, Klin and Volkmar2003; Silberberg & Silberberg Reference Silberberg and Silberberg1967; Reference Silberberg and Silberberg1971), is found almost exclusively in conjunction with autism (Burd & Kerbeshian Reference Burd and Kerbeshian1988; Just et al. Reference Just, Cherkassky, Keller and Minshew2004; Turkeltaub et al. Reference Turkeltaub, Flowers, Verbalis, Miranda, Gareau and Eden2004). The neural basis for hyperlexia was investigated in a single case study using fMRI: Hyperlexic reading was associated with hyperactivation of the left superior temporal cortex, a region that is hypoactivated in dyslexia (Turkeltaub et al. Reference Turkeltaub, Flowers, Verbalis, Miranda, Gareau and Eden2004).
6.2. Neurodevelopment
Many of the diverse traits in Table 1 are developmentally and functionally related, with a central role for coordinated allometric development of brain size, relative sizes of brain regions, brain grey and white matter composition, intra- versus inter-hemispheric connectivity, corpus callosum size, and cerebral asymmetry (Burns Reference Burns2006a). Thus, autism commonly involves an acceleration of brain development, increased childhood brain size (especially in the frontal lobe), and relatively increased white matter (Carper et al. Reference Carper, Moses, Tigue and Courchesne2002; Courchesne Reference Courchesne2004; Courchesne & Pierce Reference Courchesne and Pierce2005a; Reference Courchesne and Pierce2005b; McCaffery & Deutsch Reference McCaffery and Deutsch2005; Schumann et al. Reference Schumann, Hamstra, Goodlin-Jones, Lotspeich, Kwon, Buonocore, Lammers, Reiss and Amaral2004). In humans and other primates, increased overall brain size normally involves an increased relative proportion of white matter (Schoenemann et al. Reference Schoenemann, Sheehan and Glotzer2005), relatively increased intra-hemispheric (compared to inter-hemispheric) connectivity (see Burns Reference Burns2006a), and a corpus callosum small relative to brain size (Rilling & Insel Reference Rilling and Insel1999; Vidal et al. Reference Vidal, Nicolson, Devito, Hayashi, Geaga, Drost, Williamson, Rajakumar, Sui, Dutton, Toga and Thompson2006). Brain-growth acceleration in autism may also engender faster growth of the developmentally leading right-hemisphere prior to age three (see Chi et al. Reference Chi, Dooling and Gilles1977; Chiron et al. Reference Chiron, Leboyer, Leon, Jambaqué, Nuttin and Syrota1995; Pilcher et al. Reference Pilcher, Hammock and Hopkins2001; Seldon Reference Seldon2005), resulting in a higher frequency of reversed (rightward) asymmetry especially in language areas such as the planum temporale, and contributing to the general pattern in autism of apparent relative deficits in right-hemispheric and relatively global tasks (Gunter et al. Reference Gunter, Ghaziuddin and Ellis2002; Previc Reference Previc2007). By contrast, as described earlier, schizophrenia apparently involves slower early brain development, smaller brain size, and relative reductions in white matter, which may lead to differentially impaired development of the slower-developing left hemisphere (e.g., Crow Reference Crow1997; Flor-Henry Reference Flor-Henry1969; Galaburda Reference Galaburda, Geschwind and Galaburda1984; Harrison Reference Harrison1999; Hulshoff Pol et al. Reference Hulshoff Pol, Schnack, Mandl, Brans, van Haren, Neeltje, Baaré, van Oel, Collins, Evans and Kahn2006; Mohr et al. Reference Mohr, Röhrenbach, Laska and Brugger2001). This contrast between relative left hemisphere dysfunction in schizophrenia (involving relative deficits in sequential, serial, and more-local encoding and processing tasks) versus relative right-hemisphere impairment in autism (involving relative strengths in some local and linear processing tasks but relative deficits in gestalt, more-global, holistic tasks such as social interaction; see Bradshaw & Nettleton Reference Bradshaw and Nettleton1983, pp. 162–72) is a considerable oversimplication, but it potentially helps to link dysregulated early brain growth rates with important aspects of lateralized cognition (Han et al. Reference Han, Weaver, Murray, Kang, Yund and Woods2002). We stress that both autism and schizophrenia involve absolute deficits in functions ascribed to both hemispheres – for example, schizophrenics show impaired pragmatics of language (Langdon et al. Reference Langdon, Coltheart, Ward and Catts2002) – and that the “normal” functions of the two hemispheres may not always be unambiguously attributed to their usual cerebral locations in these two conditions.
The hypothesis that autism involves general effects of accelerated early brain development, and psychosis involves slower development, is supported by three additional lines of evidence.
First, autism involves overactivation of some growth-signalling pathways and some key brain growth factors such as BDNF (Carper et al. Reference Carper, Moses, Tigue and Courchesne2002; Connolly et al. Reference Connolly, Chez, Streif, Keeling, Golumbek, Kwon, Riviello, Robinson, Neuman and Deuel2006; McCaffery & Deutsch Reference McCaffery and Deutsch2005; Miyazaki et al. Reference Miyazaki, Narita, Sakuta, Miyahara, Naruse, Okado and Narita2004; Nelson Reference Nelson2001; Nishimura et al. Reference Nishimura, Nakamura, Anitha, Yamada, Tsujii, Iwayama, Hattori, Toyota, Takei, Miyachi, Iwata, Suzuki, Matsuzaki, Kawai, Sekine, Tsuchiya, Sugihara, Suda, Ouchi, Sugiyama, Yoshikawa and Mori2007a; Tsai Reference Tsai2005). In contrast to autism, schizophrenia involves deficiencies in growth factors (Gunnell & Holly Reference Gunnell and Holly2004; Hashimoto et al. Reference Hashimoto, Bergen, Nguyen, Xu, Monteggia, Pierri, Sun, Sampson and Lewis2005; Klejbor et al. Reference Klejbor, Myers, Hausknecht, Corso, Gambino, Morys, Maher, Hard, Richards, Stachowiak and Stachowiak2006; Moises et al. Reference Moises, Zoega and Gottesman2002; Niculescu Reference Niculescu2005; Weickert et al. Reference Weickert, Hyde, Lipska, Herman, Weinberger and Kleinman2003; Reference Weickert, Ligons, Romanczyk, Ungaro, Hyde, Herman, Weinberger and Kleinman2005), which can in some cases be traced directly to the disorder (e.g., Pieper et al. Reference Pieper, Wu, Han, Estill, Dang, Wu, Reece-Fincanon, Dudley, Richardson, Brat and McKnight2005).
Second, undergrowth versus overgrowth effects on human brain development are strongly mediated by the phosphatidylinositol 3-kinase (PI3K) signalling pathway (Brunet et al. Reference Brunet, Datta and Greenberg2001), which is strikingly downregulated in schizophrenia and bipolar disorder via alterations in growth-enhancing genes (Emamian et al. Reference Emamian, Hall, Birnbaum, Karayiorgou and Gogos2004; Kalkman Reference Kalkman2006; Stopkova et al. Reference Stopkova, Saito, Papolos, Vevera, Paclt, Zukov, Bersson, Margolis, Strous and Lachman2004) but exhibits hyperactivation in some autistic conditions due to reduced expression or activity of genes that negatively regulate PI3K pathway activation, including PTEN, NF1, TSC1, TSC2, and PRKCB1 (Belmonte & Bourgeron Reference Belmonte and Bourgeron2006; Butler et al. Reference Butler, Dasouki, Zhou, Talebizadeh, Brown, Takahashi, Miles, Wang, Stratton, Pilarski and Eng2005; Crespi, under revision; Kwon et al. Reference Kwon, Luikart, Powell, Zhou, Matheny, Zhang, Li, Baker and Parada2006; McCall et al. Reference McCall, Chin, Salzman and Fults2006; Philippi et al. Reference Philippi, Roschmann, Tores, Lindenbaum, Benajou, Germain-Leclerc, Marcaillou, Fontaine, Vanpeene, Roy, Maillard, Decaulne, Saraiva, Brooks, Rousseau and Hager2005; Serajee et al. Reference Serajee, Nabi, Zhong and Mahbubul Huq2003). In turn, PI3K signalling is mediated in part by the growth-regulating imprinted genes GRB10, IGF2, H19, and GNAS (Charalambous et al. Reference Charalambous, Smith, Bennett, Crew, Mackenzie and Ward2003; Chen et al. Reference Chen, Haluzik, Wolf, Lorenzo, Dietz, Reitman and Weinstein2004; Deng et al. Reference Deng, Bhattacharya, Swamy, Tandon, Wang, Janda and Riedel2003; Fults Reference Fults2005; Hartmann et al. Reference Hartmann, Koch, Brune, Waha, Schuller, Dani, Denkhaus, Langmann, Bode, Wiestler, Schilling and Pietsch2005; Mahmoud & Grover Reference Mahmoud and Grover2006; Riedel Reference Riedel2004; Smith et al. Reference Smith, Garfield and Ward2006; Wick et al. Reference Wick, Werner, Langlais, Ramos, Dong, Shoelson and Liu2003).
Third, brain development may also be strongly affected by altered thresholds for apoptosis and synaptic pruning, as excessive pruning during adolescence has been suggested as a strong risk factor for schizophrenia (Burns Reference Burns2004; de la Fuente-Sandoval et al. Reference de la Fuente-Sandoval, Portillo, Fresán and Apiquian2005; Walker & Bollini Reference Walker and Bollini2002; Woo & Crowell Reference Woo and Crowell2005). By contrast, several studies suggest that decreased apoptosis may be related to the accelerated early neurodevelopment found in autism (Courchesne & Pierce Reference Courchesne and Pierce2005a; Engstrom et al. Reference Engstrom, Ohlson, Stubbs, Maciulis, Caldwell, Odell and Torres2003; Fatemi & Halt Reference Fatemi and Halt2001). We suggest that the pathology of negative symptoms of schizophrenia in particular may be mediated by excessive loss of synapses and grey matter, caused in part by a bias towards maternally expressed imprinted genes, many of which act as tumor suppressors that enhance apoptosis, whereas paternally expressed genes enhance cell survival and growth (e.g., Kurita et al. Reference Kurita, Kuwajima, Nishimura and Yoshikawa2006; Margetts et al. Reference Margetts, Astuti, Gentle, Cooper, Cascon, Catchpoole, Robledo, Neumann, Latif and Maher2005).
The hypothesis that neurodevelopment is accelerated in autism but slowed in schizophrenia is congruent with neurodevelopmental models of these conditions (Courchesne & Pierce Reference Courchesne and Pierce2005a; Reference Courchesne and Pierce2005b; Kalkman Reference Kalkman2006; Rapoport et al. Reference Rapoport, Addington, Frangou and Psych2005; Redcay & Courchesne Reference Redcay and Courchesne2005). The hypothesis also fits with expectations from the conflict theory of imprinting: That autism, driven by imbalance towards increased effects of paternal gene expression, involves faster growth and increased demands on the mother, whereas psychosis engenders the reverse. We have illustrated this hypothesis in Figure 3, which depicts its continuity with classic neurodevelopmental models. Further analyses of this neurodevelopmental hypothesis require demonstrating effects of imprinted genes on early brain growth, altered lateralization (e.g., Francks et al. Reference Francks, Maegawa, Lauren, Abrahams, Velayos-Baeza, Medland, Colella, Groszer, McAuley, Caffrey, Timmusk, Pruunsild, Koppel, Lind, Matsumoto-Itaba, Nicod, Xiong, Joober, Enard, Krinsky, Nanba, Richardson, Riley, Martin, Strittmatter, Moller, Rujescu, St Clair, Muglia, Roos, Fisher, Wade-Martins, Rouleau, Stein, Karayiorgou, Geschwind, Ragoussis, Kendler, Airaksinen, Oshimura, Delisi and Monaco2007; Sun et al. Reference Sun, Patoine, Abu-Khalil, Visvader, Sum, Cherry, Orkin, Geschwind and Walsh2005), and position along a cognitive spectrum from autism to psychosis.
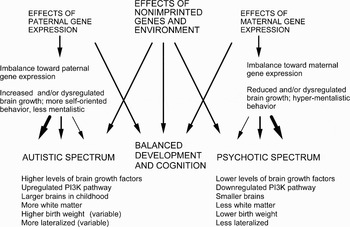
Figure 3. Our neurodevelopmental model of autism and psychosis is compatible with previous theory and data on dysregulated neurodevelopment in these two sets of conditions, which posits diverse effects from many genes as well as environmental effects (e.g., valproic acid; see Rinaldi et al. Reference Rinaldi, Silberberg and Markram2008), but it contrasts these conditions as diametric opposites modulated by differential brain growth and development, with notable effects from genetic and epigenetic alterations of imprinted genes. The degree to which imprinted and non-imprinted genes contribute to effects on brain growth and development remains unclear, but only imprinted genes are expected to exert effects on cognitive architecture that reflect dysregulated adaptations related to the conflict hypothesis of imprinting.
6.3. Cognition
Diverse hypotheses have been proposed to explain how neurological functions are altered in psychotic and autistic-spectrum conditions (e.g., Baron-Cohen et al. Reference Cohen, Pichard, Tordjman, Baumann, Burglen, Excoffier, Lazar, Mazet, Pinquier, Verloes and Héron2005; Brüne Reference Brüne2004; Burns Reference Burns2004; Gray Reference Gray1998; Happé & Frith Reference Happé and Frith2006; J. D. Johnson Reference Johnson2005). A point of near-consensus in these ideas is that cognitive dysfunctions are driven by altered interactions between and within components of the human social brain, which comprises the amygdala, superior temporal sulcus, orbitofrontal cortex, anterior congulate cortex, temporoparietal junction, temporal poles, medial prefrontal cortex, and the mirror-neuron systems (Brüne Reference Brüne2004; Burns Reference Burns2004, Reference Burns2006a; Happé & Frith Reference Happé and Frith2006). Our hypothesis predicts that integrated social brain systems are disrupted in diametrically opposite ways in autistic- and psychotic-spectrum conditions. This hypothesis can be evaluated via analysis of how aspects of gaze, intentionality, agency, theory of mind, and the mirror-neuron systems are underdeveloped or dysregulated in autism and in schizophrenia, the condition with the most information available.
6.3.1. Gaze and intention
Humans are unique among primates in exhibiting a white sclera (the “whites of the eyes”) (Emery Reference Emery2000), which allows direct observation of the form and directionality of gaze (e.g., Whalen et al. Reference Whalen, Kagan, Cook, Davis, Kim, Polis, McLaren, Somerville, McLean, Maxwell and Johnstone2004). Gaze is a fundamental component of social interactions, with most humans exquisitely cognizant of the gaze of others and perceptive of direction and form of gaze, and expressions around the eyes, as conveying a considerable amount of social information concerning mental state and intention (Emery Reference Emery2000). Specific neural regions, in the neocortex and amygdala, are dedicated to perception and interpretation of gaze and facial expression in humans and other primates (Emery Reference Emery2000; M. H. Johnson Reference Johnson2005). The amygdala in particular is highly activated when humans gaze upon one another, as emotional valence of the interaction is inferred from features of the eyes and surrounding face, with especially notable sensitivity to fear (Adolphs et al. Reference Adolphs, Gosselin, Buchanan, Tranel, Schyns and Damasio2005; Castelli Reference Castelli2005; Schwartz et al. Reference Schwartz, Wright, Shin, Kagan and Rauch2003a; Reference Schwartz, Wright, Shin, Kagan, Whalen, McMullin and Rauch2003b; Whalen et al. Reference Whalen, Kagan, Cook, Davis, Kim, Polis, McLaren, Somerville, McLean, Maxwell and Johnstone2004).
Autistic individuals exhibit notable avoidance of gaze, and reduced or absent following of gaze (Asperger Reference Asperger and Frith1991; Emery Reference Emery2000; Frith Reference Frith2003, p. 105; Ristic et al. Reference Ristic, Mottron, Friesen, Iarocci, Burack and Kingstone2005). Such behavior has motivated the development of theories for autism based on impairment of the amygdala (as in cases of amygdala damage), with consequent reduction in ability to detect and assimilate social and emotional information from the eyes and faces of other humans (Adolphs et al. Reference Adolphs, Baron-Cohen and Tranel2002; Baron-Cohen et al. Reference Baron-Cohen, Ring, Bullmore, Wheelwright, Ashwin and Williams2000; Brambilla et al. Reference Brambilla, Hardan, di Nemi, Caverzasi, Soares, Perez and Barale2004; Shaw et al. Reference Shaw, Lawrence, Radbourne, Bramham, Polkey and David2004). First-person accounts by autistic individuals (Lawson Reference Lawson1998, pp. 11–12; O'Neill Reference O'Neill1999, p. 26) suggest that amygdala “impairment” may involve hyperactivation, such that direct gaze is sufficiently uncomfortable, overwhelming, and fear-inspiring to warrant avoidance. This inference is also supported by the larger size of the amygdala in autism (at least during early childhood) (Schumann et al. Reference Schumann, Hamstra, Goodlin-Jones, Lotspeich, Kwon, Buonocore, Lammers, Reiss and Amaral2004), lower levels of the fear-reducing neuropeptide oxytocin in autism (Green et al. Reference Green, Fein, Modahl, Feinstein, Waterhouse and Morris2001; Kirsch et al. Reference Kirsch, Esslinger, Chen, Mier, Lis, Siddhanti, Gruppe, Mattay, Gallhofer and Meyer-Lindenberg2005), the ability of autistic children to recognize basic emotions (though not social, self-conscious emotions) from eyes and facial features (Castelli Reference Castelli2005; see also Adolphs et al. Reference Adolphs, Gosselin, Buchanan, Tranel, Schyns and Damasio2005), reduced inhibition of the amygdala in autism (Rubenstein & Merzenich Reference Rubenstein and Merzenich2003), a functional-imaging study that shows heightened amygdala activation in response to gaze fixation in autism (Dalton et al. Reference Dalton, Nacewicz, Johnstone, Schaefer, Gernsbacher, Goldsmith, Alexander and Davidson2005a), and relatively strong neurological links between the amygdala and the right hemisphere in normal subjects compared to autistics (Noesselt et al. Reference Noesselt, Driver, Heinze and Dolan2005). Long-term avoidance of gaze and faces presumably reduces opportunities for autistic children to develop skills related to this core component of social behavior, via cognitive appraisal of eye and facial features during interactions (Gläscher & Adolphs Reference Gläscher and Adolphs2003; M. H. Johnson Reference Johnson2005; Skuse et al. Reference Skuse, Morris and Dolan2005; see also Adolphs et al. Reference Adolphs, Gosselin, Buchanan, Tranel, Schyns and Damasio2005). Such deficits, as well as other components of the social brain, may be involved in the underdeveloped theory of mind found in Kanner autism and to a lesser extent in Asperger syndrome (Baron-Cohen Reference Baron-Cohen2002; Reference Baron-Cohen2003; Bowler Reference Bowler1992; Downs & Smith Reference Downs and Smith2004).
Langdon et al. (Reference Langdon, Corner, McLaren, Coltheart and Ward2006b) demonstrated that schizophrenia involves abnormal over-responsiveness in attentional orienting to gaze (see also Emery Reference Emery2000; Franck et al. Reference Franck, Daprati, Michel, Saoud, Daléry, Marie-Cardine and Georgieff1998), which is the opposite pattern to that observed in autism. Although impairments in tests of recognizing expression from eyes and faces have been described in schizophrenia (e.g., Kington et al. Reference Kington, Jones, Watt, Hopkin and Williams2000) as for autism, such deficits appear to be a function of inaccurate inferences concerning gaze of others as directed towards them (Hooker & Park Reference Hooker and Park2005), and mistaken inference of mental states from gaze (Langdon et al. Reference Langdon, Coltheart and Ward2006a; Reference Langdon, Corner, McLaren, Coltheart and Ward2006b), rather than the underdevelopment or absence of mental-state attribution ascribed to autistic cognition (Baron-Cohen Reference Baron-Cohen2002; Craig et al. Reference Craig, Hatton, Craig and Bentall2004; Frith Reference Frith2003). Paranoia, a prominent feature in the positive symptoms of psychosis, also commonly involves obsession with the gaze of others as directed towards one's self, often concomitant to delusions of persecution and conspiracy (e.g., Badcock Reference Badcock, Crawford and Salmon2004; Franck et al. Reference Franck, Daprati, Michel, Saoud, Daléry, Marie-Cardine and Georgieff1998; Green & Phillips Reference Green and Phillips2004; LaRusso Reference LaRusso1978; McKay et al. Reference McKay, Langdon and Coltheart2005).
We interpret these features of psychosis as forms of hyper-mentalizing, such that theory of mind is dysregulated via impaired, inflexible, or extreme inferences regarding social cues and over-attribution of mental states and intentions (Abu-Akel Reference Abu-Akel1999; Abu-Akel & Bailey Reference Abu-Akel and Bailey2000; Badcock Reference Badcock, Crawford and Salmon2004; Blackwood et al. Reference Blackwood, Howard, Bentall and Murray2001; C. D. Frith Reference Frith2004; Harrington et al. Reference Harrington, Langdon, Siegert and McClure2005a; Reference Harrington, Siegert and McClure2005b; Langdon & Coltheart Reference Langdon and Coltheart1999; Langdon et al. Reference Langdon, Coltheart and Ward2006a; Phillips et al. Reference Phillips, Senior and David2000; Steiner Reference Steiner2004). Indeed, the evolutionary psychiatrist Randolph Nesse noted that “those who have worked with schizophrenics know the eerie feeling of being with someone whose intuitions are acutely tuned to the subtlest unintentional cues, even while the person is incapable of accurate empathic understanding” (Nesse 2004, p. 62), and Claridge et al. (Reference Claridge, Pryor and Watkins1990, p. 221) noted that “anyone who has interacted with psychotics will know of their uncanny capacity to respond to subtle social cues, believed to have been concealed from them.” These patterns contrast with the extreme literal-mindedness found in autistic individuals, in comparison to the common overinterpretation of linguistic and visual input in schizophrenia and to some degree in schizotypy (Langdon & Coltheart Reference Langdon and Coltheart2004; Langdon et al. Reference Langdon, Coltheart, Ward and Catts2002; Russell et al. Reference Russell, Reynaud, Herba, Morris and Corcoran2006), although schizophrenia can also involve deficits that engender impaired processing of figurative language (Thoma & Daum Reference Thoma and Daum2006). Both autistic literal-mindedness and mechanistic cognition, and psychotic-spectrum overinterpretation can lead, though by different mechanisms, to deficits in theory of mind tasks and understanding of pragmatic language such as metaphor and humor (C. D. Frith Reference Frith2004; Frith & Allen Reference Frith, Allen, Bebbington and McGuffin1988; Mitchell & Crow Reference Mitchell and Crow2005). Taken together, this evidence suggests that reaction to gaze, as well as tendency to attribute mental states and intentions to others, exhibit contrasting patterns of hyperdevelopment and underdevelopment in psychosis and autism (Table 1).
The neurophysiological and psychological mechanisms underlying hyperdevelopment of gaze, inference of intention, and theory of mind in schizophrenia require further study. As in autism, the amygdala plays an important role in the interactions that mediate theory of mind skills in schizophrenia (Benes & Berretta Reference Benes and Berretta2001; Brüne Reference Brüne2004; Brüne & Brüne-Cohrs Reference Brüne and Brüne-Cohrs2006). Many (though by no means all) functional-imaging studies demonstrate that schizophrenia engenders underactivation of the amygdala compared to controls in tests of recognizing facial expression and emotional valence (Brunet-Gouet & Decety Reference Brunet-Gouet and Decety2006; Das et al. Reference Das, Kemp, Flynn, Harris, Liddell, Whitford, Peduto, Gordon and Williams2007; Gur et al. Reference Gur, McGrath, Chan, Schroeder, Turner, Turetsky, Kohler, Alsop, Maldjian, Ragland and Gur2002; Holt et al. Reference Holt, Kunkel, Weiss, Goff, Wright, Shin, Rauch, Hootnick and Heckers2006; Kucharska-Pietura et al. Reference Kucharska-Pietura, Russell and Masiak2003; Paradiso et al. Reference Paradiso, Andreasen, Crespo-Facorro, O'Leary, Watkins, Boles Ponto and Hichwa2003; Phillips et al. Reference Phillips, Williams, Senior, Bullmore, Brammer, Andrew, Williams and David1999; Schneider et al. Reference Schneider, Weiss, Kessler, Salloum, Posse, Grodd and Müller-Gärtner1998). These findings are consistent with the emotional dysregulation commonly found in schizophrenia (Aleman & Kahn Reference Aleman and Kahn2005), although the connections between emotion and cognition in this and other psychotic-spectrum conditions remain unclear. Underactivation of the amygdala may also be a manifestation of reduced cognitive effects from the paternal brain (Keverne Reference Keverne2001a; Reference Keverne2001b) in negative-symptom schizophrenia.
Finally, gaze and intentionality are intimately associated with the expression of emotion, which is generally recognized as reduced in schizophrenia and major depression (e.g., Gaebel & Wölwer Reference Gaebel and Wölwer2004; Troisi et al. Reference Troisi, Pompili, Binello and Sterpone2007), although lack of facial expression need not indicate a lack of emotional experience (Trémeau et al. Reference Trémeau, Malaspina, Duval, Corrêa, Hager-Budny, Coin-Bariou, Macher and Gorman2005); indeed, a relatively high level of positive schizotypy appears to be associated with more intense emotionality (Kerns Reference Kerns2005). Parental reports provide evidence that autistic children are highly expressive, especially for negative emotion, although they “communicated feeling states idiosyncratically, in a manner only their mother understood” (Capps et al. Reference Capps, Kasari, Yirmiya and Sigman1993) (p. 475). Losh and Capps (Reference Losh and Capps2006) also report that autistic children are no less emotionally expressive than controls for basic emotions, though not self-conscious ones. In autism, levels of basic emotionality may thus be as high or higher than normal (in keeping with relatively enhanced paternal-brain functions), but emotional reactions lack social context and sensitivity due to reductions in the ability of the neocortical, maternal brain to integrate social-cognitive understanding and control with emotion and behavior.
6.3.2. Agency and self
Monitoring of gaze and inference of intention involve application of theory of mind skills to other individuals (Emery Reference Emery2000; Tomasello et al. Reference Tomasello, Carpenter, Call, Behne and Moll2005). The other domain for theory of mind is reflexive – that is, self-consciousness, mentalized awareness of one's self as an agent acting upon the external world, and exhibiting spontaneous self-directed thought. Several lines of evidence suggest that autism involves a diminished or altered sense of self: (1) selective impairments of episodic and autobiographical memory – the mental constructs that generate and maintain a sense of self (Gardiner Reference Gardiner, Baddeley, Aggleton and Conway2002; Gardiner et al. Reference Gardiner, Bowler and Grice2003) – while rote, factual memory is spared or enhanced (O'Shea et al. Reference O'Shea, Fein, Cillessen, Klin and Schultz2005; Toichi & Kamio Reference Toichi and Kamio2002; Williams et al. Reference Williams, Goldstein and Minshew2006b); (2) a tendency to think in visual pictures (Grandin Reference Grandin1995; Hurlburt et al. Reference Hurlburt, Happé and Frith1994; Kana et al. Reference Kana, Keller, Cherkassky, Minshew and Just2006), suggesting a shift to the right hemisphere for brain regions generating spontaneous thought (Christoff et al. Reference Christoff, Ream and Gabrieli2004); (3) a reduced sense of self-consciousness and personal agency, perhaps due to reduced central coherence at this level (Ben Shalom Reference Ben Shalom2000; Fitzgerald Reference Fitzgerald2005, p. 78; Frith & Happé Reference Frith and Happé2005; Grandin Reference Grandin1995; Reference Grandin2004; Johnson Reference Johnson2003; Lawson Reference Lawson1998, p. i; Lombardo et al. Reference Lombardo, Barnes, Wheelwright and Baron-Cohen2007; Toichi et al. Reference Toichi, Kamio, Okada, Sakihama, Youngstrom, Findling and Yamamoto2002); (4) a less developed imagination, as noted earlier (Craig & Baron-Cohen Reference Craig and Baron-Cohen1999; Happé Reference Happé1994, p. 37; Losh & Capps Reference Losh and Capps2003); and (5) less developed experience of social and self-conscious emotion (Heerey et al. Reference Heerey, Keltner and Capps2003; Losh & Capps Reference Losh and Capps2006). Autism also involves cognition highly focused on few particular non-social aspects of the environment, which is expressed in repetitive behavior, intense preoccupations about specific and narrow topics, and difficulties in shifting attention (Courchesne et al. Reference Courchesne, Townsend, Akshoomoff, Saitoh, Yeung-Courchesne, Lincoln, James, Haas, Schreibman and Lau1994; Goldstein et al. Reference Goldstein, Johnson and Minshew2001a; Happé Reference Happé1994; Landry & Bryson Reference Landry and Bryson2004; Murray et al. Reference Murray, Lesser and Lawson2005). These features may interact via positive feedback to reduce the degree to which these individuals become socially enculturated (Murray et al. Reference Murray, Lesser and Lawson2005), a central process in human development.
In contrast to autism, schizotypy and schizophrenia are characterized by high levels of distractability, reduced filtering of sensory input, and “loose” associations between external stimuli and between components of thought (e.g., Bellgrove et al. Reference Bellgrove, Vance and Bradshaw2003; Brugger & Graves Reference Brugger and Graves1997a; Reference Brugger and Graves1997b; Claridge & Beech Reference Claridge, Beech, Raine, Lencz and Mednick1995; Grossberg Reference Grossberg2000b; Mathes et al. Reference Mathes, Wood, Proffitt, Stuart, Buchanan, Velakoulis, Brewer, McGorry and Pantelis2005; Nakamura et al. Reference Nakamura, Matsushima, Ohta, Ando and Kojima2003; Pizzagalli et al. Reference Pizzagalli, Lehmann, Gianotti, Koenig, Tanaka, Wackermann and Brugger2000). The positive symptoms of schizophrenia often involve delusions, such as persecution and grandiosity, that “reflect individuals' preoccupations about their position in the social universe” (Bentall Reference Bentall, Kircher and David2003b, p. 293) in creating complex and fanciful mental autobiographies (Salazar-Fraile et al. Reference Salazar-Fraile, Tabarés-Seisdedos, Selva-Vera, Balanzá-Martinez, Martínez-Aran, Catalán, Baldeweg, Vilela-Soler, Leal-Cercós, Vieta and Gomez-Beneyto2004), and an increased awareness of self and intentionality is found in schizophrenic delusions of control (Frith Reference Frith2005b). Social and reflexive preoccupations can be described in terms of exaggerated self-consciousness (Sass & Parnas Reference Sass and Parnas2003), and indeed, auditory hallucination, thought insertion, and passivity phenomena (e.g., Behrendt Reference Behrendt2004; Crow Reference Crow2004a; Reference Crow2004b) can be interpreted as functions of a breakdown in cognitive distinction between self and other, as in some spiritual experiences of schizotypal individuals (Brugger Reference Brugger, Houran and Lange2001). Breakdowns in self-monitoring, such as interpreting inner speech as external, or delusions of control of hand movements, have an apparent neurological basis in impaired ability to self-monitor the coordination of intended (premotor) actions (mainly speech and gesture) with one's current and predicted state (Frith et al. Reference Frith, Blakemore and Wolpert2000; Jones & Fernyhough Reference Jones and Fernyhough2007). By contrast, autism appears to involve absent or reduced use of inner speech (Whitehouse et al. Reference Whitehouse, Maybery and Durkin2006).
Finally, auditory hallucinations commonly involve social dialogue, commentary, and commands, with a focus on complex, self-conscious emotions such as shame, contempt, derogation, and embarrassment (e.g., Legg & Gilbert Reference Legg and Gilbert2006); Bentall (Reference Bentall2003a, p. 354) notes how such hallucinations often involve the voices of “significant family members,” and Birchwood et al. (Reference Birchwood, Gilbert, Gilbert, Trower, Meaden, Hay, Murray and Miles2004) describes them as operating “like external social relationships.” These patterns, and the common feelings of guilt, deserved punishment, and worthlessness in major depression (Keller et al. Reference Keller, Schatzberg and Maj2007), suggest that social emotions and interactions are often overdeveloped in psychotic-spectrum disorders, in contrast to their reduction in autism, as described earlier.
6.3.3. Functional imaging of the social brain
The neurological basis for dysregulation of gaze, intention, agency, and theory of mind in autism and psychosis provides a mechanistic level of analysis that should dovetail with psychological descriptions of these processes. Thus, autistic- and psychotic-spectrum disorders may be expected to exhibit contrasting patterns of brain activation in functional-imaging studies that involve analyzing specific components of the social brain, as described earlier for the amygdala. We have found evidence for specific contrasts involving four brain regions or networks, although differences in the tasks utilized limit the strength of inferences in some cases.
First, Greicius et al. (Reference Greicius, Krasnow, Reiss and Menon2003) have described the brain's “default mode” or “resting state” network, which includes the medial prefrontal cortex, posterior cingulate cortex, ventral anterior cingulate cortex, and precuneus. This midline-structure network exhibits high metabolic rate at rest, with functions related to self-referential processing, processing of information related to theory of mind, inner speech, retrieving and manipulating memories, and developing future plans (Garrity et al. Reference Garrity, Pearlson, McKiernan, Lloyd, Kiehl and Calhoun2007; Greicius et al. Reference Greicius, Krasnow, Reiss and Menon2003; Kennedy et al. Reference Kennedy, Redcay and Courchesne2006). This network is then “deactivated” when attention is redirected to a specific cognitive task. Kennedy et al. (Reference Kennedy, Redcay and Courchesne2006) have described a “failure to deactivate” in autistics, which they attribute to a lower level of baseline resting-state activity, and default-mode cognition directed more towards obsessive interests and sensory-environment processing than towards self-reflective, theory-of-mind, and memory-retrieval activities. Moreover, a higher level of social impairment was associated with a lower degree of deactivation among subjects, which suggests a direct link between social cognition and activation of the resting network. Ohnishi et al. (Reference Ohnishi, Matsuda, Hashimoto, Kunihiro, Nishikawa, Uema and Sasaki2000) and Castelli et al. (Reference Castelli, Frith, Happé and Frith2002) also found reduced activation in autism for regions involved in theory of mind, notably the medial prefrontal cortex.
In contrast to these results for autism, Garrity et al. (Reference Garrity, Pearlson, McKiernan, Lloyd, Kiehl and Calhoun2007) and Harrison et al. (Reference Harrison, Yücel, Pujol and Pantelis2007) have described greater task-induced deactivation in schizophrenics than in controls, for some regions. Garrity et al. (Reference Garrity, Pearlson, McKiernan, Lloyd, Kiehl and Calhoun2007) have also reported that stronger positive symptoms are associated with greater deactivation (due to increased activity at rest), and that schizophrenics included a larger area of the parahippocampal gyrus in the default mode than did controls. Higher deactivation has also been reported in anxiety disorder than in controls (Zhao et al. Reference Zhao, Wang, Li, Hu, Xi, Wu and Tang2007); and in major depression, a specific, affect-processing region of the default network, the subgenual cingulate, exhibits increased metabolic rate and higher functional connectivity with the medial prefrontal cortex than in controls (Greicius et al. Reference Greicius, Flores, Menon, Glover, Solvason, Kenna, Reiss and Schatzberg2007), which the authors ascribe to increased self-referential and emotional processing in this condition. This set of studies suggests that psychotic-spectrum conditions may involve alterations in resting-state network activation specific to increased mentalistic functions, and that they also show a pattern of greater deactivation of the default network than in controls or in autism.
Second, one of the primary areas involved in mental attribution and processing of agency is Brodmann's area (BA) 8 in the dorsomedial frontal cortex (Finger et al. Reference Finger, Marsh, Kamel, Mitchell and Blair2006; Fletcher et al. Reference Fletcher, Happé, Frith, Baker, Dolan, Frackowiak and Frith1995; Goel et al. Reference Goel, Grafman, Sadato and Hallett1995; Marjoram et al. Reference Marjoram, Job, Whalley, Gountouna, McIntosh, Simonotto, Cunningham-Owens, Johnstone and Lawrie2006). Increased activity in this region has been associated with increased genetic risk of schizophrenia by Whalley et al. (Reference Whalley, Simonotto, Flett, Marshall, Ebmeier, Owens, Goddard, Johnstone and Lawrie2004), and Frith (Reference Frith1996) describes evidence that this region is involved in auditory hallucinations in schizophrenia. By contrast, Happé et al. (Reference Happé, Ehlers, Fletcher, Frith, Johansson, Gillberg, Dolan, Frackowiak and Frith1996) found normal activity in this area among controls in a theory-of-mind task, but a complete lack of activation in an Asperger syndrome group.
Third, Luna et al. (Reference Luna, Minshew, Garver, Lazar, Thulborn, Eddy and Sweeney2002) and Silk et al. (Reference Silk, Rinehart, Bradshaw, Tonge, Egan, O'Boyle and Cunnington2006) described reduced activation in autism for BA 46 in the dorsolateral prefrontal cortex in a spatial working memory task, which the former authors interpreted in terms of reduced “top-down” executive function. By contrast, significantly increased activation of right-hemisphere BA 46 was found by Seidman et al. (Reference Seidman, Thermenos, Poldrack, Peace, Koch, Faraone and Tsuang2006) in adolescents at high risk for schizophrenia, and these authors describe parallel findings of exaggerated activity in this region from three previous studies. A role for this region in social cognition is indicated by Knoch et al. (Reference Knoch, Pascual-Leone, Meyer, Treyer and Fehr2006), who found activation in this area during implementation of fairness-related behaviors in social-reciprocity tasks.
A final set of core regions of the social brain is the mirror neuron systems. These systems, comprising regions of the superior temporal sulcus, inferior prefrontal cortex, inferior parietal cortex, and other regions, provide neural substrates for inference of intention, theory of mind and empathy, via activation of the same premotor circuitry during observation and execution of specific facial, manual, or other movements (Iacoboni & Dapretto Reference Iacoboni and Dapretto2006). Such matching of premotor to observed motor activations allows for automatic inferences regarding the intention, disposition, and emotional state of another individual, which can generate cognitive and emotional resonance during social interactions. In autism, a key component of this system, BA 44 (the pars opercularis), was hypoactivated in tasks involving imitation or viewing emotional expressions (Dapretto et al. Reference Dapretto, Davies, Pfeifer, Scott, Sigman, Bookheimer and Iacoboni2006); by contrast, Quintana et al. (Reference Quintana, Davidson, Kovalik, Marder and Mazziotta2001) found overactivation of BA 44 in schizophrenia compared to controls for tasks involving affective facial expression, which they described in terms of “increased mirror-like representational mechanisms . . . with cues of obvious social value” (p. 923).
Hadjikhani et al. (Reference Hadjikhani, Joseph, Snyder and Tager-Flusberg2007) and Dapretto et al. (Reference Dapretto, Davies, Pfeifer, Scott, Sigman, Bookheimer and Iacoboni2006) describe evidence that face- and gaze-processing deficits in autism are due to impairments in the integrated activity of the mirror-neuron system use in facial processing. Patterns of hypoactivation in mirror-system regions in autism are paralleled by evidence for cortical thinning of these areas in autism, which is directly related to the severity of autistic symptoms (Hadjikhani et al. Reference Hadjikhani, Joseph, Snyder and Tager-Flusberg2006; Zilbovicius et al. Reference Zilbovicius, Meresse, Chabane, Brunelle, Samson and Boddaert2006). Reduced activation of mirror neuron systems in autistics appears specific to tasks dependent upon mentalistic cognition, rather than representing a more general impairment that also includes conscious, deliberate imitation or inference of intention from functional gestures (Hamilton et al. Reference Hamilton, de, Brindley and Frith2007).
Taken together, these findings indicate that underdevelopment of the integrated social brain in general, and social aspects of the mirror neuron system in particular, are associated with some of the social-behavioral deficits found in autism (Williams et al. Reference Williams, Whiten, Suddendorf and Perrett2001; cf. Dapretto et al. Reference Dapretto, Davies, Pfeifer, Scott, Sigman, Bookheimer and Iacoboni2006; Hadjikhani et al. Reference Hadjikhani, Joseph, Snyder and Tager-Flusberg2006; Reference Hadjikhani, Joseph, Snyder and Tager-Flusberg2007). Schizophrenia also clearly engenders impairments in theory-of-mind skills and mentalizing (Brunet-Gouet & Decety Reference Brunet-Gouet and Decety2006; Harrington et al. Reference Harrington, Langdon, Siegert and McClure2005a; Reference Harrington, Siegert and McClure2005b; Pinkham et al. Reference Pinkham, Penn, Perkins and Lieberman2003), which commonly involve “over-mentalizing,” such as delusional ideation, inferring false intentions, and a general pattern of confabulation of subjective experience in the face of misinterpreted objective reality (C. D. Frith Reference Frith1992; Reference Frith2004; Frith & Frith Reference Frith and Frith1999). Arbib and Mundhenk (Reference Arbib and Mundhenk2005) link such impairments to the mirror neuron system, in suggesting that functional dissociations between action or speech imagination, and enactment of movement or speech, lead to misattribution of agency and consequent confabulation and rationalizing, which manifests as auditory hallucination, delusions, and paranoia. Similar considerations may apply to the mirror-neuron system underlying face perception and emotional resonance, which is also dysregulated in schizophrenia in the context of emotion inappropriate to social context and flat affect (e.g., Aleman & Kahn Reference Aleman and Kahn2005; van Rijn et al. Reference van Rijn, Aleman, Swaab and Kahn2005). A recent review of functional-imaging studies of social brain dysfunction in schizophrenia also suggests that two mirror-neuron regions – the inferior frontal cortex and the inferior parietal lobe (see Arbib & Mundhenk Reference Arbib and Mundhenk2005) – are selectively responsible for some core cognitive manifestations of this disorder, as well as strongly implicating the medial prefrontal cortex, anterior cingulate cortex, and amygdala (Brunet-Gouet & Decety Reference Brunet-Gouet and Decety2006).
These findings indicate that in contrast to autism, where the mirror-neuron system does not develop to full, integrated functional maturity, in schizophrenia this system develops but is subject to diverse forms of selective malfunction. Thus, aspects of theory of mind, and mirror-neuron system skills, are selectively impaired in both autism and schizophrenia (Brüne & Brüne-Cohrs Reference Brüne and Brüne-Cohrs2006; C. D. Frith Reference Frith1992; Reference Frith2004; Lee et al. Reference Lee, Farrow, Spence and Woodruff2004; Mazza et al. Reference Mazza, De Risio, Surian, Roncone and Casacchia2001; Pickup & Frith Reference Pickup and Frith2001; Russell et al. Reference Russell, Reynaud, Herba, Morris and Corcoran2006; Shamay-Tsoory et al. Reference Shamay-Tsoory, Shur, Barcai-Goodman, Medlovich, Harari and Levkovitz2007; see also McCabe et al. Reference McCabe, Leudar and Antaki2004), but, we believe, for different reasons.
6.4. Behavior and adaptive significance
Conflicts involving imprinted genes, and mother–offspring conflict more generally, are most obvious during prenatal and early childhood development where fitness-limiting resources from placenta and breast can be quantified. But how does one address and quantify costs imposed via cognition and behavior, after early infancy? One prediction of the imprinted brain hypothesis for autism and psychosis is that an increased tendency towards autistic traits entails higher costs imposed on mothers, and that healthy schizotypal behavior in offspring might benefit the mother and other salient maternal relatives, presuming that pathological effects of altered development are not too extreme.
The autistic spectrum, which literally refers to “self-ishness” in cognition, involves a large suite of traits that can be interpreted as imposing additional costs on mothers (Badcock & Crespi Reference Badcock and Crespi2006). Indeed, each of the three main classes of behavior that define autism, (1) impaired social interaction, (2) impaired language development, and (3) repetitive behavior and insistence on sameness, are likely to engender increased demands. Some prevalent traits, such as tantrums, attempts to control others, lack of cooperative behavior, and the notable lack of empathy that characterizes Asperger syndrome (Arbelle et al. Reference Arbelle, Sigman and Kasari1994; Asperger Reference Asperger and Frith1991; Baron-Cohen Reference Baron-Cohen2002; Lawson et al. Reference Lawson, Baron-Cohen and Wheelwright2004; Soderstrom et al. Reference Soderstrom, Rastam and Gillberg2002), appear especially demanding of maternal time and other resources. Similar considerations are expected to apply to non-clinical individuals on the autistic spectrum, showing to some degree any of the traits in Figure 1.
The development of autistic behavior in early childhood means that increased parental costs extend over many years. Psychoses normally develop in late adolescence or early adulthood, but schizotypal individuals, and juveniles who later develop schizophrenia, exhibit distinctive cognitive profiles, and some features of these profiles can be interpreted to involve reduced demands on parents. We note first that research on the so-called premorbid personality of schizophrenics has focused almost exclusively on identifying cognitive deficits as predictors of later disease development (e.g., Ellison et al. Reference Ellison, van Os and Murray1998; Sorensen et al. Reference Sorensen, Mortensen, Parnas and Mednick2006). Moreover, schizophrenia itself, like Kanner autism, involves a considerable degree of pathology, and as such it offers much less-direct insight into the dysregulated adaptive mechanisms that underlie psychosis than does healthy schizotypy (or the equivalents for bipolar disorder and major depression), which exhibits genetic and phenotypic continuity with disorder at one extreme and normality at the other.
Given that psychosis and autism can be characterized as cognitive spectra grading into normality, and that non-clinical (“healthy”) individuals with autistic traits and relatively unimpaired individuals on the autistic spectrum exhibit clear patterns of relative cognitive strengths in some aspects of mechanistic and sensory cognition (e.g., Gernsbacher et al. Reference Gernsbacher, Dawson and Mottron2006; Mottron et al. Reference Mottron, Dawson, Soulières, Hubert and Burack2006; Wheelwright & Baron-Cohen Reference Wheelwright and Baron-Cohen2001), does “healthy schizotypy” also involve specific cognitive strengths (Claridge Reference Claridge1997)? We suggest, based on the overdeveloped mentalistic cognition commonly found in schizophrenia, that healthy schizotypy may often involve enhanced mentalistic abilities and empathy relative to normal individuals (Fig. 4). Enhanced theory of mind in schizotypy is generally consistent with four lines of evidence: (1) associations between positive schizotypy scores and measures of empathy (Dinn et al. Reference Dinn, Harris, Aycicegi, Greene and Andover2002; Rim Reference Rim1994; see also Sullivan & Allen Reference Sullivan and Allen1999); (2) better performance by schizophrenic children compared to controls in a task involving deception of others (Pilowsky et al. Reference Pilowsky, Yirmiya, Arbelle and Mozes2000); (3) more accurate recognition of genuine emotions in paranoid schizophrenia than in normal controls (LaRusso Reference LaRusso1978), or in depressed patients (Davis & Gibson Reference Davis and Gibson2000); and (4) enhanced social-emotional creativity and imagination in individuals with increased levels of schizotypal traits (reviewed in Claridge et al. Reference Claridge, Pryor and Watkins1990; Nettle Reference Nettle2001). In its usual young-adult study subjects, “healthy” positive schizotypy can also involve higher verbal fluency, “openness” to the environment, and more-developed empathy and altruistic feelings and behavior in the context of spirituality (Fisher et al. Reference Fisher, Mohanty, Herrington, Koven, Miller and Heller2004; Jackson Reference Jackson and Claridge1997; Tsakanikos & Claridge Reference Tsakanikos and Claridge2005). By contrast, a number of studies have reported reduced or similar levels of empathic perspective-taking, or other theory-of-mind tasks, in schizotypy, schizophrenia, or first-order relatives of schizophrenics (Jahshan & Sergi Reference Jahshan and Sergi2007; Janssen et al. Reference Janssen, Krabbendam, Jolles and van Os2003; Kelemen et al. Reference Kelemen, Kéri, Must, Benedek and Janka2004; Langdon et al. Reference Langdon, Coltheart and Ward2006a; Montag et al. Reference Montag, Heinz, Kunz and Gallinat2007; Pickup Reference Pickup2006). Such diversity of results may be related to variation in the tasks, populations, schizotypy criteria utilized, and the positions of subjects on the mentalistic continuum (Fig. 4). An important question for future empirical work is determining the neurological, psychological, and behavioral correlates of theory of mind and empathy enhanced over “normal” levels in non-clinical populations.

Figure 4. Autistic and psychotic spectrum conditions can be conceptualized as extremes on a continuum of cognitive architecture from mechanistic to mentalistic cognition. The heights of the curves represent relative performance within and between the two cognitive domains, for individuals at any point along the continuum. Autistic-spectrum cognition thus involves enhanced mechanistic cognition but reduced mentalistic skills, while psychotic-spectrum cognition engenders the converse. Schizophrenia is hypothesized to involve notably hyper-developed mentalistic cognition, which is associated with a suite of impairments, and autism can be characterized in terms of maladaptively hyper-mechanistic and hypo-mentalistic cognition. The actual shapes of the curves are unknown, but their relative orientation should be roughly as shown.
Positive associations of empathy, theory-of-mind skills, and other traits such as mirror-neuron system function with scores on scales of positive schizotypy in non-clinical populations represent a useful prediction of the hypothesis that the autistic and psychotic spectra represent broadly diametric sets of conditions, with the caveat that existing scales of schizotypy may be geared more towards characterizing impairments than analyzing cognitive-affective architecture.
We thus expect that especially in non-clinical populations of children, mildly above-average levels of positive schizotypy may be associated with easier enculturation (see Brown Reference Brown2001) and fewer behavioral demands on the mother – traits that should reduce the costs of child rearing. This prediction fits with a central role for the Kevernian maternal brain in the evolution of human cognition and enculturation of offspring (Badcock Reference Badcock2000), and a generally heightened receptivity to the environment in schizotypy and schizophrenia (Claridge Reference Claridge1997; Dykes & McGhie Reference Dykes and McGhie1976; Park et al. Reference Park, Lenzenweger, Püschel and Holzman1996) compared to avoidance of novel stimuli in autism (Gomot et al. Reference Gomot, Bernard, Davis, Belmonte, Ashwin, Bullmore and Baron-Cohen2006).
An alternative to this “healthy positive schizotypy” hypothesis, which is not mutually exclusive, is that the relatively low birth weight and slow development typical of psychotic-spectrum children are beneficial to mothers (and maternal genes), but the later consequences of such relative energetic benefits in gestation and early childhood may impose costs on both mother and offspring (Fig. 5). By this hypothesis, deleterious behavioral aspects of psychotic-spectrum conditions represent, in part, maladaptive by-products of the tug-of-war between maternal and paternal imprinted-gene effects being disrupted towards a maternal-gene bias. Converse considerations apply to autistic conditions. Disorders of pregnancy provide clear and direct parallels here, in that gestational diabetes and pre-eclampsia, both of which are mediated by maternal–fetal conflict, can involve benefits to the offspring (such as higher birth weight) in mild cases, but severe costs to both mother and child when the dysregulated developmental tug-of-war is more severe (e.g., Catalano & Kirwan Reference Catalano and Kirwan2001; Haig Reference Haig1993; Oudejans et al. Reference Oudejans, Mulders, Lachmeijer, van Dijk, Könst, Westerman, van Wijk, Leegwater, Kato, Matsuda, Wake, Dekker, Pals, ten Kate and Blankenstein2004)

Figure 5. Alternative models for the costs and benefits to mothers and offspring of psychotic-spectrum and autistic-spectrum phenotypes can help to explain a range of possible relationships between imprinted gene expression, fetal and child development, and behavior. The psychotic-spectrum case applies most closely to schizotypy and schizophrenia. Here, mothers may benefit from reduced early parental investment, but in later development they may either garnish fitness benefits or suffer costs, depending upon the nature and strength of the effects on offspring cognition and behavior, such as more-pronounced impairments in negative schizotypy. The autistic-spectrum case involves increased costs imposed on mothers in early offspring development; and in later development offspring may either benefit from more-egoistical cognition and behavior, or suffer relative costs, depending on the form and magnitude of the developmental disruptions. All of these costs and benefits should be considered in the context of fitness-mediating interactions between kin.
There is currently little directly useful data on the nature of mother–offspring interactions, and the presumably reduced behavioral and energetic costs involved in child rearing, for individuals exhibiting healthy schizotypy. One line of evidence is increased fertility of individuals in one or more category of non-affected first-degree relatives of schizophrenics, which has been reported in multiple studies (Avila et al. Reference Avila, Thaker and Adami2001; Bassett et al. Reference Bassett, Bury, Hodgkinson and Honer1996; Fañanás & Bertranpetit Reference Fañanás and Bertranpetit1995; Haukka et al. Reference Haukka, Suvisaari and Lönnqvist2003; McGlashen et al. 2006; Srinivasan & Padmavati Reference Srinivasan and Padmavati1997; Waddington & Youssef Reference Waddington and Youssef1996), although other studies report a lack of such differences (Buck et al. Reference Buck, Hobbs, Simpson and Wanklin1975; Rimmer & Jacobsen Reference Rimmer and Jacobsen1976). Thus, Fañanás and Bertranpetit (Reference Fañanás and Bertranpetit1995) reported that the mothers (but not fathers) of schizophrenics had significantly more siblings than did controls; Bassett et al. (Reference Bassett, Bury, Hodgkinson and Honer1996) found no significant difference in number of children between siblings of schizophrenics and controls, except for female siblings exhibiting schizotypal traits, who had significantly more offspring; and Waddington and Youssef (Reference Waddington and Youssef1996) reported more siblings for male schizophrenics with a family history of the disorder, compared to those without a family history, and a higher risk of schizophrenia in brothers of probands with more than seven siblings compared to fewer than seven. Srinivasan and Padmavati (Reference Srinivasan and Padmavati1997) reported higher numbers of offspring in parents of schizophrenics compared to the general population, and a “non-significant trend” for higher numbers of offspring produced by siblings of schizophrenics, Avila et al. (Reference Avila, Thaker and Adami2001) found a significantly higher number of siblings in schizophrenics, compared to a community sample, and Haukka et al. (Reference Haukka, Suvisaari and Lönnqvist2003) reported that sisters of schizophrenics produced significantly more offspring than controls, while brothers produced significantly fewer. Finally, McGlashan et al. (Reference McGlashan, Pedersen, Hoffman and Mortensen2006) showed with large samples that fertility in parents of individuals with schizophrenia (and with bipolar disorder) was “substantially and significantly” increased. These findings also suggest that effects of increased fertility in relatives of schizophrenics are relatively strong for female, compared to male, relatives (but see also Lane et al. Reference Lane, Byrne, Mulvany, Kinsella, Waddington, Walsh, Larkin and O'Callaghan1995), which fits with schizotypy being more compatible with female than male development and cognition, as described further on. Schizophrenia itself engenders reduced fertility compared to healthy controls, especially for males (Fañanás & Bertranpetit Reference Fañanás and Bertranpetit1995; Haukka et al. Reference Haukka, Suvisaari and Lönnqvist2003; McGrath et al. Reference McGrath, Hearle, Jenner, Plant, Drummond and Barkla1999), although the presence and strength of this effect varies culturally (Hutchinson et al. Reference Hutchinson, Bhugra, Mallett, Burnett, Corridan and Leff1999).
Some of the epidemiological patterns described above may represent not increased fitness in first-order relatives of schizophrenics, but effects of family size on schizophrenia risk instead. Thus, Wahlbeck et al. (Reference Wahlbeck, Osmond, Forsén, Barker and Eriksson2001b) reported that increased schizophrenia risk involves higher numbers of siblings (controlling for maternal age), and low body mass index at age seven, and Westergaard et al. (Reference Westergaard, Mortensen, Pedersen, Wohlfahrt and Melbye1999; Reference Westergaard, Mortensen, Pedersen, Wohlfahrt and Melbye2001) found a similar pattern of schizophrenia being more common in larger sibships, with an additional effect from short intervals between siblings. These findings suggest that in some populations, larger families and shorter interbirth intervals engender physiological and metabolic stresses on mothers and offspring that increase schizophrenia risk. This mechanism cannot, however, explain reports of higher fitness in siblings of schizophrenics compared to controls.
Schizotypy may also be linked with other components of reproduction, given that Nettle and Clegg (Reference Nettle and Clegg2006) report an association in a non-clinical population between increased numbers of mating partners and measures of schizotypy. These authors provide evidence that this association is mediated by a positive correlation of the “unusual experiences” dimension of schizotypy with creative activity, and we suggest that enhanced theory-of-mind and language skills in healthy schizotypal individuals (especially relatively creative ones) may facilitate the efficacy of social interactions in determining mating success.
Our hypothesis also predicts that parents with more-autistic offspring should tend to have fewer children due to their increased costs. This prediction appears obvious for cases of Kanner autism due to its high level of impairment at an early age, but cases involving high-functioning autism or Asperger syndrome may provide useful tests. Males also appear to be physiologically more costly to gestate and rear than females (Gibson & Mace Reference Gibson and Mace2003; Rickard et al. Reference Rickard, Russell and Lummaa2007; Tamimi et al. Reference Tamimi, Lagiou, Mucci, Hsieh, Adami and Trichopoulos2003), which is consistent with a relatively higher cost of autistic children for mothers, to the extent that such children exhibit relatively male-typical phenotypic traits. Conversely, relatively female phenotypes in children on the psychotic spectrum, as described in more detail later, may make them less costly to rear.
Whether behavioral aspects of the autistic-psychotic spectrum involve trade-offs between mentalistic and mechanistic cognition and abilities remains unclear. Jarrold et al. (Reference Jarrold, Butler, Cottington and Jimenez2000) found negative correlations, for a non-clinical population and for autistics, between theory-of-mind skills and visual-spatial skills (block design and embedded-figures tests) when verbal mental age was controlled, Johnson and Bouchard (Reference Johnson and Bouchard2007) found a negative association between verbal skills and spatial-imagery skills, when the effects of general intelligence were removed, and Nettle (Reference Nettle2007) reported a significant negative correlation between Systemizing Quotient scores and Empathizing Quotient scores in a non-clinical population. By contrast, Carroll and Chiew (Reference Carroll and Chiew2006) found that Systemizing Quotient and Empathizing Quotient scores were not significantly related across individuals, with or without controlling for age or verbal ability. In mice, an apparent gain of function mutation in the autism-associated NLGN3 gene is associated with impaired social interaction but enhanced spatial learning ability (Tabuchi et al. Reference Tabuchi, Blundell, Etherton, Hammer, Liu, Powell and Sudhof2007), which suggests that such trade-offs may be mediated by single loci and relatively simple mechanisms, such as ratios of excitatory to inhibitory synaptic transmission. The neurocognitive basis for links of mechanistic and spatial skills with relatively “selfish” cognition and behavior in autism also require investigation, although Chen et al. (Reference Chen, Planche, Lemonnier and Lazartigues2007) describe how enhanced spatial skills, and reduced linguistic and mentalistic skills, may be jointly mediated by the egocentric cognition characteristic of autism and Asperger syndrome. Similarly, Langdon et al. (Reference Langdon, Coltheart and Ward2006a) describe preserved spatial perspective-taking in autism but impairments in perspectives involving beliefs and intentions.
7. Sex differences
If psychosis represents a phenotypic and genomic converse to autism, then it may exhibit a pattern of covariation with sex opposite to the pattern observed in autism. Thus, as males score higher on tests of autistic tendencies (Baron-Cohen Reference Baron-Cohen2002; Baron-Cohen et al. Reference Cohen, Pichard, Tordjman, Baumann, Burglen, Excoffier, Lazar, Mazet, Pinquier, Verloes and Héron2005), females tend to score higher on standard indexes of schizotypal cognition, which quantify such traits as magical ideation and paranormal or spiritual experiences (Venables & Bailes Reference Venables and Bailes1994; Williams & Barry Reference Williams and Barry2003; see also Claridge et al. Reference Claridge, Clark and Davis1997). Females also exhibit increased orienting to gaze cues compared to males (Bayliss et al. Reference Bayliss, di Pellegrino and Tipper2005), a more highly developed mirror-neuron system (Cheng et al. Reference Cheng, Tzeng, Decety, Imada and Hsieh2006), and enhanced autobiographic memory (Piefke & Fink Reference Piefke and Fink2005). Positive symptoms are relatively more common in females than males with schizophrenia or schizotypy, and negative symptoms, more frequent in males, are also more recalcitrant to treatment (Caligiuri et al. Reference Caligiuri, Hellige, Cherry, Kwok, Lulow and Lohr2005; Halbreich & Kahn Reference Halbreich and Kahn2003; Leung & Chue Reference Leung and Chue2000; Maric et al. Reference Maric, Krabbendam, Vollebergh, de Graaf and van Os2003; Mata et al. Reference Mata, Sham, Gilvarry, Jones, Lewis and Murray2000; Moriarty et al. Reference Moriarty, Lieber, Bennett, White, Parrella, Harvey and Davis2001; Räsänen et al. Reference Räsänen, Pakaslahti, Syvälahti, Jones and Isohanni2000; Salem & Kring Reference Salem and Kring1998; Sharma et al. Reference Sharma, Dowd and Janicak1999; Venables & Bailes Reference Venables and Bailes1994; Williams & Barry Reference Williams and Barry2003). However, the overall sex ratio in schizophrenia is somewhat biased towards males (Aleman et al. Reference Aleman, Kahn and Selten2003). Whereas autism tends to be much more severe when expressed in females (see Badcock & Crespi Reference Badcock and Crespi2006; Holtmann et al. Reference Holtmann, Bölte and Poustka2007), schizophrenia is, on average, considerably more severe on average in males than females (Halbreich & Kahn Reference Halbreich and Kahn2003; Maric et al. Reference Maric, Krabbendam, Vollebergh, de Graaf and van Os2003; Mata et al. Reference Mata, Sham, Gilvarry, Jones, Lewis and Murray2000; Moriarty et al. Reference Moriarty, Lieber, Bennett, White, Parrella, Harvey and Davis2001; Räsänen et al. Reference Räsänen, Pakaslahti, Syvälahti, Jones and Isohanni2000; Sharma et al. Reference Sharma, Dowd and Janicak1999; Walder et al. Reference Walder, Seidman, Cullen, Su, Tsuang and Goldstein2006b; Williams & Barry Reference Williams and Barry2003).
These diverse findings suggest that sex differences in autism versus schizotypy and schizophrenia mirror some of the differences between males and females. Baron-Cohen (Reference Baron-Cohen2003, p. 173) discussed the “extreme female brain” as exhibiting high empathy and low systemizing ability (in contrast to the reverse in autism), but he dismissed its role in psychological disorders on the presumption that hyperdeveloped theory-of-mind skills would be accurate and adaptive rather than pathological.
Sex differences in schizotypy and schizophrenia appear to be related to sex differences in neuroanatomy. Thus, normal females exhibit lower levels of cerebral asymmetry on average than males (Good et al. Reference Good, Johnsrude, Ashburner, Henson, Friston and Frackowiak2001; Kovalev et al. Reference Kovalev, Kruggel and von Cramon2003; Shaywitz et al. Reference Shaywitz, Shaywitz, Pugh, Constable, Skudlarski, Fulbright, Bronen, Fletcher, Shankweiler and Katz1995; Yücel et al. Reference Yücel, Stuart, Maruff, Velakoulis, Crowe, Savage and Pantelis2001), especially in language areas (Blanton et al. Reference Blanton, Levitt, Peterson, Fadale, Sporty, Lee, To, Mormino, Thompson, McCracken and Toga2004; Josse & Tzourio-Mazoyer Reference Josse and Tzourio-Mazoyer2004; Kovalev et al. Reference Kovalev, Kruggel and von Cramon2003), a robust pattern that matches the lower anatomical and functional asymmetry in schizotypy and schizophrenia compared to controls. Females also may exhibit a relatively large corpus callosum (for brain size) compared to males (especially in the splenia), although this difference is complex and disputed (e.g., Good et al. Reference Good, Johnsrude, Ashburner, Henson, Friston and Frackowiak2001; Highley et al. Reference Highley, DeLisi, Roberts, Webb, Relja, Razi and Crow2003; Mitchell et al. Reference Mitchell, Free, Merschhemke, Lemieux, Sisodiya and Shorvon2003; Schoenemann Reference Schoenemann2006). Amygdala size is notably associated with sex, psychosis, and autism in the expected pattern. Thus, normal males have a larger amygdala than females (Goldstein et al. Reference Goldstein, Seidman, Horton, Makris, Kennedy, Caviness, Faraone and Tsuang2001b; Good et al. Reference Good, Johnsrude, Ashburner, Henson, Friston and Frackowiak2001), and controlling for sex, the amygdala is larger in autism and smaller in schizophrenia, as described earlier. Gur et al. (Reference Gur, Kohler, Turetsky, Siegel, Kanes, Bilker, Brennan and Gur2004) describe how, with regard to orbitofrontal cortex to amygdala ratios, schizophrenic men show “feminization,” while women exhibit “masculinization.” These patterns are especially telling given the importance of the amygdala and orbitofrontal cortex in functioning of the social brain (Burns Reference Burns2004; Skuse et al. Reference Skuse, Morris and Lawrence2003), and the enhanced abilities of females in interpretation of some social cues, such as those related to fear (McClure et al. Reference McClure, Monk, Nelson, Zarahn, Leibenluft, Bilder, Charney, Ernst and Pine2004). Finally, Mendrek Reference Mendrek2007) describes a suite of evidence that schizophrenia engenders a pattern of reversed cerebral sexual dimorphism in structure and function, mainly involving more “female-typical” traits in males; and Nakayama et al. (2007) report significantly higher cortisol levels in males with (female-typical) high scores on the Empathy Quotient test, as well as in females with (male-typical) high scores on the Systemizing Quotient test.
Contrasts have also been documented between some visual-spatial skills (relative to verbal skills) being enhanced in autistic individuals and their first-order relatives (Baron-Cohen et al. Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley2001, Reference Baron-Cohen, Knickmeyer and Belmonte2005; Bertone et al. Reference Bertone, Mottron, Jelenic and Faubert2005; Bölte & Poustka Reference Bölte and Poustka2006), and specifically impaired in psychosis and schizotypy (Kravariti et al. Reference Kravariti, Toulopoulou, Mapua-Filbey, Schulze, Walshe, Sham, Murray and McDonald2006; Langdon & Coltheart Reference Langdon and Coltheart2001; Toulopoulou et al. Reference Toulopoulou, Mapua-Filbey, Quraishi, Kravariti, Morris, McDonald, Walshe, Bramon and Murray2005), as well as velocardiofacial syndrome (Lajiness-O'Neill et al. Reference Lajiness-O'Neill, Beaulieu, Asamoah, Titus, Bawle, Ahmad, Kirk and Pollack2006; Simon et al. Reference Simon, Bearden, Moss, McDonald-McGinn and Wang2002; Zinkstok & van Amelsvoort Reference Zinkstok and van Amelsvoort2005) and Prader-Willi syndrome disomy cases (Roof et al. Reference Roof, Stone, MacLean, Feurer, Thompson and Butler2000; Whittington & Holland Reference Whittington and Holland2004). Parallel differences in visual-spatial versus verbal skills have been described between normal males and females (Baron-Cohen et al. Reference Cohen, Pichard, Tordjman, Baumann, Burglen, Excoffier, Lazar, Mazet, Pinquier, Verloes and Héron2005; Browne Reference Browne2005; Crow et al. Reference Crow, Crow, Done and Leask1998; Gur et al. Reference Gur, Alsop, Glahn, Petty, Swanson, Maldjian, Turetsky, Detre, Gee and Gur2000; Kramer et al. Reference Kramer, Ellenberg, Leonard and Share1996; Lawrence Reference Lawrence2006; Schoenemann Reference Schoenemann2006; Walder et al. Reference Walder, Seidman, Cullen, Su, Tsuang and Goldstein2006b). Some of these neuroanatomical and cognitive differences may be mediated by differential effects of testosterone and estradiol in fetal development (Lutchmaya et al. Reference Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer and Manning2004; Mendrek Reference Mendrek2007). Thus, autistic individuals exhibit a relatively more male-typical pattern of 2:4 digit length ratio, but schizophrenics and subjects with schizotypal personality disorder show a relatively more female-typical pattern (Arató et al. Reference Arató, Frecska, Beck, An and Kiss2004; Manning et al. Reference Manning, Baron-Cohen, Wheelwright and Sanders2001; Reference Manning, Bundred and Flanagan2002; Reference Manning, Stewart, Bundred and Trivers2004; Milne et al. Reference Milne, White, Campbell, Swettenham, Hansen and Ramus2006; Walder et al. Reference Walder, Andersson, McMillan, Breedlove and Walker2006a); more-female digit lengths are also associated with higher scores on a depression scale in a non-clinical population (Bailey & Hurd Reference Bailey and Hurd2005).
Based on the evidence described above, we hypothesize that a maternal–paternal imprinting axis of cognition, and an axis based on male–female differences, jointly explain key aspects of the sex biases and differences found for autistic- and psychotic-spectrum conditions (Fig. 6). By this model, the two axes overlap partially but broadly with one another, such that the so-called male brain is relatively similar, neuroanatomically and cognitively, to a brain biased towards increased influence of paternally expressed imprinted genes. This overlap explains the male sex-ratio bias and male-typical traits found in autism, and the association between less-extreme impairment and a more male-biased sex ratio (in high-functioning autism and Asperger syndrome). Conversely, a “more-female” brain is similar to a brain developing under a relatively strong influence of maternally expressed imprinted genes, which explains the female bias in positive-symptom schizotypy and schizophrenia. The most severe neurological and cognitive impairments are found, in both disorders, where the direction of genomic-imprinting dysregulation opposes the sex difference: in females with autism, and in males with schizophrenia. This hypothesis may also help to explain some of the many striking neuroanatomical and other differences between females and males with schizophrenia (e.g., DeLisi et al. Reference DeLisi, Svetina, Razi, Shields, Wellman and Crow2002; Highley et al. Reference Highley, DeLisi, Roberts, Webb, Relja, Razi and Crow2003; Troisi et al. Reference Troisi, Pasini and Spalletta2001; see also Cahill et al. Reference Cahill, Uncapher, Kilpatrick, Alkire and Turner2004), as well as the more female-like hormonal profiles of male schizophrenics with predominantly negative symptoms (Akhondzadeh et al. Reference Akhondzadeh, Rezaei, Larijani, Nejatisafa, Kashani and Abbasi2006; Simpson et al. Reference Simpson, de la Cruz, Swerdloff, Samango-Sprouse, Skakkebaek, Graham, Hassold, Aylstock, Meyer-Bahlburg, Willard, Hall, Salameh, Boone, Staessen, Geschwind, Giedd, Dobs, Rogol, Brinton and Paulsen2003), a high prevalence of homosexual ideation in male schizophrenics (Planansky & Johnston Reference Planansky and Johnston1962), the female-biased sex ratio in major depression (e.g., Piccinelli & Wilkinson Reference Piccinelli and Wilkinson2000), and the relatively high incidences of psychosis in Klinefelter syndrome (Boks et al. Reference Boks, de Vette, Sommer, van Rijn, Giltay, Swaab and Kahn2007a; DeLisi et al. Reference DeLisi, Maurizio, Svetina, Ardekani, Szulc, Nierenberg, Leonard and Harvey2005; van Rijn et al. Reference van Rijn, Aleman, Swaab and Kahn2005) and autism in Turner syndrome (Skuse Reference Skuse2005).

Figure 6. The interaction of sex differences and genomic-imprinting effects can clarify major features of the autistic and psychotic spectra. The worst impairments in these conditions are found where the direction of sex-difference effects opposes the direction of genomic-imprinting effects: in males with schizophrenia, and females with autism.
8. Discussion
Julian Huxley and Ernst Mayr, two architects of the Modern Synthesis of biology, stated in 1964 that “schizophrenia constitutes not a purely medical or psychiatric but a biological problem, with opportunities for a combined attack in many fields – genetics, biochemistry, selection theory, psychology, psychiatry, public health, demography, social science, pathology, and general environmental and reproductive physiology” (Huxley et al. Reference Huxley, Mayr, Osmond and Hoffer1964, p. 220). We have tried to marshall such a coordinated attack, on both psychotic and autistic conditions, with evolutionary biology as guide and inspiration, and genomic imprinting as a simple mechanism that helps to connect genetic and epigenetic variation with cognition.
Our hypothesis can be conceptualized at two interacting levels: (1) the diametric architecture of autistic- and psychotic-spectrum conditions (Badcock Reference Badcock, Crawford and Salmon2004), and (2) the underpinnings of this structure in dysregulated genomic imprinting. A diametric structure to autism and schizophrenia has been considered for some traits before: thus, Abu-Akel (Reference Abu-Akel1999) and Abu-Akel and Bailey (Reference Abu-Akel and Bailey2000) implied that autism and schizophrenia represent extremes on a continuum of theory-of-mind skills ranging from the deficits commonly found in autism to “hyper-theory of mind” in schizophrenia, C. D. Frith (Reference Frith2004) described “under-mentalizing” in autism and “over-mentalizing” in paranoia, and Nettle (Reference Nettle2006) anticipated an autism-psychosis spectrum in noting that “autistic traits are in many ways the converse of the unusual experiences component of schizotypy.” Similarly, the well-known autistic Donna Williams (Reference Williams1992, p. 204) considered autism and schizophrenia as opposite conditions along a scale of “degree of sensitivity of an automatic cut-off mechanism that stops emotional overload,” with the mechanism underdeveloped, oversensitive, and triggered too easily in autism. Most previous research and writing on autism and psychosis has, by contrast, considered the disorders to be etiologically unrelated (or has considered the negative symptoms of schizophrenia in terms of autism), although both disorders are believed to be underlain by dysregulated development of the social brain (Broks Reference Broks and Claridge1997; Burns Reference Burns2004; Reference Burns2006a; Emery Reference Emery2000). By our hypothesis, autism and psychosis represent extremes on a continuum of human cognitive architecture from mechanistic to mentalistic cognition, with balanced cognition at the center (Fig. 4). Each set of conditions is extremely heterogeneous but also highly convergent, in that diverse genetic, epigenetic, and environmental effects can generate similar cognitive phenotypes (Badcock & Crespi Reference Badcock and Crespi2006; Happé Reference Happé1994, p. 2; Happé et al. Reference Happé, Ronald and Plomin2006; Keverne Reference Keverne1999; Seeman et al. Reference Seeman, Weinshenker, Quirion, Srivastava, Bhardwaj, Grandy, Premont, Sotnikova, Boksa, El-Ghundi, O'Dowd, George, Perreault, Männistö, Robinson, Palmiter and Tallerico2005). These striking convergences are mediated, in our view, by the dynamics of social brain development, with underdevelopment in autistic conditions and hyperdevelopment in psychotic conditions (Badcock Reference Badcock, Crawford and Salmon2004), as shown at the cores of Figures 1 and 2. Further tests of this hypothesis should focus on assessing the breadth and depth of diametric phenotypic structure to autistic- and psychotic-spectrum conditions, and testing for trade-offs between mentalistic and mechanistic thought and ability.
A role for genomic imprinting in the diametric structure of autistic and psychotic conditions is based on inclusive fitness theory, which explains how alleles affecting interactions between kin have evolved (Alexander Reference Alexander1979; Hamilton Reference Hamilton1964). Inclusive fitness theory provides the evolutionary foundation for understanding animal and human social behavior (Alexander Reference Alexander1979; Reference Alexander1987; Reference Alexander, Mellars and Stringer1989), so applying it to understanding disorders of the social brain, the core of human behavior, may lead to useful insights. Such applications are not always straightforward. In particular, developmental systems subject to genomic imprinting effects are expected to resemble dynamic tugs-of-war, whose disruption should lead to relative maternal-gene or paternal-gene benefit only for small deviations, beyond which we expect pathological effects beneficial to no one. Separating adaptation from maladaptation can be extremely difficult for psychological traits (Nesse Reference Nesse2005), and requires knowledge of genetic, epigenetic, and developmental mechanisms (Crespi Reference Crespi2000).
Both non-imprinted and imprinted genes obviously contribute to the neurodevelopmental and physiological processes involved in autistic- and psychotic-spectrum conditions (Crespi, under revision). Our hypothesis should therefore be considered in terms of both genomic imprinting effects on growth, development, cognition, and behavior, and causal factors that are distinct from genomic imprinting but may also include maternal–fetal conflict mediated by non-imprinted genes. The relative contributions of these processes to the etiologies of autistic- and psychotic-spectrum conditions are as yet unclear, but evidence for parent-of-origin effects in these disorders is extensive (Crespi, under revision). Thus, although few of the genes linked so far with autism are known to be imprinted, recent evidence implicates the imprinted gene UBE3A and the imprinting-regulation gene MeCP2 in autism, Angelman syndrome, and Rett Syndrome (Samaco et al. Reference Samaco, Nagarajan, Braunschweig and LaSalle2004; Reference Samaco, Hogart and LaSalle2005). Similarly, a strong parent-of-origin effect for both schizophrenia risk and relative hand skill has been found for the chromosomal region 2p12-q11 by Francks et al. (Reference Francks, DeLisi, Fisher, Laval, Rue, Stein and Monaco2003a; Reference Francks, DeLisi, Shaw, Fisher, Richardson, Stein and Monaco2003b), who noted that their findings suggest that “lateralized development of the human brain, and complex human cognitive abilities, have been subject to a parental conflict over investment” (Francks et al. Reference Francks, DeLisi, Shaw, Fisher, Richardson, Stein and Monaco2003b, p. 3227). One of the largest meta-analyses of schizophrenia genome scans to date showed that this region of chromosome 2 exhibited the only linkage signal reaching genome-wide significance (Lewis et al. Reference Lewis, Levinson, Wise, DeLisi, Straub, Hovatta, Williams, Schwab, Pulver, Faraone, Brzustowicz, Kaufmann, Garver, Gurling, Lindholm, Coon, Moises, Byerley, Shaw, Mesen, Sherrington, O'Neill, Walsh, Kendler, Ekelund, Paunio, Lönnqvist, Peltonen, O'Donovan, Owen, Wildenauer, Maier, Nestadt, Blouin, Antonarakis, Mowry, Silverman, Crowe, Cloninger, Tsuang, Malaspina, Harkavy-Friedman, Svrakic, Bassett, Holcomb, Kalsi, McQuillin, Brynjolfson, Sigmundsson, Petursson, Jazin, Zoëga and Helgason2003), and Francks et al. (Reference Francks, Maegawa, Lauren, Abrahams, Velayos-Baeza, Medland, Colella, Groszer, McAuley, Caffrey, Timmusk, Pruunsild, Koppel, Lind, Matsumoto-Itaba, Nicod, Xiong, Joober, Enard, Krinsky, Nanba, Richardson, Riley, Martin, Strittmatter, Moller, Rujescu, St Clair, Muglia, Roos, Fisher, Wade-Martins, Rouleau, Stein, Karayiorgou, Geschwind, Ragoussis, Kendler, Airaksinen, Oshimura, Delisi and Monaco2007) have recently demonstrated the apparent genetic basis of this linkage, in showing that a haplotype of the imprinted, paternally expressed gene LRRTM1 at 2p12 is associated with increased schizophrenia risk and left-handedness. Our imprinted-brain hypothesis predicts that the risk haplotype should involve lower expression of this gene, or reduced activity of its product, either of which would generate a relative bias towards maternal-gene effects. Further tests of a role for genomic imprinting in the development of autistic and psychotic conditions should focus on identification and functional characterization of brain-expressed imprinted genes, and tests for parent of origin effects on the inheritance of autistic and psychotic phenotypes, especially those that underlie mentalistic skills.
Several important theoretical and clinical implications follow from the conceptualization of a continuum between autistic- and psychotic-spectrum conditions. First, diametric phenotypes in autism and psychosis provide a simple predictive framework for future studies that may reciprocally illuminate the causes and correlates of both sets of conditions. Is the cholinergic system differentially affected in each disorder, given high rates of self-medication via smoking in schizophrenics (De Luca et al. Reference De Luca, Wong, Muller, Wong, Tyndale and Kennedy2004; Ripoll et al. Reference Ripoll, Bronnec and Bourin2004), but apparent low rates of smoking in autistic adults (Bejerot & Nylander Reference Bejerot and Nylander2003; Lippiello Reference Lippiello2006)? Is the well-replicated lower cancer risk in schizophrenia (Barak et al. Reference Barak, Achiron, Mandel, Mirecki and Aizenberg2005; Dalton et al. Reference Dalton, Mellemkjaer, Thomassen, Mortensen and Johansen2005b; Goldacre et al. Reference Goldacre, Kurina, Wotton, Yeates and Seagroat2005; Levav et al. Reference Levav, Lipshitz, Novikov, Pugachova, Kohn, Barchana, Ponizovsky and Werner2007) paralleled by a higher risk in autism (Ingudomnukul et al. Reference Ingudomnukul, Baron-Cohen, Wheelwright and Knickmeyer2007)? Do reductions in olfactory and nociceptive sensitivity in schizophrenia and schizotypy (Mohr et al. Reference Mohr, Röhrenbach, Laska and Brugger2001; Singh et al. Reference Singh, Giles and Nasrallah2006) represent a neurologically based contrast with apparently enhanced smell and pain perception in autistic children (Bursch et al. Reference Bursch, Ingman, Vitti, Hyman and Zeltzer2004; Cascio et al. Reference Cascio, McGlone, Folger, Tannan, Baranek, Pelphrey and Essick2008; Rogers et al. Reference Rogers, Hepburn and Wehner2003)? Can autism be characterized as a condition strongly mediated by early childhood overgrowth of the body and brain (Mraz et al. Reference Mraz, Green, Dumont-Mathieu, Makin and Fein2007; Sacco et al. Reference Sacco, Militerni, Frolli, Bravaccio, Gritti, Elia, Curatolo, Manzi, Trillo, Lenti, Saccani, Schneider, Melmed, Reichelt, Pascucci, Puglisi-Allegra and Persico2007), while schizophrenia-risk is associated with childhood undergrowth (Saugstad Reference Saugstad1999; Wahlbeck et al. Reference Wahlbeck, Forsén, Osmond, Barker and Eriksson2001a)? Second, some conditions such as obsessive-compulsive disorder (OCD) and ADHD have been reported as highly comorbid in both autism and schizophrenia (e.g., Bejerot Reference Bejerot2007; Hattori et al. Reference Hattori, Ogino, Abiru, Nakano, Oka and Ohtsuka2006; Kayahan et al. Reference Kayahan, Ozturk, Veznedaroglu and Eraslan2005; Leyfer et al. Reference Leyfer, Folstein, Bacalman, Davis, Dinh, Morgan, Tager-Flusberg and Lainhart2006; Ross et al. Reference Ross, Heinlein and Tregellas2006b). By our hypothesis, OCD or ADHD in autistic-spectrum conditions should be fundamentally distinct from OCD or ADHD in psychotic-spectrum conditions (see, e.g., Bürgy Reference Bürgy2007; Goos et al. Reference Goos, Ezzatian and Schachar2007), a prediction that, if supported, strongly impacts on nosology, diagnosis, and treatment. Similar conditions may apply to psychopathy, which has been attributed to subtypes of schizophrenia as well as to Asperger syndrome (e.g., Abu-Akel & Abushua'leh Reference Abu-Akel and Abushua'leh2004; Blair Reference Blair2005; Haskins & Silva Reference Haskins and Silva2006). Finally, therapies to reduce hyper-mentalistic cognition in subjects with psychotic-spectrum conditions may be just as useful as the encouragement of mentalistic abilities in autistics, given that impaired theory of mind in schizophrenia is not a deficit so much as a suite of alternative hyperdevelopments (Abu-Akel Reference Abu-Akel1999; Badcock Reference Badcock, Crawford and Salmon2004).
As W. D. Hamilton (Reference Hamilton and Ridley2005, p. 205) has noted, we live in a world of things and a world of people. An autistic-psychotic continuum can usefully be conceptualized in these overly simplistic terms, although the real world is much more nuanced and complex than our broad, crude strokes can depict. The usefulness of inclusive fitness theory and evolutionary biology in psychiatry, psychology, and neuroscience may ultimately be gauged by the insights that they can provide into the genetic, developmental, and evolutionary bases of the social brain and its disorders.
ACKNOWLEDGMENTS
Bernard Crespi is grateful to NSERC and the Canada Council for the Arts for financial support. Both authors thank Paul Bloom, Martin Brüne, Will Davies, Clyde Francks, David Haig, Randy Jirtle, Randy Nesse, Daniel Nettle, Sophie van Rijn, and Alfonso Troisi for helpful comments and discussions.









