Introduction
Quantifying growth and its seasonal patterns is integral to understanding the life history of euphausiids. Growth in krill, like other crustaceans, is not a continuous process but occurs periodically, punctuated by moulting of the exoskeleton. Growth is intrinsically tied to the environment, influenced by temperature and food availability in addition to the partitioning of energy between storage, reproduction and somatic growth (Kawaguchi et al. Reference Kawaguchi, Candy, King, Naganobu and Nicol2006, Tarling et al. Reference Tarling, Shreeve, Hirst, Atkinson, Pond, Murphy and Watkins2006). Consequently, growth rates of krill are highly variable both spatially and temporally. Measuring the somatic growth of krill is often achieved by one of two methods: the analysis of length-frequency distributions of single populations or live experimentation (Nicol Reference Nicol2000).
Thysanoessa macrura G.O. Sars is a small, highly abundant species of krill in the Southern Ocean. Despite the recognition as the second most abundant krill species in this region and trends suggesting an increase in abundance in some areas including the Western Antarctic Peninsula, estimates of its growth are currently restricted to repeated sampling length-frequency analyses (Haraldsson & Siegel Reference Haraldsson and Siegel2014, Steinberg et al. Reference Steinberg, Ruck, Gleiber, Garzio, Cope and Bernard2015). Due to the uncertainties and limitations associated with this method, including the assumption of the same population being repeatedly sampled with no immigration or emigration occurring, growth rates are reported as averages for periods that span 4–13 months, providing little information on seasonal cycles (Nordhausen Reference Nordhausen1992, Haraldsson & Siegel Reference Haraldsson and Siegel2014). Thysanoessa macrura is thought to live for up to four years, growing to a maximum size of 35 mm and reaching sexual maturity at 13 mm in length (Haraldsson & Siegel Reference Haraldsson and Siegel2014, Wallis et al. Reference Wallis, Kawaguchi and Swadling2018). Recent investigations have provided valuable information on the winter reproductive cycle of T. macrura and the role of stored lipids in fuelling gonad maturation (Wallis et al. Reference Wallis, Kawaguchi and Swadling2018). Understanding of seasonal growth patterns of T. macrura represents a remaining fundamental gap in knowledge of the life history of this species that is essential for quantifying the partitioning of energy between somatic growth, lipid storage and reproduction, and establishing conceptual models of population dynamics.
The instantaneous growth rate (IGR) method has proved to be a valuable means of quantifying growth at fine spatial and temporal scales (Nicol et al. Reference Nicol, Stolp, Cochran, Geijsel and Marshall1992). Whilst this method requires the incubation of large numbers of krill (a minimum of 100 individuals) the information gained allows for a more accurate in situ determination of growth, accounting for environmental influences and mortality (Nicol Reference Nicol2000). Furthermore, the IGR method allows for the determination of the intermoult period (IMP) - the number of days between moulting events - which is influenced by temperature and krill length, and daily growth rates (DGR) which incorporate both the IGR and IMP of the experiment (Tarling et al. Reference Tarling, Shreeve, Hirst, Atkinson, Pond, Murphy and Watkins2006). Despite providing valuable, fine scale information, allowing for both regional and seasonal comparisons, this method is consistently only used for Euphausia superba in the Southern Ocean, with T. macrura often considered too fragile to use in live experiments (Haraldsson & Siegel Reference Haraldsson and Siegel2014). The IGR method however, has been successfully applied to Thysanoessa species in the Northern Hemisphere (Pinchuk & Hopcroft Reference Pinchuk and Hopcroft2007). Furthermore, Nicol (Reference Nicol2000) indicates its effectiveness on other Southern Ocean euphausiids, including T. macrura, although quantitative data were not provided.
Advances in the methods and capabilities to provide fine scale estimates of growth and population structure are currently limited to E. superba in the Southern Ocean due to both their importance as a keystone species and the focus of large-scale fisheries (Melvin et al. Reference Melvin, Kawaguchi, King and Swadlingin press). These techniques, especially the IGR method, are transferrable to other Southern Ocean euphausiid species, but have yet to be implemented to begin to fill major knowledge gaps in growth patterns, both spatially and seasonally. The present work demonstrates the successful application of the IGR method to T. macrura and provides the first in situ T. macrura growth parameters including IGR, IMP and DGR, information that is critical for future evaluations of the population dynamics of T. macrura and its role in the Southern Ocean ecosystem.
Methods
Sampling
Specimens of T. macrura were collected in the Kerguelen Plateau region of the Southern Ocean, as part of the Kerguelen Axis marine science voyage undertaken by the RV Aurora Australis during late January 2016. Live T. macrura were collected using a rectangular midwater trawl net (RMT 8) equipped with a flow meter (Baker et al. Reference Baker, Clarke and Harris1973). The ship-equipped scientific echo sounder was used to identify regions of potentially high T. macrura abundance, with aggregations of T. macrura appearing as dense ‘patches’ or ‘mats’ at the ocean surface (10–30 m depth), verified by routine trawls of the RMT. A dense mat of T. macrura was identified at 63.5°S, 93.6°E, located over the Princess Elizabeth Trough at a depth of 15 m. The RMT was subsequently deployed to this depth with a trawl time of seven minutes at a ship speed of < 2.0 knots to ensure minimal damage to individuals during trawling.
Upon collection, live T. macrura were immediately transferred into an on-board holding tank equipped with continuously flowing surface seawater. Two hundred and eighty-eight freely swimming individuals were then randomly selected and transferred into individual 250 ml jars equipped with small holes to allow water exchange and placed in a specially built flow-through system to maintain ambient ocean temperature for the duration of the experiment. For detailed technical information on the flow-through system see Kawaguchi et al. (Reference Kawaguchi, Candy, King, Naganobu and Nicol2006). The experiment was conducted for a total of four days, with jars checked for moults every 24 hours. If an individual was found to have moulted, the animal and its moult were immediately frozen together and stored in liquid nitrogen for later measurement.
The remainder of the krill were then collected and a Motoda box plankton splitter (Motoda Reference Motoda1959) was used to fraction the sample, with a proportion immediately frozen at -80°C and the remainder stored in 10% buffered formalin. Preserved krill were counted, measured for their total length and sexed according to the descriptions provided by Wallis et al. (Reference Wallis, Kawaguchi and Swadling2018). Direct counts of T. macrura were also performed, accompanied by the flow meter reading to provide the abundance of the dense mat of T. macrura sampled.
Growth estimates
The relationship between uropod length and total body length was determined for T. macrura. A total of 50 frozen individuals caught during the sampling of live T. macrura were measured for their total body length, from the tip of the rostrum to the base of the uropod using digital callipers under a stereo microscope, and uropods were measured using a calibrated eyepiece micrometre (±0.01 mm). ANCOVA was used to evaluate the difference in this relationship between males and females.
Instantaneous growth rates were calculated using measurements of both the left and right uropod exopodites of both the moult and post-moult individual. Moulted individuals were sexed according to Wallis et al. (Reference Wallis, Kawaguchi and Swadling2018) and uropods analysed using a Leica MZ9.5 microscope equipped with a DFC380 digital colour camera. Moults and moulted individuals were placed ventral side down and the uropod arranged at an approximate 45° angle to the telson and photographed. The Leica Application Suite V2.4 image analysis software was then used to measure the length of the uropod. Damaged uropods were not measured, and an average of the length of left and right uropod was used for subsequent calculations if both were found to be intact and undamaged. The IGR of each individual was then calculated as the change in the length of the uropod from the moult (Um) and the animal post-moult (Ua) by the following equation (Tarling et al. Reference Tarling, Shreeve, Hirst, Atkinson, Pond, Murphy and Watkins2006):
To establish the changes in total body length (IGRTL) based upon changes in uropod length, the following equation is used:
where TL a is the total length of the post-moult krill and TL m is the total length of the moult. The total length of krill was first determined by applying the derived relationship between uropod length and total body length (Eq. 5). ANOVA was subsequently used to test for differences in estimates of IGR based upon the change in uropod length and total body length for males, females and juveniles.
Due to the surviving non-moulted krill not being sexed, the intermoult period (IMP) of T. macrura was calculated for the whole experiment. IMP was determined by the equation (Tarling et al. Reference Tarling, Shreeve, Hirst, Atkinson, Pond, Murphy and Watkins2006):
where N is the number of surviving krill at the conclusion of the experiment, m is the number of krill that moulted, and d is the duration of the experiment (days).
IGR calculations provide growth information in terms of proportional change. The daily growth rate (DGR) of T. macrura was subsequently calculated by the follow equation (Tarling et al. Reference Tarling, Shreeve, Hirst, Atkinson, Pond, Murphy and Watkins2006):
Theoretical DGRs were also calculated for male, female and juvenile T. macrura. Despite not having quantitative information on the number of maturity stages of T. macrura within the IGR experiment (male, female and juvenile), the maturity stage ratio of the population obtained was used as a proxy for the ratio of males, females and juveniles used within the experiment. It is assumed that due to the random sampling of the 288 freshly caught individuals the IGR population was reflective of the ratio of maturity stage in the wild population, whilst also assuming that mortality within the experiment was constant across males, females and juveniles. Using this information, the IMPs for each sex and juveniles were calculated using Eq. 3 and a subsequent sex- and stage-specific DGRs determined (Eq. 4).
Results
The aggregation of T. macrura had a density of 30 ind. m−3. The ratio of maturity stages of T. macrura within this surface mat indicated a female dominant aggregation, with 67% female, 29% male and 4% juveniles identified. The length frequency of T. macrura indicated a relatively similar size distribution for both males and females, albeit with females at higher frequencies than males (Fig. 1).
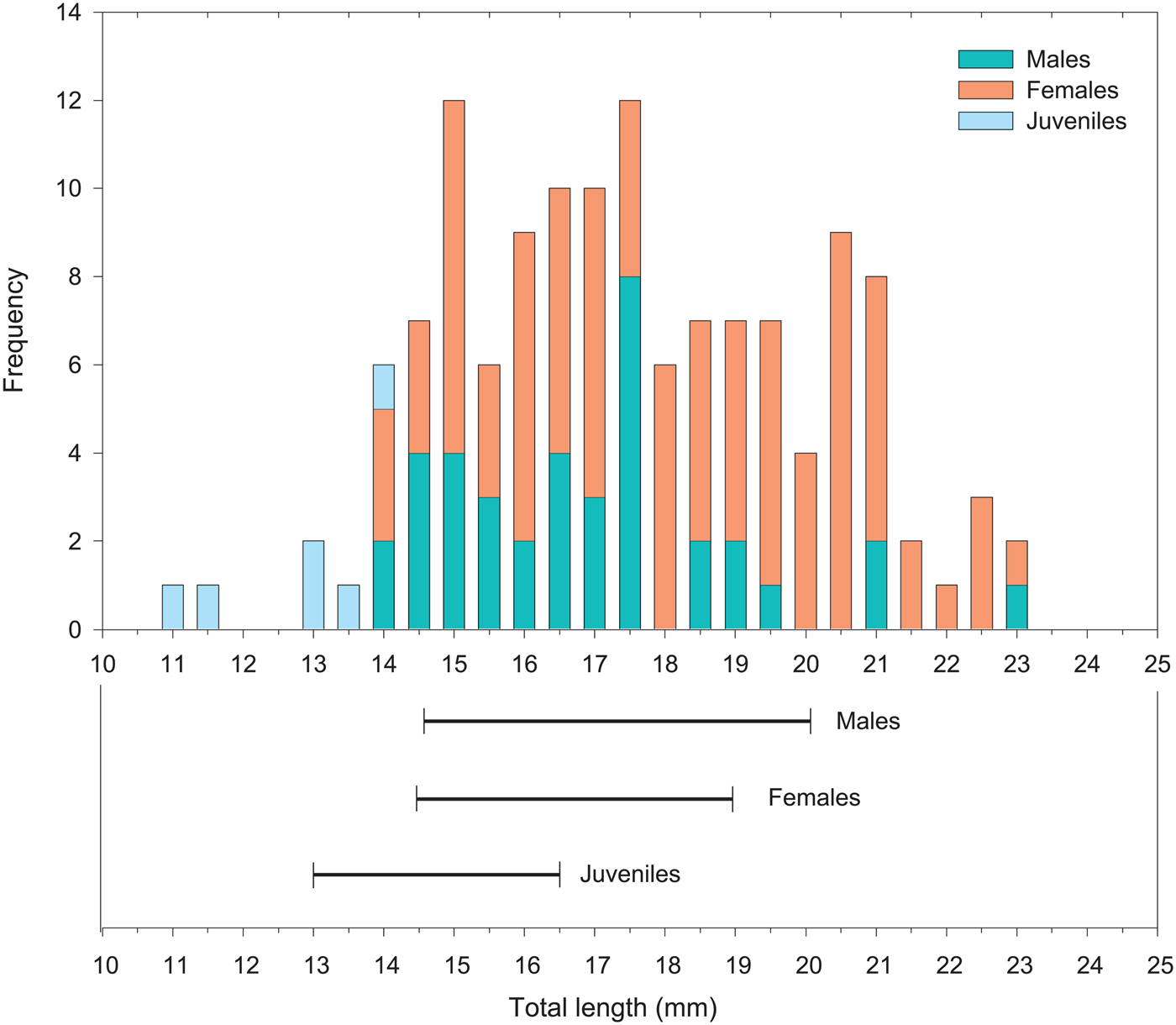
Fig. 1. Size-frequency distribution of Thysanoessa macrura for the maturity stages; male, female and juvenile from the aggregation sampled for the IGR experiment. The solid lines indicate the range in size of pre-moult individuals used in the IGR experiment.
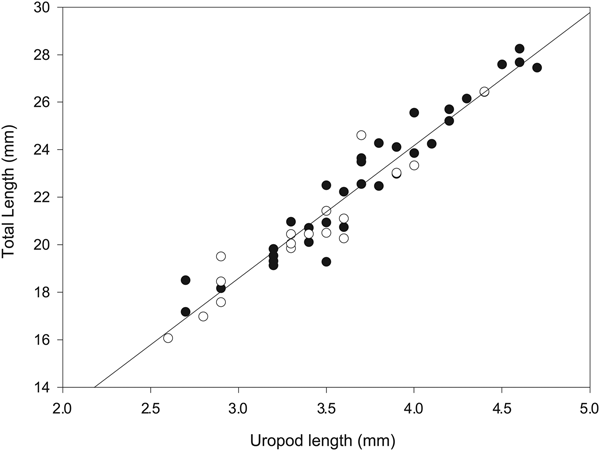
Analysis of the relationship between total length and uropod length for males and females, shown in Fig. 2 indicated no significant difference based upon sex (F = 0.23, P > 0.2). Consequently, both males and females were combined to provide a single relationship to estimate total length based upon uropod length. This relationship was found to have a strong linear relationship (R 2 = 0.93, P < 0.001) and is described by:

Fig. 2. Relationship between total body length and uropod length of adult Thysanoessa macrura males (empty circles) and females (filled circles). Total length = 5.595 × Uropod length + 1.804 (R 2 = 0.93, P < 0.001).
Instantaneous growth rates
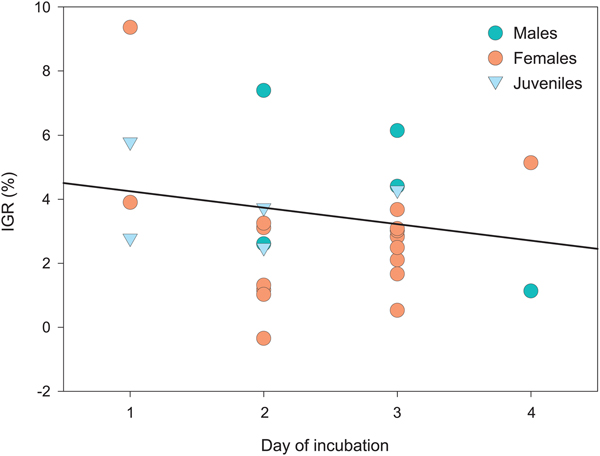
Twenty-eight individuals had moulted by the end of the IGR experiment (Table I). The number of individuals that moulted per day was variable between days of incubation, with the most individuals moulting during the second and third days of incubation (Fig. 3). As shown in Fig. 4, the IGR calculated for each day of the incubation indicated a non-significant decrease of IGR over the duration of the experiment (F = 1.52, P > 0.2). Due to the limited number of juveniles and males that moulted over the course of the experiment, this relationship was assessed based upon cumulative IGR values for each day of the experiment rather than maturity stage trends. During the course of the experiment, 30 individuals died, accounting for 10.4% of the experimental T. macrura used.

Fig. 3. Number of each Thysanoessa macrura maturity stage moulted for each day of the IGR experiment.

Fig. 4. IGR recorded for each day of the experiment for males, females and juveniles. The solid line indicates the linear regression relationship between cumulative IGR and day of incubation.
Table I. Summary of the growth parameters for Thysanoessa macrura based upon the four-day experiment. Instantaneous growth rates (IGR) are provided based upon changes in uropod length (IGRuro) and the total length of moulted T. macrura (IGRTL). Subsequent intermoult period (IMP) and daily growth rate (DGR) are provided. Theoretical estimates of growth are based upon the ratio of developmental stages present within the aggregation of Thysanoessa macrura sampled. TL = total body length.

IGR calculations based upon the change in uropod length and total length using the relationship derived from frozen individuals (Eq. 5) were both used to estimate the percentage growth of male, female and juvenile T. macrura. Based upon both methods for IGR calculation, juveniles were found to have the highest proportional growth of all three maturity stages (Table I). Juveniles had an average IGRuro of 4.9% and IGRTL of 4.3%, followed by males with an IGRuro and IGRTL of 4.3% and 3.8% respectively. Females had the lowest growth estimates at 3.1% (IGRuro) and 2.8% (IGRTL). Comparisons of IGR estimates based upon the change in length of uropods and total body length between moulting indicated that there was no significant difference in these estimates for males (F = 0.28, p = 0.61), females (F = 0.66, p = 0.66) or juveniles (F = 0.12, p = 0.74). Consequently, calculations of DGR for T. macrura were based upon IGRuro to avoid increasing unnecessary errors associated with applying total length conversions.
Comparisons of the total lengths of males, females and juveniles used in the IGR experiment indicate that the spread in the size is relatively consistent with the size range of each maturity stage identified in the aggregation sampled, although males and females above 21 mm were not represented in the experiment (Fig. 1).
Intermoult period and daily growth rates
The IMP of T. macrura based upon cumulative information from the entire IGR experiment was found to be 41 days. The subsequent DGR of T. macrura irrespective of maturity stage ranged from 0.003–0.04 mm day−1 with a mean DGR of 0.009 mm day−1 (Table I). In order to provide quantitative growth rates for the different maturity stages present in the sample, the proportion of males, females and juveniles present in the T. macrura aggregation sampled was used to estimate the number of each present in the IGR experiment. The IMPs of all three maturity stages were then calculated assuming that mortality rates were consistent for males, females and juveniles within the experiment (Table I).
The theoretical IMPs were highly variable for the different maturity stages. Males had the largest IMP at 62, with a corresponding mean DGR of 0.011 mm day−1. Females had a smaller IMP period than males, with an estimated IMP of 42 days, however DGR for females with a mean of 0.012 mm day−1 was not significantly different from the value for males (F = 0.07, P = 0.77). Juveniles had the lowest IMP of 14 days, with a corresponding mean DGR of 0.55 mm day−1, significantly greater than both males and females (F = 24.6, P < 0.001).
Discussion
The results of this study demonstrate the practicability of applying the IGR method successfully to T. macrura in the field. The dense aggregation of T. macrura, appearing as a mat in surface waters demonstrated that this species can reach high densities in discrete regions, indicating behaviour not previously recorded for the species. Thysanoessa macrura is often thought of as a fragile species that does not survive net sampling (Haraldsson & Siegel Reference Haraldsson and Siegel2014). Although T. macrura often do not survive the manual handling of long, standard trawls, often double oblique tows to 200 m, our observations demonstrate that T. macrura can survive short, targeted trawls, reducing the damage incurred during regular trawling by large nets. Furthermore, T. macrura were maintained alive in an on-board holding tank for one month (J. Wallis, personal observation). The structure of the T. macrura aggregation is consistent with other observations of T. macrura populations with a size range of 13–23 mm with a skewed sex ratio, dominated by females (Haraldsson & Siegel Reference Haraldsson and Siegel2014).
The IGR method, established for the use of measuring in situ growth of E. superba, assumes that somatic growth is directly proportional to the change in uropod length between pre-moult (measured directly from the moult) and the moulted individual due to the linear relationship between uropod length and total length (Nicol Reference Nicol2000, Kawaguchi et al. Reference Kawaguchi, Candy, King, Naganobu and Nicol2006). The relationship between uropod and total length was also found to follow a linear relationship for T. macrura. No sexual differentiation was identified in this relationship, consistent with other somatic relationships for T. macrura (Fäber-Lorda Reference Fäber-Lorda1990). Somatic relationships for T. macrura have been shown to display regional variability due to the influence of temperature and food availability and their influence on growth (Fäber-Lorda Reference Fäber-Lorda1994). Accordingly, uropod-length relationships should be derived for each population of T. macrura sampled. A comparison of IGRs derived from uropod measurements and total body length, calculated from the relationship derived from the population sampled indicated no difference in IGR estimates and therefore indicates the feasibility of using uropods to estimate growth for T. macrura as per the established method for E. superba (Quetin & Ross Reference Quetin and Ross1991, Nicol et al. Reference Nicol, Stolp, Cochran, Geijsel and Marshall1992)
Due to the lack of quantitative information regarding the ratio of maturity stages used in the experiment, only simple estimates of IMP and DGR of T. macrura (regardless of maturity stage) could be confidently calculated. The simple IMP for T. macrura of 41 days indicates a period of very little and slow somatic growth. Tarling et al. (Reference Tarling, Shreeve, Hirst, Atkinson, Pond, Murphy and Watkins2006) highlight the potential error associated with the calculation of IMP from a single IGR experiment if moult synchronicity is present. Due to the restrictions of only a single IGR experiment being performed, it was not possible to apply alternative methods of determining the IMP of T. macrura. Given that less than 10% of the incubated individuals moulted within the four-day experiment, it is likely that there was little moult synchronicity present and therefore the calculated IMP closely reflects the wild population. The large IMP is reflected by the low IGR values determined for T. macrura with females exhibiting the smallest growth increment (IGRuro) at 3.1% followed by males at 4.3% and juveniles showing the highest growth at 4.9%. A growth rate of 0.009 mm day−1 is lower than yearly average growth rates previously reported for T. macrura (Haraldsson & Siegel Reference Haraldsson and Siegel2014, Driscoll et al. Reference Driscoll, Reiss and Hentschel2015). Given that these previously reported growth estimates are yearly averages based upon repeated sampling length-frequency analysis, seasonal variation is not accounted for. Similar DGRs of northern hemisphere congener species, T. inermis and T. spinifera estimated using the IGR method have been determined during the onset of autumn, a period of low to negative growth for both species (Pinchuk & Hopcroft Reference Pinchuk and Hopcroft2007). Thysanoessa macrura probably shows similar seasonal growth patterns, highlighting the need for the application of the IGR method during different times of the year in assessing these seasonal cycles.
The growth rate estimates of T. macrura, based upon cumulative information of the IGR experiment provide a broad overview of T. macrura growth and highlight the power of the IGR method. Despite the lack of quantitative information on the maturity stages present in the IGR experiment, using the assumptions of an equal maturity ratio to the wild population sampled allows for the first insights into the variation in growth for males, females and juveniles. The pattern in sex-specific IMPs observed by Tarling et al. (Reference Tarling, Shreeve, Hirst, Atkinson, Pond, Murphy and Watkins2006) for E. superba, with males possessing longer IMPs than females was reflected in the theoretical IMPs calculated for T. macrura in this study, with males having an IMP of 62 days and females 42 days. Juveniles had a much shorter IMP of only 13 days, indicating much higher growth than adult T. macrura and is reflective of the IGRs determined. Despite differences in the IMPs, males and females displayed highly similar DGRs. The DGRs determined for both males and females are very similar to previously reported growth estimates (Table II), however the DGR of juveniles, up to five times greater than adults, is substantially larger than current estimates for T. macrura (Haraldsson & Siegel Reference Haraldsson and Siegel2014). These IMPs and DGRs of T. macrura are also consistent with the observed growth of E. superba during the same voyage (Melvin et al. in press). Whilst both patterns in IMP and growth rates are consistent with E. superba from the same time of year, the underlying mechanisms to explain these growth rates differ. During summer, E. superba is still actively reproducing, with energy used to fuel reproduction rather than somatic growth, resulting in longer IMP and lower growth (IGR and DGR) (Tarling et al. Reference Tarling, Shreeve, Hirst, Atkinson, Pond, Murphy and Watkins2006, Melvin et al. in press). Thysanoessa macrura however has finished its reproductive period by spring, accruing lipid reserves in the free space of the cavity during summer (Hagen & Kattner Reference Hagen and Kattner1998, Wallis et al. Reference Wallis, Kawaguchi and Swadling2018). The preferential partitioning of energy to lipid storage rather than somatic growth during late summer could help explain the growth identified for T. macrura and its similarity to that of E. superba. Furthermore, juveniles, with growth rates up to five times faster than adults, do not accumulate large lipid reserves until autumn, capitalizing on the high food availability in spring and summer for a sustained growth period, a similar trend as observed in E. superba (Hagen & Kattner Reference Hagen and Kattner1998, Kawaguchi et al. Reference Kawaguchi, Candy, King, Naganobu and Nicol2006).
Table II. Estimates of Thysanoessa macrura growth rates based upon repeated measured length-frequency analysis.

1Nordhausen Reference Nordhausen1992.
2Haraldsson & Siegel Reference Haraldsson and Siegel2014.
3Driscoll et al. Reference Driscoll, Reiss and Hentschel2015.
4This study.
The results of the IGR experiment provided in this study demonstrates the success of the IGR method for T. macrura, providing the first IGR, quantitative IMP and DGR estimates for the species. Whilst the power of the IGR method is contingent upon multiple sampling locations to assess regional variations in growth and the ability to understand the influence of temperature and food availability on IMP and DGR, the limited sample size of our study currently precludes this ability. The theoretical growth parameters established for males, females and juveniles provide an interesting insight into variation between these maturity stages and prompt the need for further investigation. Answering the fundamental questions of the influence of sex and developmental stage, and environmental conditions on somatic growth rates is vital for producing a comprehensive understanding of the seasonal and regional patterns in T. macrura growth and should be the target of future investigations by employing the IGR method.
Acknowledgements
We would like to thank the captain and crew of the Aurora Australis and the members of the research expedition for their help and support during sample collection and the running of the experiment. We would also like to thank the reviewers for their help and expertise. This research was supported by the Antarctic Climate and Ecosystems Cooperative Research Centre (ACE CRC) and funded through the Australian Antarctic Science (AAS) grants 4331, 4344 and 4037.
Author contribution
J.R. Wallis performed the data analysis and was assisted by S. Kawaguchi with preparing the manuscript; R. King and J.E. Melvin performed the live experiment at sea.