Principles of field investigation
Field investigations are studies of causation. We conduct field investigations for the purposes of (1) reducing the losses associated with existing cases; and (2) preventing new cases from occurring. The challenge is to conduct an investigation that leads to a solution to the problem. Unfortunately, in veterinary medicine, we often spend considerable time and money to name the pathogen without solving any problems. To be fair, knowing the pathogens involved in a disease outbreak can sometimes be useful; however, outbreak investigations can become sidetracked in the sole pursuit of an etiologic agent rather than identifying more useful explanations for the outbreak. Knowing the etiologic agent may provide an explanation for the proximal cause of disease and might provide therapeutic insight. However, that knowledge only rarely explains the course of events that led to the outbreak, or provides a solution for preventing future problems. Often, we are more successful at meeting the objectives of a field investigation if we can identify the actions or behaviors associated with the production system that led up to (caused) the problem. For example, recognizing that the incidence of mastitis on a dairy is the highest on Mondays might give an investigator reason to investigate the weekend milking process; recovering coliform bacteria from mastitic milk would not be as informative.
Systematic approach to field investigation
It can be difficult to provide solutions to disease outbreaks, but success is more likely if an organized, epidemiologic approach to outbreak investigation is followed (Hancock and Wikse, Reference Hancock and Wikse1988; Waldner and Campbell, Reference Waldner and Campbell2006). Field investigations involve an orderly process to characterize the outbreak (Smith, Reference Smith2012):
(1) Interview key individuals (e.g. owners, caretakers, veterinarians, and other stakeholders)
(2) Verify the clinical diagnosis and assure that treatments are appropriate
(3) Identify the factors responsible for the outbreak
(4) Develop strategies to prevent new cases and future outbreaks
(5) Communicate observations and recommendations with the key individuals
Detailed discussions on these steps of the field investigation have been published elsewhere (Smith, Reference Smith2012).
Risk assessment
Risk assessment is a process of: (1) evaluating the likelihood and costs (or benefits) of potential hazards (or opportunities) – termed risk analysis; (2) determining what actions, at what relative cost, can be taken to mitigate those hazards – termed risk management; and (3) sharing the action plan with all members of the team, as well as keeping records to show what was done and whether the actions were successful – termed risk communication.
From a risk assessment standpoint, field investigation steps 1–3 above are risk analysis, step 4 is risk management, and step 5 is part of the documentation and risk communication phase.
During the risk analysis phase, it may be useful to supplement published data with herd-specific data from health records (Rae, Reference Rae2006), outbreak investigation (Smith, Reference Smith2012), or clinical trials (Sanderson, Reference Sanderson2006). It may be possible to recognize important hazards and estimate their costs without ranch data, but it is more difficult to evaluate progress or compliance in the risk management stage without using records. Unfortunately, few cow–calf operations collect animal health data in an easily analyzable format (USDA, 2008). The lack of a simple record keeping system on many farms hinders the process of recognizing important hazards and their costs and makes it difficult to document that risk management actions were implemented and to evaluate if those actions were effective.
Causal inference
On-farm investigations of cattle diseases are more often qualitative than quantitative because useful quantitative data (e.g. from health records) are often not available for analysis. A qualitative investigation relies on more subjective observations including partial records, memory, and perceptions of relationships. Causal inferences in qualitative investigations are largely based on the logic of: (1) method of agreement; (2) method of difference; or (3) concomitant variations (Gay, Reference Gay2006). A causal factor might be identified by the method of agreement if the factor is common to multiple instances of the outcome when other factors are dissimilar (e.g. finding that bovine respiratory disease (BRD) outbreaks are common to herds using a particular receiving ration, even though other management practices differ). A causal factor might also be identified by the method of difference if a particular factor differs while others remain the same (e.g. if incidence of BRD is greater among calves receiving one particular vaccine than calves on the same farm receiving a different vaccine). Finally, causal relationships may be revealed by the method of concomitant variations if the risk for the outcome changes with the level of the risk factor, all other factors being the same (e.g. the longer calves are in transit to the feedyard, the greater the incidence of BRD).
Outbreak characterization
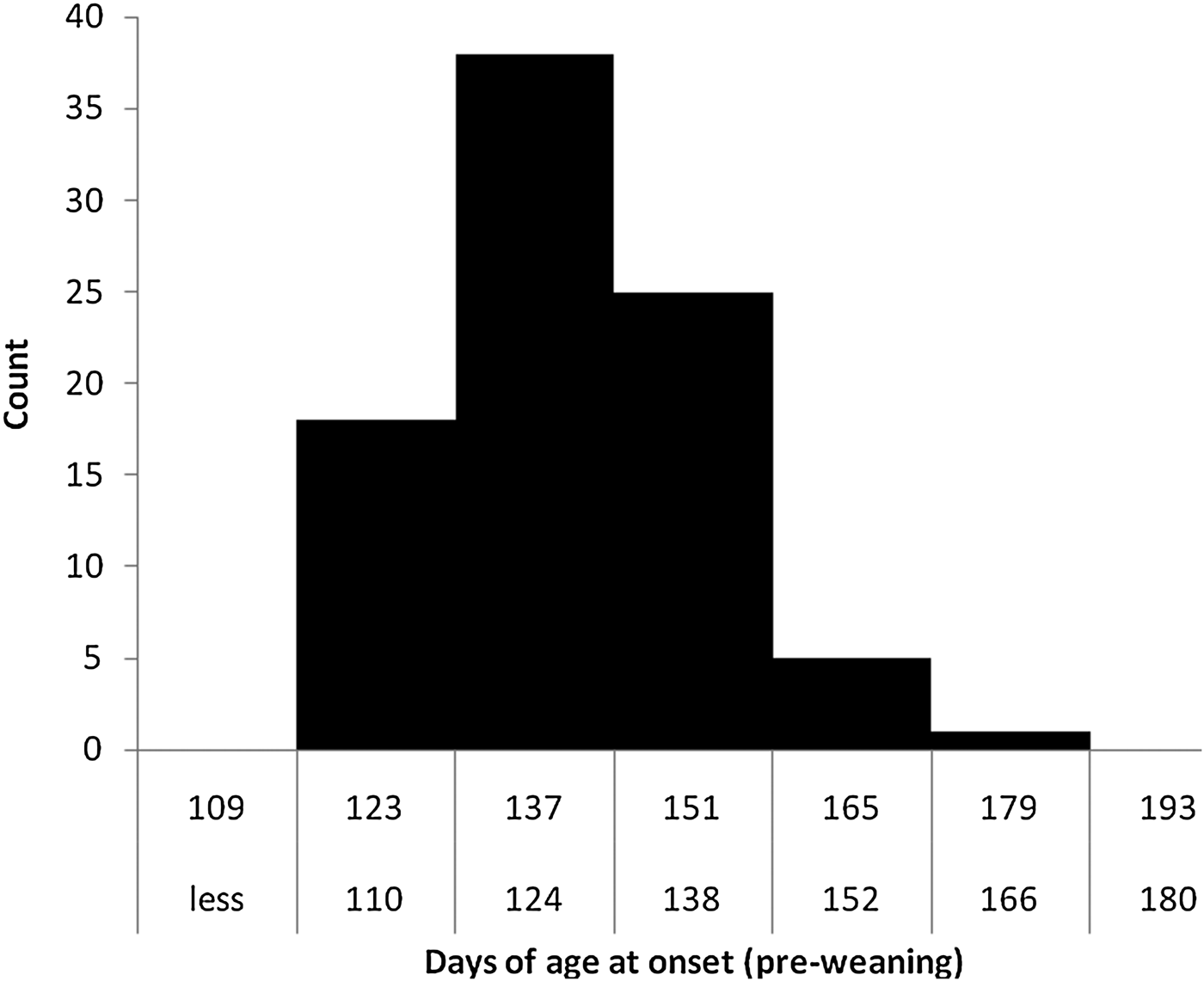
Field investigation relies heavily on pattern recognition to generate causal hypotheses. The most common way of doing this would be to characterize the outbreak by subject, time, and space. The ‘subject’ may be specifically identified individuals, or it may be group-level information (e.g. herds or pens). Time may be by the calendar or it may be relative to a relevant point in time (e.g. age, or time since feedlot entry). It may be useful to graphically portray the data; for example, in the form of maps or frequency histograms (Fig. 1). A frequency histogram of particular interest is the count of cases plotted by calendar time, also known as an epidemic curve (Fig. 2). Disease outbreaks may occur because of a common exposure (point-source epidemic) (e.g. the bacteria-contaminated potato salad at the community picnic). These types of outbreaks are typically rapid in development and resolution, or they may become propagated epidemics (e.g. you got sick from the community picnic, then you infected your family and they passed the infection on to friends at school). Propagated epidemics are typically characterized by less rapid, but ongoing, transmission from animal to animal. Observing the shape of the epidemic curve may reveal the nature of the disease process, identify potential risk factors preceding the onset of clinical signs, or suggest methods to prevent new cases (e.g. by removing the potato salad or isolating sick individuals) (Lessard, Reference Lessard1988). For example, outbreaks that occur as a point-source epidemic (e.g. because of a sudden exposure to a pathogen, the sudden loss of immunity, or something that suddenly facilitates pathogen transmission) may be evidenced as an epidemic curve with a high peak in a relatively short period of time. When the outbreak is propagated (e.g. when the disease process is one of ongoing transmission, or there is a continuous presence of risk factors) the epidemic curve may appear flatter over a longer period of time.

Fig. 1. Age distribution of 87 pneumonia cases from among 296 pre-weaned calves in a Nebraska ranch.

Fig. 2. Epidemic curve of 87 pneumonia cases from among 296 calves in a Nebraska ranch. Lines represent the proportion of calves at least 100 (solid) or 120 (dashed) days of age each week.
Quantitative study designs
When data are available, a quantitative approach is often more useful for discovering causal relationships and evaluating the effectiveness of interventions. The best study design for evaluating causal relationships depends on the circumstances. There are three basic observational study designs: (1) case-control; (2) cohort or longitudinal; and (3) cross-sectional. Case-control studies compare odds of exposure among cases to the odds of exposure among non-cases. Case-control studies excel when the disease is rare and when there are many potential exposures to test. Cohort and longitudinal studies compare incidence of disease among subjects with an exposure to the incidence of those without the exposure. Cohort and longitudinal studies are best when it is possible to follow subjects over time, either prospectively or retrospectively. Cross-sectional studies look at the relationship between disease and exposure prevalence at a point in time (Shott, Reference Shott2011).
Measures of association
The measure of association is an important statistic because it helps quantify the strength of the relationship between the risk factor and the occurrence of disease. When the outcome is dichotomous (e.g. diseased or not diseased), the measure of association is the odds ratio (or, in some situations, relative risk). These are comparisons of the odds, probability, or incidence of observing disease with one exposure level compared to another. If the odds ratio (or relative risk) has a value of one, then the exposure is not associated with the disease. If the odds ratio is greater than one, then that exposure is associated with the disease. If the odds ratio is less than one then the exposure is associated with the absence of disease (e.g. it is protective from disease). The further the odds ratio is from one the stronger the association.
Epidemiologic principles relevant to BRD
Component causes
In disease causal theory, each factor that contributes to the development of disease is a component cause (Rothman, Reference Rothman1976). Clinical signs of disease are expressed when various component causes add up to complete a sufficient cause. This explains why some component causes are observed in the absence of disease (e.g. we might recover Mannheimia haemolytica from nasal secretions of calves without signs of BRD). This concept also explains why the manager of a herd that reliably vaccinates against respiratory pathogens might blame BRD on bad weather, while the manager of a herd in a moderate climate might observe BRD when they have been lax on timely vaccinations. Removing one component cause means that the sufficient cause is not completed and thus disease is not observed. Therefore, in a field investigation we hope to determine which possible component causes are completing a sufficient cause, and determine which of those component causes (also known as causal factors or risk factors) are key determinants. Key determinants are those causal factors which are under management control.
Herd immunity
Herd immunity occurs when the proportion of immune individuals in a population is large enough to inhibit ongoing transmission of the pathogen; therefore individuals in the population who are not immune may be protected against disease because they do not experience an effective contact with the pathogen. So, in populations where herd immunity may be playing a protective role, it may not be sufficient to know an individual's immune status – it may be necessary to interpret individual immunity in the context of the immune status of others in the immediate population. An effective contact is an exposure to sufficient pathogen dose-load for an adequate duration of time to transmit the infection. To the author's knowledge, the proportion of a herd that must have protective immunity to prevent transmission of the common BRD pathogens in a herd of cattle has not been defined.
Using the results of field investigation to manage BRD in beef cattle systems
One of the perplexing animal health problems on some beef cattle ranches is the occurrence of pneumonia in calves prior to weaning. Studying patterns of disease occurrence may provide clues to disease causation, and this knowledge can be used to develop methods to prevent or control important livestock diseases. Since 2008 we have conducted field investigations in ranch herds experiencing summer pneumonia. The results of this work may be relevant to cattle producers, veterinarians, and others interested in the health and well-being of cattle elsewhere.
In the course of our investigations we recognized two patterns of occurrence of summer pneumonia – either sporadically in young calves or as sudden outbreaks in older calves. We have observed that calves 90–150 days of age are at greatest risk for BRD in the absence of other management stressors, such as weaning (Fig. 1). We note that the sudden outbreaks of BRD in older calves usually occur when a large proportion of the calves in the herd exceed 100–120 days of age (Fig. 2). We speculate that the causes of these two patterns may be failure of passive transfer resulting in more sporadic cases in very young calves, or a large proportion of the population losing maternal antibody protection (i.e. losing herd immunity) resulting in rapid and widespread onset of pneumonia in older calves. An additional factor was the influence of the age of the dam.
Each of these factors suggests that maternal immunity (or lack of it) may play an important role in the occurrence of pneumonia in ranch calves. We also recognized the need for better methods to record health data to investigate these types of health events.
Acknowledgments
A contribution of the Beef Cattle Population Health and Reproduction Program at Mississippi State University. Supported by the Hall-Davis Endowment for Beef Cattle Health and grants from Zoetis, Inc (formerly Pfizer Animal Health, Inc.).