INTRODUCTION
Dientamoeba fragilis is a trichomonad parasite of the human gastrointestinal tract that is associated with gastrointestinal disease (Stark et al. Reference Stark, Barratt, Van Hal, Marriott, Harkness and Ellis2009b, Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010b). Despite its discovery over a century ago, the life cycle of Dientamoeba is not understood (Fig. 1). The only known stage in the life cycle of Dientamoeba is the trophozoite, which is extremely fragile once passed from the host. No environmentally resistant cyst stage has been identified. While it is possible that Dientamoeba trophozoites are transmitted directly from host to host, the fragile nature of the trophozoite stage has led some researchers to suggest that this mode of transmission is unlikely (Yang and Scholten, Reference Yang and Scholten1977). Consequently, several theories have emerged which attempt to explain how Dientamoeba trophozoites could survive outside their host for a sufficient period to allow their transmission. One possibility is that Dientamoeba is transmitted via the ova of a helminth. Another possibility is that a resistant cyst stage exists for Dientamoeba though remains undiscovered. Unfortunately, none of these theories has been sufficiently proven.
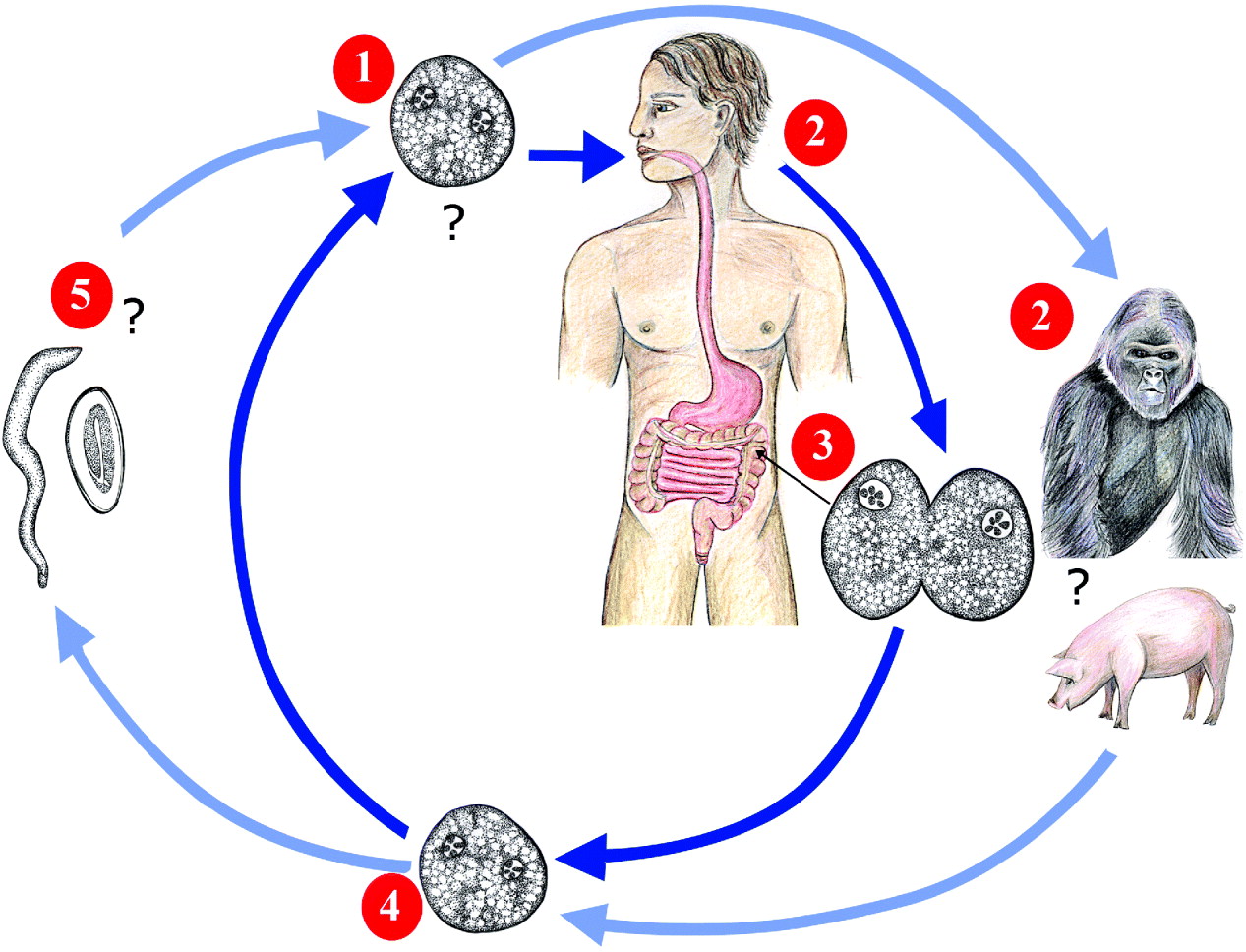
Fig. 1. The proposed life cycle of Dientamoeba fragilis. Dientamoeba trophozoites (or an undiscovered transmissible stage) are ingested from the external environment by a host species (1). Humans are thought to be the preferred host of Dientamoeba, though gorillas, pigs, sheep and other primate species may also be potential hosts (2). Once ingested, Dientamoeba travels to the large intestine where it multiplies by binary fission (3). Dientamoeba organisms are then passed into the environment in the faeces (4) where they contaminate food and/or water sources. Dientamoeba is then ingested by a new host, completing the cycle. Some authors propose that; due to the fragile nature of Dientamoeba trophozoites outside their host and the apparent lack of a cyst stage, it is unlikely that Dientamoeba can infect humans directly. It has been suggested that Dientamoeba may be transmitted in the ova of the helminth; Enterobius vermicularis (5) though the role of Enterobius in the life cycle of Dientamoeba is controversial.
In the initial description of Dientamoeba, Jepps and Dobell (Reference Jepps and Dobell1918) commented on the fragile nature of the trophozoite stage though were unable to identify a cyst stage in the stools of infected human subjects. Subsequently, these authors theorized that Dientamoeba may produce cysts in an unidentified species of animal (Jepps and Dobell, Reference Jepps and Dobell1918). While no cyst stage has been identified in humans or animals, several species of animal are reported to carry Dientamoeba (Knowles and DasGupta, Reference Knowles and DasGupta1936; Dobell, Reference Dobell1940; Noble and Noble, Reference Noble and Noble1952; Myers and Kuntz, Reference Myers and Kuntz1968; Crotti et al. Reference Crotti, Sensi, Crotti, Grelloni and Manuali2007; Stark et al. Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008; Lankester et al. Reference Lankester, Kiyang, Bailey and Unwin2010).
Following the initial description of Dientamoeba (Jepps and Dobell, Reference Jepps and Dobell1918) several authors described what appeared to be cysts, pseudocysts or cyst-like stages of Dientamoeba (Kofoid, Reference Kofoid1923; Greenway, Reference Greenway1928; Wenrich, Reference Wenrich1936; Knoll and Howell, Reference Knoll and Howell1945; Piekarski, Reference Piekarski1948; Silard et al. Reference Silard, Colea, Panaitescu, Florescu and Roman1979). However, these apparent cyst-like forms were found to be degenerate trophozoites or their true identity could not be confirmed (Johnson et al. Reference Johnson, Windsor and Clark2004). Despite the relatively high incidence of D. fragilis infection reported in recent studies (Millet et al. Reference Millet, Spencer, Chapin, Garcia, Yatabe and Stewart1983b; Girginkardesler et al. Reference Girginkardesler, Coskun, Cuneyt Balcioglu, Ertan and Ok2003; Bruijnesteijn van Coppenraet et al. Reference Bruijnesteijn Van Coppenraet, Wallinga, Ruijs, Bruins and Verweij2009; Schuster and Jackson, Reference Schuster and Jackson2009), a cyst stage has not been reported. It is now generally accepted that D. fragilis does not have a cyst stage (Johnson et al. Reference Johnson, Windsor and Clark2004).
Dobell (Reference Dobell1940) was the first to postulate that Dientamoeba may be transmitted in the ova of a helminth. This theory was based on Dientamoeba's similarity to Histomonas meleagridis which is transmitted in the ova of the poultry helminth Heterakis gallinarum. While several authors provide support for this theory (Burrows and Swerdlow, Reference Burrows and Swerdlow1956; Ockert, Reference Ockert1972a, Reference Ockertb, Reference Ockert1975; Ockert and Schmidt, Reference Ockert and Schmidt1976; Yang and Scholten, Reference Yang and Scholten1977; Girginkardesler et al. Reference Girginkardesler, Kurt, Kilimcioglu and Ok2008), other researchers report no association between helminths and Dientamoeba (Vandenberg et al. Reference Vandenberg, Peek, Souayah, Dediste, Buset, Scheen, Retore, Zissis and Van Gool2006; Stark et al. Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010b). As such, the role of helminths in the transmission of Dientamoeba remains a matter of debate.
According to phylogenetic studies Dientamoeba, H. meleagridis and Parahistomonas wenrichi share a recent common ancestor with members of the genus Tritrichomonas (Gerbod et al. Reference Gerbod, Edgcomb, Noel, Zenner, Wintjens, Delgado-Viscogliosi, Holder, Sogin and Viscogliosi2001, Reference Gerbod, Noel, Dolan, Edgcomb, Kitade, Noda, Dufernez, Ohkuma, Kudo, Capron, Sogin and Viscogliosi2002; Ohkuma et al. Reference Ohkuma, Iida, Ohtoko, Yuzawa, Noda, Viscogliosi and Kudo2005) (Fig. 2). The life cycles of Histomonas, Parahistomonas and Tritrichomonas spp. are generally well characterized and it is postulated that the lives of these species’ could provide some clues as to how Dientamoeba is transmitted. However, the lives of Histomonas, Parahistomonas and Tritrichomonas are quite different. Histomonas and Parahistomonas are gastrointestinal parasites of poultry (Levine, Reference Levine1985; McDougald, Reference McDougald2005) while members of the genus Tritrichomonas include a sexually transmitted pathogen of cattle (Felleisen et al. Reference Felleisen, Lambelet, Bachmann, Nicolet, Muller and Gottstein1998), the aetiological agent of a feline diarrhoeal disease (Levy et al. Reference Levy, Gookin, Poore, Birkenheuer, Dykstra and Litaker2003; Corbeil et al. Reference Corbeil, Campero, Van Hoosear and Bondurant2008), and parasites of the porcine (Tachezy et al. Reference Tachezy, Tachezy, Hampl, Sedinova, Vanacova, Vrlik, Van Ranst, Flegr and Kuldaa2002), simian (Culberson et al. Reference Culberson, Pindak, Gardner and Honigberg1986), reptilian and amphibian (Borges et al. Reference Borges, Wiltuschnig, Tasca and De Carli2004) gut. Despite the apparent differences between the life cycles of these organisms, similarities do exist which may provide some insights into the life cycle of Dientamoeba.

Fig. 2. Phylogenetic tree showing the relative phylogenetic positions of Dientamoeba fragilis genotype 1 (GenBank Accession: AY730405.1), Dientamoeba fragilis genotype 2 (U37461.1), Histomonas meleagridis (AJ920323.1), Parahistomonas wenrichi (EU647889.1) Tritrichomonas foetus (M81842.1), Monocercomonas colubrorum (AY319278.1), Trichomonas vaginalis (AY338475.1) and Trichomonas tenax (U37711.1) based on Small Subunit Ribosomal DNA (SSU rDNA) sequences. Support values for branches are shown as a percentage. The length of the distance scale bar is equivalent to a sequence difference of 5%. This tree was constructed using the software available on the website; www.phylogeny.fr/
This manuscript critically reviews the theories relating to Dientamoeba's transmission by discussing the strengths and weaknesses of each. Where gaps in current knowledge exist, suggestions are made on how future research could improve our understanding on the life cycle of Dientamoeba. Also, the life cycles of Histomonas, Parahistomonas and T. foetus are explored to identify similarities which may aid in the further characterization of Dientamoeba's life cycle.
DIENTAMOEBA's MODE OF TRANSMISSION IS UNKNOWN
Based on the absence of a cyst stage and the fragility of Dientamoeba trophozoites once passed from their host, some researchers suggest that direct faecal oral transmission of Dientamoeba is unlikely (Yang and Scholten, Reference Yang and Scholten1977). Dientamoeba's mode of transmission presents a problem for parasitologists. Despite years of research all efforts to elucidate the details of Dientamoeba's life cycle have been mostly unsuccessful.
Attempts to infect humans with cultured D. fragilis trophozoites via the oral route failed (Dobell, Reference Dobell1940), suggesting that they do not survive the acidic conditions of the stomach. Furthermore, Dientamoeba trophozoites are reported to survive from 6 to 48 h after being passed from the host, which is too short a period to make transmission efficient (Kean and Malloch, Reference Kean and Malloch1966; Stark et al. Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010b). To complicate matters further, Dientamoeba trophozoites are said to burst when placed in boiled pond water (Wenrich, Reference Wenrich1944) or tap water (Butler, Reference Butler1996). This suggests that water sources contaminated with human faeces are unlikely to be a source of Dientamoeba infection. Furthermore, Dientamoeba trophozoites do not grow at ambient room temperature (Brug, Reference Brug1938; Barratt et al. Reference Barratt, Banik, Harkness, Marriott, Ellis and Stark2010), indicating that Dientamoeba is not a free-living organism which infects humans opportunistically.
THE ROLE OF ANIMALS IN DIENTAMOEBA's LIFE CYCLE
Animal hosts play an important role in the transmission of many enteric protozoa that infect humans (Schlundt et al. Reference Schlundt, Toyofuku, Jansen and Herbst2004; Smith et al. Reference Smith, Caccio, Cook, Nichols and Tait2007; Pozio, Reference Pozio2008). Animal reservoirs are also a potential source of many human parasitic infections (Yoshikawa et al. Reference Yoshikawa, Wu, Nagano and Takahashi2003; Inpankaew et al. Reference Inpankaew, Traub, Thompson and Sukthana2007; Robertson, Reference Robertson2009; Traub et al. Reference Traub, Inpankaew, Reid, Sutthikornchai, Sukthana, Robertson and Thompson2009). As such, it is possible that animals are involved in the transmission of Dientamoeba. As few studies have explored this possibility, the role of animals remains uncertain. In most cases the finding of Dientamoeba in animals was incidental.
Knowles and Das Gupta (Reference Knowles and DasGupta1936) detected Dientamoeba in the stools of captive macaques (1/30) using an iron haematoxylin staining technique. According to these authors, the organism was encountered in ‘scanty numbers’ and was of ‘typical appearance’ (Knowles and DasGupta, Reference Knowles and DasGupta1936). Hegner and Chu (Reference Hegner and Chu1930) reported Dientamoeba infections in 2/44 wild monkeys from the Philippines. Myers and Kuntz (Reference Myers and Kuntz1968) detected D. fragilis in <1% of captive baboons and <2% of those trapped in the wild. Microscopic examination of stool samples was the method employed though the specific staining technique was not described (Myers and Kuntz, Reference Myers and Kuntz1968). Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008) identified Dientamoeba in the stools of 3 western lowland gorillas using an iron haemotoxylin staining technique and confirmed these results by PCR. More recently, Lankester et al. (Reference Lankester, Kiyang, Bailey and Unwin2010) described a case of irritable bowel-like disease in a western lowland gorilla. The illness described by Lankester (Reference Lankester, Kiyang, Bailey and Unwin2010) was later attributed to Dientamoeba by identification of trophozoites in faecal smears stained with a Field's stain.
Noble and Noble (Reference Noble and Noble1952) observed D. fragilis trophozoites in stained smears (haematoxylin and/or Giemsa stains) made from the stools of sheep though make no mention of the prevalence. In contrast, Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008) reported Dientamoeba infections in 0/50 sheep using an iron haematoxylin technique. Crotti et al. (Reference Crotti, Sensi, Crotti, Grelloni and Manuali2007) detected Dientamoeba trophozoites in the stools of 53/121 farmed pigs using a Giemsa staining technique. In contrast, Noble and Noble (Reference Noble and Noble1952) examined stools from 30 pigs and made no mention of D. fragilis in these specimens. Similarly, Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008) found no evidence of D. fragilis in the stools of 135 swine. Interestingly, one study described contact with rabbits as a risk factor for Dientamoeba infection (Stensvold et al. Reference Stensvold, Lewis, Hammerum, Porsbo, Nielsen, Olsen, Arendrup, Nielsen and Molbak2009). However, Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008) examined the stools of 20 rabbits and did not detect D. fragilis.
Attempts to induce experimental infections in a range of animals have been unsuccessful (Mollari and Anzulovic, Reference Mollari and Anzulovic1938; Dobell, Reference Dobell1940; Wenrich, Reference Wenrich1944; Knoll and Howell, Reference Knoll and Howell1945; Kean and Malloch, Reference Kean and Malloch1966). Mollari and Anzulovic (Reference Mollari and Anzulovic1938) failed in their attempt to infect kittens with Dientamoeba. Dobell (Reference Dobell1940) tried to infect 6 chicks by rectal inoculation of cultured Dientamoeba trophozoites. A transient infection was achieved in 1 chick though the infection was spontaneously cleared after 1 week. At the end of this experiment, the chick was sacrificed and examination of the caeca and liver revealed no pathological changes (Dobell, Reference Dobell1940). This author also tried to infect himself and 2 macaques orally with cultured Dientamoeba trophozoites though without success. Efforts made to infect 1 of these macaques with cultured Dientamoeba trophozoites via rectal injection also failed (Dobell, Reference Dobell1940).
Wenrich (Reference Wenrich1944) tried to infect laboratory rats with cultured Dientamoeba trophozoites orally and via rectal injection, also without success. Knoll and Howell (Reference Knoll and Howell1945) were unable to infect kittens with cultured Dientamoeba trophozoites via rectal injection and the oral route. According to Knoll and Howell (Reference Knoll and Howell1945), no Dientamoeba trophozoites were recovered at autopsy and no gross pathological changes in the gastrointestinal tract were noted. Knoll and Howell (Reference Knoll and Howell1945) also examined the entrails of 12 laboratory rats obtained from an unrelated study and found no trace of Dientamoeba infection. Attempts were also made by Kean and Malloch (Reference Kean and Malloch1966) to infect laboratory rats. Apparently, preliminary observations showed that Dientamoeba does ‘attach to the caecal mucosa and cause damage to the underlying cells’ and, ‘oedema of the mucosa [was] evident, but actual ulceration [had] not yet been produced’ (Kean and Malloch, Reference Kean and Malloch1966). However, no later reference was made pertaining to these experiments (Kean and Malloch, Reference Kean and Malloch1966). Studies that report the finding of Dientamoeba in animals are summarized in Table 1.
Table 1. Studies that report the finding of Dientamoeba in animals

* The diagnostic technique employed is important to note due to differences in sensitivity and specificity. Usually, molecular techniques such as PCR are more sensitive and specific than light microscopy (Stark et al. Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010a).
THE ROLE OF HELMINTHS IN THE TRANSMISSION OF DIENTAMOEBA
It was originally postulated by Dobell (Reference Dobell1940) that D. fragilis could be transmitted via the ova of a nematode such as Trichuris trichuria or Ascaris lubricoides. This was based on Dientamoeba's similarity to Histomonas and a noted association between Dientamoeba and helminth infections (Dobell, Reference Dobell1940). Burrows and Swerdlow (Reference Burrows and Swerdlow1956) were the first to propose that Enterobius vermicularis (pinworm) was the probable vector of Dientamoeba and described what appeared to be Dientamoeba trophozoites in the ova of E. vermicularis. Several years later, Ockert and coworkers published a series of reports which supported the opinion that D. fragilis was transmitted in the ova of E. vermicularis (Ockert, Reference Ockert1972a, Reference Ockertb, Reference Ockert1975; Ockert and Schmidt, Reference Ockert and Schmidt1976). Ockert claimed that he had infected himself and 2 other subjects with Dientamoeba using pinworm eggs derived from a boy who was infected with both pinworm and Dientamoeba (Ockert, Reference Ockert1972b; Ockert, Reference Ockert1975). Isoelectric studies performed by Ockert and Schmidt (Reference Ockert and Schmidt1976) showed that the nuclei and cytoplasm of amoeboid bodies which occurred in Enterobius ova and trophozoites of Dientamoeba from culture had almost identical isoelectric points (Ockert and Schmidt, Reference Ockert and Schmidt1976; Johnson et al. Reference Johnson, Windsor and Clark2004). In a later study, Yang and Scholten (Reference Yang and Scholten1977) noted a strong association between D. fragilis infections and infections with E. vermicularis in a large survey examining 43 000 individuals. Girginkardesler et al. (Reference Girginkardesler, Kurt, Kilimcioglu and Ok2008) recently reported a relationship between the incidence of Dientamoeba and Enterobius infections. Interestingly, Sukanahaketu (Reference Sukanahaketu1977) also identified Dientamoeba-like structures within the ova of A. lumbricoides isolated from the stools of subjects with mixed infections of Dientamoeba and Ascaris.
In contrast, several studies found no relationship between Dientamoeba and E. vermicularis (Kean and Malloch, Reference Kean and Malloch1966; Walker et al. Reference Walker, Bahr and Ehl1985; Oxner et al. Reference Oxner, Paltridge, Chapman, Cook and Sheppard1987; Cuffari et al. Reference Cuffari, Oligny and Seidman1998; Menghi et al. Reference Menghi, Makiya and Gatta2005; Stark et al. Reference Stark, Beebe, Marriott, Ellis and Harkness2006, Reference Stark, Barratt, Ellis, Harkness and Marriott2009a, Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010b). In the study by Kean and Malloch (Reference Kean and Malloch1966), only 2/100 patients with pure Dientamoeba infections had a history of pinworm infection. A study performed in the Sydney suburb of French's Forest found that only 2/125 subjects had E. vermicularis ova in their stools while 21/125 were infected with Dientamoeba. The authors noted, however, that the prevalence of E. vermicularis in this group could have been under-represented as only stools were examined and E. vermicularis ova are rarely observed in stools (Walker et al. Reference Walker, Bahr and Ehl1985). In a study performed at Christchurch Hospital Microbiology department (New Zealand) (Oxner et al. Reference Oxner, Paltridge, Chapman, Cook and Sheppard1987) the incidence of D. fragilis was 41/1350 (3%). At the same time, the incidence of helminth infections was only 1/1350 (Oxner et al. Reference Oxner, Paltridge, Chapman, Cook and Sheppard1987). In another study, DNA extracted from E. vermicularis ova derived from people infected with D. fragilis failed to produce a PCR product using Dientamoeba specific primers (Menghi et al. Reference Menghi, Makiya and Gatta2005). Stark et al. (Reference Stark, Barratt, Ellis, Harkness and Marriott2009a) found no current pinworm infection in D. fragilis-infected patients in 2 unrelated families from Sydney, Australia. Stark et al. (Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010b) also found no co-infections with Dientamoeba and any helminth in a group of 19 patients infected with Dientamoeba.
THE LIFE CYCLES OF HISTOMONAS, PARAHISTOMONAS AND TRITRICHOMONAS
According to phylogenetic studies, the closest relatives of Dientamoeba include Histomonas, Parahistomonas and members of the genus Tritrichomonas (Gerbod et al. Reference Gerbod, Edgcomb, Noel, Zenner, Wintjens, Delgado-Viscogliosi, Holder, Sogin and Viscogliosi2001, Reference Gerbod, Noel, Dolan, Edgcomb, Kitade, Noda, Dufernez, Ohkuma, Kudo, Capron, Sogin and Viscogliosi2002; Ohkuma et al. Reference Ohkuma, Iida, Ohtoko, Yuzawa, Noda, Viscogliosi and Kudo2005; Mantini et al. Reference Mantini, Dalia-Cornette, Noda, Van Der Heijden, Capron, Dei-Cas, Landman, Ohkuma and Viscogliosi2009). A simple phylogenetic tree constructed for the purposes of this discussion summarizes these relationships (Fig. 2). While the lives of these related trichomonads seem quite different, some similarities do exist which appear to be inherent in members of this group. It is postulated that these similarities may provide some information on the life cycle of Dientamoeba.
Tritrichomonas foetus
Members of the genus Tritrichomonas infect a broad range of animals including reptiles, mammals and birds. The most important member of the genus Tritrichomonas is Tritrichomonas foetus due to its economic significance in the cattle-raising industries. As such, T. foetus will be discussed here as a representative of the genus Tritrichomonas.
Tritrichomonas foetus has a broad host range though is best known as a sexually transmitted pathogen of cattle (Felleisen et al. Reference Felleisen, Lambelet, Bachmann, Nicolet, Muller and Gottstein1998). However, based on recent reports the host range of T. foetus has expanded to include other animals.
Until recently, Pentatrichomonas hominis was considered to be the cause of a diarrhoeal disease in cats (Gookin et al. Reference Gookin, Breitschwerdt, Levy, Gager and Benrud1999; Romatowski, Reference Romatowski2000; Levy et al. Reference Levy, Gookin, Poore, Birkenheuer, Dykstra and Litaker2003). However, later reports utilizing DNA sequence analysis, DNA restriction analysis and electron microscopy confirmed that the aetiological agent was actually T. foetus (Levy et al. Reference Levy, Gookin, Poore, Birkenheuer, Dykstra and Litaker2003; Tolbert and Gookin, Reference Tolbert and Gookin2009). However, experimental infections in cows have demonstrated that T. foetus isolates derived from cats induce a similar yet slightly different disease in cows when compared to T. foetus isolates derived from cattle (Stockdale et al. Reference Stockdale, Rodning, Givens, Carpenter, Lenz, Spencer, Dykstra, Lindsay and Blagburn2007). Similarly, T. foetus isolates derived from cattle may be used to infect cats experimentally, though infectivity is reduced compared to isolates derived from cats (Stockdale et al. Reference Stockdale, Dillon, Newton, Bird, Bondurant, Deinnocentes, Barney, Bulter, Land, Spencer, Lindsay and Blagburn2008). As such, these organisms probably represent different subtypes of the same species.
Based on molecular, biochemical and morphological evidence, Tritrichomonas suis was also found to be identical to T. foetus (Tachezy et al. Reference Tachezy, Tachezy, Hampl, Sedinova, Vanacova, Vrlik, Van Ranst, Flegr and Kuldaa2002; Lun et al. Reference Lun, Chen, Zhu, Li and Xie2005). Therefore T. foetus is now considered an inhabitant of the porcine gut and snout (Levine, Reference Levine1985; Tachezy et al. Reference Tachezy, Tachezy, Hampl, Sedinova, Vanacova, Vrlik, Van Ranst, Flegr and Kuldaa2002). However, Cobo et al. (Reference Cobo, Cano and Campero2001) were unable to induce colonization of the genito-urinary tract of 9 heifers with T. suis via vaginal inoculation. As with the T. foetus isolates derived from cats, it is possible that the organism known as T. suis is actually a different subtype of T. foetus which has adapted to specifically infect swine. Interestingly, phylogenetic studies based on ribosomal RNA genes suggest that Tritrichomonas mobilensis which was originally described in the Bolivian squirrel monkey (Culberson et al. Reference Culberson, Pindak, Gardner and Honigberg1986), is also synonymous with T. foetus and T. suis (Felleisen, Reference Felleisen1997; Kleina et al. Reference Kleina, Bettim-Bandinelli, Bonatto, Benchimol and Bogo2004).
Tritrichomonas foetus has also been isolated from the faeces of dogs with diarrhoea (Gookin et al. Reference Gookin, Birkenheuer, St John, Spector and Levy2005). According to Levine (Reference Levine1985), T. foetus-like organisms have also been found in the genito-urinary tract and aborted foetuses of pigs, horses and roe deer. These reports could have important ramifications to the epidemiology and control of trichomoniasis. This is because interspecies transmission of T. foetus may become a future problem. The finding of a T. foetus-type organism in non-human primates could also have implications for human health. However, given the failure to establish a T. suis infection in cattle (Cobo et al. Reference Cobo, Cano and Campero2001) and the limited infectivity of cattle T. foetus isolates in cats (Stockdale et al. Reference Stockdale, Dillon, Newton, Bird, Bondurant, Deinnocentes, Barney, Bulter, Land, Spencer, Lindsay and Blagburn2008), it is more likely that different strains or subtypes of T. foetus exist, each with a fairly restricted host range. Given the number of animals reported to carry T. foetus, it is possible that other animals may be identified as hosts of T. foetus in the future.
The life cycle of T. foetus is thought to involve 2 forms; a tear-shaped trophozoite form and a recently described pseudocyst form (Pereira-Neves and Benchimol, Reference Pereira-Neves and Benchimol2009). The T. foetus trophozoite is 10–25 μm long and possesses 3 posterior flagella, 1 anterior flagellum and an undulating membrane (Levine, Reference Levine1985). Trophozoites multiply asexually by binary fission (Levine, Reference Levine1985).
Pseudocysts usually appear in response to unfavourable conditions though a small percentage of pseudocysts exist under normal conditions (Pereira-Neves et al. Reference Pereira-Neves, Ribeiro and Benchimol2003). Pseudocysts occur when T. foetus trophozoites round up and internalize their flagella in response to various stimuli (Granger et al. Reference Granger, Warwood, Benchimol and De Souza2000; Pereira-Neves et al. Reference Pereira-Neves, Ribeiro and Benchimol2003; Mariante et al. Reference Mariante, Lopes and Benchimol2004). This form lacks a protective cyst wall and does not represent a true cyst form (Granger et al. Reference Granger, Warwood, Benchimol and De Souza2000). No true cyst stage exists (Levine, Reference Levine1985).
In cattle, T. foetus is known as a cause of infertility and abortion (Felleisen et al. Reference Felleisen, Lambelet, Bachmann, Nicolet, Muller and Gottstein1998) and infections are usually transferred during coitus. Infections may also be transferred to cows during gynaecological examinations or artificial insemination (Rae and Crews, Reference Rae and Crews2006; Mardones et al. Reference Mardones, Perez, Martinez and Carpenter2008). In bulls, infections are usually chronic and asymptomatic (Mardones et al. Reference Mardones, Perez, Martinez and Carpenter2008) and spontaneous recoveries are rare (Levine, Reference Levine1985). There is no legal treatment for bovine trichomoniasis in several countries and as a result infected bulls are often slaughtered (Cobo et al. Reference Cobo, Canton, Morrell, Cano and Campero2004, Reference Cobo, Corbeil, Agnew, Vanhoosear, Friend, Olesen and Bondurant2007; Agnew et al. Reference Agnew, Munson, Cobo, Olesen, Corbeil and Bondurant2008). Infected cows will experience vaginitis which may or may not resolve spontaneously. Infections which exist during pregnancy will often result in foetal loss. In some cases endometritis as a result of T. foetus infection can result in complete sterility (Levine, Reference Levine1985) (Fig. 3).
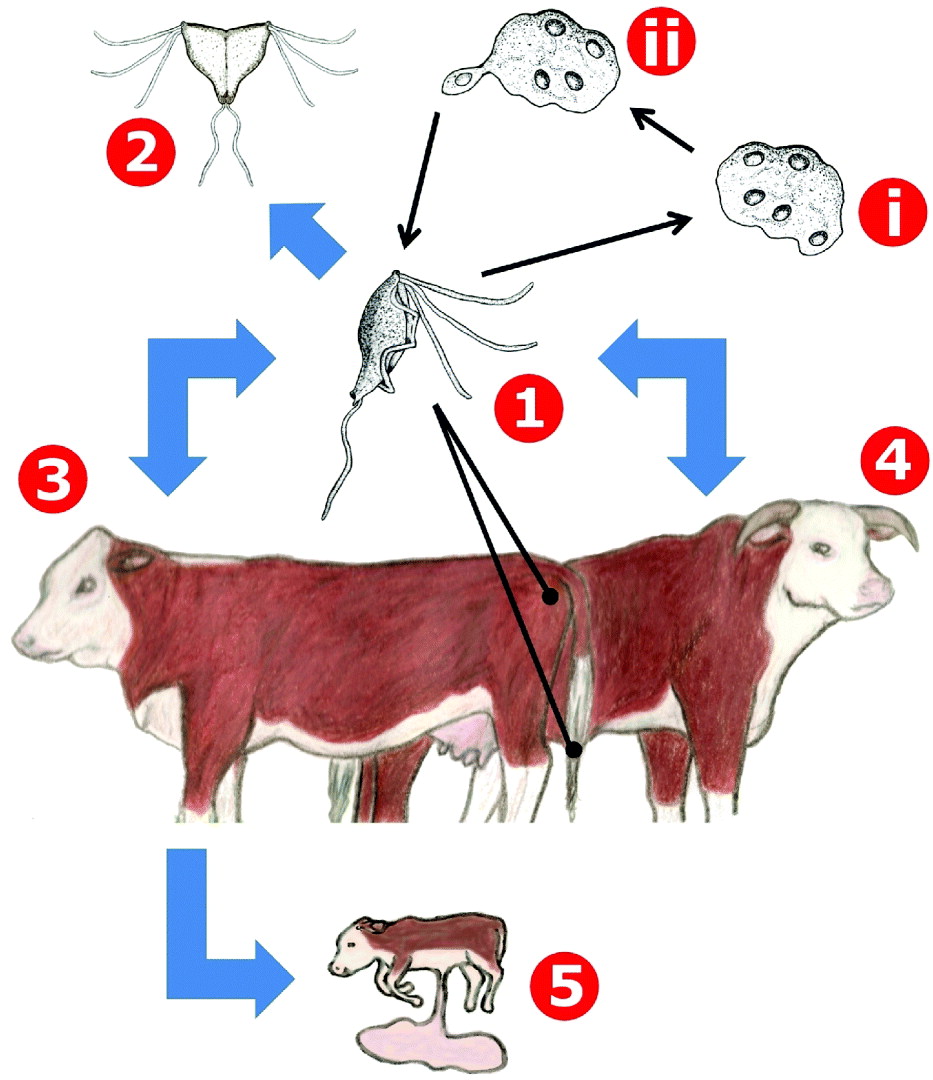
Fig. 3. The life cycle of Tritrichomonas foetus in cattle. Trophozoites of Tritrichomonas are transmitted between cows and bulls during coitus (1) and remain in the genito-urinary tract where they multiply by longitudinal binary fission (2). Under stress conditions trophozoites will internalize their flagella and replication of the nuclei and other cellular structures will occur, resulting in a multinucleated pseudocyst form (i). When conditions become desirable once more, mononucleate trophozoites will bud from the pseudocyst (ii). In bulls (4), infections are usually chronic and asymptomatic and often persist for the life of the animal. Infected cows (3) will initially experience vaginitis which may or may not resolve spontaneously. In some cases, endometritis can occur resulting in complete sterility. Tritrichomonas infections may also result in foetal loss during pregnancy (5).
Diagnosis of bovine trichomoniasis is often complicated by the presence of non-pathogenic T. foetus-like organisms (namely, Pentatrichomonas hominis and Tetratrichomonas spp.) in the genito-urinary tract of cattle (Cobo et al. Reference Cobo, Canton, Morrell, Cano and Campero2004, Reference Cobo, Corbeil, Agnew, Vanhoosear, Friend, Olesen and Bondurant2007; Dufernez et al. Reference Dufernez, Walker, Noel, Caby, Mantini, Delgado-Viscogliosi, Ohkuma, Kudo, Capron, Pierce, Villanueva and Viscogliosi2007; Agnew et al. Reference Agnew, Munson, Cobo, Olesen, Corbeil and Bondurant2008; Huby-Chilton et al. Reference Huby-Chilton, Scandrett, Chilton and Gajadhar2009). It is postulated that the presence of these non-pathogenic organisms is most likely due to sodomy practiced amongst young bulls (BonDurant et al. Reference Bondurant, Gajadhar, Campero, Johnson, Lun, Nordhausen, Hoosear, Villanueva and Walker1999; Cobo et al. Reference Cobo, Campero, Mariante and Benchimol2003, Reference Cobo, Canton, Morrell, Cano and Campero2004). As such, it is recommended that PCR and culture techniques are employed in conjunction with light microscopy for an accurate diagnosis of bovine trichomoniasis (Campero et al. Reference Campero, Rodriguez Dubra, Bolondi, Cacciato, Cobo, Perez, Odeon, Cipolla and Bondurant2003; Hayes et al. Reference Hayes, Anderson and Walker2003; Cobo et al. Reference Cobo, Canton, Morrell, Cano and Campero2004).
In cats, T. foetus infection is acquired via ingestion of trophozoites from material contaminated with faeces. Trophozoites then travel to the intestines where they remain, inducing chronic diarrhoea (Holliday et al. Reference Holliday, Deni and Gunn-Moore2009; Stockdale et al. Reference Stockdale, Givens, Dykstra and Blagburn2009; Tolbert and Gookin, Reference Tolbert and Gookin2009). Tritrichomonas infections in cats show no preference in terms of breed or sex (Stockdale et al. Reference Stockdale, Givens, Dykstra and Blagburn2009). Trophozoites are reported to remain culturally viable in cat faeces for up to 6 h after defecation (Hale et al. Reference Hale, Norris and Slapeta2009) which allows only a short period for re-infection to take place (Fig. 4).
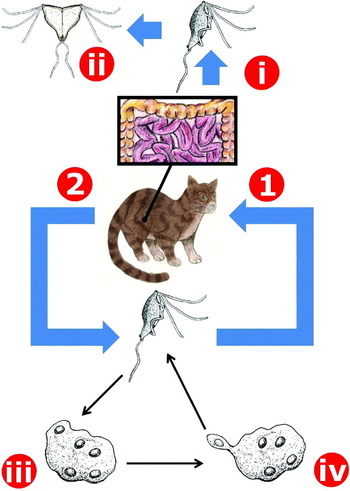
Fig. 4. The life cycle of Tritrichomonas foetus in cats. Tritrichomonas trophozoites are ingested by a feline host (1) and travel to its colon (i) where they multiply by longitudinal binary fission (ii). The presence of Tritrichomonas trophozoites in the colon induces chronic diarrhoea. Trophozoites are then passed in the faeces to the external environment (2) where they contaminate food and water sources of other potential hosts. Trophozoites of T. foetus are reported to survive for up to 6 h after being passed from the host. Under stress conditions trophozoites will internalize their flagella and replication of the nuclei and other cellular structures occurs, resulting in a multinucleated pseudocyst form (iii). When conditions become desirable once more, mononucleate trophozoites will bud from the pseudocyst (iv).
Histomonas meleagridis and Parahistomonas wenrichi
Histomonas meleagridis is the closest known relative of D. fragilis and is the only species within the genus Histomonas. Histomonas infects a broad range of gallinaceous birds including chickens, pheasants, quails, guinea fowl and peafowl, although it is most renowned for its ability to decimate commercial turkey flocks resulting in huge economic losses (McDougald, Reference McDougald2005; Bleyen et al. Reference Bleyen, De Gussem, Pham, Ons, Van Gerven and Goddeeris2009; Leberl et al. Reference Leberl, Hess and Bilic2009). Compared to other gallinaceous production birds, turkeys are the most susceptible to histomoniasis (McDougald, Reference McDougald2005; Powell et al. Reference Powell, Rothwell, Clarkson and Kaiser2009). The disease caused by Histomonas in turkeys is often referred to as ‘blackhead disease’ (McDougald, Reference McDougald2005).
Levine (Reference Levine1985) described 4 distinct stages within the Histomonas life cycle; a non-flagellated ‘invasive stage’, a non-flagellated ‘vegetative stage’, a non-flagellated ‘resistant stage’ and a flagellated ‘caecal stage’. For the purposes of this manuscript, these 4 stages will be condensed into 2 stages; the flagellated caecal stage and the amoeboid tissue stage which comprises the 3 non-flagellated stages described by Levine (Reference Levine1985). The spherical non-flagellated stage is between 8 and 21 μm in diameter. The caecal stage is spherical, between 5 and 30 μm in diameter and has a single flagellum. The early invasive stage and the caecal stage both possess active pseudopodia and multiply by binary fission (Levine, Reference Levine1985; Mielewczik et al. Reference Mielewczik, Mehlhorn, Al-Quraishy, Grabensteiner and Hess2008; Munsch et al. Reference Munsch, Lotfi, Hafez, Al-Quraishy and Mehlhorn2009a).
Transmission of H. meleagridis is known to occur via 2 routes. Firstly and most simply, the flagellated caecal stage can be transmitted directly by the faecal-oral route (Levine, Reference Levine1985; Hu and McDougald, Reference Hu and McDougald2003; McDougald and Fuller, Reference McDougald and Fuller2005; Liebhart and Hess, Reference Liebhart and Hess2009) which is thought to occur mostly in turkeys (McDougald, Reference McDougald2005). The second route of infection involves the helminth H. gallinarum.
In the event of a co-infection between Histomonas and H. gallinarum, Histomonas takes advantage of H. gallinarum to improve its survival in the external environment. In the caeca, Histomonas trophozoites are ingested by H. gallinarum. Histomonas then travels to the reproductive organs of the helminth. In the female worm, Histomonas enters the ovaries and eventually penetrates the undeveloped oocytes. The helminth ova containing Histomonas become embryonated, and are shed by the female worm into the caeca where they are eventually passed in the hosts faeces (Lee, Reference Lee1969b; Ruff et al. Reference Ruff, McDougald and Hansen1970). Heterakis ova harbouring Histomonas are able to survive in the soil for up to 2 years (Levine, Reference Levine1985) (Fig. 5). The protozoa are liberated when the helminth ova are ingested by an appropriate host and hatch, releasing both immature worms and the protozoa. In the soil, Heterakis ova containing Histomonas may also be ingested by the common earthworm and still remain infective for both Histomonas and Heterakis (Lund et al. Reference Lund, Wehr and Elli1966; Kemp and Franson, Reference Kemp and Franson1975). A gallinaceous bird can become infected with Histomonas by eating an earthworm that has ingested Heterakis ova containing Histomonas (Fig. 5). The earthworm is thought to play an important role in the long-term survival of Histomonas in the soil (Levine, Reference Levine1985). Several studies exist which describe the relationship between Histomonas and H. gallinarum in greater detail (Lund and Burtner, Reference Lund and Burtner1957; Kendall, Reference Kendall1959; Gibbs, Reference Gibbs1962; Lee, Reference Lee1969a; Ruff et al. Reference Ruff, McDougald and Hansen1970; Lee, Reference Lee1971; Lund and Chute, Reference Lund and Chute1973). While it is generally accepted that a cyst stage does not exist for Histomonas, some researchers report the formation of cyst-like structures in vitro which may represent another transmissible stage of Histomonas (Munsch et al. Reference Munsch, Lotfi, Hafez, Al-Quraishy and Mehlhorn2009a) (Fig. 5).
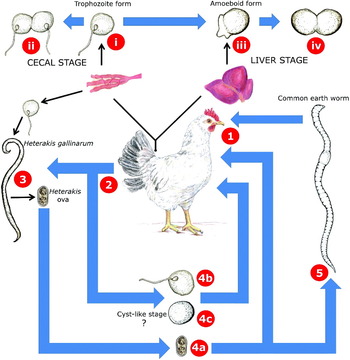
Fig. 5. The life cycle of Histomonas meleagridis. Histomonas trophozoites are ingested from the external environment in various forms by a gallinaceous bird (1). The flagellated trophozoite form of Histomonas travels to the caeca (i) where it multiplies by longitudinal binary fission (ii). Infections with Histomonas usually result in lesions on the caecal wall accompanied by a yellowish diarrhoea. Eventually, Histomonas trophozoites penetrate the caecal mucosa and travel to the liver, where they take on an amoeboid form (iii). The amoeboid form also multiplies by binary fission (iv). The damage caused to liver tissues during the invasive liver stage is often so severe that death will ensue. Histomonas organisms in various forms are then passed in the host's faeces (2) and contaminate food and water sources of other gallinaceous birds. In the event of a Heterakis/Histomonas coinfection, Histomonas trophozoites are ingested by a female Heterakis worm and invade its ovaries. Once in the Heterakis ovaries, Histomonas can then penetrate the developing Heterakis ova (3). These ova are then shed into the host's caeca by the female worm and are eventually passed in the host's faeces. Histomonas can remain viable outside the host within these ova for up to 2 years (4a). Alternatively, free flagellated trophozoites of Histomonas which were shed in the faeces may be directly ingested by a new host resulting in a Histomonas infection (4b). Recently, cyst-like structures of Histomonas have been described (4c) which could represent a newly discovered transmissible stage. However, the infectivity of these cyst-like structures is yet to be demonstrated. In the soil, Heterakis ova containing Histomonas organisms (4a) may also be ingested by the common earth worm (5) which may then be consumed by a gallinaceous bird, resulting in a Histomonas infection. Earthworms are believed to play a significant role in the survival of Histomonas organisms in the soil.
After an incubation period of 15–21 days, infected birds become weak and drowsy in appearance (Levine, Reference Levine1985). This is accompanied by the appearance of a yellowish diarrhoea and ulcerative lesions in the caeca and liver (Huber et al. Reference Huber, Reynaud, Callait and Zenner2006). Other tissues such as the kidneys and lungs may also be involved (Levine, Reference Levine1985). In turkey flocks, the mortality rate approaches 100% in some cases, while in chicken flocks the mortality rate approaches 10–20% though with high morbidity (McDougald, Reference McDougald2005). Interestingly, experiments involving gnotobiotic birds indicate that in the absence of certain bacteria Histomonas loses its pathogenicity (McDougald, Reference McDougald2005).
Parahistomonas wenrichi [synonym: Histomonas wenrichi (Mantini et al. Reference Mantini, Dalia-Cornette, Noda, Van Der Heijden, Capron, Dei-Cas, Landman, Ohkuma and Viscogliosi2009)] is similar to Histomonas in terms of its life cycle and biology. Like Histomonas, Parahistomonas is spherical though is approximately 1·5 times larger than Histomonas (Levine, Reference Levine1985). Parahistomonas possesses 4 flagella as opposed to Histomonas’ single flagellum though its movement and feeding are mostly dependent on its pseudopodia. Unlike Histomonas, Parahistomonas is non-pathogenic (Lund, Reference Lund1963). Parahistomonas’ preferred host range also includes gallinaceous birds such as turkeys, chickens and pheasants (Lund, Reference Lund1963; Levine, Reference Levine1985). Parahistomonas infections can also be transmitted in the ova of H. gallinarum (Lund, Reference Lund1968, Reference Lund1971).
Pseudocyst forms and cyst-like structures in Trichomonads
Under stress conditions several trichomonads enter a pseudocyst stage which is characterized by ‘rounding up’ of trophozoites and internalization of flagella (Lipman et al. Reference Lipman, Lampen and Nguyen1999; Granger et al. Reference Granger, Warwood, Benchimol and De Souza2000; Boggild et al. Reference Boggild, Sundermann and Estridge2002; Ribeiro et al. Reference Ribeiro, Pereira-Neves and Benchimol2002; Pereira-Neves et al. Reference Pereira-Neves, Ribeiro and Benchimol2003; Borges et al. Reference Borges, Wiltuschnig, Tasca and De Carli2004; Mariante et al. Reference Mariante, Lopes and Benchimol2004; Hussein and Atwa, Reference Hussein and Atwa2008; Pereira-Neves and Benchimol, Reference Pereira-Neves and Benchimol2009). During this process, duplication of the nuclei and other cellular structures also occurs resulting in a multinucleated giant cell. When conditions become favourable again, flagellated trophozoites begin to bud from the multinucleated cell (Pereira-Neves and Benchimol, Reference Pereira-Neves and Benchimol2009). Pseudocysts of trichomonads are generally described as compact, spherical, multinucleated forms which lack flagella and do not have a true cyst wall (Pereira-Neves et al. Reference Pereira-Neves, Ribeiro and Benchimol2003).
Pseudocysts were once thought to be degenerative forms though are now known to represent a true stage in the life cycle of some trichomonads. This is because mitosis occurs in the pseudocyst and the process of pseudocyst formation is reversible (Pereira-Neves et al. Reference Pereira-Neves, Ribeiro and Benchimol2003). Pseudocysts from various trichomonads are also infective to their respective hosts (Friedhoff et al. Reference Friedhoff, Kuhnigk and Muller1991; Lipman et al. Reference Lipman, Lampen and Nguyen1999; Pereira-Neves et al. Reference Pereira-Neves, Ribeiro and Benchimol2003; Pereira-Neves and Benchimol, Reference Pereira-Neves and Benchimol2009). Moreover, pseudocysts of T. foetus are able to adhere to vaginal epithelial cells more effectively than the trophozoite stage (Mariante et al. Reference Mariante, Lopes and Benchimol2004; Pereira-Neves and Benchimol, Reference Pereira-Neves and Benchimol2009). Usually, a small portion of the normal cell population will exist as pseudocysts (Pereira-Neves et al. Reference Pereira-Neves, Ribeiro and Benchimol2003).
Various stress conditions are known to trigger pseudocyst formation. In cultures of Monocercomonas, the largest number of pseudocyts were produced when cultures were incubated for 4–5 days at pH levels between 5 and 6 (Borges et al. Reference Borges, Gottardi, Stuepp, Larré, Vieira, Tasca and Carli2007). Fewer pseudocysts were produced by nutrient depletion and incubation at 20°C compared to 37°C (Borges et al. Reference Borges, Gottardi, Stuepp, Larré, Vieira, Tasca and Carli2007). Pseudocyst formation can also be induced in Tritrichomonas by the cooling of cultures to just below 16°C (Granger et al. Reference Granger, Warwood, Benchimol and De Souza2000). The addition of certain drugs to growth media can also trigger pseudocyst formation (Pereira-Neves and Benchimol, Reference Pereira-Neves and Benchimol2009). Mariante et al. (Reference Mariante, Lopes and Benchimol2004) found that incubation of cultures with the drug colchicine, incubation with dimethyl sulfoxide and submitting trophozoites to cycles of temperature oscillation will also induce pseudocyst formation.
Several flagellates are also capable of producing true cysts including members of the genera Retortomonas, Chilomastix and Enteromonas (Levine, Reference Levine1985) as well as Trichomitus batachorum, Trichomitus sanguisugae and Monocercomonas tipulae (Brugerolle, Reference Brugerolle1973; Pereira-Neves et al. Reference Pereira-Neves, Ribeiro and Benchimol2003). Interestingly, Mielewczik et al. (Reference Mielewczik, Mehlhorn, Al-Quraishy, Grabensteiner and Hess2008) also observed cyst-like structures in the faeces of chickens infected with Histomonas. However, these structures could not be attributed to H. meleagridis definitively, as all methods of purification failed (Mielewczik et al. Reference Mielewczik, Mehlhorn, Al-Quraishy, Grabensteiner and Hess2008). A number of later studies also report the finding of cyst-like stages in cultures of Histomonas (Munsch et al. Reference Munsch, Lotfi, Hafez, Al-Quraishy and Mehlhorn2009a, Reference Munsch, Mehlhorn, Al-Quraishy, Lotfi and Hafezb; Zaragatzki et al. Reference Zaragatzki, Hess, Grabensteiner, Abdel-Ghaffar, Al-Rasheid and Mehlhorn2010). According to Zaragatzki et al. (Reference Zaragatzki, Hess, Grabensteiner, Abdel-Ghaffar, Al-Rasheid and Mehlhorn2010), formation of these structures can be induced by cultivating Histomonas trophozoites at pH values between 7 and 8. However, the infectivity of these structures is yet to be demonstrated.
FURTHER RESEARCH IS REQUIRED
With respect to animals
Given the close relationship between humans and primates, it is not surprising that most reports in Table 1 describe the finding of Dientamoeba in monkeys and apes. The evidence for D. fragilis infections in gorillas is the most recent and is also well supported. Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008) provided molecular evidence to support their finding of D. fragilis in the stools of western lowland gorillas. The recent report by Lankester et al. (Reference Lankester, Kiyang, Bailey and Unwin2010) describing an irritable bowel-like illness in a western lowland gorilla also provides support for the findings of Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008). While monkeys and apes may be among the preferred hosts of Dientamoeba, transmission of Dientamoeba between humans and other primates is not a suitable model in parts of the British Isles (Schuster and Jackson, Reference Schuster and Jackson2009), the USA (Millet et al. Reference Millet, Spencer, Chapin, Stewart, Yatabe, Brewer and Garcia1983a) and the Netherlands (van Gool and Dankert, Reference Van Gool and Dankert1996). This is because Dientamoeba infections are quite common in these places, although human contact with monkeys and apes is virtually non-existent. In these regions, if an animal reservoir is ever identified it is more likely to be a pet or livestock animal, as these are more commonly integrated into those societies.
Dientamoeba has also been reported in the stools of sheep (Noble and Noble, Reference Noble and Noble1952) and swine (Crotti et al. Reference Crotti, Sensi, Crotti, Grelloni and Manuali2007). In regions where human contact with apes is low, these animals are more plausible as reservoirs of Dientamoeba infection. However, Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008) found no evidence of Dientamoeba in the stools of 50 sheep and 135 swine. According to Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008), a number of reasons could have attributed to these non-concordant reports. Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008) suggested that the Giemsa stain employed by Crotti et al. (Reference Crotti, Sensi, Crotti, Grelloni and Manuali2007) may not have been ideal for visualization of the nuclear structure of D. fragilis. Johnson et al. (Reference Johnson, Windsor and Clark2004) noted that the fragmented nuclear structure of D. fragilis enables one to distinguish it from organisms such as Endolimax nana which can appear quite similar to Dientamoeba in stained preparations. In the study by Noble and Noble (Reference Noble and Noble1952), the staining technique which detected Dientamoeba was not disclosed and no image of Dientamoeba was provided. Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008) did note, however, that differences in farming practices such as caged farming as opposed to free-range style farming or the use of anti-protozoal compounds could have attributed to these conflicting reports. The screening of wild or feral animals for the presence of Dientamoeba may be informative as differences in farming practices do not apply and these animals are unlikely to have been treated with anti-protozoal compounds.
In light of these conflicting reports, the role of swine and sheep in the life cycle of Dientamoeba is uncertain. However, their potential role in the life cycle of Dientamoeba cannot be dismissed. In the study by Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008), a two-step screening approach was employed where stained faecal smears were prepared and those found to contain Dientamoeba were then confirmed with PCR. It is well documented that PCR is more sensitive than light microscopy (Stark et al. Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010a). The intermittent shedding of Dientamoeba trophozoites in humans is also well documented (Stark et al. Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010b). It has been shown that the results of molecular tests are less likely to be influenced by the phenomenon of intermittent shedding (Stark et al. Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010a). As such, the study performed by Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008) could have been improved by testing specimens with PCR or real-time PCR prior to microscopic analysis. The use of molecular tests as the first step in the screening process would have reduced the chances of obtaining false negatives that occur as a result of low parasite loads, intermittent shedding and human error.
While the use of PCR by Stark et al. (Reference Stark, Phillips, Peckett, Munro, Marriott, Harkness and Ellis2008) provides strong evidence that gorillas are a true host for Dientamoeba, DNA sequence data derived from the SSU rDNA of these gorilla isolates would have been ideal. Similarly, the study by Crotti et al. (Reference Crotti, Sensi, Crotti, Grelloni and Manuali2007) could have been greatly improved had their results been supported by molecular evidence in the form of a PCR product and preferably, some DNA sequence data. In order to improve future studies, it is extremely important that researchers utilize molecular techniques to substantiate all findings. Researchers should also obtain sequence data to ensure that their PCR products are specific for Dientamoeba. Sequence data would also be useful for genotyping purposes.
Regarding the experimental infections in humans and animals, these reports are also non-concordant. For instance, Dobell's attempt to infect macaques by rectal inoculation failed (Dobell, Reference Dobell1940). However, 2 reports describe the finding of Dientamoeba in the stools of macaques (Hegner and Chu, Reference Hegner and Chu1930; Knowles and DasGupta, Reference Knowles and DasGupta1936). There are several plausible explanations for Dobell's failed attempt to infect macaques. One possibility is that the organisms described in these reports (Hegner and Chu, Reference Hegner and Chu1930; Knowles and DasGupta, Reference Knowles and DasGupta1936) were misidentified and macaques are not a true host for Dientamoeba. Another plausible explanation is that Dobell's cultured isolates had been attenuated over time and lost their ability to infect new hosts. However, Dobell's success in infecting 1 of 6 chicks by rectal inoculation is surprising when considering that infections could not be achieved in macaques using the same technique (Dobell, Reference Dobell1940). However, we do not know whether these experiments were carried out at the same time using the same isolate of Dientamoeba. As mentioned previously, Kean and Malloch (Reference Kean and Malloch1966) also reported some success in their attempt to experimentally infect laboratory rats with Dientamoeba although these experiments are only briefly discussed and appear to be incomplete. Other than the reports by Kean and Malloch (Reference Kean and Malloch1966) and Dobell (Reference Dobell1940), all additional attempts to experimentally infect animals with Dientamoeba have failed.
Taken as a whole, these conflicting reports are difficult to interpret. However, it seems that animal experiments like those performed by Dobell (Reference Dobell1940) and Kean and Malloch (Reference Kean and Malloch1966) must be repeated. The development of a simian model of dientamoebiasis could represent a breakthrough in Dientamoeba research. This would not only allow researchers to explore Dientamoeba's mode of transmission in a controlled manner, but could also be used to determine whether Dientamoeba satisfies Koch's postulates as a cause of gastrointestinal illness.
Given Dobell's success in inducing a transient infection in a chick (Dobell, Reference Dobell1940), the role of poultry in the transmission of Dientamoeba is also worth exploring further. Interestingly, the optimum temperature for growth of Dientamoeba in vitro is 41–42 °C rather than 37°C (Dobell, Reference Dobell1940; Barratt et al. Reference Barratt, Banik, Harkness, Marriott, Ellis and Stark2010). Therefore, the optimum growth temperature for Dientamoeba is closer to the body temperature of birds rather than humans. Given that Dientamoeba's closest relative is a poultry pathogen; it is plausible that poultry could be involved in Dientamoeba's transmission.
With respect to helminths
While Ockert claimed to have infected himself with Dientamoeba using the ova of E. vermicularis, this is difficult to substantiate without the aid of molecular or electron-microscopic evidence. Ideally, if structures resembling Dientamoeba trophozoites are observed in the ova of a helminth, electron microscopic images of these Dientamoeba-like bodies should be taken and compared to those produced by Camp et al. (Reference Camp, Mattern and Honigberg1974) and Silard et al. (Reference Silard, Burghelea, Panaitescu and Burcos1984). Such evidence would provide strong support for Ockert's claims. Furthermore, without molecular evidence it is difficult to ascertain whether Ockert's Dientamoeba was of the same genotype as the Dientamoeba in the child from which he had infected himself. Given Ockert's frequent handling and processing of stool specimens containing Dientamoeba, it is possible that he infected himself from another source. The most compelling support for the lack of an association between Enterobius and Dientamoeba is the molecular evidence provided by Menghi et al. (Reference Menghi, Makiya and Gatta2005) who failed to amplify a Dientamoeba-specific PCR product from Enterobius ova derived from a patient who was also infected with Dientamoeba.
While associations may have been observed between Enterobius and Dientamoeba, similar associations between Dientamoeba and other enteric parasites have also been reported (Johnson et al. Reference Johnson, Windsor and Clark2004). Ayadi and Bahri (Reference Ayadi and Bahri1999) noted, that Dientamoeba infections were most often associated with Blastocystis. Stark et al. (Reference Stark, Beebe, Marriott, Ellis and Harkness2005) also found that Dientamoeba infections were most often associated with Blastocystis. Ozcakir et al. (Reference Ozcakir, Gureser, Erguven, Yilmaz, Topaloglu and Hascelik2007) noted that Blastocystis was more frequently detected alongside D. fragilis compared to any other enteric protozoa. Stensvold et al. (Reference Stensvold, Lewis, Hammerum, Porsbo, Nielsen, Olsen, Arendrup, Nielsen and Molbak2009) found that 34·8% (n=32) of patients with Blastocystis infection were also infected with D. fragilis. As such, it is possible that the associations between Dientamoeba and Enterobius described by Yang and Scholten (Reference Yang and Scholten1977) and Girginkardesler et al. (Reference Girginkardesler, Kurt, Kilimcioglu and Ok2008) may represent nothing more than a shared mode of transmission between these organisms.
The report by Sukanahaketu (Reference Sukanahaketu1977) provides support for the existence of a relationship between Dientamoeba and Ascaris. While the images presented by Sukanahaketu (Reference Sukanahaketu1977) are interesting, the finding of Dientamoeba-like structures in the ova of Ascaris is not supported by the occurrence of Dientamoeba infections in non-tropical, western countries where Ascaris infections are very uncommon (Walker et al. Reference Walker, Bahr and Ehl1985; Stensvold et al. Reference Stensvold, Arendrup, Molbak and Nielsen2007; Schuster and Jackson, Reference Schuster and Jackson2009). Therefore, the transmission of Dientamoeba by means of an Ascaris vector is not a suitable model for Dientamoeba transmission in these regions.
Given the molecular evidence described by Menghi et al. (Reference Menghi, Makiya and Gatta2005) and the lack of an association between pinworm and Dientamoeba observed in several studies, the role of Enterobius in the life cycle of Dientamoeba remains controversial. Despite this, Ockert's hypotheses regarding the role of Enterobius in the transmission of Dientamoeba should not be disregarded. Johnson et al. (Reference Johnson, Windsor and Clark2004) noted that spontaneous remissions of Enterobius infection do occur and it is often not clear in some reports whether patients were tested correctly for the presence of Enterobius. Clearly, if future researchers observe Dientamoeba-like bodies in the ova of a helminth, support for these findings in the form of molecular evidence and electron microscopic images is essential.
With respect to Dientamoeba's relatives
Given the existence of pseudocysts in T. foetus and the recent evidence for cyst-like structures in Histomonas, it is plausible that similar structures could exist for Dientamoeba. Pseudocyst formation as observed in some trichomonads is easily noted by the invagination of the flagella. Unfortunately, such an event cannot be observed in Dientamoeba because it completely lacks flagella. Furthermore, another feature of pseudocysts in trichomonads is that they are multinucleated (Pereira-Neves et al. Reference Pereira-Neves, Ribeiro and Benchimol2003; Borges et al. Reference Borges, Gottardi, Stuepp, Larré, Vieira, Tasca and Carli2007). Dientamoeba trophozoites are often multinucleated (Johnson et al. Reference Johnson, Windsor and Clark2004) which would also make it difficult to identify pseudocyst forms in Dientamoeba if they did exist.
To explore the possible existence of a pseudocyst stage in Dientamoeba, the experiments performed by Borges et al. (Reference Borges, Gottardi, Stuepp, Larré, Vieira, Tasca and Carli2007), Pereira-Neves et al. (Reference Pereira-Neves and Benchimol2009), and Mariante et al. (Reference Mariante, Lopes and Benchimol2004) should be repeated for Dientamoeba. Staining Dientamoeba cells with a specific nuclear stain such as DAPI (Noel et al. Reference Noel, Gerbod, Delgado-Viscogliosi, Fast, Younes, Chose, Roseto, Capron and Viscogliosi2003; Al-Adhami et al. Reference Al-Adhami, Nichols, Kusel, O'grady and Smith2007; Taniwaki et al. Reference Taniwaki, Da Silva, Da Silva and Mortara2007) would be helpful in these experiments to identify if any changes occur with respect to the number of nuclei in the cells. It is possible that the Dientamoeba cells occasionally reported to contain 4 or more nuclei (Johnson et al. Reference Johnson, Windsor and Clark2004) could represent a pseudocyst form.
As previously mentioned, several flagellates are also capable of producing true cysts (Brugerolle, Reference Brugerolle1973; Levine, Reference Levine1985; Pereira-Neves et al. Reference Pereira-Neves, Ribeiro and Benchimol2003). Therefore, the possibility that a cyst stage could exist for Dientamoeba should not be dismissed. Given the recent discovery of the cyst stage of Blastocystis in 1991 (Stenzel and Boreham, Reference Stenzel and Boreham1991) it is not impossible that a cyst stage for Dientamoeba is yet to be discovered. Moreover, given the recent reports of a cyst-like stage for Histomonas (Mielewczik et al. Reference Mielewczik, Mehlhorn, Al-Quraishy, Grabensteiner and Hess2008; Munsch et al. Reference Munsch, Lotfi, Hafez, Al-Quraishy and Mehlhorn2009a; Zaragatzki et al. Reference Zaragatzki, Hess, Grabensteiner, Abdel-Ghaffar, Al-Rasheid and Mehlhorn2010), the existence of a similar stage for Dientamoeba is plausible. Should a cyst-like stage be identified for Dientamoeba, the findings must be substantiated through the use of electron microscopic comparisons of these structures to the cyst stages of other trichomonads. Molecular support for such a substantial claim is also important. Finally, the infectivity of these structures must also be demonstrated.
When examining the life cycles of Histomonas and Tritrichomonas, one major similarity becomes apparent. That is that these organisms can be transmitted directly between their preferred hosts. As such, the trophozoite stage of Dientamoeba may still be the only stage in its life cycle. It was noted previously that T. foetus does not possess a cyst stage and remains culturally viable for up to 6 h after being excreted in the faeces of cats (Hale et al. Reference Hale, Norris and Slapeta2009). Also, direct bird-to-bird transmission of the trophozoite stage of Histomonas in the absence of a Heterakis vector has been well documented (Levine, Reference Levine1985; Hu and McDougald, Reference Hu and McDougald2003; McDougald and Fuller, Reference McDougald and Fuller2005; Liebhart and Hess, Reference Liebhart and Hess2009). Given that Dientamoeba trophozoites are reported to remain viable for up to 8 times longer than those of T. foetus (Kean and Malloch, Reference Kean and Malloch1966; Stark et al. Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010a), it is not unreasonable to suggest that Dientamoeba trophozoites may be transmitted directly from human to human. Nevertheless, if trophozoites are the transmissible form, it is uncertain as to why Dobell (Reference Dobell1940) was unable to infect himself and 2 macaques orally with cultured trophozoites. This could be the result of attenuation of the trophozoites after long-term culture though, without information on the passage number and source of Dobell's cultures it is difficult to speculate. The development of an animal model for dientamoebiasis would greatly assist in addressing these problems.
CONCLUDING REMARKS
Dientamoeba fragilis is an inhabitant of the human gastrointestinal tract for which the mode of transmission is unknown. As no cyst stage has been identified for Dientamoeba (at the time of writing), the trophozoite form is generally accepted as the only stage in its life cycle. However, the fragile nature of Dientamoeba trophozoites once passed from their host implies that direct human-to-human transmission of the trophozoite form seems unlikely. Numerous investigations have been carried out in an attempt to better understand Dientamoeba's life cycle. Despite these efforts our knowledge of this organism has progressed very little. Clearly, more research is required.
While several reports attempt to address the lack of knowledge on this organism, most are lacking in one crucial component; that is evidence in the form of molecular and/or electron microscopic data. It is imperative that claims relating to the life cycle and transmission of Dientamoeba, are substantiated using these techniques for several reasons. As discussed previously, light microscopic analysis is less sensitive and less specific than PCR. Moreover, light microscopy introduces a greater element of human error when compared to molecular techniques. Sequencing of PCR products allows researchers to accurately determine the presence and/or identity of an organism almost beyond a doubt. Electron microscopy is a powerful tool which allows detailed observations to be made on the intracellular architecture of cells which cannot be matched by light microscopy. It has become apparent that if modern techniques such as PCR, DNA sequencing and electron microscopy are not employed to substantiate findings related to the life cycle of Dientamoeba, it is unlikely that they will be accepted by the broader scientific community.
Unfortunately, our lack of an animal model for this organism is also a short coming which must be addressed. The lack of an animal model for Dientamoeba infection hampers our ability to study its biology, mode of transmission and mechanisms of pathogenicity in a well-controlled manner. Such a model may also help in the fulfilment of Koch's postulates for Dientamoeba.
Dientamoeba has recently emerged as a significant cause of gastrointestinal illness in humans (Girginkardesler et al. Reference Girginkardesler, Coskun, Cuneyt Balcioglu, Ertan and Ok2003; Johnson et al. Reference Johnson, Windsor and Clark2004; Lagace-Wiens et al. Reference Lagace-Wiens, Vancaeseele and Koschik2006; Crotti and D'Annibale, Reference Crotti and D'annibale2007; Stark et al. Reference Stark, Barratt, Ellis, Harkness and Marriott2009a, Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010b). As such, the lack of research being performed on this organism limits our capacity to introduce appropriate control methods. As the importance of this neglected parasite becomes increasingly recognized, the need for more research on this organism becomes more apparent.
The life cycles of Dientamoeba's closest relatives are generally well characterized and it is postulated that these organisms could provide some clues in relation to Dientamoeba's mode of transmission. Based on the life cycles of Histomonas and Tritrichomonas, it is plausible that helminths and/or animals could play a role in the transmission of Dientamoeba. Moreover, the recent reports of pseudocysts and cyst-like structures in some trichomonads imply that similar structures could exist for Dientamoeba. Direct human-to-human transmission of Dientamoeba is also plausible given that Histomonas and Tritrichomonas can be transmitted between their respective hosts in this way. As none of these theories has been sufficiently proven or disproven, none can be dismissed at this stage.
Unfortunately, our lack of knowledge on the life cycle of Dientamoeba makes prevention and control of infections extremely difficult. Moreover, the fact that Koch's postulates have not yet been fulfilled for this organism means that some still consider its pathogenicity as a matter of question. Ultimately, it is essential that more research is carried out on Dientamoeba to better understand the life cycle and biology of this neglected albeit important organism.








