Kawasaki disease is an acute febrile childhood illness that is classified as a systemic vasculitis syndrome. Together with aspirin, high-dose intravenous immunoglobulin therapy is generally effective to resolve inflammation in Kawasaki disease and reduce the occurrence of coronary artery abnormalities.Reference Newburger, Takahashi and Burns 1 – Reference Newburger, Takahashi and Beiser 3 Although “giant” and “supergiant” coronary aneurysms of ≥8 and ≥10 mm, respectively, are uncommon, Kawasaki disease patients may be predisposed to acute thrombotic occlusion leading to acute myocardial infarction and mortality.Reference Ekici, Varan, Kocabaş, Erdoğan, Eminoğlu and Aktaş 4 , Reference Motozawa, Uozumi and Maemura 5 Patients with giant coronary artery aneurysms therefore need lifelong anticoagulant therapy. In a recent Japanese study of 245 patients, 15 deaths were caused by Kawasaki disease, among which 13 were directly due to myocardial infarction.Reference Tsuda, Hamaoka and Suzuki 6
We here report the clinical findings of an infant boy who suffered a myocardial infarction caused by a thrombus in a “supergiant” aneurysm. Since his symptoms were mild, routine electrocardiography played a crucial role in myocardial infarction identification. Anticoagulant treatment including combined heparin injection and high-dose warfarin was effective.
Case report
A 14-month-old Japanese boy with Kawasaki disease was admitted to our hospital for suspected coronary artery aneurysms. Initial treatment with prednisolone 2 mg/kg/day, aspirin 30 mg/kg/day, and intravenous immunoglobulin therapy 2 g/kg was administered on day 4, as he was predicted to be a non-responder to intravenous immunoglobulin therapy by conventional risk scores. On day 8, he was unresponsive to the second round of intravenous immunoglobulin and soon after developed coronary aneurysms of 4.6 mm in diameter in the proximal left anterior descending coronary artery and 4.7 mm in diameter in the right coronary artery. On day 12, he commenced infliximab 5 mg/kg through intravenous injection. Although he became afebrile and other Kawasaki disease symptoms disappeared, he eventually exhibited “supergiant” coronary aneurysms that were 8.6 mm in diameter in the proximal left anterior descending coronary artery and 12.6 mm in diameter in the right coronary artery (Fig 1a and b) that required urgent anticoagulant therapy with warfarin. At discharge, he was prescribed aspirin, warfarin, and dipyridamole. Propranolol and enalapril maleate were also added to control heart rate and blood pressure and prevent aneurysm rupture. According to the guidelines for Kawasaki disease management in Japan, warfarin intake was adjusted to maintain a therapeutic range of prothrombin time based on the international normalised ratio of 2.0:2.5. 7
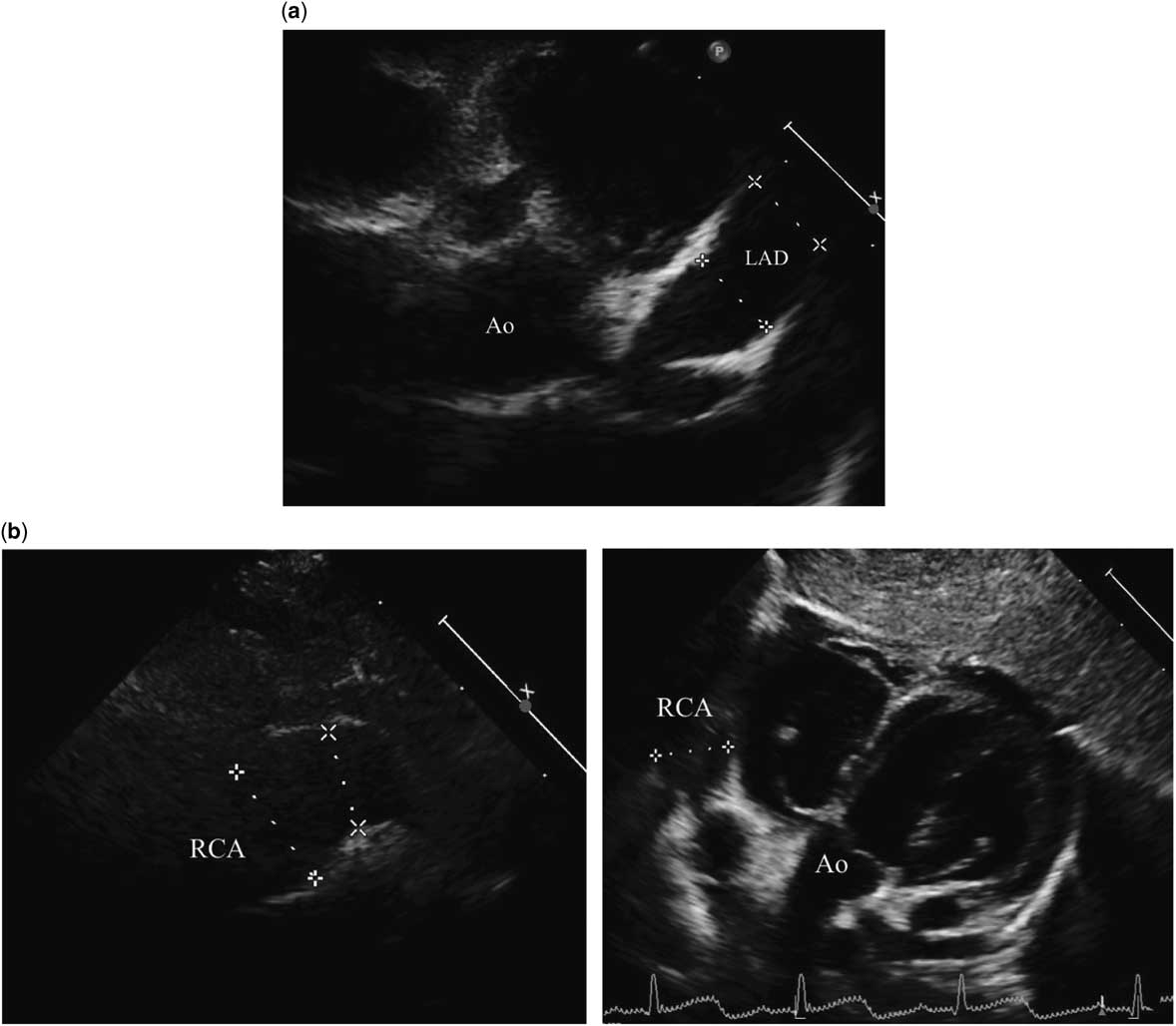
Figure 1 Echocardiography at the acute phase of Kawasaki disease. Echocardiography revealed diffuse dilatation and “supergiant” aneurysms in the left anterior descending coronary artery (a) and right coronary artery (b) on the 20th day of acute Kawasaki disease. The left anterior descending coronary artery was 8.6 mm and the right coronary artery was 12.6 mm in maximum diameter.
At the age of 2 years, the patient experienced upper abdominal pain and sudden vomiting but recovered spontaneously in several hours. One week later, he visited our hospital for scheduled coronary angiography and showed no obvious symptoms suggestive of myocardial infarction; however, standard 12-lead electrocardiography revealed abnormal Q-waves in V1-3 and giant negative T-waves in V2-4 (Fig 2a). Laboratory data showed brain natriuretic peptide of 188.9 pg/ml, the normal range being <20 pg/ml, prothrombin time based on the international normalised ratio of 2.17, and normal cardiac enzymes. Echocardiography also uncovered a large thrombus of 7.9 × 17.3 mm in the left anterior descending coronary artery aneurysm (Fig 2b) without any apparent dyskinesia of the left ventricular wall. Left ventricular systolic and diastolic functions were preserved. Subsequent 99m-technetium myocardial perfusion scintigraphy revealed decreased myocardial blood flow in the anterior wall both at rest and during adenosine stress. He was diagnosed of having suffered an anterior myocardial infarction without apparent symptoms or cardiac dysfunction. We decided against thrombolytic therapy, such as urokinase or tissue-plasminogen activator, since more than 1 week had passed from the myocardial infarction, indicating that his minor gastrointestinal symptoms had been caused by myocardial infarction and his cardiac condition had been preserved. Anticoagulant therapy with combination of heparin injection and warfarin was initiated. Warfarin dose was adjusted to maintain a prothrombin time based on the international normalised ratio between 2.5 and 3.0. One week later, angiography of the left coronary artery confirmed a thrombus in the aneurysm of the left anterior descending coronary artery and total occlusion of the distal left anterior descending coronary artery (Fig 2c). Furthermore, angiographic imaging of the right coronary artery disclosed collateral circulation from the right coronary artery to the distal left anterior descending coronary artery. Intensive anticoagulant therapy with heparin and warfarin was continued to resolve the remaining thrombus. Carvedilol instead of propranolol was administered to prevent myocardial fibrosis.
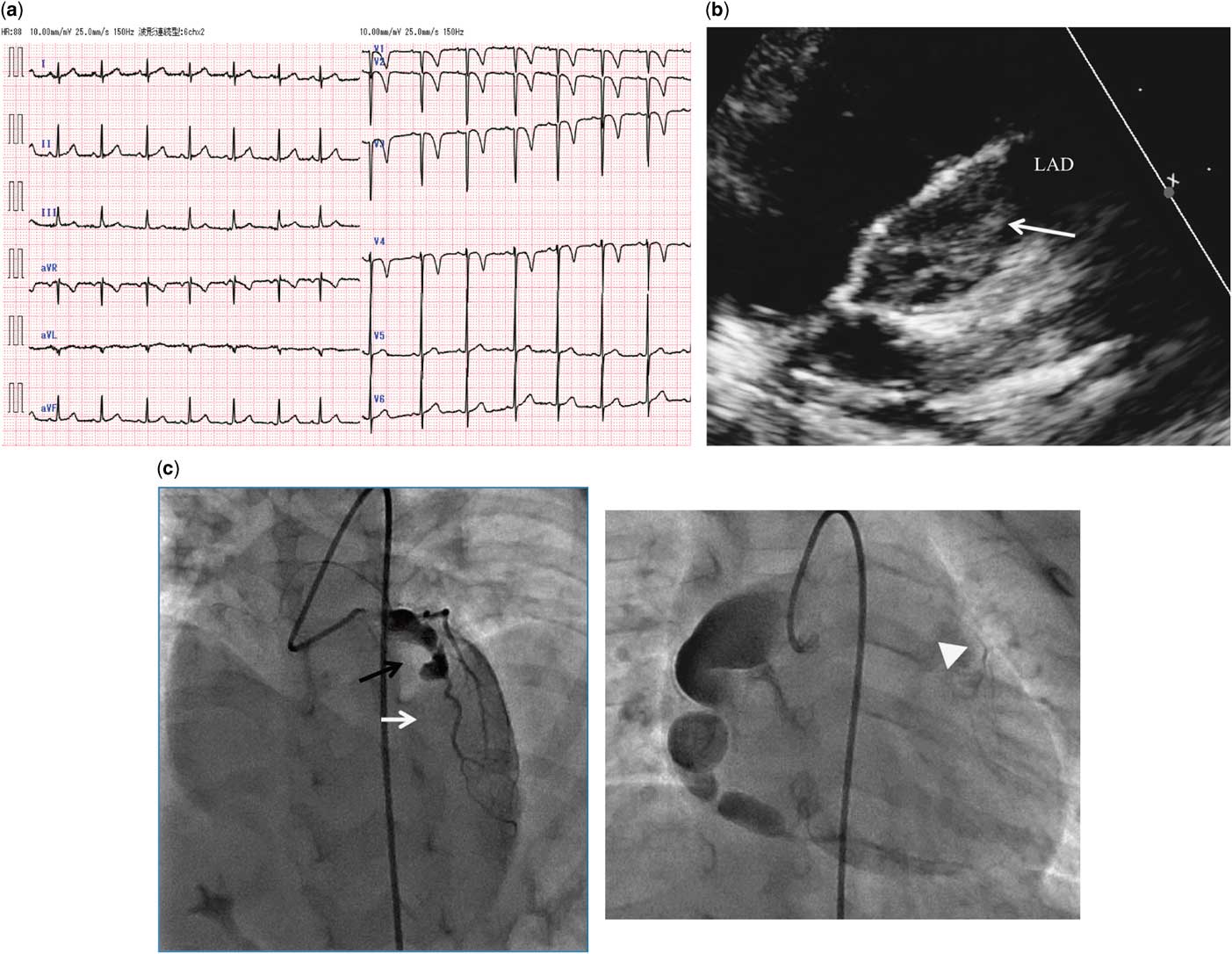
Figure 2 Cardiovascular examination on admission for myocardial infarction due to a thrombus in a coronary aneurysm. (a) Standard 12-lead electrocardiography at scheduled coronary angiography. Abnormal Q waves in V1-3 and giant negative T waves in V2-4 were evident. (b) Echocardiography revealed a large thrombus (white arrow; 7.9 × 17.3 mm) in the giant aneurysm of the left anterior descending coronary artery. (c) Coronary angiography a week after starting anticoagulation therapy disclosed a large thrombus in the left anterior descending coronary artery aneurysm (black arrow) and total occlusion of the distal left anterior descending coronary artery (white arrow). Collateral circulation had extended from the right coronary artery to the distal left anterior descending coronary artery (white arrowhead).
Daily electrocardiography measurements indicated shrinking of the thrombus in the left anterior descending coronary artery. It was also notable that the abnormal Q-waves and negative T-waves in the precordial leads had completely disappeared in 2 weeks (Fig 3a). Owing to the success of the aggressive anticoagulant treatment, heparin was discontinued and high-dose warfarin was maintained by prothrombin time based on the international normalised ratio 2.5:3.0. Three months after the myocardial infarction, follow-up coronary angiography revealed that the thrombus had almost completely disappeared and that perfusion of the left anterior descending coronary artery was restored (Fig 3b). Finally, his myocardial infarction because of a thrombus in a giant aneurysm was successfully managed without any apparent ischaemic signs or cardiac dysfunction, although he ultimately required continuous therapy with warfarin.

Figure 3 Cardiovascular examination after anticoagulant therapy. (a) Standard 12-lead electrocardiography 2 weeks after starting aggressive anticoagulant therapy. The abnormal Q waves in V1-3 and negative T waves in V4 had disappeared. (b) Coronary angiography 3 months after the myocardial infarction. The thrombus had shrunk (black arrow), and perfusion of the distal left anterior descending coronary artery was restored (white arrow).
Discussion
Giant aneurysms secondary to Kawasaki disease increase the risk of sudden cardiac death because of myocardial infarction caused by a massive thrombus.Reference Kato, Sugimura and Akagi 8 Myocardial infarction occurs most commonly in the first year after the disease onset.Reference Tsuda, Hamaoka and Suzuki 6 However, its diagnostic difficulty stems from the rarity of acute myocardial infarction in young patients and is compounded by its diverse clinical features and the inability of infants to describe their symptoms. Consequently, the diagnosis of acute myocardial infarction is likely to be delayed in infants and young children, resulting in a diminished chance of timely reperfusion.Reference Tsuda, Hamaoka and Suzuki 6 Because most cases of acute myocardial infarction involve Kawasaki disease patients with giant aneurysms, close follow-up is needed, particularly within the first year after Kawasaki disease diagnosis.
In the present case, changes in electrocardiography findings helped diagnose asymptomatic myocardial infarction and enable successful reperfusion. In the absence of QRS confounders, abnormal Q-waves are usually diagnostic of myocardial necrosis. However, they may regress or disappear over time in as many as 25–63% of patients with a history of Q-wave myocardial infarction.Reference Yasuda, Iida and Itagane 9 , Reference Voon, Chen, Hsu, Lai and Sheu 10 Some investigators have reported that Q-wave appearance after myocardial infarction depends on infarct size.Reference Delewi, Ijff and van de Hoef 11 , Reference Nagase, Tamura, Mikuriya and Nasu 12 Patients with myocardial infarction showed Q-wave regression trend towards a smaller amount of necrotic myocardium and a significantly larger amount of stunned myocardium.Reference Nagase, Tamura, Mikuriya and Nasu 12 Transient ischaemic Q-waves may appear acutely or be present chronically with the potential of disappearing when coronary perfusion is restored. Oedema and inflammation may also play a part in rendering the myocardium electrically inert, and their disappearance may explain a loss of Q-waves.Reference Barold, Falkoff, Ong and Heinle 13 Electrical stunning, which presents as transient abnormal Q-waves, may be associated with the myocardial “stunning” following episodes of acute transient coronary ischaemia. In our case, at least 1 week had passed from the suspected asymptomatic myocardial infarction. He could have had a stunned myocardium due to ischaemia rather than an infarction, and therefore the abnormal Q-waves disappeared by reperfusion using anticoagulant therapy. However, the infarct size was small and it was thought that myocardial viability remained preserved in the stunned myocardium.
Conclusion
We described in this study a Kawasaki disease patient with “supergiant” coronary aneurysms, who suffered an asymptomatic myocardial infarction because of a large thrombus in the left anterior descending coronary artery. Abnormal Q-waves in electrocardiography played a key role in diagnosing the disease, as well as in the early monitoring of anticoagulant therapy progress. Electrocardiography is a simple, non-invasive, widely available tool. Combination heparin and warfarin treatment enabled successful reperfusion without additional catheter or surgical intervention.
Acknowledgements
The authors sincerely thank Dr Ryuji Fukazawa at Nippon Medical School for his suggestions regarding the present patient and for providing clinical data. We also thank Dr Takashi Shimizu at Saku Central Hospital for his routine medical care, as well as Yūka Miyajima and Trevor Ralph for their language assistance.
Financial Support
No funding was received for this study. The authors have no financial relationships relevant to this article to disclose.
Conflicts of Interest
The authors have no conflicts of interest to disclose.





