Introduction
The planthopper Delphacodes kuscheli Fennah (Hemiptera: Delphacidae) is vector of three viruses affecting cereal crops in Argentina, Mal de Río Cuarto virus (MRCV, Fijivirus, Reoviridae) (Remes Lenicov et al., Reference Remes Lenicov, Tesón, Dagoberto and Huguet1985), Barley yellow striate mosaic virus (BYSMV, Cytorhabdovirus, Rhabdoviridae) (Dumón et al., Reference Dumón, Argüello-Caro, Alemandri, Bainotti, Mattio, Rodríguez, del Vas and Truol2011) and a recently described rhabdovirus closely related to Maize yellow striate virus (Cytorhabdovirus, Rhabdoviridae) (Maurino et al., Reference Maurino, Laguna, Giolitti, Nome and Gimenez Pecci2012; Dumón et al., Reference Dumón, Mattio, Argüello Caro, Alemandri, Puyané, del Vas, López Lambertini and Truol2015). Plant reoviruses and rhabdoviruses are transmitted in a persistent propagative manner by their planthopper vectors (Hogenhout et al., Reference Hogenhout, Ammar, Whitfield and Redinbaugh2008; Ammar et al., Reference Ammar, Tsai, Whitfield, Redinbaugh and Hogenhout2009). Acquisition is followed by a latency period, during which the virus particles enter the insect midgut epithelial cells, replicate, reach the hemocoel and move to the salivary glands from where the virus is transmitted to a plant host upon feeding (Hogenhout et al., Reference Hogenhout, Ammar, Whitfield and Redinbaugh2008; Ammar et al., Reference Ammar, Tsai, Whitfield, Redinbaugh and Hogenhout2009). Most of those viruliferous vectors infected with persistent-propagative viruses are not able to transmit them, and are therefore known as non-transmitting viruliferous vectors. Accordingly, as reviewed by Hogenhout et al. (Reference Hogenhout, Ammar, Whitfield and Redinbaugh2008) demonstrated that viruses acquired orally have a lower transmission rate than when injected directly into the hemolymph, suggesting that the insect gut is a major morphophysiological barrier to transmission. To reach the salivary glands, the persistent-propagative virus must overcome numerous barriers; hence, the inability of a vector to transmit a virus may be due to a failure of the virus to replicate, enter or leave the insect cells or organs (Ohnishi et al., Reference Ohnishi, Knight, Hosokawa, Fujisawa and Tsuda2001; de Assis Filho et al., Reference de Assis Filho, Stavisky, Reitz, Deom and Sherwood2005; Hogenhout et al., Reference Hogenhout, Ammar, Whitfield and Redinbaugh2008; Ammar et al., Reference Ammar, Tsai, Whitfield, Redinbaugh and Hogenhout2009). This inability may also be explained by the action of the vector innate immune response, which interferes at different stadia of the viral infection cycle (Ammar et al., Reference Ammar, Tsai, Whitfield, Redinbaugh and Hogenhout2009).
MRCV causes an important disease mainly in maize (Zea mays L.). However, wheat (Triticum aestivum L.) has been proposed as a useful model to study virus–vector interactions because it is a natural host of the virus (Rodríguez Pardina et al., Reference Rodríguez Pardina, Giménez Pecci, Laguna, Dagoberto and Truol1998) and a suitable rearing host of the vector D. kuscheli (Truol et al., Reference Truol, Usugi, Hirao, Arneodo, Giménez Pecci and Laguna2001); in addition wheat develops MRCV symptoms earlier than maize (Truol et al., Reference Truol, Usugi, Hirao, Arneodo, Giménez Pecci and Laguna2001; Arneodo et al., Reference Arneodo, Guzmán, Conci, Laguna and Truol2002). Under experimental conditions, only 30% of the planthoppers fed on an MRCV-infected wheat plant are capable of transmitting the virus to healthy wheat plants (cv. ProINTA Federal), after a latency period of 17 days (Arneodo et al., Reference Arneodo, Guzmán, Conci, Laguna and Truol2002). Moreover, higher transmission efficiency was observed when the virus was acquired by a first-instar nymph (Arneodo et al., Reference Arneodo, Guzmán, Ojeda, Ramos, Laguna, Conci and Truol2005; Argüello Caro et al., Reference Argüello-Caro, Maroniche, Dumón, Sagadin, del Vas and Truol2013). Argüello Caro et al. (Reference Argüello-Caro, Maroniche, Dumón, Sagadin, del Vas and Truol2013) found a higher viral load in MRCV-transmitting D. kuscheli, indicating a positive correlation between viral accumulation and transmission capacity. However, the mechanisms underlying MRCV transmission and the factors that determine that only some individuals feeding on an infected plant are capable of transmitting the virus remain to be elucidated.
MRCV and wheat rhabdovirus were found in mixed infections in nature (Dumón et al., Reference Dumón, Mattio, Argüello Caro, Alemandri, Puyané, del Vas, López Lambertini and Truol2015). It is known that mixed infections may lead to a modification of viral titers and therefore, in some cases, influence the transmission efficiency of the viruses involved (Rentería-Canett et al., Reference Rentería-Canett, Xoconostle-Cázares, Ruiz-Medrano and Rivera-Bustamante2011). For example, in mixed infections with two reoviruses, Southern rice black-streaked virus (SRBSDV, Fijivirus, Reoviridae) and Rice ragged stunt virus (RRSV, Oryzavirus, Reoviridae), an increase of viral titers was observed for both viruses (Li et al., Reference Li, Wang and Zhou2014). Furthermore, it was observed that a structural protein (protein 6, P6) coded by Rice yellow stunt rhabdovirus (RYSV, Nucleorhabdovirus, Rhabdoviridae) can enhance the virulence of PVX in N. benthamiana and N. tabacum plants (Guo et al., Reference Guo, Song, Xie, Huo, Zhang, Chen, Geng and Fang2013).
To date, no studies have been conducted to elucidate the consequences of MRCV and rhabdovirus interactions in their natural vector insect. In the current study, we analyzed the relationship between transmission efficiency and MRCV load in D. kuscheli from single and mixed infections assays at different developmental stadia of the vector and latency periods.
Materials and methods
Source and maintenance of insects and viruses
Insects were obtained from a colony maintained on wheat plants, at the Vector's Laboratory of Instituto de Patología Vegetal-Centro de Investigaciones Agropecuarias-Instituto Nacional de Tecnología Agropecuaria (IPAVE-CIAP-INTA) since 2008. Controlled conditions of temperature (23 ± 2°C), RH (50%) and photoperiod (16L: 8D) were followed as described by Truol et al. (Reference Truol, Usugi, Hirao, Arneodo, Giménez Pecci and Laguna2001). MRCV (MRCV-2008) and a rhabdovirus (Rh 2013) isolate closely related to Maize yellow striate virus (98% identity with GenBank Accession No. JQ715419) used as source of inoculum were obtained from symptomatic plants present in the MRCV endemic area (Río Cuarto, Córdoba province, Argentina). These isolates were maintained on wheat (Triticum aestivum, cv. ProINTA Federal, for MRCV) and barley (Hordeum vulgare, cv. Goldie, for rhabdovirus) by serial vector transmissions using D. kuscheli (Truol et al., Reference Truol, Usugi, Hirao, Arneodo, Giménez Pecci and Laguna2001; Dumón et al., Reference Dumón, Sagadín, Truol and Truol2009). The mixed inoculum was obtained also performing serial transmission to oat (Avena sativa, cv Bonaerense Payé) by D. kuscheli infected with both viruses (Dumón, Reference Dumón2013). Dually infected planthoppers were obtained from a plant with mix infection (MRCV-rhabdovirus).
Measurement of MRCV transmission efficiency
Groups of D. kuscheli males and females were allowed to reproduce on non-infected wheat plants. Adults were removed 24 h after oviposition and plants were maintained in breeding chambers under controlled conditions for egg development (Truol et al., Reference Truol, Usugi, Hirao, Arneodo, Giménez Pecci and Laguna2001). First-(N1) and third-instar nymphs (N3) were obtained 4 and 9 days after egg hatching, respectively, and used for subsequent transmission assays.
Measurement in single infection assays
Groups of 100 nymphs of each instar were fed on MRCV-infected wheat for 48 h (Acquisition Access Period [AAP]). The insects were then moved to chambers containing non-infected wheat plants during 9 or 17 days (latency period). Next, 1:1 transmission assays were performed by individually transferring one insect to a single non-infected wheat seedling cv. Pro INTA Federal (Truol et al., Reference Truol, Usugi, Hirao, Arneodo, Giménez Pecci and Laguna2001) (Inoculation Access Period [IAP]). All these assays were carried out under the same environmental conditions described for planthopper rearing. After 24 h, planthoppers were individually stored in absolute ethanol at −20°C until total RNA extraction. Finally, plants were transferred to a greenhouse with temperature controlled conditions (23 ± 2°C). MRCV infection was analyzed by DAS-ELISA assays 30 days post-inoculation (dpi) (Truol et al., Reference Truol, Usugi, Hirao, Arneodo, Giménez Pecci and Laguna2001). Each combination of nymph stadium and latency period was considered a treatment: first-instar nymphs, 9 days after AAP (N1L9); first-instar nymphs, 17 days after AAP (N1L17); third-instar nymphs, 9 days after AAP (N3L9); and third-instar nymphs, 17 days after AAP (N3L17). Three replicates of 15 insects each were performed for each treatment (n = 45).
Measurement in mixed infection assays
Groups of 100 nymphs of each instar were allowed to feed on MRCV-rhabdovirus infected oat plants for 48 h (AAP). Then, the same procedure as that used for single infections was applied using wheat seedlings as test plants (cv. Pro INTA Federal). MRCV and rhabdovirus infections were analyzed by DAS-ELISA and indirect ELISA, respectively, 30 dpi (Truol et al., Reference Truol, Usugi, Hirao, Arneodo, Giménez Pecci and Laguna2001; Dumón, Reference Dumón2013). Each combination of nymph stadium and latency period was considered a treatment as described above. Three replicates of 15 insects each were performed for each treatment (n = 45).
For both single and mixed infections, control seedlings consisted of 10 seedlings that had been mock-inoculated with virus-free vectors (i.e., planthoppers submitted to a 48-h ‘mock’ AAP on non-infected plants). MRCV titer was measured by DAS-ELISA (Rodríguez Pardina et al., Reference Rodríguez Pardina, Giménez Pecci, Laguna, Dagoberto and Truol1998) and infected plants with equivalent absorbance values were used as inoculum for all trials.
In single and mixed infection assays we respectively used wheat and oat plant species as a source of virus, considering that these species are the best hosts for each virus. Both plant species are preferential breeding and feeding hosts of the vector (Remes Lenicov & Virla, Reference Remes Lenicov and Virla1999). Therefore, sustained feeding occurs in the two species ensuring virus acquisition from both hosts.
Symptom development in infected plants
At 15, 20 and 30 dpi, plants were examined for the presence of some of the characteristic symptoms of MRCV (Arneodo et al., Reference Arneodo, Guzmán, Conci, Laguna and Truol2002) and rhabdovirus (Dumón et al., Reference Dumón, Mattio, Argüello Caro, Alemandri, Puyané, del Vas, López Lambertini and Truol2015). For MRCV we evaluated the appearance of short, erect, dark green-colored leaves, number of tillers, shortening of the internodes and dwarf plants. These symptoms are manifested in wheat (cv. Pro INTA Federal) about 30 dpi (Arneodo et al., Reference Arneodo, Guzmán, Conci, Laguna and Truol2002). For the rhabdovirus-infected plants, the symptoms included: mild chlorotic streaking on leaves, dwarfing and yellowing. Cereals infected with rhabdovirus exhibit the first symptoms about 10–15 dpi (Dumón et al., Reference Dumón, Mattio, Argüello Caro, Alemandri, Puyané, del Vas, López Lambertini and Truol2015). All plants of each treatment were observed and compared with control plants.
MRCV quantification in planthoppers by real time quantitative polymerase chain reaction (RT qPCR)
Total RNA was extracted individually from each planthopper (five insects per repetition), using a modified Trizol (Invitrogen, CA, USA) protocol (Maroniche et al., Reference Maroniche, Sagadin, Mongelli, Truol and del Vas2011). RNA concentration and purity were measured using a spectrophotometer (NanoDrop ND-1000, NanoDrop Technologies, USA). Synthesis of cDNA was carried out from 500 ng of total RNA using the ImProm-II Reverse Transcription System kit (Promega, USA), according to the manufacturer's protocol. The synthesized cDNAs were used for subsequent qPCR in an IQ 5 iCycler (BIORAD, USA), using a QuantiTec SYBR Green PCR kit (QIAGEN, Germany), according to the manufacturer's instructions. MRCV quantification was carried out by amplifying a fragment of MRCV segment S3 (MRCV-S3). Segment S3 codes for the major core capsid protein (Distéfano et al., Reference Distéfano, Maldonado, Hopp and del Vas2009) and is the most conserved genomic segment (Distéfano et al., Reference Distéfano, Conci, Muñoz Hidalgo, Guzmán, Hopp and del Vas2003). As internal control, the D. kuscheli ubiquitin gene (UBI) was amplified (Maroniche et al., Reference Maroniche, Sagadin, Mongelli, Truol and del Vas2011). For S3 and UBI amplifications, we used the same primers as those used by Argüello Caro et al. (Reference Argüello-Caro, Maroniche, Dumón, Sagadin, del Vas and Truol2013). qPCR reactions were carried out in a 20-μl final volume and using 1 µl of cDNA. cDNA from non-infected insects was used as a negative control of MRCV-S3 amplification, whereas no template was added to the UBI negative controls. The qPCR cycling conditions were: an initial step at 95°C for 10 min followed by 40 cycles composed of a 15-s denaturalization step at 95°C and 1-min annealing and elongation step at 60°C. A final dissociation step was carried out as a control of the PCR amplification specificity. All the reactions were performed in triplicate. Output results were processed with the LinReg software (Ruijter et al., Reference Ruijter, Ramaker, Hoogaars, Bakker, van den Hoff, Karlen and Moorman2009), for calculations of threshold cycle values (Ct) and PCR efficiencies.
Statistical analyses
Data of transmission trials were treated as categorical (transmitting: 1 and non-transmitting: 0) and analyzed using contingency tables and generalized models under a binary distribution using Infostat (Di Rienzo et al., Reference Di Rienzo, Casanoves, Balzarini, Gonzalez, Tablada and Robledo2012). Difference in Proportions Test (Di Rienzo et al., Reference Di Rienzo, Casanoves, Balzarini, Gonzalez, Tablada and Robledo2012) was applied to data of the timing of the expression of MRCV symptoms. Statistical analyses of relative qPCR quantification data were performed using the fgStatics software (Di Rienzo, Reference Di Rienzo2010), which uses the Pfaffl method for calculation of the expression ratios (Pfaffl et al., Reference Pfaffl, Horgan and Dempfle2002). Data obtained from acquisition events from single inoculum were considered a control group, and data obtained from acquisition events from mixed inoculum were considered the treatment group.
Results
Transmission efficiency of MRCV by D. kuscheli in single and mixed infections
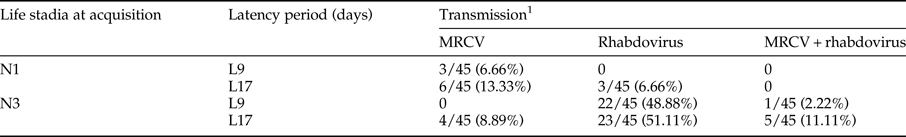
To evaluate the influence of rhabdovirus presence on MRCV transmission efficiency, experimental transmission assays were performed considering four different treatments: virus acquisition by first-(N1) or third-(N3) instar nymphs and latency periods of nine-(L9) or 17-(L17) days. Insects from these assays were classified as transmitting (T) or non-transmitting (NT) based on visual observations of MRCV symptom development in wheat and MRCV detection by DAS-ELISA in all inoculated plants. MRCV transmission efficiency of each treatment and from single (MRCV source) or from mixed infections assays (MRCV-rhabdovirus source) is presented in tables 1 and 2, respectively.
Table 1. Number of MRCV-transmitting Delphacodes kuscheli individuals obtained from single infection assays, involving different treatments defined by life stadia at acquisition (first or third instars) and latency periods (9 or 17 days).

N1 = first instar; N3 = third instar; L9 and L17 = latency period (9 or 17 days after AAP, respectively).
1 Different letters indicate significant differences at P < 0.05.
2 Number of transmitting planthoppers over the total numbers of individuals tested.
Table 2. Number of Delphacodes kuscheli transmitting MRCV, rhabdovirus or both MRCV + rhabdovirus, obtained from a mixed inoculum, involving different treatments defined by life stadia at acquisition (first or third instars) and latency period (9 or 17 days).
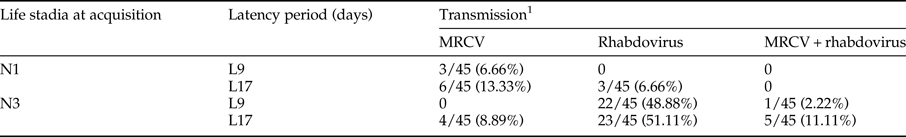
The treatments were the same as those considered in table 1.
1 Number of transmitting planthoppers over the total numbers of individuals tested.
When MRCV was acquired from a single inoculum (table 1), the highest virus transmission efficiency was obtained with the third-instar nymphs and a latency period of 17 days (N3L17), followed by treatments N3L9 and N1L17. The lowest transmission efficiency was observed with N1L9. The relationship between transmission efficiency and treatments was found to be significant (P = 0.0306) after analysis using X 2 test for independence. This result indicates that MRCV transmission efficiency from single infection assays was related to treatments (life stadia at acquisition and latency periods).
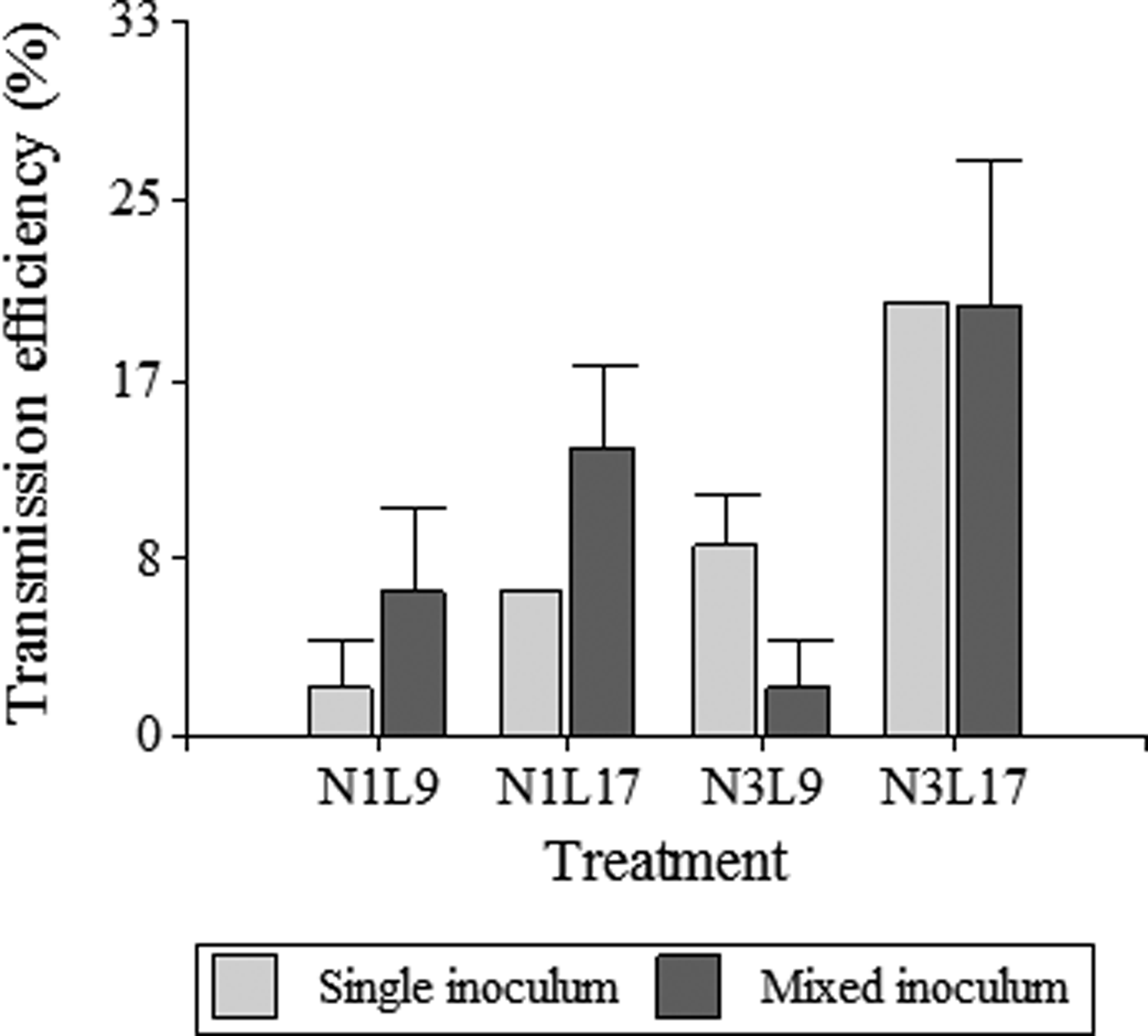
When MRCV was acquired from a mixed MRCV-rhabdovirus inoculum, transmitting insects were able to transmit only MRCV or rhabdovirus, or both viruses (MRCV + rhabdovirus) (table 2). MRCV transmission efficiency for each treatment and from single (MRCV) or mixed (MRCV-rhabdovirus) infection assays is summarized in fig. 1.
Treatment N3L17 also showed the highest MRCV transmission efficiency (20%), considering the total MRCV transmission efficiency of D. kuscheli as the sum of MRCV transmitting insects plus MRCV + rhabdovirus transmitting insects (table 2; fig. 1). Treatment N3L17 was followed by N1L17 and N1L9 in transmission efficiency. Unlike single infection assays, treatment N3L9 from mixed infection assays had the lowest MRCV transmission efficiency. Vector transmission efficiency was also related to treatments (life stadia at acquisition and latency periods) (P = 0.025). Nevertheless, no correlation between transmission efficiency and type of inoculum used to infect insects with MRCV was found (P = 0.725). These result showed that N3L17 was the most efficient treatment for MRCV transmission, regardless of the type of inoculum. Moreover, the MRCV transmission efficiency was not influenced by the rhabdovirus presence within the vector. Interestingly, acquisition by first-instar nymphs did not result in transmission of both viruses simultaneously in any of the two latency periods considered (table 2).
Symptom expression
Wheat plants co-infected with MRCV and rhabdovirus exhibited typical MRCV symptoms earlier (15–20 dpi; P = 0.004–0.00055) than plants singly infected with MRCV (30 dpi; P = 1). No differences in the time of symptom appearance (10–15 dpi) were observed between co-infected plants and plants singly infected with rhabdovirus. Control plants did not show symptoms of viral diseases. This result indicates that the timing of the expression of MRCV symptoms is altered by co-infection with the rhabdovirus.
MRCV symptoms in wheat include erect, dark green colored and twisted leaves, presence of tillers and plants with a dwarfism appearance. In turn, rhabdovirus symptoms are the presence of chlorotic streaking on some leaves and yellowing. Interestingly, plants co-infected with both viruses showed a combination of the symptoms observed in single infections for each virus. These results indicate that MRCV and rhabdovirus interacted in an additive manner and co-infection did not induce new or more severe symptoms.
Relative quantification of MRCV titers in D. kuscheli
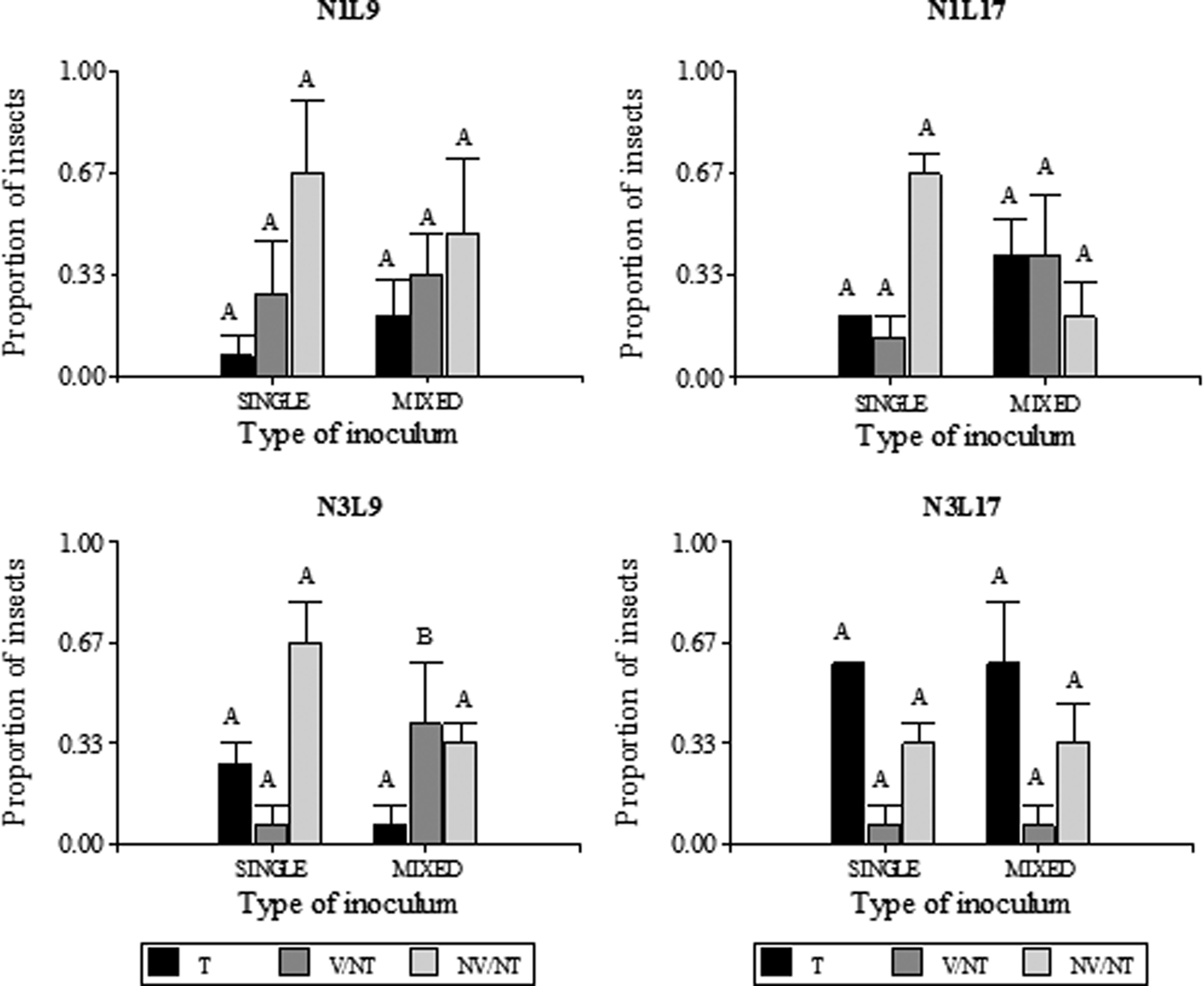
To compare the MRCV titers between transmitting and non-transmitting planthoppers obtained from single or mixed infection assays, the relative accumulation of MRCV was analyzed. Although all the MRCV-transmitting insects (T) were qPCR-positive, some were viruliferous (qPCR-positive) but non-transmitters (NT). This group of insects was classified as viruliferous non-transmitting (V/NT). The NT insects with no detectable levels of MRCV-S3 were denominated non-viruliferous/non-transmitting (NV/NT). The proportion of T, V/NT and NV/NT insects for each life stadium at acquisition (N1: first instars and N3: third instars), latency period (9 or 17 days after AAP) and type of inoculum at the moment of acquisition (single or mixed) are shown in fig. 2.
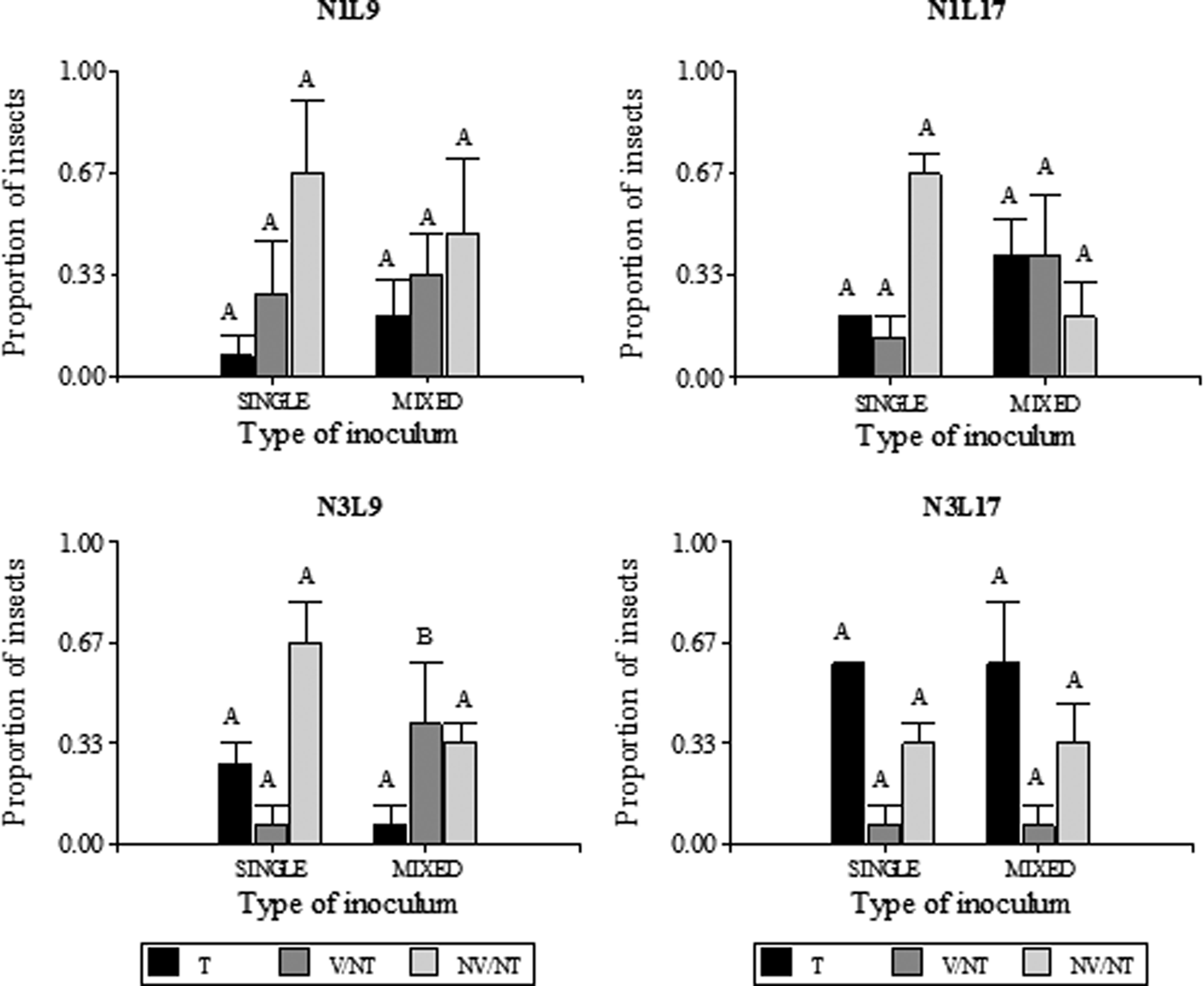
Fig. 2. Proportion of MRCV transmitter (T), viruliferous non-trasmitter (V/NT) and non-viruliferous non-transmitter (NV/NT) D kuscheli obtained from single and mixed infection assays. Different life stadia at acquisition (N1 and N3) and different latency periods (9 or 17 days) were considered (n = 15). Planthoppers were classified as MRCV viruliferous or non-viruliferous according to RT qPCR amplification of a portion of the MRCV-S3 genomic segment. MRCV viruliferous planthoppers were further classified as transmitter or non-transmitter, according to symptom development and ELISA test of the plant tissues. Different letters above bars indicate significant differences (single vs. mixed) at P < 0.05.
The proportion of T insects for each type of inoculum at the moment of acquisition (single or mixed) was similar among treatments. No significant association was observed among treatments N1L9 (P = 0.3173), N1L17 (P = 0.1573), N3L9 (P = 0.1797), N3L17 (P = 0.6171) and type of inoculum used for vector infection. This trend was also observed for NV/NT, which also did not show a significant relationship among treatments and type of inoculum (N1L9: P = 0.7963; N1L17: P = 0.0833; N3L9: P = 0.7963; N3L17: P > 0.999). However, regarding the proportion of V/NT insects, the relationship between N3L9 treatment and type of inoculum was significant (P = 0.0339), indicating that the V/NT proportion is related to the type of inoculum used to obtain the infected insects. This result was not observed for the other treatments, since no association was observed between the V/NT proportion and type of inoculum (N1L9: P = 0.7389; N1L17: P = 0.1573; N3L17: P > 0.999). Again, we observed that the MRCV transmission efficiency is not influenced by the rhabdovirus presence within the vector. On the other hand, these analyses revealed that the proportion of V/NT in N3L9 treatment, obtained from a mixed infection assay, is higher than that obtained from single infection.
Then, the data obtained by qPCR was statistically analyzed. Quantification of MRCV did not show significant differences in MRCV viral load of T insects among treatments (first- and third- instar nymphs at 9 or 17 days of latency period), with either type of inoculum (single or mixed). No differences were also observed between viruliferous non-transmitters; therefore, insects were classified according to their MRCV transmission capacity (T and NT). To perform the statistical analysis between types of inocula using the Pfaffl method (Pfaffl et al., Reference Pfaffl, Horgan and Dempfle2002), we considered non-transmitter insects and transmitter insects as ‘control’ and ‘treatment’ groups, respectively. The results of this analysis showed that T insects exhibited higher relative abundance of MRCV-S3 (viral load) than NT, in insects that acquired MRCV both from a single inoculum (P = 0.0004) and from a mixed inoculum (MRCV-rhabdovirus) (P = 0.0042), indicating a strong association between viral load and MRCV transmission capacity of D. kuscheli.
Viral load of T insects was also analyzed according to the source of MRCV acquisition (single or mixed inoculum), considering MRCV load in T insects from a single inoculum as ‘control’ group and MRCV load in T insects from a mixed inoculum as ‘treatment’. Here, T insects from single infection assays resulted in higher MRCV load than T insects from mixed infection assays (P = 0.0018) (table 3; fig. 3). By contrast, the same analysis performed for the NT insect group showed no significant differences between types of inoculum (single or mixed) used for vector infection (P = 0.1892) (table 3). This result indicates that the rhabdovirus causes a reduction of MRCV titers in transmitting planthoppers co-infected with both viruses.

Fig. 3. Relative MRCV load in Delphacodes kuscheli transmitters obtained from single and mixed infection assays (n = 60), represented by mean relative abundance of MRCV-S3 relative to expression of the endogenous gene Ubiquitin. SINGLE: insects that acquired MRCV from a single inoculum and transmitted MRCV; MIXED Rh+: insects that acquired MRCV from a mixed inoculum and transmitted MRCV and rhabdovirus; MIXED Rh-: insects that acquired MRCV from a mixed inoculum and transmitted MRCV but did not transmit rhabdovirus.
Table 3. MRCV-S3 relative abundance in MRCV transmitting (T) and non-transmitting (NT) D. kuscheli planthoppers comparing single (MRCV-infected plant) and mixed (MRCV-rhabdovirus-infected plant) sources as determined by RT qPCR.

1 Relative to expression of the endogenous gene Ubiquitin.
2 Standard Error.
Regarding the viral load in MRCV-transmitting insects from mixed infection assays, we observed that MRCV accumulation was similar between MRCV + rhabdovirus transmitters (Rh+) and MRCV transmitters that did not transmit rhabdovirus (Rh-) (fig. 3). This result suggests that the transmission capacity of the rhabdovirus by D. kuscheli does not modify the MRCV titers in a co-infection.
Discussion
This work explored the possible interactions between two important viruses affecting cereal crops within their natural planthopper vector species. Both viruses are transmitted by Delphacid vectors in a persistent propagative manner (Arneodo et al., Reference Arneodo, Guzmán, Conci, Laguna and Truol2002; Dumón et al., Reference Dumón, Mattio, Argüello Caro, Alemandri, Puyané, del Vas, López Lambertini and Truol2015); hence, throughout the transmission cycle of these viruses, there is a close association between the proteins coded by the virus and vector constituents, based mainly on the specific protein–protein interactions. Current knowledge of the vector components involved in viral infection for persistent propagative viruses is still limited (Barandoc-Alviar et al., Reference Barandoc-Alviar, Badillo-Vargas, Whitfield, Czosnek and Ghanim2016). Several molecular studies have focused on evidencing the mechanisms underlying transmission efficiency to develop more efficient strategies of disease control (Hogenhout et al., Reference Hogenhout, Ammar, Whitfield and Redinbaugh2008).
There are previous reports on differences in MRCV transmission efficiency by D. kuscheli related to the vector development stadium at virus acquisition and latency period (Arneodo et al., Reference Arneodo, Guzmán, Conci, Laguna and Truol2002; Argüello Caro et al., Reference Argüello-Caro, Maroniche, Dumón, Sagadin, del Vas and Truol2013). Here, we evaluated whether those differences in vectorial capacity persist when insects acquire MRCV from a mixed inoculum (source plants infected with MRCV and rhabdovirus). We also explored possible differences in MRCV load between those treatments by comparing infected insects fed from single or mixed inoculum. MRCV load in transmitting insects were not different treatments (first and third instar nymphs; 9 and 17 days latency period). The same occurred in non-transmitting insects, indicating that viral load in MRCV transmitting and non-transmitting insects did not vary with the nymphal stadia submitted to virus acquisition or latency periods.
Furthermore, the highest MRCV transmission efficiency by D. kuscheli was found when virus was acquired by third-instar nymphs and after latency of 17 days (table 1). This result does not agree with findings by Arneodo et al. (Reference Arneodo, Guzmán, Conci, Laguna and Truol2002) and Argüello Caro et al. (Reference Argüello-Caro, Maroniche, Dumón, Sagadin, del Vas and Truol2013), who reported the highest transmission efficiency after acquisition by first-instar nymphs and 17 days of latency period. Accordingly, Hardy et al. (Reference Hardy, Houk, Kramer and Reeves1983) observed intraspecific variations in virus transmission capacity in different mosquito populations, which were attributed to barrier systems that avoid/facilitate infections of various cells or tissues, or that hinder dissemination in tissues, affecting disease epidemiology. Lambrechts et al. (Reference Lambrechts, Quillery, Noël, Richardson, Jarman, Scott and Chevillon2013) showed that different mosquito populations are resistant or sensitive to dengue virus (DENV) because they present a natural polymorphism in the Dicer-2 gene (dcr2). Since this gene codes for a protein that acts early in the event cascade leading to gene silencing, these results suggest that this defense mechanism is determinant in defining virus-insect specificity in this system. These variations observed between individuals of a single species in the barrier system or the existence of different alleles of the Dicer-2 among insect populations might explain the different MRCV transmission efficiency of D. kuscheli, since the populations used in this study were different from those used by Arneodo et al. (Reference Arneodo, Guzmán, Conci, Laguna and Truol2002) and Argüello Caro et al. (Reference Argüello-Caro, Maroniche, Dumón, Sagadin, del Vas and Truol2013). Moreover, in this study we also used a MRCV inoculum different from that used by these authors.
D. kuscheli individuals that acquired MRCV from a mixed inoculum (MRCV-rhabdovirus) at the first-stadia were then unable to transmit both viruses to non-infected plants. This result suggests that at this nymph stadium, both viruses in co-infection within the vector would be unable to reach the salivary glands together. Very few combinations of persistent propagative viruses have been studied in relation to the possible interaction within the vector (Ammar, Reference Ammar and Harris1994). A study conducted in Maize mosaic virus (MMV, Nucleorhabdovirus) and Maize stripe virus (MSpV, Tenuivirus,) revealed that when the vector Peregrinus maidis acquires MMV before acquiring MSpV, MSpV transmission is significantly reduced. By contrast, acquisition of MSpV by P. maidis did not influence MMV acquisition and/or transmission (Ammar et al., Reference Ammar, Gingery and Nault1987). Furthermore, no evidences of interaction were found between Wound tumor virus (WTV, Phytoreovirus, Reoviridae) and Potato yellow dwarf virus (PYDV, Nucleorhabdovirus, Rhabdoviridae) when transmitted simultaneously by the leafhopper Agallia constricta (Nagaraji & Black, Reference Nagaraji and Black1962). Further research is necessary to determine the type of interaction occurring in mixed infections of MRCV and rhabdovirus, specifically when both viruses are acquired simultaneously at the first-instar nymph stadium.
Wheat plants co-infected with MRCV and rhabdovirus showed MRCV symptoms earlier (15–20 dpi) than plants infected only with MRCV (20–30 dpi), indicating that co-infection anticipates the appearance of symptoms. Similar results were reported for mixed infections with SRBSDV and RRSV (both viruses of the family Reoviridae), with 50% of co-infected rice plants showing the typical symptoms of both viral diseases at 15 dpi, whereas plants infected with a single virus did not show symptoms at that early time after infection (Li et al., Reference Li, Wang and Zhou2014). The early appearance of MRCV symptoms in mixed infections might be due to physiological effects associated with an increase in the load of one or both viruses in the plant during co-infection, as reported for other mixed infections (Wintermantel, Reference Wintermantel2005). Furthermore, Guo et al. (Reference Guo, Song, Xie, Huo, Zhang, Chen, Geng and Fang2013) reported that plants of N. benthamiana and N. tabacum co-infected with a chimerical PVX expressing protein P6 of RYSV rhabdovirus showed more severe symptoms than plants infected only with PVX. These results suggest that P6 could have a role as an RNA silencing suppressor (RSS), and that the increase of virulence would be associated with an increase in viral RNA accumulation (Guo et al., Reference Guo, Song, Xie, Huo, Zhang, Chen, Geng and Fang2013). Likewise, protein P (phosphoprotein) of Lettuce necrotic yellows virus (LNYV, Cytorhabdovirus, Rhabdoviridae) has been found to exhibit RNA silencing activity in N. benthamiana plants (Mann et al., Reference Mann, Johnson and Dietzgen2015). To date, no suppressors of gene silencing active in plants or insects were identified after analyzing 10 of the 13 MRCV virus proteins (Mongelli, Reference Mongelli2010), whereas these suppressors of gene silencing have been reported for other viruses of the family Reoviridae (Cao et al., Reference Cao, Zhou, Zhang, Zhu, Zhong, Xiao, Ding and Li2005; Liu et al., Reference Liu, Zhao, Ruan, He and Li2008; Bo et al., Reference Bo, Guo, Gao, Zhou, Wu, Meng, Wei and Li2010; Wu et al., Reference Wu, Wang, Du, Cai, Hu, Wu, Li and Xie2011).
The analysis of D. kuscheli T, V/NT and NV/NT obtained from single or mixed infection assays showed that the highest proportion of V/NT with treatment N3L9 was obtained when MRCV was acquired from a mixed inoculum (fig. 2). This failure in transmission could be that MRCV cannot reach the salivary glands during a short latency period (9 days), even when the virus is actively replicating in other tissues. However, further studies will be necessary to elucidate the type of interaction established between these viruses at the molecular level that consequently affect the proportion of V/NT insects and transmission efficiency of the vector D. kuscheli.
Regarding MRCV quantification in the vector, it was established that D. kuscheli MRCV transmitters have a higher viral load than non-transmitters (Argüello Caro et al., Reference Argüello-Caro, Maroniche, Dumón, Sagadin, del Vas and Truol2013). Here, we compared viral load of both D. kuscheli MRCV transmitters and non-transmitters between individuals that acquired the virus from a single inoculum and from a mixed inoculum. Our results are consistent with those of Argüello Caro et al. (Reference Argüello-Caro, Maroniche, Dumón, Sagadin, del Vas and Truol2013) for single infections with MRCV. MRCV transmitter planthoppers had significantly higher viral load values than non-transmitters, regardless of the source inoculum (single or mixed). Accordingly, viral load is known to be directly associated with insect susceptibility (Contamaine et al., Reference Contamaine, Petitjean and Ashburner1989; Ziegler & Morales, Reference Ziegler and Morales1990) and immunity mechanisms play an important role in viral infection of insects vectors (Ammar et al., Reference Ammar, Tsai, Whitfield, Redinbaugh and Hogenhout2009). Transcriptomic analyses of different insect vectors using high throughput approaches allowed the identification of transcripts that might play a role in viral infection and replication cycle, such as those participating in immune pathways, endocytosis and exocytosis (Barandoc-Alviar et al., Reference Barandoc-Alviar, Badillo-Vargas, Whitfield, Czosnek and Ghanim2016).
Interestingly, we observed that viral load of MRCV-transmitting insects was higher in individuals that acquired the virus from a single inoculum than in those infected with a mixed inoculum (table 3), regardless their rhabdovirus transmission capacity (fig. 3). The lower viral load achieved in insects from a mixed infection assays might be related to a decrease in the capacity of MRCV to accumulate in the presence of another virus. Regarding that, it is known that viral titer depends on the balance between virus replication and vector's defense mechanisms (Contamaine et al., Reference Contamaine, Petitjean and Ashburner1989; Zambon et al., Reference Zambon, Nandakumar, Vakharia and Wu2005) and, in turn, the activation of these mechanisms differs with the infecting agent. In Drosophila, humoral response to microbial infections consists of activation of the Toll and IMD pathways (De Gregorio et al., Reference De Gregorio, Spellman, Rubin and Lemaitre2001). Using Drosophila and Drosophila X virus (DXV, a non-enveloped virus) as models, it has been determined that, while viral infection activates both pathways, only activation via Toll leads to a reduction in viral titer (Zambon et al., Reference Zambon, Nandakumar, Vakharia and Wu2005). By contrast, upon Drosophila to infection with the rhabdovirus Sigma virus (with a lipid envelope) the expression of several antimicrobial peptides was induced but did not affect the expression of genes of the Toll pathway (Tsai et al., Reference Tsai, Mc Graw, Ammar, Dietzgen and Hogenhout2008). Activation of different defense mechanisms against diverse viruses might limit or favor replication of one or both viruses during co-infection.
The similar load of MRCV detected in MRCV V/NT insects that acquired the virus from single or mixed inoculum (table 3) supports the assumption that viral accumulation is directly associated with insect susceptibility; therefore, whether an insect is transmitter or not will depend on whether the insect exceeds a given accumulation threshold (Argüello Caro et al., Reference Argüello-Caro, Maroniche, Dumón, Sagadin, del Vas and Truol2013). Thus, in non-transmitters, the vector's defense mechanisms would keep titer levels low, regardless of whether the infection is single or mixed. Future high throughput molecular studies will allow the identification of molecular pathways involved in planthopper defense to virus infection and will aid in the design of novel strategies to limit transmission.
Acknowledgements
This work was supported by Research projects PICT 2006 No. 0358 and PICT 2012 No. 0391 from the Argentine Agency for Promotion of Science and Technology, PID 2010 (Res.113/2011) from the Ministry of Science and Technology of Córdoba Province, AEPV 214012 from the National Institute of Agricultural Technology and ArgenInta Foundation. A.D.D. holded doctoral and post-doctoral fellowships from the Fondo para la Investigación Científica y Tecnológica (FONCyT).