Introduction
Recent advances in biomedical studies have enabled the modification of various important genes in whole animals, resulting in the explosive increase in the numbers of genetically modified (GM) mice to examine functions of specific genes and their interactions (Simpson et al., Reference Simpson, Linder, Sargent, Davisson, Mobraaten and Sharp1997; Critser & Mobraaten, Reference Critser and Mobraaten2000). In order to maintain these mice, assisted reproductive technologies (ARTs) have become important tools for laboratory animal facilities. Among various techniques of ARTs, in vitro fertilization (IVF) plays an important role to efficiently obtain large numbers of embryos. However, the current IVF conditions, which are based on studies using outbred or hybrid mice (Toyoda et al., Reference Toyoda, Yokoyama and Hoshi1971; Fraser, Reference Fraser1993), are not always successfully applied to inbred stains of mice used in biomedical research, such as C3H, BALB/c and 129 (Thornton et al., Reference Thornton, Brown and Glenister1999; Sztein et al., Reference Sztein, Farley and Mobraaten2000; Byers et al., Reference Byers, Payson and Taft2006; Hino et al., Reference Hino, Oda, Nakamura, Tateno, Toyoda and Yokoyama2011). Various studies by our laboratory and others have indicated that IVF conditions need to be determined on a strain by strain basis (Kito & Ohta, Reference Kito and Ohta2005, Byers et al., Reference Byers, Payson and Taft2006; Vasudevan et al., Reference Vasudevan, Raber and Sztein2010). For example, in C57BL/6 and BALB/c mice, an increased concentration of calcium during sperm:ova coincubation was reported to improve fertilization (Itagaki & Toyoda, Reference Itagaki and Toyoda1992; Kito & Ohta, Reference Kito and Ohta2008a,Reference Kito and Ohtab). In the 129 strain, the interval between ovulation and sperm insemination has been shown to be important because precocious release of cortical granules before sperm penetration through the ooplasm causes a modification of the zona pellucida resulting in reduced fertilization levels (Hino et al., Reference Hino, Oda, Nakamura, Tateno, Toyoda and Yokoyama2011).
In this study we focused on IVF in the inbred C3H/He strain, which has been widely used for more than half a century in areas of biomedical research such as immunology and inflammation, sensorineural, cardiovascular biology as well as radiation biology (detail information can be obtained at http://www.informatics.jax.org/external/festing/mouse/docs/C3H.shtml). As with other major strains such as C57BL/6, BALB/c and 129, mutants and many GM strains have been developed. Although the C3H/He strain shows good reproductive performance among inbred strains (Nagasawa et al., Reference Nagasawa, Miyamoto and Fujimoto1973), the use of IVF to obtain large number of zygotes for cryopreservation and colony expansion has not been necessarily successful (Thornton et al., Reference Thornton, Brown and Glenister1999; Byers et al., Reference Byers, Payson and Taft2006). We previously showed that when a high concentration of calcium (5.13 mM) was used in the IVF of cumulus intact C3H/He ova, sperm penetration through the zona pellucida and resulting fertilization improved compared with IVF using modified Kreb–Ringer's bicarbonate solution, called TYH (Kito et al., Reference Kito, Hayao, Noguchi-Kawasaki, Ohta, Hideki and Tateno2004). However, when cumulus-free ova were fertilized under these conditions in the preliminary studies, we noticed an increased deficiency in the extrusion of the second polar body (PBII), resulting in the triploid ova with two female and one male pronuclei.
Calcium has been known to play important roles in almost all processes of fertilization including sperm capacitation, sperm interaction with zona pellucida and oolemma and the following egg activation (Yanagimachi, Reference Yanagimachi, Knobil and Neill1994). During egg activation, signals from sperm induce sustained oscillational changes in the intracellular concentration of calcium, which then leads to major events of egg activation for further development including exocytosis of cortical granules, exit from the metaphase of the second meiotic division, extrusion of the second polar body (PBII) and embryonic genome activation (Reviewed in Ducibella et al., Reference Ducibella, Schultz and Ozil2006). The role of calcium during IVF at lower than physiological levels (i.e. ≤1.71 mM) has been studied in various mammalian species (Yanagimachi, Reference Yanagimachi, Knobil and Neill1994). However, the few studies on the effects of high concentrations of calcium on IVF have produced inconsistent results; some authors showed reduced fertilization (Miyamoto & Ishibashi, Reference Miyamoto and Ishibashi1975; Fraser, Reference Fraser1987), while others showed increased fertilization (Kaplan & Kraicer, Reference Kaplan and Kraicer1978; Huneau & Crozet, Reference Huneau and Crozet1989; Itagaki & Toyoda, Reference Itagaki and Toyoda1992; Kito & Ohta, Reference Kito and Ohta2008a). All of these reports showed increases or decreases in fertilization judged by sperm penetration through the zona pellucida and ooplasm or development to 2-cell stage, but the role of increased calcium in inducing abnormal fertilization processes was not assessed. Our study may be the first to report that increased calcium induces abnormalities in fertilization process per se.
Materials and methods
Animals
Inbred C3H/He mice were obtained from the specific-pathogen-free breeding facility of the National Institute of Radiological Sciences, Japan. C57BL/6N × DBA/2N (BDF1) mice were purchased from CLEA Japan, Inc. (Tokyo, Japan). Animals were maintained at 23 ± 2°C under a light regimen of 12 L:12 D condition (lights on from 07:00 to 19:00). All animals were handled and treated according to the Recommendations for Handling of Laboratory Animals for Biomedical Research, compiled by the Institutional Animal Care and Use Committee for Laboratory Animal Experiments, the National Institute of Radiological Sciences, Japan.
Medium preparation
All salts were purchased from Nacalai Tesque Inc. (Kyoto, Japan), unless indicated otherwise. The osmolarities of media were measured by a freezing-point depression osmometer (OM802, Vogel, Giessen, Germany). Media lacking pyruvate, glutamine and bovine serum albumin (BSA) were stored at 4°C for no longer than 1 week. Pyruvate (P4562, Sigma-Aldrich Co., St. Louis, MO, USA), glutamine (G5763, Sigma-Aldrich Co.), BSA (A3311, Sigma-Aldrich Co.), minimal essential amino acid solution (11130051, Invitrogen, Carlsbad, CA, USA) and non-essential amino acid solution (11140050, Invitrogen) were added as necessary immediately before equilibration. Media for IVF were equilibrated overnight under 5% CO2 in air at 37°C and saturated humidity and embryo culture medium was equilibrated for more than 2 h under 5% CO2, 5% O2 and 90% N2 at 37°C and saturated humidity.
Gamete preparation
The procedure of gamete collection was described previously (Kito & Ohta, Reference Kito and Ohta2008a). Briefly, females 8 to 16 weeks of age were injected with 5 IU pregnant mares serum gonadotropin (PMSG, Serotropin; Aska Pharmaceutical Co., Ltd, Tokyo, Japan) and 5 IU human chorionic gonadotropin (hCG, Gonadotropin; Aska Pharmaceutical Co., Ltd) 47–49 h apart. The cumulus–oocyte complexes (COCs) were collected 15–16 h post hCG injection. Cumulus cells were removed by flushing and handling medium (FHM) (Lawitts and Biggers, Reference Lawitts and Biggers1993) supplemented with 1 mg/ml bovine testis hyaluronidase (type I-S, H3506, Sigma-Aldrich Co.) and 0.01 mg/ml soybean trypsin inhibitor (202–09221, Wako Pure Chemical Industries, Ltd, Osaka, Japan). In the study using cumulus-free ova, ova from individual animals were equally distributed into each treatment. When COCs were inseminated, the similar mass of the COCs from individual animals were distributed into each treatment. Sperm were collected from the distal cauda epididymides of males 10–24 weeks of age under mineral oil (M8410, Sigma-Aldrich Co.). The medium used for sperm capacitation was modified Krebs–Ringer's bicarbonate solution (mKRB), which includes 127.67 mM NaCl, 4.78 mM KCl, 1.19 mM KH2PO4, 1.71 mM CaCl2, 1.19 mM MgSO4, 25.07 mM NaHCO3, 5.56 mM glucose, 1 mM Na-pyruvate, 0.05 mg/ml streptomycin sulfate (S1277, Sigma-Aldrich Co.), 100 IU/ml penicillin G (K-salt, P4679, Sigma-Aldrich Co.) and 4.0 mg/ml BSA. Sperm were capacitated at the concentration of 1–2 × 107 cells/ml for 1.5 to 2 h under 5% CO2 in air at 37°C.
IVF and evaluation of fertilization
Basic medium used for sperm:ova coincubation was modified human tubal fluid (mHTF) (Kito & Ohta, Reference Kito and Ohta2008a) with various concentrations of calcium. The composition of mHTF was 101.61 mM NaCl, 4.69 mM KCl, 0.40 mM KH2PO4, 0.20 mM MgSO4, 25.00 mM NaHCO3, 18.36 mM Na-lactate (L7900, Sigma-Aldrich Co.), 2.78 mM glucose, 0.34 mM Na-pyruvate, 0.05 mg/ml streptomycin sulfate (S1277, Sigma-Aldrich Co.), 100 IU/ml penicillin G (P7794, Sigma-Aldrich Co.) and 4 mg/ml BSA. Calcium concentration was adjusted by changing the concentration of CaCl2 between 1.71 and 6.84 mM with constant osmotic pressure (303 ± 5 mOsmol) adjusted by NaCl. Capacitated sperm were inseminated into 100 µl drops of media covered with the mineral oil in 60 mm Petri dish (no. 1007, Becton Dickinson, Franklin Lakes, NJ, USA) under 5% CO2 in air at 37°C at the final concentration of 1–2 × 105 sperm/ml. When ova needed to be transferred from one to another conditions during 5 h sperm:ova coincubation, they were gently moved using wide bore pipettes (>150 μm diameter) without disturbing sperm attached to the zona pellucida. At 5 h post-insemination (PI), ova were washed a few times to remove attached but non-penetrating sperm and fixed in 2% formaldehyde and 2% glutaraldehyde (Kito & Ohta, Reference Kito and Ohta2008a). Fixed ova were mounted on glass slides and overlaid with coverslips supported by a 3:1 mixture of paraffin wax:vaseline. Ova were stained with aceto-orcein and examined for the PBII extrusion and formation of male and female pronuclei (PNs) by Nomarski interference microscopy (Nikon Optiphot, Tokyo, Japan). Fertilized ova were defined as those which had incorporated sperm nuclei or male PN, and normally fertilized ova were defined as those with a single extruded PBII in the perivitelline space, single female PN, and male PN associated with sperm tail in the ooplasm.
Parthenogenetic activation of ova by ethanol
Activation of cumulus-free ova by ethanol was based on the method of Kaufman (Reference Kaufman1982). Concentration of ethanol and incubation time was determined in the preliminary experiments and incubation with 5.25% of ethanol for 4.5 min was applied because this condition resulted in the highest percentage of ova activation. After activation, ova were incubated in various concentrations of calcium for 5 h and examined for PN formation and PBII extrusion after fixation as described above.
Embryo culture
At 5 h PI, inseminated ova were examined under inverted microscope (Leica DMI 3000B, Wetzlar Germany) equipped with micromanipulator for ovum selection based on the PBII status. Then, the ova were cultured in 50 µl of KSOMaa (Ho et al., Reference Ho, Wigglesworth, Eppig and Schultz1995) under 5% CO2, 5% O2, and 90% N2 at 37°C for 96 h to examine developmental competence. After 96 h of culture, some blastocysts were used for chromosome counts or for differential staining.
Chromosome counts
Air-dried slides were prepared by method described by Takagi et al. (Reference Takagi, Wake and Sasaki1978). Blastocysts were treated with 0.05 µg/ml colcemid (D7385, Sigma-Aldrich Co.) for another 6 h and were removed from the zona pellucida in acid Tyrode's solution at pH 2.5. Then, they were incubated in 1% sodium citrate for 10 min at room temperature, and fixed with a 3:1 mixture of methanol:acetic acid at −20°C. After overnight fixation, a single embryo in a small drop of fixative was placed on a clean slide glass and a drop of lactate solution (1:1:6 mixture of distilled water:sodium lactate:acetic acid) was added immediately. When blastomeres began to dissociate, a drop of 9:3:4 mixture of methanol:acetic acids:distilled water was applied a few times. The slides were allowed to dry in the air after application of 3:1 mixture of methanol:acetic acid and stained by conventional Giemsa staining to count chromosomes.
Differential staining of inner cell mass (ICM) and trophectoderm (TE)
Some expanded blastocysts were stained to count ICM and TE nuclei. Method of staining was as described in Biggers et al. (Reference Biggers, Lynda, McGinnis and Raffin2000). Blastocysts were removed of the zona pellucida by brief exposure to acid Tyrode's solution and washed a few times in FHM. Then, blastocysts were incubated in FHM-PVA with 10% rabbit antiserum to mouse red blood cell (210-4139, Rockland Immunochemicals Inc., Limerick, PA, USA) for 30 min. After three 5-min washes in FHM-PVA, they are transferred into FHM-PVA with 10% guinea pig complement (55854, MP Biomedicals Inc., Aurora, OH, USA), 1 μg/ml Hoechst 33258 (B2883, Sigma-Aldrich Co.), and 1 μg/ml propidium iodide (P4864, Sigma-Aldrich Co.) for 15 min at 37°C, then fixed in 4% paraformaldehyde in PBS-PVP with 1 μg/ml Hoechst 33258 and 1 μg/ml propidium iodide for 1 h. The embryos were moved to glass slide compressed with a coverslip and nuclei were counted under fluorescence microscope (Olympus IX-70, Tokyo, Japan).
Immunofluorescence staining of microtubules and actin filaments
Ova were fixed and permeabilized in PBS with 4% paraformaldehyde and 0.2% Triton X-100 for 1 h at room temperature (RT), followed by washing in blocking solution (PBS with 10% goat serum) at 4°C overnight. The ova were incubated with a mice-monoclonal anti-α-tubulin antibody (diluted 1:500; T9026, Sigma-Aldrich Co.) for 1 h at RT. After washing in PBS with 3 mg/ml BSA, the samples were stained with FITC-conjugated goat anti-mice IgG antibody (diluted 1:500; F8264, Sigma-Aldrich Co.) for 1 h at RT. Then, the ova were incubated with rhodamine-conjugated phalloidin (0.2 µg/ml; P1951, Sigma-Aldrich Co.) for 1 h at RT to stain the actin filaments, which were then mounted on slide glasses and sealed by Vectashield with 4,6-diamidio-2-phenylindole (DAPI) (H-1500, Vector Labs, Burlingame, CA, USA). Images were captured ×40 magnification objectives using a confocal scanning laser microscope (FV-1000, Olympus, Tokyo, Japan) with appropriate wave lengths.
Immunofluorescence staining of beta- and gamma-cytoplasmic actins
Double staining of beta- and gamma-cytoplasmic actin (CYA) was performed following the protocol of Brockmann et al. (Reference Brockmann, Huarte, Dugina, Challet, Rey, Conne, Swetloff, Nef, Chaponnier and Vassalli2011) with modifications. Ova were fixed in PBS with 0.1% PVP (194017, ICN Biomedicals Inc., Aurora, OH, USA) and 1% paraformaldehyde for 1 h at 37°C. After 1 h washing in PBS–PVP, ova were permeabilized for 5 min in ice cold methanol, washed three times in PBS–PVP and then incubated in PBS with 10% goat serum (G9023, Sigma-Aldrich Co.) for 1 h at RT. After washing three times in PBS-PVP, ova were incubated with primary antibodies of 1:50 dilution of beta-CYA (4C2 IgG1, ab123020, Abcam, Cambridge, UK) and 1:100 dilution of gamma-CYA (2A3 IgG2b, ab123034, Abcam) for 1 h at 37°C, then with a 1:50 dilution of secondary antibodies for 1 h at 37°C. The secondary antibodies were FITC-conjugated goat anti-mouse IgG1 (1070–02, Southern Biotech, Birmingham, AL, USA) for beta-CYA and Rhodamine-conjugated goat anti-mouse IgG2b (1090–03, Southern Biotech). Next, ova were stained with 1 μg/ml Hoechst 33258 for 1 h and mounted on a slide glass with mounting medium (100 mg n-propyl gallate in 0.5 ml 2.3% Na2-citrate and 4.5 ml glycerol) and images were recorded with the confocal microscope as described above.
Statistical analysis
Experiments with numerical data were replicated at least four times for statistical analysis. Percentage data were transformed using arcsin transformation to control unequal variances (Tukey–Freeman transformation) (Zar, Reference Zar1996), and were analyzed by analysis of variance (ANOVA) with a random block design using the SAS program with each male assigned as a block. Multiple comparisons were made using the Least Significant Difference (LSD) test. A probability of P < 0.05 was considered to be statistically significant.
Results
Morphologies of fertilized ova with deficient PBII extrusion
Figure 1 shows representative pictures of ova of normal fertilization (Fig. 1 a) and polyspermic fertilization (Fig. 1 b) found in IVF under 1.71 mM CaCl2, and an ovum without PBII extrusion (Fig. 1 c) found in IVF under 6.84 mM CaCl2 at 5 h PI. Ova without PBII extrusion were easily distinguished from polyspermic ova by the number of sperm tail(s) and existence of PBII.

Figure 1 Representative pictures of C3H/He fertilized ova at 5 h post-insemination (PI). Scale bar indicates approximately 20 μm. PBI: the first polar body. PBII: the second polar body. ♀: female pronucleus (PN). ♂: male pronucleus (PN). In normal fertilized ova under insemination at 1.71 mM calcium (a), single female PN, male PN, PBII and sperm tail (arrows) are clearly observed. In ova of polyspermic fertilization (b), single female PN and two male PNs with associated two sperm tails (out of focus) and PBII are observed. This particular polyspermic ova was found under 1.71 mM calcium. In ova without PBII extrusion fertilized under at 6.84 mM calcium (c), two female PNs and single male PN associated with a single sperm tail (out of focus) were observed.
Extrusion of PBII under various concentrations of calcium
When cumulus-free ova were inseminated at various concentrations of calcium (1.71, 2.57, 3.42, 5.13 and 6.84 mM), no significant difference in total percentages of fertilization was observed (Table 1). However, there were significant increases in the percentages of ova without PBII extrusion from 3.42 mM and above concentrations of calcium (16% at 3.42 mM, 19% at 5.13 mM and 29% at 6.84 mM) compared with controls (2% at 1.71 mM; P < 0.05). Calcium concentration higher than 6.84 mM was not examined because the medium precipitated easily during equilibration. The correlation between calcium concentrations and incidences of ova without PBII was significant (r 2 = 0.5906; P < 0.01).
Table 1 Effects of various concentrations of calcium during sperm:ova coincubation on PBII extrusion of C3H/He ova a
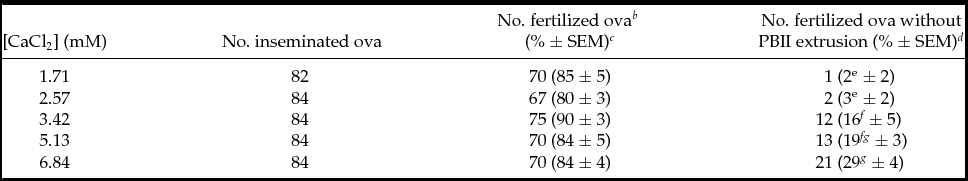
a Total of six replicates of experiments. Cumulus-free ova were inseminated in mHTF with various concentrations of calcium. At 5 h PI, ova were fixed and stained with aceto-orcein for examination under Nomarski interference microscopy.
b Fertilized ova were those with sperm in the ooplasm.
c Percentages of the total ova inseminated. Percentage data were transformed by arcsin transformation and were analyzed by ANOVA.
d Percentages of the fertilized ova. Percentage data were transformed by arcsin transformation for ANOVA.
e-g Percentages with different superscripts within the same column are significantly different (P < 0.05).
Time windows sensitive to high calcium during fertilization
To examine time window when ova are sensitive against high calcium, ova during IVF were transferred from 1.71 mM to 6.84 mM CaCl2 or vice versa at 1, 2 or 3 h PI. When ova were moved from 1.71 mM to 6.84 mM CaCl2, exposure to high calcium before 3 h PI significantly increased percentages of ova without PBII (P < 0.05; Fig. 2 a). When opposite conditions were tested, exposing to high calcium for the first 3 h PI or longer significantly increased percentages of ova without PBII (P < 0.05; Fig. 2 b). These results together indicate that high calcium influences PBII extrusion during 2–3 h PI when the meiotic division resumes after activation of C3H/He ova (see Fig. 3).
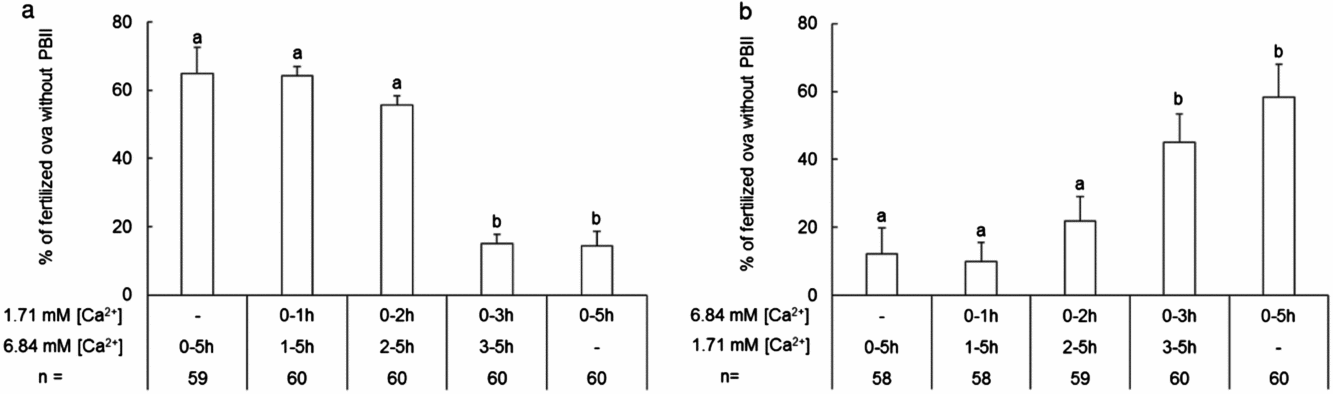
Figure 2 Time windows of calcium effect on the PBII extrusion of C3H/He ova during sperm:ova coincubation. Empty bars and error bars indicate ova without PBII extrusion in percentages and standard error of the mean (SEM) of fertilized ova, respectively. Sperm and ova were coincubated first in low (1.71 mM) calcium for 1, 2, or 3 h then moved to high (6.84 mM) calcium (a), or vice versa (b), and fixed 5 h PI for observation of fertilization. Percentage data were transformed arcsin transformation for ANOVA. n: total number of fertilized ova examined in five replicates. a,bDifferent letters above each bar indicate significant differences (P < 0.05).

Figure 3 Confocal images of cytoskeletal organization of C3H/He ova fertilized in 1.71 or 6.84 mM calcium. The scale bar indicates approximately 20 μm. PBI: the first polar body. PBII: the second polar body. ♀: ova chromosome(s) or female PN(s). ♂: decondensing sperm head or male pronucleus (PN). Microtubules, actin filaments and chromosomes are shown by green, red and blue, respectively. (a) In an ovum at metaphase II before insemination, chromosomes are arranged at the equatorial plate and long axis of the spindle was parallel to the nearest oolemma. (b) At 2 h PI in 1.71 mM calcium, the second meiosis resumes as a result of sperm penetration. The chromosomes separate toward the two spindle poles. The spindle has oriented with its long axis perpendicular to the oolemma, and actin filaments start to form the contractile ring (arrowhead). (c) At 3 h PI in 1.71 mM calcium, the PBII already extruded and contractile ring (arrowhead) has shrunk with the spindle located in the ring. (d) At 2 h PI in 6.84 mM calcium, the ovum has almost completed the second meiosis, while the spindle located with its long axis parallel to the nearest oolemma. Actin filaments start to localize around the oolemma over the equatorial plate of the spindle (arrowhead). (e) At 3 h PI in 6.84 mM calcium, the spindle is still located with its long axis parallel to the nearest oolemma and no sign of PBII extrusion is observed. A clump of actin enlarges compared with 2 h PI (d), but the ring structure is not formed. (f) At 4 h PI in 6.84 mM calcium, the PBII fails to extrude and two female PNs and a single male PN (out of focus) are observed. In this particular ovum, actin filaments form contractile ring-like structure (arrowhead) in the cytoplasm without a sign of cytokinesis.
In vitro developmental ability of ova without PBII
Almost all fertilized ova (≥ 95%) developed to blastocysts after 96 h culture irrespective of PBII extrusion (Table 2). When blastocysts derived from ova without PBII were examined for chromosome count they were all triploid embryos. The numbers of ICM nuclei, but not numbers of TE and total nuclei, of blastocysts fertilized in 6.84 mM CaCl2 were significantly lower than those fertilized in 1.71 mM CaCl2 (P < 0.05).
Table 2 In vitro development, karyotype and nuclear numbers of blastocysts developed from ova with and without PBII extrusion a
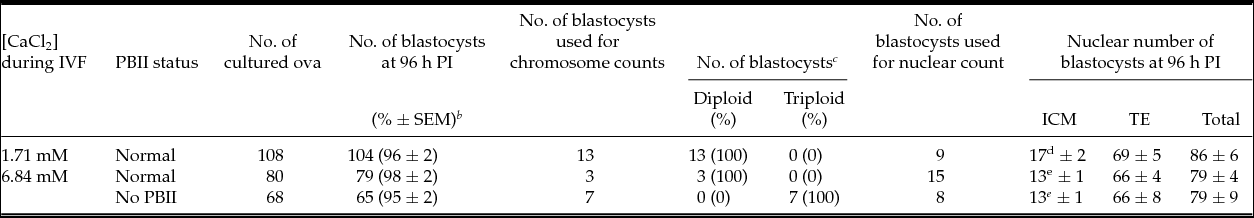
a Total of eight replicates of experiments. After 5 h PI, ova were examined for fertilization and PBII status under inverted Nomarski microscope equipped with micromanipulator. Fertilized ova were cultured in KSOMaa for 96 h.
b Percentage data were transformed by arcsin transformation for ANOVA.
c A part of blastocysts was selected for karyotyping or counting nuclei.
d, e Numbers with different superscripts within the same column are significantly different (P < 0.05).
Effects of cumulus cells on deficiency in PBII extrusion
To examine how cumulus cells influence PBII extrusion at high calcium concentration, cumulus-free ova and COCs were inseminated at 1.71 and 6.84 mM calcium (2 × 2 factorial design). The results are listed in Table 3. Insemination to COCs resulted in significantly higher percentages of fertilization than those of cumulus-free ova (P < 0.05). In addition, the percentages of ova without PBII extrusion in 6.84 mM calcium (30% in COCs and 49% in cumulus-free ova) were significantly higher than those in 1.71 mM calcium (1% in COCs and 10% in cumulus-free ova; P < 0.05) irrespective of the presence or absence of cumulus cells. However, statistical analysis showed a significant effect of cumulus cells (P = 0.002), providing the evidence that the presence of cumulus cells significantly alleviates PBII extrusion deficiency by the high concentration of calcium.
Table 3 Effects of cumulus cells and high concentrations of calcium during IVF on PBII extrusion of C3H/He ova a
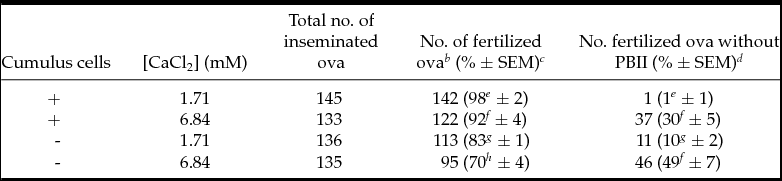
a Total of four replicates of experiments. The cumulus–oocyte complexes (COCs) and the cumulus-free ova were inseminated with capacitated sperm in mHTF under 1.71 and 6.84 mM calcium. At 5 h PI, ova were stained with aceto-orcein for examination under Nomarski interference microscopy.
b Fertilized ova were these with sperm in cytoplasm.
c Percentages of total ova inseminated. Percentage data were transformed by arcsin transformation for ANOVA.
d Percentages of fertilized ova. Percentage data were transformed by arcsin transformation for ANOVA.
e-h Percentages with different superscripts within the same column are significantly different (p < 0.05). Main effects of cumulus cells and calcium concentrations on PBII extrusion are P = 0.002 and P < 0.001, respectively. Interaction between cumulus cells and calcium concentrations about PBII extrusion is not significant (P = 0.061).
Strain specificity of deficiency in PBII extrusion under high calcium concentration
To examine whether disturbed PBII extrusion by a high calcium concentration is a specific phenomenon to C3H/He strain, a hybrid experiment between C3H/He and B6D2F1 was performed by 2 (female gametes) × 2 (male gametes) factorial design at 6.84 mM CaCl2. We chose BDF1 because in our preliminary study this hybrid strain did not show abnormality in PBII extrusion under high calcium concentration at all. Results are shown in Table 4. We found that whenever female gamete were derived from C3H/He, the percentages of ova without PBII extrusion significantly increased irrespective of the inseminated male strains (P < 0.05). Statistical analysis indicated highly significant main effects of female gamete (P < 0.001) whereas no significant main effect of male gamete and their interaction was detected (P = 0.364 and 0.223, respectively).
Table 4 Effects of gamete strains on PBII extrusion under 6.84 mM calcium by hybrid experiment between C3H/He and BDF1 a
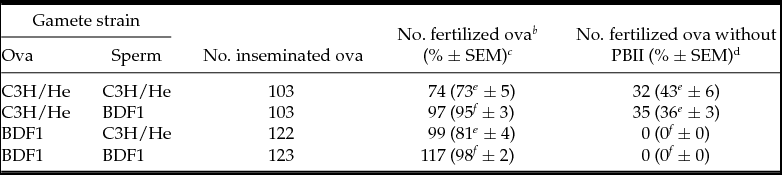
a Total of six replicates of experiments. The cumulus-free C3H or BDF1 ova were inseminated with either C3H or BDF1 sperm at 6.84 mM calcium. At 5 h PI, ova were stained with acetorcein for examination under Nomarski interference microscopy.
b Fertilized ova were these with sperm in cytoplasm.
c Percentages of total ova inseminated. Percentage data were transformed by arcsin for ANOVA.
d Percentages of fertilized ova. Percentage data were transformed by arcsin transformation for ANOVA.
e-f Percentages with different superscripts within the same column are significantly different (p ≤0.01). Main effect of female gametes on PBII extrusion is highly significant (p < 0.001). Main effects of male gametes and interaction between female and male gametes about PBII extrusion are not significant (P = 0.364 and P = 0.223, respectively).
The PBII extrusion in ova parthenogenetically activated by ethanol
To determine whether the male gamete plays a role in deficient PBII extrusion under high calcium concentration, PBII extrusion was examined in ova activated by ethanol and incubated either in 1.71 or 6.84 mM calcium for 5 h. Unexpectedly, irrespective of calcium concentrations, the percentages of ova without PBII extrusion were observed in only one of 62 instances (1.6%, n = 5) and two of 65 instances (3%, n = 7) of parthenogenetically activated ova in 1.71 and 6.84 mM calcium, respectively (seven replicates; P > 0.05).
Comparison of cytoskeletal organization of fertilizing ova with and without PBII extrusion
Ova with and without PBII extrusion were compared to examine localization of microtubules and actin filaments during fertilization by using immunofluorescent confocal microscopy. At metaphase II before fertilization, the long axis of the metaphase spindle was arranged parallel to the nearest oolemma (Fig. 3 a). At 2 h PI, the spindles of 8 of 10 ova fertilized in 1.71 mM calcium rotated so that the long axis was perpendicular to the nearest oolemma, and contractile ring was formed around the equatorial plate, while sperm nucleus decondensed and ova chromosomes segregated to the spindle poles (Fig. 3 b). At 3 h PI, in all examined ova with PBII extrusion (n = 10), both male and female pronuclei were formed and the spindle localized in the middle of the cleavage furrow and the contractile ring shrunk (Fig. 3 c). Conversely, in 19 of 39 ova (49%) inseminated under 6.84 mM CaCl2, the spindle did not rotate and it was still localized with its long axis parallel to the nearest oolemma at 2 h PI, even though ova chromosomes segregated to the spindle poles and sperm nucleus started to decondense (Fig. 3 d). At 3 h PI, ova without PBII extrusion were distinguished from ova with PBII extrusion. All ova with PBII extrusion (n = 20) rotated the spindle and formation of the contractile ring was complete, while ova without PBII extrusion (n = 19) failed to rotate the spindle and the actin filaments aggregated at the region of the oolemma over the spindle without forming the ring structure (Fig. 3 e). At 4 h PI, in ova without PBII extrusion two female PNs and one male PN formed and were located near the center of the ova, and in 6 of 22 of such ova (27%), the ring-like structure of actin filaments was observed in the cytoplasm without any sign of cytokinesis (Fig. 3 f).
A recent study has shown that two isotypes of cytoplasmic actin (CYA), namely beta-CYA and gamma-CYA, are present in the mouse ova with distinguished localization during the meiosis (Brockmann, et al., Reference Brockmann, Huarte, Dugina, Challet, Rey, Conne, Swetloff, Nef, Chaponnier and Vassalli2011). This study also showed that beta-CYA localized at the cleavage furrow during the first and second meiotic division and that gamma-CYA distributed cortices of ova and PBII of fertilizing ova, suggesting that both isotypes of CYA may contribute the formation and anchorage of the second meiotic spindle to the cortical region. We examined the localization of beta- and gamma-CYA in ova without PBII extrusion at 3 h PI when the status of PBII extrusion becomes clear. In ova with PBII extrusion (n = 40), beta-CYA was localized at the cleavage furrow and gamma-CYA was localized at the cortices of ova and PBII (Fig. 4 a–d). However, in ova without PBII extrusion (n = 24), while gamma-CYA was localized at the cortex with intense staining at the region over the decondensing chromatin, only faint signal of beta-CYA was observed (Fig. 4 e–h).

Figure 4 Localization of beta- and gamma-cytoplasmic actins (CYA) in ova with and without PBII extrusion at 3 h PI. The scale bar indicates approximately 20 μm. Ova were inseminated either in 1.71 mM calcium (with PBII extrusion) or in 6.84 mM calcium (without PBII extrusion), and at 3 h PI ova were fixed and double-stained with beta-CYA (green) and gamma-CYA (red). Merged images are shown in the right panels (d, h). In ova with PBII extrusion (a–d), beta actin is mainly localized in the region of the cleavage furrow [arrow in (d)] and gamma-actin are localized both the cortices of ova and PBII (c). In ova without PBII (e–h), only faint signal of beta- CYA is observed (f), while gamma- CYA is localized at cortex with slightly intense staining at the region over the chromatins (g).
Discussion
With the development of a large variety of methods for the genetic manipulation of gametes and embryos, the number of mouse strains, especially GM strains, has increased explosively during the last a few decades and maintenance and distribution of these mice by cryopreserved embryos and/or sperm has become essential part of ARTs in laboratory animals (Critser & Mobraaten, Reference Critser and Mobraaten2000). GM mice are produced using either inbred strains or hybrid/outbred strains and, when the latter were used, they are backcrossed to desired inbred strains for use in biomedical research. Therefore, basic information about ARTs of inbred strains is quite useful for the application for GM mice. In vitro fertilization is an essential part of ARTs because it allows for the production of large numbers of embryos for cryopreservation and embryo manipulation. However, successful IVF is not reported in all inbred strains. In some strains, it is still difficult to obtain high percentages of normal fertilization; this includes strains such as BALB/c, C3H and 129, all of which are heavily used in research (Thornton et al Reference Thornton, Brown and Glenister1999; Sztein et al., Reference Sztein, Farley and Mobraaten2000; Byers et al., Reference Byers, Payson and Taft2006). Thus, accomplishing successful IVF is an important issue for the efficient use of animals. We have previously reported that successful IVF in COCs of C3H/He can be accomplished using mHTF, which increases calcium concentration to 5.1 mM (Kito et al., Reference Kito, Hayao, Noguchi-Kawasaki, Ohta, Hideki and Tateno2004). However, we found that when cumulus-free C3H/He ova were used for IVF under such conditions, significant percentages of ova failed PBII extrusion, which can be distinguished from normal and polyspermic fertilization under the microscope (Fig. 1). Thus, we have attempted more detail examination of the effects of high concentration of calcium during IVF using cumulus-free C3H/He ova.
Initially, we studied whether there is dose dependency in deficient PBII extrusion against calcium concentrations. Incidence of fertilized ova with no PBII extrusion significantly increased in a dose dependent manner (r 2 = 0.5906; P < 0.01) without influencing on total percentage of fertilization (Table 1). Then, we showed that the sensitive timing of ova against high calcium was between 2 and 3 h PI (Fig. 2). At this time period ova resume meiosis and dynamic changes in localization of the spindle occur, therefore some processes of meiotic division are thought to be influenced by high concentration of calcium (discussed below). We noticed that percentages of ova without PBII vary among series of experiments from 0 to 15% in 1.71 mM and from 10 to 80% under 6.84 mM calcium. These large variability may derived from variability among individual animals and/or colonies provided from breeding facilities.
Although a high concentration of calcium, specifically at 5.13 mM, has been shown an enhancing effect in C57BL/6 and BALB/c stains (Itagaki & Toyoda, Reference Itagaki and Toyoda1992; Kito et al., Reference Kito, Hayao, Noguchi-Kawasaki, Ohta, Hideki and Tateno2004), the high incidence of abnormal fertilization we found has no practical applications. In addition, we also showed that almost all of these digynic triploid C3H/He ova developed at least to the morphologically normal blastocyst in culture (Table 2). In established IVF protocols in which ova are often moved from IVF medium to embryo culture media at 5–6 h PI or after overnight culture in IVF medium for further manipulation, it is important to carefully select normally fertilized ova under the dissecting microscope at the pronucleate stage, otherwise abnormal ova as shown in this study could be utilized inappropriately for further manipulation.
Detail studies of the blastocysts nuclei showed that ova fertilized in 6.84 mM calcium significantly reduced the number of ICM nuclei compared with ova fertilized in 1.71 mM calcium (Table 2). Composition of IVF media was reported to influence on development in rat and human and zygotes are more sensitive to culture conditions than the later stage of embryos (Quinn & Whittingham, Reference Quinn and Whittingham1982; Quinn et al., Reference Quinn, Warnes, Kerin and Kirby1985; Oh et al., Reference Oh, Miyoshi and Funahashi1998), thus suboptimal IVF conditions such as used in our study also have negative effect on the developmental competence and should be carefully avoided.
Although disturbed PBII extrusion still occurred when COCs were used for IVF with high calcium concentration, cumulus cells seem to significantly alleviate disturbed PBII extrusion (Table 3). Cumulus cells interact directly and indirectly with the oocyte via gap junctions during oocyte maturation. To provide a microenvironment suitable for oocyte maturation, fertilization and development, cumulus cells functions include metabolic cooperation, signal transduction, production of matrix abundant with hyaluronic acid and induction of acrosome reactions (Leese & Barton, Reference Leese and Barton1985; Yanagimachi, Reference Yanagimachi, Knobil and Neill1994; Kim et al., Reference Kim, Yamashita, Kimura, Honda, Kashiwabara and Baba2008). Thus, in this study, it is possible that cumulus cells themselves may alter calcium concentration or the cumulus matrix may act as a buffer to incorporate calcium ions to provide a microenvironment for fertilization.
In our preliminary studies using other strains, IVF under high calcium concentrations did not influence PBII extrusion; < 2% of ova failed PBII extrusion in C57BL/6 and 0% in BDF1 (data not shown). Thus, to study strain specificity and which gametes (sperm or ova) are responsible for the deficient PBII extrusion under high calcium concentrations, we examined the incidences of PBII extrusion in reciprocal fertilization between BDF1, whose ova were not influenced by high calcium concentration, and C3H gametes and in parthenogenetically activated ova in which sperm contribution can be eliminated. In reciprocal IVF, deficient PBII extrusion was observed only when C3H ova were used and was never found in BDF1 ova irrespective of strains of sperm (Table 4), indicating that ova rather than sperm are responsible for this phenomenon. However, an unexpected result was obtained in parthenogenetically activated ova; only 3% of ova failed to extrude the PBII at 5 h after activation under high concentration of calcium, indicating that some sperm factor(s) is likely to be involved. It is possible that such sperm factor(s) is mediating the action of calcium during IVF, because sperm plasma membrane and cytoplasm have been shown to be incorporated into and spread over the oolemma and the ooplasm at the sperm–ova fusion (Gaunt, Reference Gaunt1983; Gundersen et al., Reference Gundersen, Medill and Shapiro1986). Another possibility is that differences in the patterns of changes in cytoplasmic calcium concentrations at activation by sperm and ethanol may lead to different consequences in PBII extrusion. This possibility is less likely, but cannot be completely denied, as the sensitive period of ova against high calcium is 2–3 h PI (Fig. 2) when ova are already activated to resume meiosis.
During egg activation triggered by sperm, a series of repetitive calcium oscillations continue for several hours until formation of pronuclei (Yanagimachi, Reference Yanagimachi, Knobil and Neill1994; Marangos et al., Reference Marangos, Fitzharris and Carroll2003). The first oscillation is induced by phospholipase C-zeta (PLCζ) released from sperm which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-triphosphate (IP3); IP3 then binds the IP3 receptor onto the endoplasmic reticulum to release stored calcium into the cytoplasm (Saunders et al., Reference Saunders, Larman, Parrington, Cox, Royse, Blayney, Swann and Lai2002; Ducibella & Fissore, Reference Ducibella and Fissore2008; Wakai et al., Reference Wakai, Vanderheyden and Fissore2011; Wang & Machaty, Reference Wang and Machaty2013). In later oscillations, extracellular calcium flows into the ooplasm through so-called store operated calcium channel (Wakai et al., Reference Wakai, Vanderheyden and Fissore2011; Wang & Machaty, Reference Wang and Machaty2013). Conversely, exposure to ethanol also forces a continuous flow of extracellular calcium into the ooplasm, resulting in the continued elevation of cytoplasmic calcium for a prolonged period (Cuthbertson et al. Reference Cuthbertson, Whittingham and Cobbold1981, Rogers et al., Reference Rogers, Halet, Piao, Carroll, Ko and Swann2006). Because the parameters of oscillation including amplitude, frequency, duration and number of oscillation have been known to regulate various enzymatic activities required for egg activation and subsequent development, the inconsistent phenotypes between fertilization and ethanol activation seen in our study may be the result of different patterns of changes in cytoplasmic calcium concentrations (Ozil et al., Reference Ozil, Markoulaki, Tóth, Matson, Banrezes, Knott, Schultz, Huneau and Ducibella2005, Reference Ozil, Banrezes, Tóth, Pan and Schultz2006; Ducibella et al., Reference Ducibella, Schultz and Ozil2006; Tóth et al., Reference Tóth, Huneau, Banrezes and Ozil2006; Ducibella & Fissore, Reference Ducibella and Fissore2008; Miao et al., Reference Miao, Stein, Jefferson, Padilla-Banks and Williams2012). Activation of ova by direct injection of the physiological sperm factor, or PLCζ, may help to clarify the male contribution to deficient PBII extrusion.
Although the molecular mechanisms by which calcium oscillations regulate egg activation are not completely understood, calcium/calmodulin-dependent kinase II (CaMKII) has been shown to be a key regulator of meiotic resumption (Johnson et al., Reference Johnson, Bierle, Gallicano and Capco1998; Tatone et al., Reference Tatone, Delle Monache, Iorio, Caserta, Di Cola and Colonna2002; Ducibella et al., Reference Ducibella, Schultz and Ozil2006; Ducibella & Fissore Reference Ducibella and Fissore2008; Krauchunas & Wolfner, Reference Krauchunas and Wolfner2013). CaMKII activities are synchronized with calcium oscillations to generate discrete waves of activities during fertilization, suggesting that calcium oscillations maintain the sensitivity of CaMKII for the duration of calcium oscillation to avoid hyperactivation and resultant down-regulation (Tatone et al., Reference Tatone, Delle Monache, Iorio, Caserta, Di Cola and Colonna2002; Markoulaki et al., Reference Markoulaki, Matson, Abbott and Ducibella2003; Ducibella et al., Reference Ducibella, Schultz and Ozil2006). The calcium oscillations of the ova fertilizing in medium with high calcium levels were reported to become more frequent than those in ova fertilizing in low calcium levels (Deng & Fan, Reference Deng and Fan1996). Thus, the ova fertilized in high concentration calcium in this study may experience increased basal cytoplasmic calcium level through frequent oscillations, which possibly induce hyperactivation and down-regulation of the enzymes controlled by calcium and later resulting in disrupted regulation and/or interactions of the machineries needed for the meiotic division. These effects may further remain until the blastocyst stage as blastocysts fertilized under high calcium concentration had a reduced nuclear number of ICM.
Mature mammalian ova are highly polarized cells with asymmetrical spindle localization at the ovum cortex. This asymmetry ensures unequal cell division to extrude two small volume polar bodies during the two meiotic divisions, remaining minimum reduction of the oocyte size. This asymmetry is regulated by microfilament-microtubule cytoskeletons and various associated proteins and they are in turn regulated by mitogen-activating kinase (MAPK), myosin light-chain kinase (MLCK), as well as protein kinase C and CaMKII (Sun & Schatten, Reference Sun and Schatten2006; Azoury et al., Reference Azoury, Verlhac and Dumont2009; Brunet & Verlhac, Reference Brunet and Verlhac2011; Chaigne et al., Reference Chaigne, Verlhac and Terret2012). A recent study further showed that ova has beta and gamma isoforms of cytoplasmic actin (CYA) and that these isoforms are localized at the cleavage furrow and cortices of the ovum and the PBII, respectively, suggesting that they have a role during fertilization (Brockmann et al., Reference Brockmann, Huarte, Dugina, Challet, Rey, Conne, Swetloff, Nef, Chaponnier and Vassalli2011). We hypothesize that ova without PBII extrusion fertilized under high calcium concentration may have deficiencies in the organization and kinetics of cytoskeletal components during the second meiotic division. In the normally fertilized ovum, the meiotic spindle whose long axis underlies parallel to the closest oolemma at the second metaphase (Fig. 3 a), has already rotated 90° at 2 h PI, so that its long axis located perpendicular to the oolemma as meiosis progresses to telophase and cytokinesis (Fig. 3b). At this time period, the contractile ring has begun to form around the spindle equation where cytokinesis starts to extrude the PBII (Fig. 3 b, c). However, in ova inseminated in high calcium, abnormal cytoskeletal organization was observed at 2 and 3 h PI when ovum are sensitive against high calcium (Fig. 2). At 2 h PI about half of the ova have the spindle with its long axis located parallel the nearest oolemma (Fig. 3 d). At 3 h PI when the second meiosis progresses to telophase or later stages and failure of the PBII extrusion become distinguished (Fig. 3 c, e), all ova without the PBII extrusion fail to rotate the spindle and a mass of actin filaments aggregates over the oolemma covering the spindle without any ring structure (Fig. 3 e). At this time, beta-CYA localization, but not gamma-CYA, is also deficient in ova without PBII (Fig. 4 h), suggesting actin localization at the cleavage furrow has not properly progressed. At 4 h PI, when female and male pronuclei have already formed, in most ova without the PBII a mass of actin filaments still remains on the cortex over the spindle, and some ova (6 out of 22 ova) have ring-like structure around the centre of ooplasm with the spindle penetrating through the ring but without any appearance of cytokinesis (Fig. 3 f). These results suggest that under the high concentration of calcium cytoskeletal organization, especially the machinery related to anchoring the spindle at the cortex microfilaments may be disrupted, resulting in the deficient spindle rotation. Localization of proteins related to anchoring the meiotic spindle, such as partitioning deficient (PAR) proteins, formin-2, Ran-GTPase, Rac-GTPase, Cdc42 and actin related protein (Arp) 2/3 complex (Azoury et al., Reference Azoury, Verlhac and Dumont2009; Brunet & Verlhac, Reference Brunet and Verlhac2011; Chaigne et al., Reference Chaigne, Verlhac and Terret2012) in ova with deficient PBII extrusion will be required to conclude the mechanisms of deficient PBII extrusion induced by high concentration of calcium. It should be noted that C3H/He ova can be a candidate model to elucidate mechanisms of asymmetric cell division in relation to calcium signaling pathway during the second meiosis.
In summary, we have found that in the C3H/He strain, IVF of cumulus-free ova under high calcium concentrations increased abnormalities in the PBII extrusion in a strain specific manner, which results in triploid embryos and causing subsequent defective development. This deficiency in PBII extrusion can be somewhat alleviated by the presence of the cumulus cells. Abnormal cytoskeletal organization of ovum, probably spindle anchorage, is responsible for deficient PBII extrusion, but some unknown factor(s) from sperm still seems to be involved in this abnormality. From this study and our previous studies using various inbred mice (Kito et al., Reference Kito, Hayao, Noguchi-Kawasaki, Ohta, Hideki and Tateno2004; Kito & Ohta, Reference Kito and Ohta2008a), optimal IVF conditions such as calcium concentration, ionic strength and osmotic pressure are shown to be different among inbred mice and needed to be examined in a strain by strain basis. Our results showed specifically in IVF of C3H/He mice the use of high concentrations of calcium to enhance fertilization, or sperm penetration must be avoided in the practice of ARTs, otherwise resulting in the inefficient production of normal embryos. Our recommendation of IVF conditions for C3H/He is the use of mHTF at 1.71 mM CaCl2. More interestingly, our continued studies indicate that there are differences in the response to IVF under high calcium concentrations among C3H/He substrains. Considering the long history of the C3H strain (Altman & Katz, Reference Altman and Katz1979), genetic drifting or genetic contamination during colony maintenance may possibly change the phenotypes of this strain as in other strains such as C57BL/6 and 129 (Simpson et al., Reference Simpson, Linder, Sargent, Davisson, Mobraaten and Sharp1997; Threadgill et al., Reference Threadgill, Yee, Matin, Nadeau and Magnuson1997; Simon et al., Reference Simon, Greenaway, White, Fuchs, Gailus-Durner, Wells, Sorg, Wong, Bedu, Cartwright, Dacquin, Djebali, Estabel, Graw, Ingham, Jackson, Lengeling, Mandillo, Marvel, Meziane, Preitner, Puk, Roux, Adams, Atkins, Ayadi, Becker, Blake, Brooker, Cater, Champy, Combe, Danecek, di Fenza, Gates, Gerdin, Golini, Hancock, Hans, Hölter, Hough, Jurdic, Keane, Morgan, Müller, Neff, Nicholson, Pasche, Roberson, Rozman, Sanderson, Santos, Selloum, Shannon, Southwell, Tocchini-Valentini, Vancollie, Westerberg, Wurst, Zi, Yalcin, Ramirez-Solis, Steel, Mallon, de Angelis, Herault and Brown2013).
Acknowledgements
We thank Ms Y. Kaneko, Ms H. Yano and M. Hayashi for taking care of animals used in this study. We also thank Dr H. Maruyama and Dr K. Higuchi at the Center of Molecular Imaging for technical advice for conducting confocal microscopy, and the Department of Technical Support and Development in National Institute of Radiological Sciences for various supports. We are also grateful to Mr Craig Steger and Dr Diane Cookfair for critically reviewing this manuscript. This research received no specific grant from any funding agency.
Conflicts of interest
The authors declare no conflicts of interest.










