Introduction
Delirium is a neuropsychiatric syndrome characterized by an abrupt onset, fluctuating disturbances in consciousness and cognition, as well as a range of noncognitive domains including alterations in motor behavior, emotionality, and sleep-wake cycle, caused by an underlying etiology (American Psychiatric Association, 2000; Trzepacz et al., Reference Trzepacz1999).
Among the psychiatric syndromes, delirium is the most common across healthcare settings (Bucht et al., Reference Bucht, Gustafson and Sandberg1999; Inouye et al., Reference Inouye, Westendorp and Saczynski2014). Up to 70% of cardiosurgical patients develop this syndrome (Gottesman et al., Reference Gottesman2010; Norkiene et al., Reference Norkiene2007). Even more, in mechanically ventilated patients, the occurrence of delirium reaches 80% (Pun & Ely, Reference Pun and Ely2007). Delirium has been recognized to inflict adverse short-term (Rosen et al., Reference Rosen2002; Santos et al., Reference Santos, Velasco and Fraguas2004) and long-term consequences for patients and the healthcare system (Koster et al., Reference Koster, Hensens and van der Palen2009). These adversities include a prolonged stay on the intensive care unit (ICU) (Ely et al., Reference Ely2004; Ouimet et al., Reference Ouimet2007), higher rates and prolongation of mechanical ventilation (Heymann et al., Reference Heymann2010), increased morbidity and mortality (Balas et al., Reference Balas2009; Heymann et al., Reference Heymann2010), and, as a long term-consequence, functional impairment and cognitive disabilities (Bickel et al., Reference Bickel2008), requiring institutionalization (Ouimet et al., Reference Ouimet2007).
Several instruments have been developed to improve the screening for and detection of delirium. In the intensive care setting, one of the most commonly used screening instruments is the Intensive Care Delirium Screening Checklist (ICDSC) (Devlin et al., Reference Devlin2007).
The ICDSC was evaluated in several studies, the sensitivities and specificities ranged from 43–96% and 73–95%, respectively. In a previous study, the agreement between the ICDSC and the Diagnostic and Statistical Manual, 4th edition, text revision (DSM-IV-TR)-determined diagnosis of delirium was only moderate, while being specific and not very sensitive, however, exceeded the agreement of the confusion assessment method (CAM)-ICU (Boettger et al., Reference Boettger2017b). A meta-analysis indicated a sensitivity and specificity of 74% and 81.9%, respectively, and, overall, the accuracy was considered good (Gusmao-Flores et al., Reference Gusmao-Flores2012). Conversely, other studies indicated lower sensitivities (43–47%), whereas specificities remained very high (>94%) (Neufeld et al., Reference Neufeld2013; van Eijk et al., Reference van Eijk2009).
Although most studies focused on the ICDSC threshold for delirium, one study assessed the symptom profile of delirium with this scale (Marquis et al., Reference Marquis2007). In this sample, inattention, disorientation, and psychomotor agitation were the most frequent symptoms. Each item was highly discriminative between the delirious and nondelirious. In the nondelirious, psychomotor alterations, either agitation or retardation, and disorientation occurred at more than 20%. The individual symptom evaluation by nursing varied and was lowest for the altered level of consciousness and highest for disorientation and hallucinations.
In another study assessing delirium with the Delirium Rating Scale-Revised 1998 (DRS-R-98), sleep-wake cycle disturbances, lability of affect, thought abnormalities, inattention and disorientation, as well as short- and long-term memory impairment were the most common symptoms; in contrast, delusions were rarely recorded (Lahariya et al., Reference Lahariya2016; Sharma et al., Reference Sharma2012). In the only study assessing delirium dependent on the level of sedation using the DRS-R-98, drowsiness increased the odds for delirium eightfold and was subthreshold for delirium (Boettger et al., Reference Boettger2017a).
Thus, although the ICDSC has been commonly accepted as an appropriate screening tool for delirium in the intensive care setting, the symptom profile of its items between the delirious and nondelirious dependent on sedation still remains mostly elusive. Therefore, in the following, the symptom profile and utility of individual items in the detection of delirium were evaluated in the drowsy, as well as alert and calm.
Methods
Patients
All patients in this prospective, descriptive cohort study were recruited at the University Hospital Zurich, a level 1 trauma center, with nearly 900 beds and 39,000 admissions yearly. The cardiovascular-surgical patients in this study were recruited on a 12-bed ICU between May 2013 and April 2015. Inclusion criteria were being an adult, ability to consent, and intensive care management for more than 18 hours. Exclusion criteria were the inability to consent and a past or present substance use disorder.
Procedures
All patients in this study were informed of procedures and it was attempted to obtain written informed consent. In those patients unable to provide the consent at this initial attempt, either because of more severe delirium, their medical condition and sedation, or frailty, proxy assent from the next of kin or a responsible caregiver was obtained instead. After medical stabilization, consent was obtained or patients were excluded when participation and consent were refused.
The assessment of delirium was performed by four raters trained in the application of the DSM-IV-TR criteria and inter-rater reliability with the DSM-IV-TR diagnosis of delirium was achieved.
The aim of this study was to evaluate the symptom profile, utility and correctness of the individual ICDSC-items versus the DSM-IV-TR diagnosis of delirium with respect to the RASS levels −1 (drowsiness) and Richmond Agitation and Sedation Scale (RASS) 0 (alertness and calmness).
The baseline assessment included the interview of the patient, determination of the presence or absence of delirium according to the DSM-IV-TR criteria (American Psychiatric Association, 2000) and then administration of the ICDSC (Devlin et al., Reference Devlin2007) and RASS (Sessler et al., Reference Sessler2002), which are routinely performed by nurses and doctors specifically trained in their use. The assessment was completed by obtaining collateral information from nursing, medical-surgical staff, the electronic medical record system (Klinikinformationssystem, KISIM, CisTec AG, Zurich) and family or caregivers.
Measurements
DSM-IV-TR
The diagnosis of delirium was determined by DSM-IV-TR (American Psychiatric Association, 2000) including four criteria: –(1) disturbance of consciousness (i.e., reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention; (B) a change in cognition (such as memory deficit, disorientation, language disturbance) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia; (C) the disturbance develops over a short period (usually hours to days) and tends to fluctuate during the course of the day; and –(D) there is evidence from the history, physical examination, and laboratory findings that: (a) the disturbance is caused by the direct physiological consequences of a general medical condition, (b) the symptoms in criterion (a) developed during substance intoxication, or during or shortly after, a withdrawal syndrome, or (c) the delirium has more than one etiology.
RASS
The RASS is a medical scale developed to measure the level of sedation, alertness, and agitation (Sessler et al., Reference Sessler2002). This scale can be used in all hospitalized patients, but is mostly used in ventilated patients to avoid over- and undersedation. The RASS includes 10 points ranging from −5 to 4 and provides a detailed description for each score. The score of 0 represents the alert and cam patient, spontaneously paying attention to the caregiver. Negative scores describe the level of sedation, with −1 representing drowsiness, characterized by not being fully alert, sustained awakening as defined by more than 10 seconds, with eye contact to voice. Levels of −2 to −5 describe light, moderate, and deep sedation, as well as being unarousable. Positive scores describe the level of agitation, ranging from +1 to +4, representing restlessness, agitation, pronounced agitation, and combativeness.
ICDSC
The ICDSC (Devlin et al., Reference Devlin2007) is a screening instrument including eight items based on the DSM-IV TR criteria specifically designed for the intensive care setting with two points: absent or present. This scale was designed for patients with limited communication abilities (e.g., intubation). The items include the assessment of (1) consciousness (comatose, soporose, awake, or hypervigilant), –(2) orientation, (3) hallucinations or delusions, –(4) psychomotor activity, (5) inappropriate speech or mood, (6) attentiveness, (7) sleep-wake cycle disturbances, and –(8) fluctuation of symptomatology. The maximum score is eight; scores of more than three indicate the presence of delirium. Each item is rated on the patient's behavior over the previous 24 hours.
Statistical methods
All statistical procedures were conducted using the Statistical Package for Social Sciences, version 22. Descriptive statistics were implemented for the characterization of the sample such as sociodemographic, clinical variables, and delirium variables, in particular, the ICDSC items and total scores. In a first step, the excluded patients were compared with those that were included; in a second step, after dichotomizing the data set into those with RASS score of 1 and 0, those patients with and without delirium were compared. Variables on the interval scale, such as age or laboratory parameters, were tested for normal distribution with Shapiro-Wilk's test and the majority found to be nonparametric. Further, parameters on ordinal scales, such as the summation scores of the ICDSC, were present. In both instances, Mann-Whitney's U test was computed. These variables were described with their respective means, ranges, and standard deviations, as well as median and interquartile range. For items on categorical scales, such as the gender distribution or presence of ICDSC items, Pearson's chi-square test was performed.
The inter-rater reliability was determined by its corresponding Fleiss κ with agreement defined as >0.80 perfect (DeVellis, Reference DeVellis2012).
Following, a discriminate analysis to establish the ability of ICDSC items to correctly classify the presence versus the absence of delirium in those patients with RASS scores of −1 and 0 was computed with the function coefficient set on unstandardized. In addition, to further describe the ability of the ICDSC to distinguish between delirium and the absence of delirium, the sensitivities and specificities, as well as corresponding positive and negative predictive values (PPVs and NPVs) were calculated and their confidence intervals determined as exact Clopper-Pearson confidence intervals. For the sensitivities, specificities, PPVs, and NPVs, values of >70% were considered substantial, values of >40% as moderate, and values of ≤40% as modest.
For all implemented tests, the significance level alpha was set at 0.05.
Results
Inter-rater reliability with respect to DSM-IV-TR diagnosis
With respect to the DSM-IV-TR diagnosis of delirium, the overall rating agreement between the psychiatrists' assessment was almost perfect (Cohen's Κ 0.89; 95% confidence interval [CI 95%] = 0.69 1.1; p < 0.001) and with respect to the presence and absence of delirium perfect (Cohen's Κ 0.97; CI 95% = 0.69, 1.1; p < 0.001 and Cohen's Κ 0.93; CI 95% = 0.69, 1.1; p < 0.001).
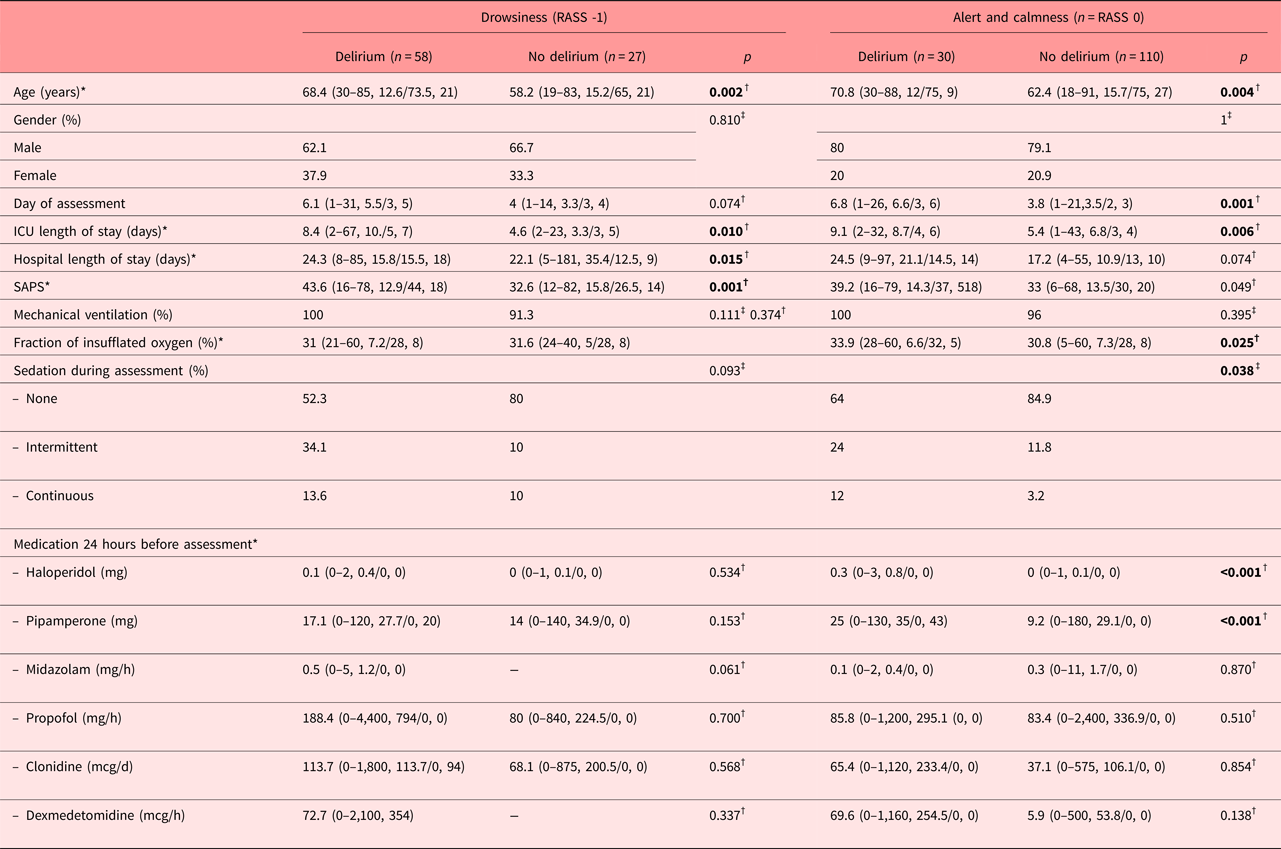
Characteristics of patients with delirium at RASS-1 (drowsiness) and RASS 0 (alert and calmness) versus patients without delirium
In the presence of drowsiness, patients with delirium were older, had a prolonged stay in both the ICU and hospital, were sicker as documented by the corresponding Simplified Acute Physiology Scores. Among laboratory parameters, they had lower indices of hemoglobin and thrombocytes (Table 1). In the presence of alertness and calmness, patients with delirium were also older, assessed at a later day, had a prolonged stay in both the ICU and hospital, were sicker as indicated by the Simplified Acute Physiology Scores, had higher fractions of insufflated oxygen, more frequently received sedation during assessment, and received higher doses of haloperidol and pipamperone in the 24 hours before assessment.
Table 1. Sociodemographic, medical, and management characteristics of delirium dependent on the RASS score -1 and 0

DSM-IV-TR, Diagnostic and Statistical Manual, 4th edition, text revision; ICU, intensive care unit; RASS, Richmond Agitation Sedation Scale.
*Mean, range/median, interquartile range; †Mann-Whitney's U test; ‡Pearson's chi-square test. Bold values significants at p < 0.05.
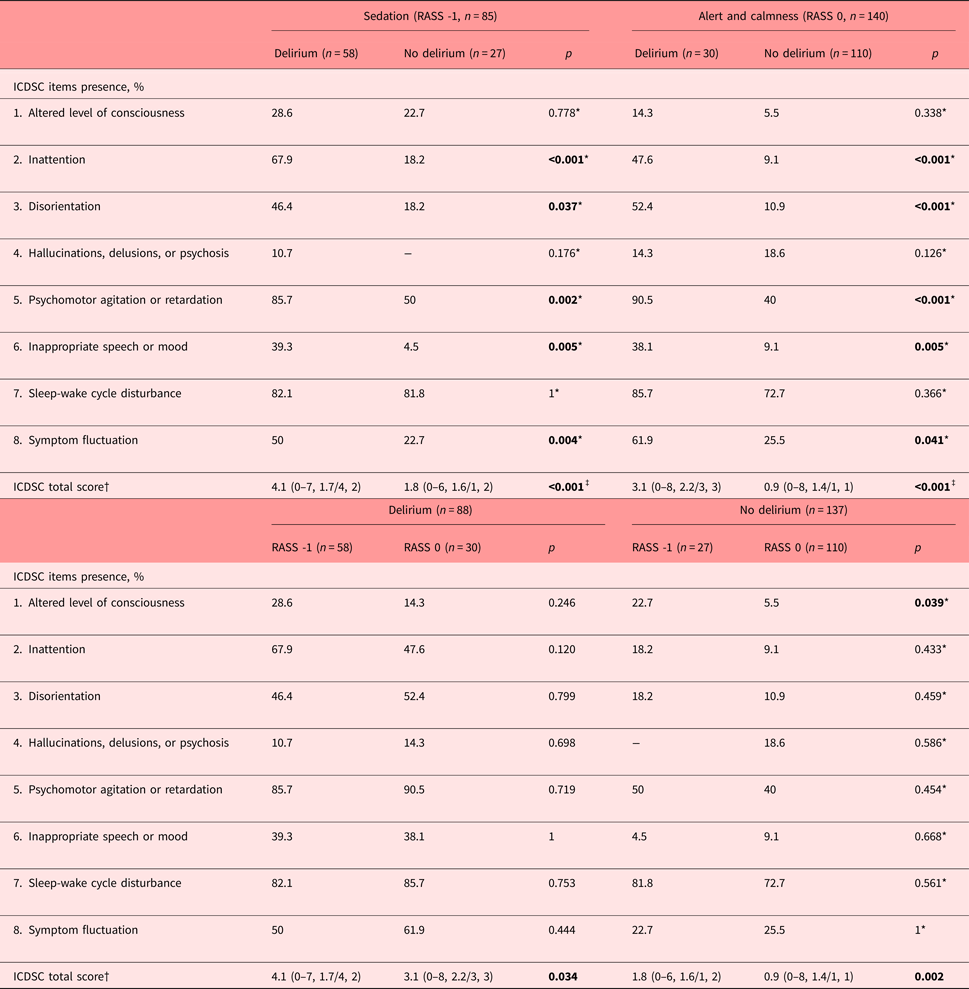
Characteristics of delirium as measured with the ICDSC items
To determine which items of the ICDSC identified delirium in the presence of drowsiness, following characteristics became apparent (Table 2). In the drowsy patient, inattention (ICDSC item 2) was the key feature for the correct identification of delirium. Further disorientation (ICDSC item 3), psychomotor agitation or retardation (ICDSC item 5), inappropriate speech or mood (ICDSC item 6), and fluctuations in symptomatology (ICDSC item 8) were characteristic of delirium. An altered level of consciousness, hallucinations, delusions or psychosis, and the sleep-wake cycle disturbance did not contribute to the correct identification of delirium.
Table 2. Characteristics of delirium as described by the ICDSC dependent on RASS scores of -1 and 0
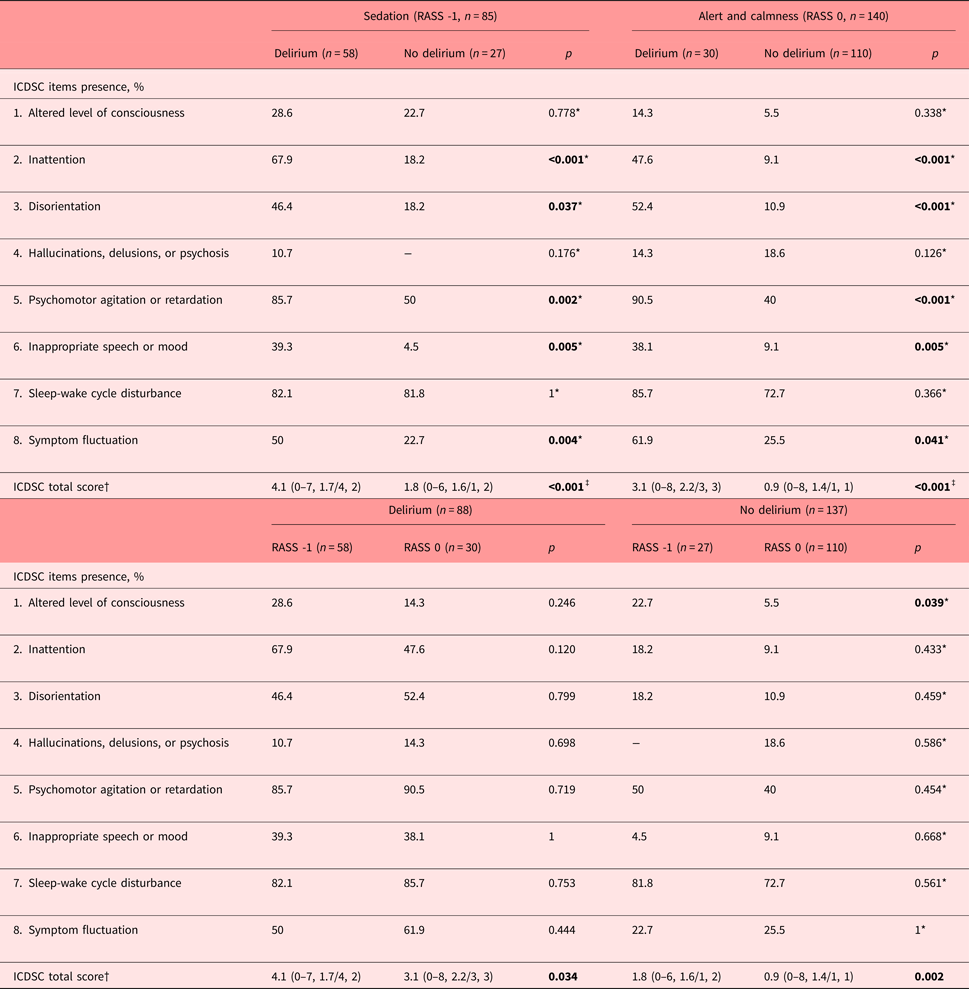
ICDSC, Intensive Care Delirium Screening Checklist; RASS, Richmond Agitation Sedation Scale.
*Pearson's chi-square test; †mean, range/median, interquartile range; ‡Mann-Whitney's U test. Bold values significants at p < 0.05.
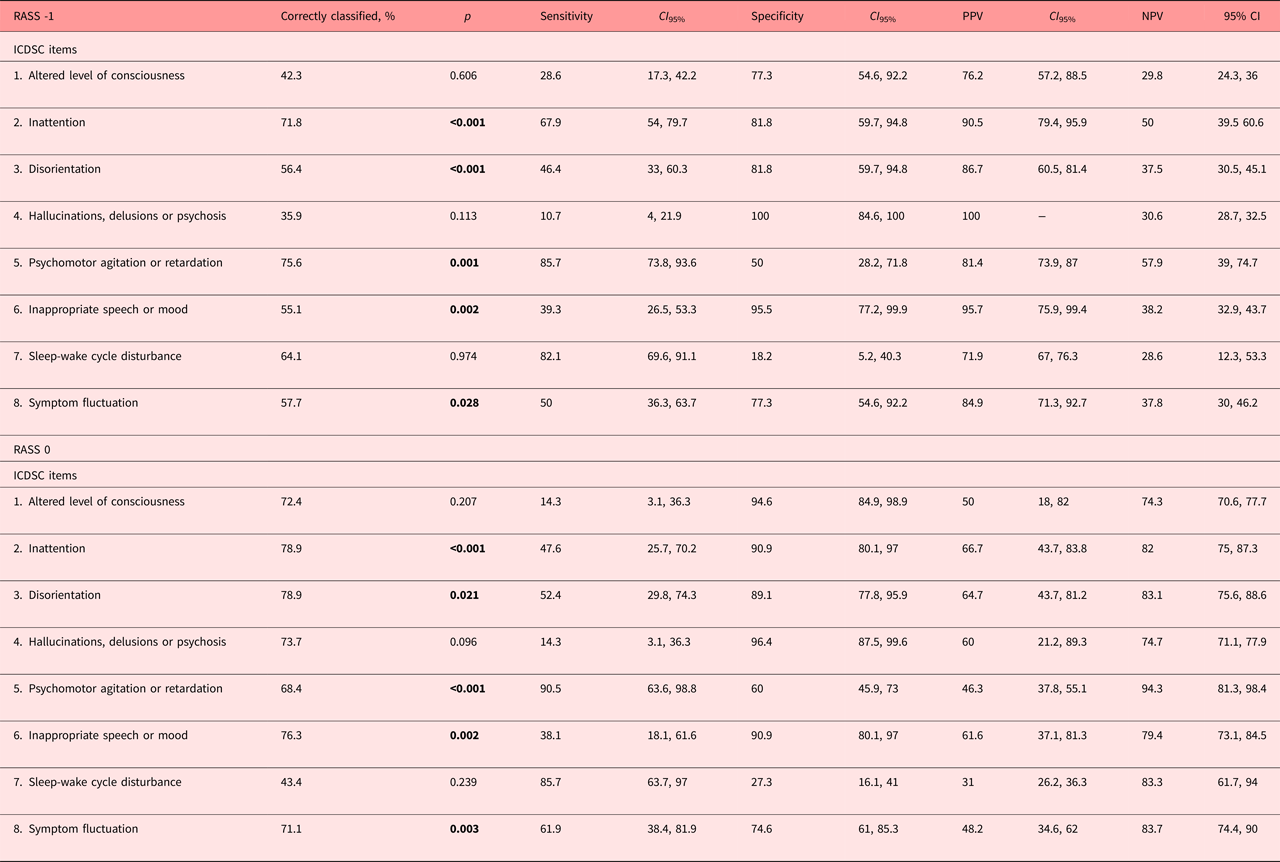
As determined by the discriminant analyses (Table 3), these same items allowed the correct classification of delirium, whereas the altered level of consciousness, hallucinations, delusions or psychosis, and sleep-wake cycle disturbance did not. The sensitivities of the individual ICDSC-items to correctly detect delirium were substantial for psychomotor agitation or retardation, sleep-wake cycle disturbance and inattention, in contrast to hallucinations, delusions, or psychosis, altered level of consciousness or symptom fluctuations were not. With exception for the sleep-wake cycle disturbances and psychomotor agitation or retardation, all items were very specific for delirium. The positive prediction was substantial for all ICDSC items, whereas the negative prediction was only modest.
Table 3. Correct classifications, sensitivities and specificities, and PPVs and NPVs at RASS -1 and 0
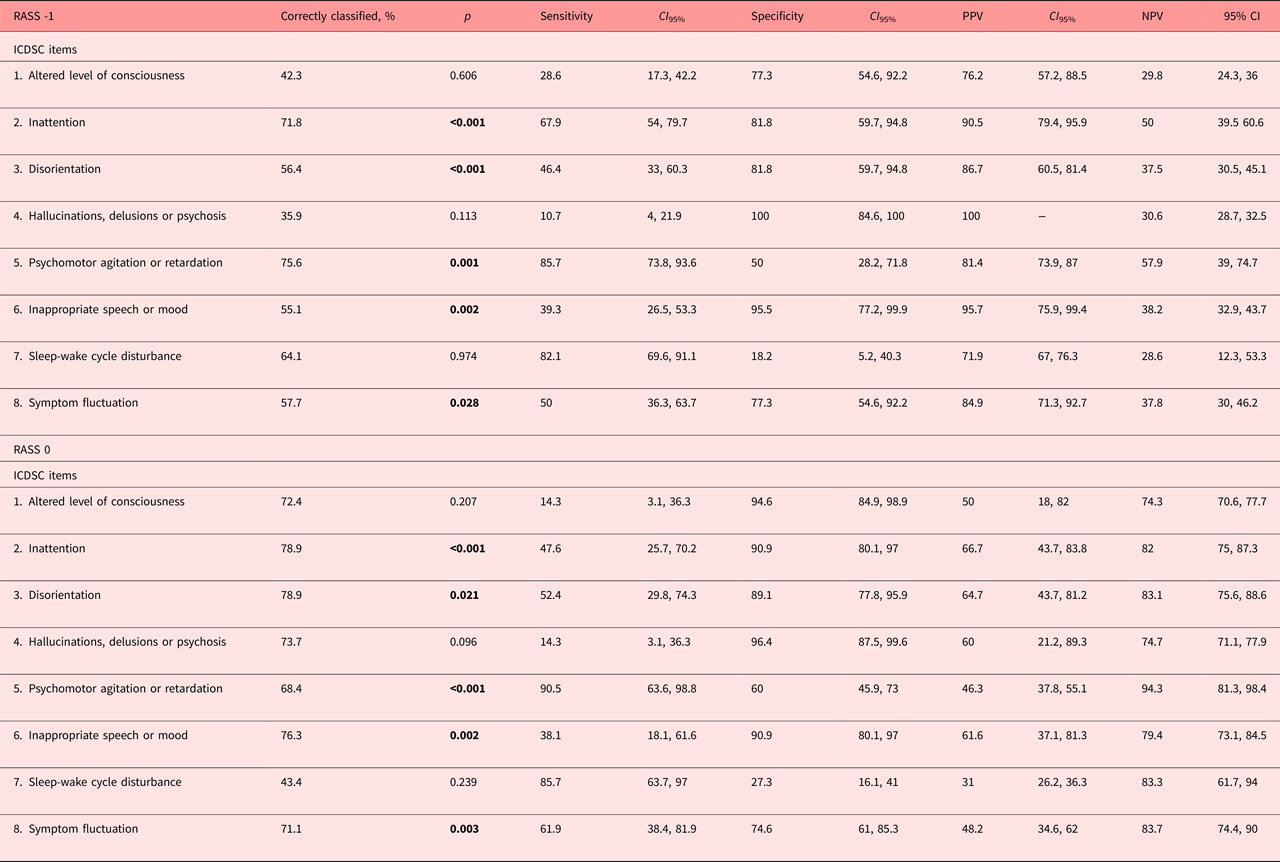
CI 95%, 95% confidence interval; ICDSC, Intensive Care Delirium Screening Checklist; NPV, negative predictive value; PPV, positive predictive value; RASS, Richmond Agitation and Sedation Scale. Bold values significants at p < 0.05.
Similarly, in the alert and calm patient, the same ICDSC items reflecting inattention, disorientation, psychomotor agitation or retardation, inappropriate speech or mood, and symptom fluctuation were characteristic for delirium (Table 2). Conversely, compared with delirium in the drowsy patient, the altered level of consciousness, hallucinations, delusions or psychosis, or sleep-wake cycle disturbance were not.
The discriminant analyses indicated that the cognitive items of the ICDSC, inattention and disorientation, as well as the psychomotor abnormalities, inappropriate speech or mood, and symptom fluctuation correctly classified delirium in the alert and calm patient (Table 3). The altered level of consciousness, hallucinations, delusions or psychosis, and sleep-wake cycle disturbances did not correctly classify delirium. The sensitivities of individual ICDSC items in the detection of delirium were substantial for psychomotor abnormalities and the sleep-wake cycle disturbances, modest for fluctuations in symptomatology and only modest with respect to the altered level of consciousness, positive symptomatology or speech and or mood inappropriateness. The ICDSC items were generally very specific for delirium. The specificities were substantial, only psychomotor alterations (ICDSC 5) reached moderate and the sleep-wake cycle disturbance modest specificities. The positive prediction was moderate throughout items, only the sleep-wake cycle disturbance reached modest positive prediction, and the negative prediction substantial across all ICDSC items.
Differences in the delirious and nondelirious patient between drowsiness and alert/calmness
To assess differences between delirious and nondelirious in the drowsy versus the alert and calm patient, the prevalence rates of the individual ICDSC items were determined (Table 2). In the delirious, between the drowsy versus alert and calm, the symptomatology of individual ICDSC items was the same. In the nondelirious, between the drowsy versus alert and calm, drowsiness was associated with more disturbances in the level of consciousness. In both the delirious and nondelirious, the summation scores reached higher values.
Discussion
Summary of main findings
From these findings, patients with delirium, whether drowsy or alert, were older, assessed at a later day, stayed longer on the ICU or hospital, had more severe disease, had an increased requirement of the fraction of insufflated oxygen, either were more commonly receiving sedation in the day before assessment, or were administered higher doses of haloperidol and pipamperone.
In the daily clinical routine, in particular, the ICDSC items for inattention, disorientation, psychomotor alterations, including agitation and retardation, as well fluctuations of symptomatology were most useful in the correct detection of delirium in the drowsy or alert and calm. In addition, as determined by the discriminant analysis, these items reliably allowed the correct classification of delirium. Items that were very sensitive toward the detection of delirium were inattention, psychomotor alterations, and sleep-wake cycle disturbances. The altered level of consciousness, hallucinations, delusions or psychosis, as well as inappropriate speech or mood proved to be less sensitive in the detection of this syndrome. Most ICDSC items very specific for delirium, only psychomotor alterations, and even more sleep-wake cycle disturbances lacked this specificity. With respect to the positive and negative prediction, an inverse pattern appeared. Although the drowsy reached substantial positive prediction and only modest negative prediction, this pattern reversed in the alert and calm.
Delirium in the drowsy versus alert and calm was the same as determined by the presence of ICDSC symptomatology; however, drowsiness caused altered levels of consciousness.
Comparison with the existing literature
There is robust evidence that patients with delirium are older, suffer from more severe sickness, require longer hospitalization on either the ICU or regular floors, which was confirmed in this study (Meagher, Reference Meagher, Trzepacz, Gelder, Andreasen and Lopez-Ibor2009).
However, there is a paucity of studies assessing delirium dependent on the level of sedation or aiming to identify the ICDSC items with particular usefulness in the correct detection of delirium in the intensive care setting.
In one study, inattention, disorientation, and psychomotor agitation were the most common symptoms of delirium (Marquis et al., Reference Marquis2007). Each item was considered highly discriminative between the delirious and nondelirious. However, this sample had high rates of psychomotor agitation indicating either the hyperactive or mixed subtype of delirium. In contrast, the sample in this study had predominantly hypoactive delirium and only inattention, disorientation, psychomotor alterations, inappropriate speech or mood, and symptom fluctuation correctly discriminated the delirious from those nondelirious at drowsiness and alert/calmness. Although, given that these items correctly discriminated in the drowsy and alert and calm patient, it was likely that these items would have also discriminated in the whole sample. In those not delirious, psychomotor alterations, either agitation or retardation, and disorientation occurred at more than 20%. Similarly, psychomotor alterations were documented at even 40 and 50%.
In the intensive care setting, delirium assessed with the DRS-R-98 features sleep-wake cycle disturbances, lability of affect, thought disorder, inattention and disorientation, as well as short- and long-term memory impairment are more common. In contrast, delusions occurred only at a marginal rate (Lahariya et al., Reference Lahariya2016; Sharma et al., Reference Sharma2012). The only study assessing delirium dependent on sedation as measured with the RASS showed that drowsiness increased the risk and was a subthreshold state for delirium (Boettger et al., Reference Boettger2017a). Although in this study, the ICDSC was used, there is an overlap in symptomatology described between both scales. Inattention and disorientation were key items allowing the correct identification and classification of delirium; however, the lability of affect reflecting ICDSC inappropriate speech or mood, were neither characteristic in drowsiness nor alert/calmness. Nonetheless, delusions as reflected by ICDSCI tem 4, hallucinations, delusions or psychosis, were also rarely noted.
There has been some debate about the accuracy and validity of the ICDSC as a scale. From a review (Gusmao-Flores et al., Reference Gusmao-Flores2012), the sensitivities as well as specificities ranged from 43–96% and 73–95%, respectively. The subsequent meta-analysis indicated a sensitivity and specificity of 74 and 81.9%, respectively, and, overall, the accuracy was considered good (Gusmao-Flores et al., Reference Gusmao-Flores2012). Conversely, further studies indicated lower sensitivities (43–47%) while maintaining high specificities (>94%) (Neufeld et al., Reference Neufeld2013; van Eijk et al., Reference van Eijk2009). Although the disturbance of consciousness is a key criterion for the DSM-IV-TR criteria of delirium, these were recorded in only less than one-third of the patients. However, the approach the ICDSC uses is different. The ICDSC requires one to three items for the classification of subsyndromal delirium and ≥4 items for delirium. A RASS score of -1 only translates into an altered level of consciousness when no recent sedatives were administered. In contrast, this decision tree applied to the nondelirious, caused an increase in the altered level of consciousness in the RASS-1 group. Conversely, the evaluation by nursing was lowest for the altered level of consciousness and highest for disorientation and hallucinations; missing altered levels of consciousness could not be excluded (Marquis et al., Reference Marquis2007).
Strengths and limitations
This study has several strengths; however, a number of limitations have to be noted. Almost 300 patients were prospectively screened and rated for delirium using the ICDSC versus the DSM-IV-TR criteria; 289 patients were finally included. With respect to diagnosis of delirium with the DSM-IV-TR criteria, the inter-rater agreement was perfect. Limitations included the lack of inter-rater reliability in the performance of the ICDSC, not allowing the exclusion of its proper administration. Further, there was a high prevalence of hypoactive delirium in this sample biasing the results toward this subtype, which was indebted to the critical care population studied. In addition, prevalence rates for hallucinations, delusions, or psychosis were low, causing a potential bias toward their limited utility in the identification of delirium in this study. Finally, the absence of baseline cognitive recording owed to the study design potentially biased in particular the cognitive items.
Conclusion
In the daily clinical routine, the ICDSC items for inattention, disorientation, psychomotor alterations, including agitation and retardation, as well fluctuations of symptomatology were very useful in the detection of delirium in the drowsy and alert and calm patient. The same items reliably allowed the correct classification of delirium and, in addition to the sleep-wake cycle disturbances, were very sensitive toward delirium. Most ICDSC items were very specific for delirium, only psychomotor alterations and sleep-wake cycle disturbances lacked this specificity. While in the drowsy, the items reached substantial positive prediction and only modest negative prediction, this pattern reversed in the alert and calm.
Drowsiness was associated with the ICDSC item for altered levels of consciousness.
Conflict of interest
None.