Introduction
Blood-flukes of the genus Schistosoma are digenean trematodes that require a freshwater snail intermediate host and a mammalian definitive host. Out of the 21 currently recognized species, only eight have been confirmed as infections of humans and, of these, only three are heavily implicated as diseases of significant public health importance (table 1). The rest of the genus is only known from animal infections, and specifically mammals; the various species have adapted to a wide variety of taxa, with some specializing on one species while others have a wide definitive host range (Pitchford, Reference Pitchford1977). For the purposes of examining cross-over of parasitic infections between humans and animals and vice versa, the term ‘zoonosis’ is used throughout this paper to describe transmission in both directions. Figure 1 shows a phylogeny of the genus Schistosoma, with main geographical distributions and primary definitive hosts marked.
Table 1 The eight species of schistosome reported in humans.

Schistosoma guineensis was only described in 2003, and so many past references to S. intercalatum may have actually been referring to S. guineensis. References include Fenwick (Reference Fenwick1969), Pitchford (Reference Pitchford1977), Christensen et al. (Reference Christensen, Mutani and Frandsen1983), Loker (Reference Loker1983), Pagès et al. (Reference Pagès, Jourdane, Southgate, Tchuem Tchuenté, Combes, Combes and Jourdane2003), Attwood et al. (Reference Attwood, Fatih, Campbell and Upatham2008) and Standley et al. (Reference Standley, Mugisha, Verweij, Adriko, Arinaitwe, Rowell, Atuhaire, Betson, Hobbs, Van Tulleken, Kane, Van Lieshout, Ajarova, Kabatereine and Stothard2011).
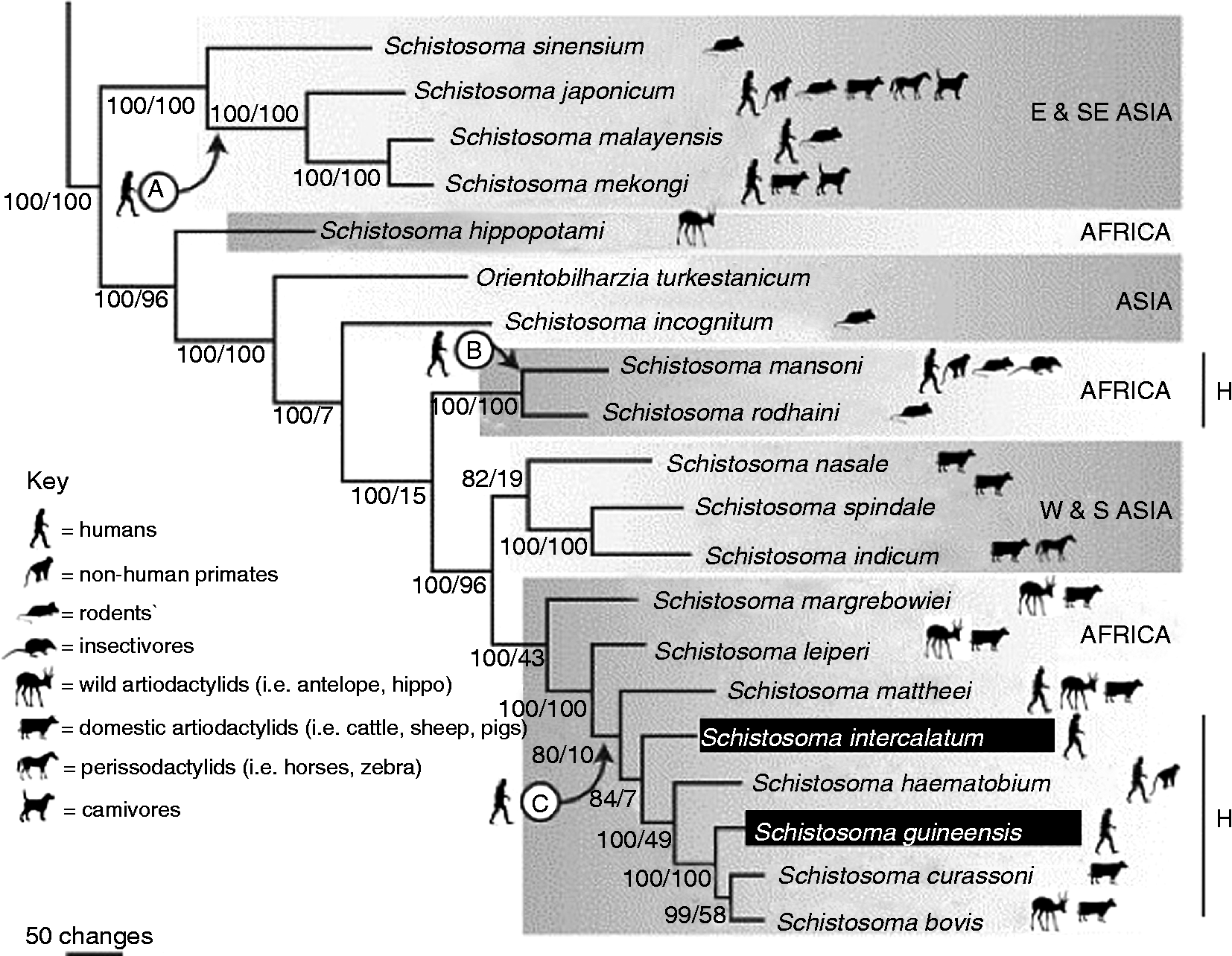
Fig. 1 Phylogeny of Schistosoma, as recognized in 2006. Schistosoma kisumuensis was only described in 2009 (Hanelt et al., Reference Hanelt, Brant, Steinauer, Maina, Kinuthia, Agola, Mwangi, Mungai, Mutuku, Mkoji and Loker2009). Known naturally infected definitive host groups are shown by the icons. The points ‘A’, ‘B’ and ‘C’ indicate the three suggested points where species adapted to infect humans; molecular clock estimates place the date of each divergence at roughly 3.8 MYA (millions of years ago) for point A and less than 1 million years ago for points B and C (Morgan et al., Reference Morgan, Dejong, Adeoye, Ansa, Barbosa, Bremond, Cesari, Charbonnel, Correa, Coulibaly, D'Andrea, De Souza, Doenhoff, File, Idris, Incani, Jarne, Karanja, Kazibwe, Kpikpi, Lwambo, Mabaye, Magalhaes, Makundi, Mone, Mouahid, Muchemi, Mungai, Sene, Southgate, Tchuente, Theron, Yousif, Magalhaes, Mkoji and Loker2005; Webster et al., Reference Webster, Southgate and Littlewood2006; Attwood et al., Reference Attwood, Fatih, Campbell and Upatham2008). The lines marked ‘H’ demonstrate clades with known hybridization between species. Figure adapted from Webster et al. (Reference Webster, Southgate and Littlewood2006).
Infections in non-human primates
In Africa, Schistosoma spp. belong either to the S. mansoni group, characterized by eggs with lateral spines, or the S. haematobium group, identified by terminal spines on the eggs. The eponymous species of these two groups are most commonly found in humans and exact a huge public health burden on many communities and regions. There are, however, other species within these groups that primarily affect non-human animals; this section will outline these other species that are found in Africa, most of which are primarily known as infections in rodents and ungulates, but some of which have been reported from non-human primates too. The rest of the review will focus on accounts of ‘human’ schistosome species, namely S. haematobium, S. intercalatum/guineensis and S. mansoni, as found in non-human primates.
Other species that make up the Schistosoma haematobium group are S. intercalatum, S. guineensis, S. bovis, S. mattheei, S. margrebowiei, S. leiperi, S. curassoni and the recently described S. kisumuensis (Webster et al., Reference Webster, Southgate and Littlewood2006; Hanelt et al., Reference Hanelt, Brant, Steinauer, Maina, Kinuthia, Agola, Mwangi, Mungai, Mutuku, Mkoji and Loker2009). Schistosoma intercalatum and S. guineensis primarily infect humans (table 1). The remaining species, with the exception of S. kisumuensis, usually parasitize artiodactylid ruminants, with some most commonly found in domestic ungulates whereas others are more frequently observed in wild bovids. There are occasional reports in the literature of S. bovis and S. mattheei from humans and baboons, although usually alongside a mixed infection with either S. mansoni or S. haematobium. Given the similarities in egg morphology between most of the various species of the terminally spined S. haematobium group, it has often been assumed that many of these observations were a case of misidentification of the schistosome species, which can vary considerably (Pitchford, Reference Pitchford1965). However, S. mattheei was recently confirmed using molecular methods from a free-ranging baboon troop in Zambia (Weyher et al., Reference Weyher, Phillips-Conroy, Fischer, Weil, Chansa and Fischer2010), suggesting that accounts of this parasite in humans should also be investigated further as a potential zoonosis.
In addition, there is one account of eggs of S. margrebowiei, traditionally considered an antelope schistosome though also found in domestic ungulates, being recovered from a human rectal biopsy in Mali, mixed with S. haematobium and S. mansoni (Pitchford, Reference Pitchford1959). The egg morphology of S. margrebowiei is unique among African schistosomes (Christensen et al., Reference Christensen, Mutani and Frandsen1983); although experimental passage has never been successful, other infections have also been reported from Zambia (Giboda et al., Reference Giboda, Dietrich and Stěrba1988), suggesting that while humans may contract incidental infections with S. margrebowiei, infections are not fully viable in humans. So far, non-human primates have not been reported to be infected with this schistosome but epidemiological coverage has been limited. Schistosoma kisumuensis is unique among the ‘non-human’ S. haematobium group species in being exclusively an infection of small mammals, such as rodents and insectivores; it was very recently described, using molecular methods, from western Kenya, and as such is not included in the 2006 phylogeny on which fig. 1 is based (Hanelt et al., Reference Hanelt, Brant, Steinauer, Maina, Kinuthia, Agola, Mwangi, Mungai, Mutuku, Mkoji and Loker2009). Further research is required before its compatibility with primates can be described with any certainty.
The S. mansoni group has traditionally been classified to consist only of three other species: S. hippopotami, S. edwardiense and S. rodhaini. Schistosoma hippopotami, on the basis of recent molecular analysis, has since been re-classified as basal to all African schistosomes (Webster et al., Reference Webster, Southgate and Littlewood2006); this species, together with S. edwardiense, is also unusual in only having been found in a single species of definitive host: the African hippopotamus (Hippopotamus amphibius). Schistosoma rodhaini, on the other hand, is primarily an infection of rodents; baboons have been successfully experimentally infected, but only when also co-infected with S. mansoni (Nelson & Teesdale, Reference Nelson and Teesdale1965). The literature only mentions one reported case of a natural infection of S. rodhaini in a human, from what is now D. R. Congo (D'Haenens & Santele, Reference D'Haenens and Santele1955); however, given the age of the reference and its isolation in the literature, it may be suggested that it is a case of false diagnosis of an egg-variant of S. mansoni.
Focus upon S. mansoni, S. haematobium and S. intercalatum/guineensis
The distribution of the four main types of human schistosome (S. haematobium, S. mansoni, S. intercalatum and S. guineensis) covers vast swathes of Africa and Madagascar, including areas of high human density such as the West African coast, the Sahel and the southern and eastern highlands (see fig. 2). Many of these regions are also highly suitable for other species of primate; given the close geographical and genetic proximity of many primate species to humans, it is no surprise that these are the groups of mammals most likely to be at risk from infection with human diseases of all kinds, including schistosomes, and infections with other trematode genera have been observed in free-ranging primate populations in Africa (Murray et al., Reference Murray, Stem, Boudreau and Goodall2000; Sleeman et al., Reference Sleeman, Meader, Mudakikwa, Foster and Patton2009). In addition, as human populations grow at a rapid rate and communities push ever further into remote forest locations, they are coming into contact with relatively pristine primate habitats, thus potentially putting new species at risk of exposure to schistosomiasis infection.
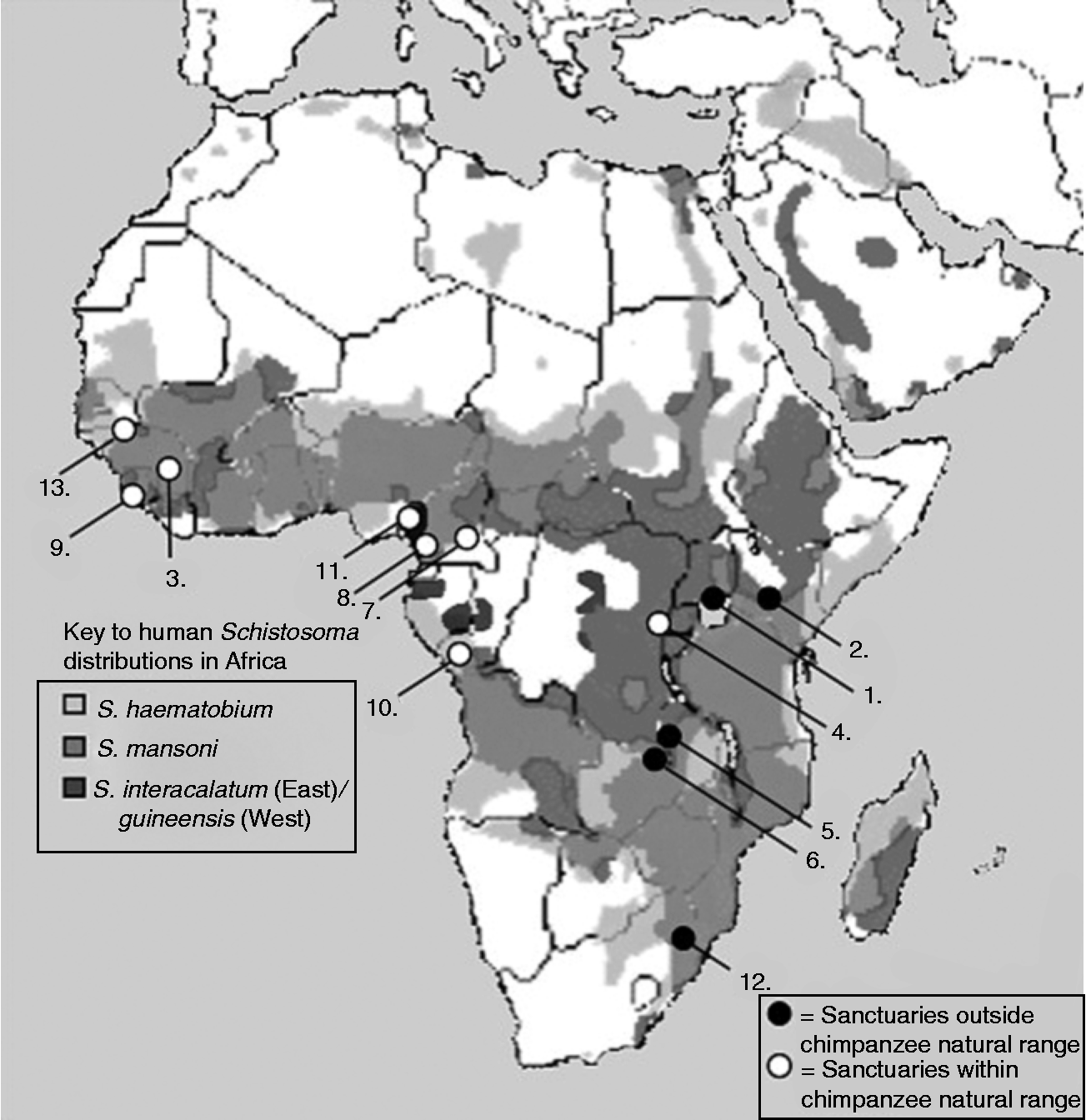
Fig. 2 Map of Africa with circles denoting the locations of the 13 Pan-African Sanctuary Alliance (PASA) sanctuaries containing chimpanzees. White circles indicate sanctuaries located within the natural range of chimpanzees, whereas black circles indicate sanctuaries outside of this range. The background layers depict the distributions of S. mansoni, S. haematobium and S. intercalatum/guineensis.
Given the endangered and threatened status of many of Africa's primate populations, these examples of human to wildlife transmission of parasites are of immediate concern to primate conservation managers. As such, and particularly considering the vast amount of research attention awarded to wild primate populations, it is surprising that until recently, relatively little concerted effort has been undertaken to characterize and diagnose parasitic infections in non-human primates, revealing a disease screening prejudice. By contrast, the early years of the 21st century have witnessed renewed interest in questions of zoonotic transmission of diseases, and thus a number of surveys have since reported on the observation of Schistosoma in a variety of non-human primates, mainly afforded by exploratory applications of new diagnostic tools concurrently being rolled-out for disease surveillance in people. The methods for identifying these infections and confirming their transmission will be discussed in the next section; here, it suffices to outline past reports of human schistosomiasis in non-human primate species, as well as more current accounts.
To this date, S. intercalatum/guineensis has yet to be observed as a natural infection in non-human primates. This may be because, on the whole, these parasites are focally distributed in relatively dense tropical forest regions, where primate species tend to be arboreal and are less likely to come into contact with terrestrial infected water sources. Evidence for this hypothesis comes from the observation that several primate species, including some that are distributed in the same countries as S. intercalatum/guineensis, are experimentally susceptible to infection (Cheever et al., Reference Cheever, Kuntz, Moore and Huang1976; Kuntz et al., Reference Kuntz, McCullough, Moore and Huang1978b, Reference Kuntz, Huang and Moore1980). However, these species might not be exposed to the parasite, due to habitat preference or behaviour; for example, the Patas monkey is known to be a good experimental host of S. intercalatum (Kuntz et al., Reference Kuntz, McCullough, Huang and Moore1978a), and is found in parts of Central and West Africa, but tends to inhabit savannah habitats which might not be suitable for Bulinus forskalii or B. africanus group snails, the intermediate snail hosts of S. guineensis and S. intercalatum, respectively. Wright et al. (Reference Wright, Southgate and Knowles1978) indeed suggested that the forest/non-forest interface might be a barrier to transmission. Given that chimpanzees are also known to be susceptible (Kuntz et al., Reference Kuntz, McCullough, Moore and Huang1978b), spend time on the ground as well as in the trees, and are distributed in patches throughout the region where S. intercalatum/guineensis are found, future parasitological surveys of Pan troglodytes and P. paniscus (the bonobo) should be alert to the possibility of encountering S. intercalatum/guineensis.
In contrast, S. haematobium has been reported as a natural infection of non-human primates, although S. mansoni is still the more common human schistosome in this taxon. Based on the collated records presented by Ouma & Fenwick (Reference Ouma, Fenwick, Macpherson and Craig1991), by the early 1990s both parasites had been observed in vervet monkeys (Cercopithecus aethiops, also known as grivet monkeys), Sykes monkeys (C. mitis) and baboons (Papio spp.). These accounts have spanned Africa; they include surveys from Kenya, Tanzania, Uganda, Zimbabwe, Senegal and Ethiopia. In addition, a chimpanzee imported into the USA from Senegal was diagnosed with S. haematobium (De Paoli, Reference De Paoli1965), although at the time of Ouma & Fenwick's review (Reference Ouma, Fenwick, Macpherson and Craig1991) no S. mansoni had ever been observed as a natural infection of any ape other than humans. It is no coincidence that all of these localities are known areas with high endemic prevalence of schistosomiasis in human communities, suggesting that a combination of environmental transmission suitability and high levels of infection in humans is putting non-human primates at risk of exposure.
Changing disease landscapes and epidemiological potential
It should therefore come as no surprise that human-mediated landscape change and population growth may explain the increasing number of observations of human schistosomiasis in non-human primates in recent years. By far the most common reported non-human primate host has been the baboon, and the dominant parasite in these instances has been S. mansoni, although one observation of a baboon infected with S. haematobium was made from South Africa in the 1990s (Appleton & Henzi, Reference Appleton and Henzi1993). Observations have been equally as widespread in the past two decades as in earlier years, with accounts of infection from Kenya (Munene et al., Reference Munene, Otsyula, Mbaabu, Mutahi, Muriuki and Muchemi1998; Muriuki et al., Reference Muriuki, Murugu, Munene, Karere and Chai1998; Hahn et al., Reference Hahn, Proulx, Muruthi, Alberts and Altmann2003), Tanzania (Muller-Graf et al., Reference Muller-Graf, Collins, Packer and Woolhouse1997; Murray et al., Reference Murray, Stem, Boudreau and Goodall2000), Ethiopia (Phillips-Conroy, Reference Phillips-Conroy, Taub and King1986; Legesse & Erko, Reference Legesse and Erko2004), Senegal (McGrew et al., Reference McGrew, Tutin, Collins and File1989; Howells et al., Reference Howells, Pruetz and Gillespie2011) and Nigeria (Weyher et al., Reference Weyher, Ross and Semple2006); baboons have also recently been implicated as potential reservoir hosts for S. mansoni in parts of the Arabian peninsula (Ghandour et al., Reference Ghandour, Zahid, Banaja, Kamal and Bouq1995; Zahed et al., Reference Zahed, Ghandour, Banaja, Banerjee and Dehlawi1996).
In several of these cases, as well as other incidences of parasite transmission between humans and non-human primates, it has been suggested that forest fragmentation, increased proximity of humans to wild habitats and the emerging reliance of wild primates on human settlements for food (such as through crop-raiding) is at least partially responsible for increased exposure and risk of these animals contracting ‘human’ diseases (Weyher et al., Reference Weyher, Ross and Semple2006). A worrying trend is that national park and forest reserve areas, which might have been expected to afford a degree of protection against zoonotic transmission of infections, also seem to show signs of human to animal transfer of parasites, as has been seen in Bwindi National Park in Uganda with infections of intestinal parasites in mountain gorillas (Graczyk et al., Reference Graczyk, Bosco-Nizeyi, Ssebide, Thompson, Read and Cranfield2002) although transmission of schistosomiasis at least would be very unlikely in these cooler, higher altitude environments. In most of the above cases, it is unclear whether transmission is being sustained entirely by the primate population or if human populations continue to contribute significantly to the maintenance of the life cycle.
Of note is the observation that while baboons in many locations have been shown to be infected with S. mansoni, other sympatric non-human primate species were described as free of the parasite. This is particularly interesting given that in several cases, these sympatric primate species are known to be experimentally susceptible to S. mansoni infection, and have even been observed naturally infected in the wild. For example, in their survey of three species of wild primate in Kenya, Munene et al. (Reference Munene, Otsyula, Mbaabu, Mutahi, Muriuki and Muchemi1998) positively identified S. mansoni infections in baboons but not in local vervet or Sykes monkeys, despite earlier accounts of these species being infected, also in East Africa (Nelson, Reference Nelson1960; Nelson et al., Reference Nelson, Teesdale, Highton, Wolstensholme and O'Connor1962).
Similarly, Legesse & Erko (Reference Legesse and Erko2004) observed natural schistosomiasis infections in baboons in Ethiopia, but not in sympatric vervet monkeys. Infected baboons have further been reported from two localities, Fongoli in Senegal and Gombe Stream National Park in Tanzania, which are also inhabited by troops of chimpanzees; despite extensive parasitological surveys, these chimpanzees have never convincingly displayed positive infection with S. mansoni (Muller-Graf et al., Reference Muller-Graf, Collins, Packer and Woolhouse1997; Murray et al., Reference Murray, Stem, Boudreau and Goodall2000; Bakuza & Nkwengulila, Reference Bakuza and Nkwengulila2009; Howells et al., Reference Howells, Pruetz and Gillespie2011). There is an isolated, unpublished account, from the early 1990s, of S. mansoni eggs being recovered from chimpanzee stool in Gombe Stream National Park (Nutter, Reference Nutter1993); however, since both earlier and ensuing examinations failed to reconfirm the finding, it may be that this report is a case of mislabelled samples, and the stool had actually belonged to a baboon.
Likewise, there are locations where chimpanzees are known to inhabit areas that have high levels of schistosomiasis transmission to humans, and yet appear not to have contracted the disease; one such location is Rubondo Island, where prevalence in humans in island communities nearby is very high, and snails shedding S. mansoni cercariae have been observed in the shallow waters fringing the island itself (Standley et al., Reference Standley, Kabatereine, Lange, Lwambo and Stothard2010). However, despite extensive parasitological surveys, the chimpanzees, vervet monkeys and guerezas (Colobus guereza) that inhabit the island have not been reported to be infected (Petrzelkova et al., Reference Petrzelkova, Hasegawa, Moscovice, Kaur, Issa and Huffman2006; Petrášová et al., Reference Petrášová, Modrý, Huffman, Mapua, Bobáková, Mazoch, Singh, Kaur and Petrželková2010), based on stool examinations; given the insensitivity of direct faecal examinations it would be interesting to also include serological tests for evidence of exposure and infection as an alternative diagnostic tool.
The sanctity of sanctuaries: opportunities for testing captive populations
The relative absence of natural infections of Schistosoma species in chimpanzees has long suggested that despite their close phylogenetic relationship to humans, these apes are perhaps not naturally susceptible to the parasite, or do not access infected water sources in ways which would expose them sufficiently to infection. This assumption has been resolutely refuted through the confirmation of naturally acquired infections of S. mansoni in wild-born, semi-captive chimpanzees on Ngamba Island, a sanctuary for rescued and orphaned chimpanzees (Standley et al., Reference Standley, Mugisha, Verweij, Adriko, Arinaitwe, Rowell, Atuhaire, Betson, Hobbs, Van Tulleken, Kane, Van Lieshout, Ajarova, Kabatereine and Stothard2011). Over the course of four surveys in 3 years, 11% of chimpanzees tested for S. mansoni infection were stool-positive for eggs, which were later hatched and used to infect Biomphalaria snails, proving their viability. Chimpanzees were also observed close to the water's edge on a number of occasions, indicating behavioural risk of exposure that might have accrued by gradual habituation to the lake itself. Of more concern is recent evidence of significant liver pathology in these chimpanzees, comparable to humans progressing towards chronic infection status; this observation was made by using ultrasound imagery, a technology never before used for diagnosis of clinical schistosomiasis in naturally infected non-human primates (C.J. Standley & J.R. Stothard, in preparation).
In general, primate sanctuaries may provide a useful means for monitoring the risk of zoonotic transmission of schistosomiasis. In the case of chimpanzees, there are over a dozen sanctuaries across Africa dedicated to their rehabilitation and protection. While independently managed, run and funded, they are all co-ordinated by the Pan-African Sanctuary Alliance (PASA), an umbrella organization dedicated to the rehabilitation and care of non-human primates. While most of the sanctuaries are found close to or within the existing range of chimpanzees, others are further removed from their natural habitat, such as Sweetwaters in Kenya and JGI-Chimpanzee Eden in South Africa. Figure 2 denotes the locations of each of these sanctuaries, against the background of the distribution of schistosomiasis; it clearly shows that even though some of the sanctuaries are located outside of the natural range of chimpanzees, all 13 are located in regions known to be endemic for schistosomiasis transmission.
Sanctuaries possess a number of qualities that lend themselves to monitoring the emergence and progression of zoonotic diseases. First of all, the animals are all usually highly habituated to human presence, and in many cases, spend a portion of their time in a captive environment, allowing for the easy collection of various types of samples for diagnosis of disease. Similarly, the behaviour of the chimpanzees themselves is often easily observed; as such, levels of water contact, for example, can be calculated, as a proxy for transmission risk of schistosomiasis. In addition, for reasons of access to the public, several of these sanctuaries are located relatively close to human habitation, although contact with local populations is strictly controlled. Finally, if disease is detected, sanctuaries provide a controlled environment in which to administer treatment, for example via provisioning or annual health checks. The close observation of the animals and their habituated status allows for close monitoring of the effects of such medication once it has been given.
Despite these clear benefits of surveying the health of chimpanzees (and other non-human primates) in sanctuaries, and their location within transmission zones, very few sanctuaries have performed diagnostic screening for schistosomiasis in their captive animals (table 2). In addition to Ngamba Island, Sweetwaters sanctuary in Kenya recently tested the urine of some of its chimps with circulating cathodic antigen (CCA)-dipsticks, finding roughly 50% of those tested positive for the disease (unpublished data). In Guinea, only a single individual was tested; low-intensity infections may not present any discernible symptoms, so it would be recommended that if screening were to be done, it should be applied to as many of the individual chimpanzees at the sanctuary as possible. Finally, given the ease of collecting different forms of samples from captive chimps (faeces, urine and blood, for example), sanctuaries would be an ideal location to test out new field-ready diagnostic tools, which can then be applied more widely to determining the emergence of schistosomiasis as a zoonotic infection in wild primates.
Table 2 A summary of the Pan-African Sanctuary Alliance (PASA) sanctuaries containing chimpanzees and their schistosomiasis infection status, if known. ‘Map ID’ refers to the geographical location of each sanctuary, as shown in fig. 2.

DRC, Democratic Republic of Congo (Congo-Kinshasa); Rep. Congo, Republic of the Congo (Congo-Brazzaville). *Although rehabilitated with human intervention, these chimpanzees and their descendents are now free-living, though protected and monitored by the Chimpanzee Rehabilitation Trust.
Balancing diagnosis and analysis: identifying emerging zoonotic risk
A consideration of past reports of schistosomiasis in animals in Africa, together with an evaluation of the disease in captive primates would not be complete without looking at potential risks of emerging zoonotic infections in wild populations. This includes an examination of methods for researchers to determine the origin, transmission direction and causes of infections found both in humans and wildlife. In order to do so, scientists should be encouraged to employ the same up-to-date technology on animal populations as is used in humans, for consistency of results. Finally, research on zoonotic parasitic infections should embrace the use of a multidisciplinary tools in order to analyse their results and thus produce conclusions that can be used to inform other interested parties across different fields, from public health to conservation medicine and encompassing biogeography, molecular epidemiology and mathematical modelling (Stephens et al., Reference Stephens, Moxon, Adams, Altizer, Antonovics, Aral, Berkelman, Bond, Bull, Cauthen, Farley, Glasgow, Glasser, Katner, Kelley, Mittler, Nahmias, Nichol, Perrot, Pinner, Schrag, Small and Thrall1998; Daszak et al., Reference Daszak, Tabor, Kilpatrick, Epstein and Plowright2004; Morens et al., Reference Morens, Folkers and Fauci2004; Wilcox & Gubler, Reference Wilcox and Gubler2005).
The observation and identification of forms of schistosomiasis passing between humans and animals is often a non-trivial matter, given the wide variety of definitive host species at risk of infection as well as morphological similarities between different schistosome species across several life stages. A crucial obstacle facing researchers is the difficulty in accessing samples from populations of wild animals. Standardized protocols for sampling and data collection of primate groups should be employed, to ensure consistency of results between surveys; some such protocols already exist, and so should be rigorously followed (Gillespie, Reference Gillespie2006).
The next question involves the type of sample and the methods used for diagnosing infection. The traditional approach is to observe eggs passed either in the stools, for gastrointestinal species (such as S. mansoni, S. rodhaini, S. intercalatum and S. guineensis) or urine, for S. haematobium. While these methods are cheap, simple and relatively effective, they are not in line with current diagnostic efforts in human populations. For example, the CCA dipstick utilizes a tiny drop of urine, but since only feeding, adult worms produce circulating cathodic antigen, this is more indicative than eggs in stool of an active infection. This method has been tested on Ngamba Island's semi-captive chimpanzee population (Standley et al., Reference Standley, Mugisha, Verweij, Adriko, Arinaitwe, Rowell, Atuhaire, Betson, Hobbs, Van Tulleken, Kane, Van Lieshout, Ajarova, Kabatereine and Stothard2011), and was shown to have greater sensitivity than any stool-based diagnostic (apart from polymerase chain reaction), and so may be especially useful in animals that are not efficient egg excretors.
Similarly, if non-human primate IgG/IgM molecules are sufficiently similar to human IgG/IgM, blood samples can be used for schistosome egg antigen enzyme-linked immunosorbent assay (SEA-ELISA), which is highly sensitive in the diagnosis of humans (Stothard et al., Reference Stothard, Sousa-Figueiredo, Standley, Van Dam, Knopp, Utzinger, Ameri, Khamis, Khamis, Deelder, Mohammed and Rollinson2009). This method has also been tested on Ngamba Island, showing over 90% prevalence in the chimpanzees (Standley et al., Reference Standley, Mugisha, Verweij, Adriko, Arinaitwe, Rowell, Atuhaire, Betson, Hobbs, Van Tulleken, Kane, Van Lieshout, Ajarova, Kabatereine and Stothard2011). Given that antibodies to egg antigens may persist in the bloodstream up to several years after the infection has been cleared, this method may be particularly effective in gauging past exposure to Schistosoma (Doenhoff et al., Reference Doenhoff, Chiodini and Hamilton2004), but is not necessarily accurate in measuring current infection status. New research initiatives, such as SCORE (Schistosomiasis Consortium for Operational Research and Evaluation, http://score.uga.edu), specifically include a focus on field-evaluation of diagnostics, including the CCA test but also alternatives such as CAA (circulating anode antigen). In order to ensure that surveys of animals are comparable and consistent with these on-going and parallel human studies, the inclusion of such new, field-reliable and highly sensitive rapid diagnostic tests should be incorporated into future sampling efforts.
One disadvantage with these rapid diagnostic tests is that they only diagnose infection to genus rather than species level. Morphology has traditionally been the first method for species identification; the shape, size and spine location on schistosome eggs is often sufficient. Adult worms, too, have interspecies variations in body form and behaviour; cercarial identification is more difficult, but is aided by the type of intermediate snail host used and emergence behaviour. However, there have been observations of intraspecific variations in egg shape, perhaps due to host morphology; moreover, hybrids are difficult to detect through morphology alone. In these cases, the advent of molecular tools has revolutionized researchers' ability to confirm species identification (Webster et al., Reference Webster, Southgate and Littlewood2006), hybrids (Webster et al., Reference Webster, Tchuem Tchuenté and Southgate2007) and even the direction of transmission, in this setting as well as with other parasitic diseases (Graczyk et al., Reference Graczyk, Bosco-Nizeyi, Ssebide, Thompson, Read and Cranfield2002; Standley et al., Reference Standley, Mugisha, Verweij, Adriko, Arinaitwe, Rowell, Atuhaire, Betson, Hobbs, Van Tulleken, Kane, Van Lieshout, Ajarova, Kabatereine and Stothard2011).
Moreover, monitoring changes in parasite genotype over time and comparing variation between human and animal populations can determine the source and maintenance of the infection, with implications for the resultant design of control interventions (Nejsum et al., Reference Nejsum, Bertelsen, Betson, Stothard and Murrell2010). For example, molecular tools may provide insight into whether a non-human primate population is maintaining transmission independently of human communities, and thus can be considered a true reservoir of disease (as we believe is now occurring on Ngamba Island), or whether human transmission is required for continued infection, implicating public health measures as the key to breaking the infection cycle. As such, when investigating purported cases of zoonotic transmission of schistosomiasis, we recommend that molecular tools be employed alongside accurate and effective diagnoses, in order to produce best evidence for the transmission dynamics of that particular setting.
In addition to molecular tools, there are other methods that should be employed alongside traditional parasitological surveys for analysing the zoonotic potential of parasitic diseases. For example, there is a growing movement to integrate spatial epidemiological data with human and animal population distribution information, using geographical information systems (GIS), in order to evaluate high-risk areas for the cross-over of infectious diseases (Eisen & Eisen, Reference Eisen and Eisen2007). While geospatial models have been applied to schistosomiasis epidemiology in humans (Clements et al., Reference Clements, Deville, Ndayishimiye, Brooker and Fenwick2010), and to human–animal schistosomiasis transmission in Asia (Williams et al., Reference Williams, Sleigh, Li, Feng, Davis, Chen, Ross, Bergquist and McManus2002; Ishikawa et al., Reference Ishikawa, Ohmae, Pangilinan, Redulla and Matsuda2006), it would be useful to integrate animals into these models within an African setting. These can also be informed by classic models of disease transmission, as developed by Anderson & May (Reference Anderson and May1991), and used to gauge the risk of emergence of infection in particular areas, as well as to evaluate the effect of control interventions.
Implications for public health and wildlife conservation
In Africa, the human public health burden of schistosomiasis is well known, but less recognized is the risk imparted on non-human primate populations by these parasites. Better epidemiological monitoring of non-human primates is needed, especially where such animals may become important animal reservoirs maintaining disease transmission in the face of public health control. Examples are also given how endangered non-human mammals, such as chimpanzees, will profit from a specific consideration of schistosomiasis as part of their conservation strategy. Indeed, we advocate that public health initiatives and wildlife monitoring groups should work more synergistically to develop better disease control and conservation strategies.
Acknowledgements
The authors wish to thank the British Society for Parasitology for hosting a session on zoonotic diseases in their Spring 2011 meeting. We are also grateful to Professor John Lewis and the Journal of Helminthology for their kind invitation to consider publication of our work. This paper is based in part on a chapter authored by CJS, APD and JRS in Schistosomiasis, published by InTech Open Access Publishers (www.intechweb.org). Many thanks are also due to Dr Justin Crocker for assistance with preparing the figures. This research would not have been possible without the assistance and expertise of the Chimpanzee Sanctuary and Wildlife Conservation Trust staff, especially the Director, Lilly Ajarova, and all those working on Ngamba Island.






