Isolated atrial defects usually lead to left-to-right shunt and right ventricular volume load. Descriptions of cyanosis with this congenital heart defect are rare.
Whilst intrauterine flow from the inferior vena cava is directed through the foramen ovale by the eustachian valve, changes in the interatrial pressures postnatally usually lead to closure of the foramen ovale stopping this flow. In very few case reports, the eustachian valve remains functional and even after birth divides systemic venous blood towards the left atrium. Reference Manuel, Ghosh and Alex1–Reference Raffa, Al-Ibrahim, Kayali, Sorefan and Rustom3
Anatomical connections of systemic veins to the left atrium are rare. They lead to different degrees of cyanosis dependent on the amount of systemic venous blood mixing with the pulmonary venous retour. In cases of isolated liver veins connected to the left atrium, cyanosis maybe mild, whilst patients in whom the inferior or superior caval vein is connected directly to the left atrium desaturations can be severe. Reference Aliter, El-Haddad, Gallo and Al-Halees4–Reference Van Praagh, Geva and Lock6
We describe a rare case of inferior caval vein flow directed through an atrial septal defect in the fossa ovalis leading to severe cyanosis, but without any additional intracardiac anatomical abnormalities. The baby with clinical features of Marfan’s syndrome had an eventration of the right-sided diaphragm.
Case report
Male newborn was the 2nd child of healthy unrelated parents with no family history of congenital heart disease or other chronic diseases. The baby was delivered spontaneously. Clinically, there were arachnodactylia, overstretchable joints, and muscular hypotonia, suggestive of Marfan’s syndrome. Both lungs were ventilated. Heart sounds were normal, mildly tachycardic with a heart frequency of ca. 160 beats/minute, with no murmurs audible. The child was cyanotic with transcutaneously measured oxygen saturations of 60–70% in room air. The baby was intubated without improvement of the saturations and transferred to our centre. Here, we confirmed the clinical findings.
On chest X-ray, there was a mild eventration of the right hemidiaphragm. The heart was not enlarged. Vascular markings were unremarkable.
Echocardiography showed situs solitus, but a clockwise rotated heart. All valves were mildly regurgitant. Tricuspid valve prolapse was visible, as seen in Marfan’s patients. There was an atrial septal defect, which was difficult to visualise due to the abnormal position of the heart. No other intracardiac shunts were seen. Systemic and pulmonary veins seemed normally connected, as were the great arteries.
Contrast echocardiogram carried out from an arm vein showed immediate filling of the right ventricle, but no clear right-to-left shunt.
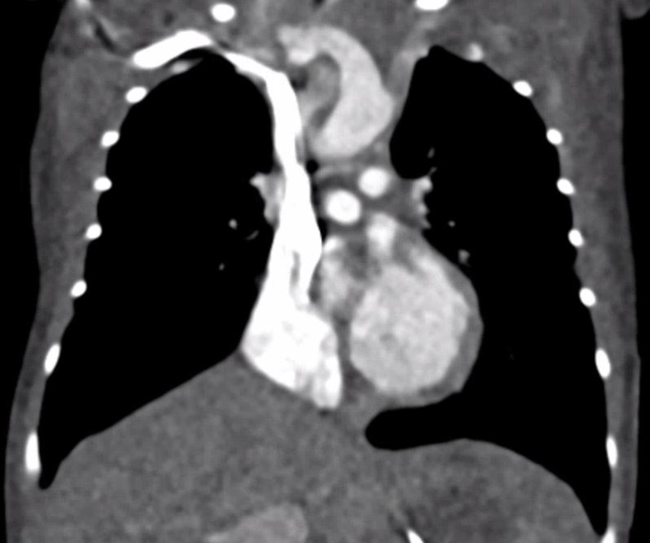
A computed tomography scan was suggestive of an atrial septal defect, but shunt direction could not be determined. Also, contrast injection was performed from the arm and hence through the superior caval vein (Fig 1).

Figure 1. Computed tomography scan, contrast injection from the upper half of the body. Note that there is no filling of the left atrium
Cardiac catheterisation was performed on the 2nd day of life, and an angiography in the inferior caval vein showed immediate filling of the left atrium (Fig 2). Injection into the superior caval vein filled the right ventricle; no dye was seen in the left atrium. Whilst crossing the tricuspid valve, the child lost output and had to be resuscitated. Cardiac output was not restored, and the child went on emergency arteriovenous extracorporeal membrane oxygenation. Unfortunately, a hemorrhage of the left cerebral ventricle developed, and hence surgery was delayed.

Figure 2. Angiography, contrast injection from the inferior caval vein. Note filling of the left atrium and ventricle.
Eventually, surgery was carried out 13 days later, and the only anatomical abnormality found was the secundum atrial septal defect. There was no prominent eustachian valve or direct connection of the inferior caval vein to the left atrium.
The child could be weaned from extracorporeal membrane oxygenation, and no further cyanotic episodes occurred.
The further cause was determined by the Marfan’s syndrome: valvar insufficiency became more severe. Ventricular function was inadequate. The child developed respiratory insufficiency due to muscular hypotonia. As there was no curative approach, palliative care was administered and the child deceased aged 10 weeks.
Discussion
There are rare descriptions of isolated atrial septal defects with cyanosis in children, but in the literature the majority of these were classified as sinus venosus defects with straddling of the superior caval vein and subsequent streaming towards the left atrium. Reference Abadeer, Luceri and Abraham5 The anatomical basis in these cases is the position of the atrial septal defect, which is basically the connection of the left atrium and the pulmonary veins, in which the superior caval vein also drains. Reference Van Praagh, Geva and Lock6 Rarely, this can be detected as early as in fetal life, Reference Vassallo, Pascotto and Pisacane7 but is sometimes diagnosed as late as in adulthood. Reference Shapiro, Al-Sadir and Campbell8
If the interatrial communication is very large, cyanosis can occur due to mixing on atrial level. This has been described, i.e., in a child with 18q deletion chromosomal anomaly. Reference Kim, Park, Han, Yoon and Choi9
True anomalous drainage of the inferior caval vein to the left atrium has rarely been described. Reference Aliter, El-Haddad, Gallo and Al-Halees4 In these patients, the mechanism of cyanosis is obvious. This entity can be detected antenatally. Reference Vassallo, Pascotto and Pisacane7
The position and size of the eustachian valve can be a determinant for direction of shunt. There are reports of a floppy eustachian valve directing the flow from the inferior caval vein to the left atrium. Reference Manuel, Ghosh and Alex1–Reference Raffa, Al-Ibrahim, Kayali, Sorefan and Rustom3 This has been described as early as in 1976. Reference Maillis, Cheng, Meyer, Crawley and Lindsay10 The very first description of atrial septal defects with cyanosis dates from 1949, where Selzer and Lewis postulate functional drainage of systemic venous blood into the left atrium. Reference Selzer and Lewis11 These assumptions were based on postmortem examinations.
Cyanosis can also occur due to additional defects, which may not be easily detected or correctly interpreted, like tricuspid regurgitation, Reference Magoon, Choudhury, Karanjkar and Singh12 in which streaming through the interatrial communication leads to clinical cyanosis. An atrial septal defect with bilateral caval veins of which the left one drained through a coronary sinus leading to intermitted cyanosis has been described. Reference Atik, Tanamati and Barbero-Marcial13 This entity could also have been missed easily. Of course in these cases the atrial septal defect is not isolated.
Right-to-left shunt over an isolated atrial septal defect has been described in adults with platypnea–orthodeoxia syndrome, but the shunt direction in these patients can be dependent on body position. Reference Kenny, Murphy, Clough and Ashley14
Shunt reversion in Eisenmenger’s syndrome can lead to right-to-left shunt, too, but here the underlying are changes in pulmonary vascular resistance with impaired right ventricular filling and subsequent high right atrial pressures. Reference Schwerzmann and Pfammatter15 Persistent hypertension of the newborn can be a potentially fatal course of cyanosis with an isolated atrial septal defect. Reference Chong, So, Fok and Gerlis16 The mechanism in both these diseases lies in high pulmonary vascular resistance, even if the cause is different.
To our recent knowledge, there is only a single case in the literature describing an anomaly of the diaphragm as the reason for right-to-left shunt through an atrial septal defect. Reference Shely, Loitz, Fox and Wells17 In this case, a congenital diaphragmatic herniation into the pericardial cavity lead to altered streaming of the inferior caval vein return through the defect towards the left atrium. We assume a comparable mechanism in our case: due to the eventration of the diaphragm, the cause of the inferior caval vein was altered resulting in a different, more oblique angle of the inferior caval vein towards the fossa ovalis. Streaming was therefore not directed towards the body of the right atrium, but directly through the atrial septal defect. As contrast/dye was injected into the upper half of the body during contrast echocardiography and computed tomography angiography, shunting could not be determined during these investigations. It may be worth performing contrast echocardiography both from the upper and lower extremity in cases with otherwise unexplained mechanism of cyanosis.
Deterioration during cardiac catheterisation probably occurred as soon as the tricuspid valve was passed. Herewith, there was increased tricuspid regurgitation and hence less forward flow through the right ventricle and the pulmonary arteries, which received superior caval vein blood only. As soon as this flow was further reduced due to the catheter splintering the tricuspid valve, cardiac output was not sufficient any more. It remains unclear why this could not be re-established after the catheter was removed. Extracorporeal membrane oxygenation stabilised the patient until operation.
As soon as the atrial septal defect was closed surgically, cyanosis was solved and did not recur.
Summary
In our patient, there was no anatomical cardiac abnormality leading to altered systemic venous return to the left atrium. Eventration of the right hemidiaphragm altered the angle of the connection of the inferior caval vein to the left atrium with subsequent flow of systemic venous blood towards the left atrium leading to cyanosis. This eventration was probably part of the underlying Marfan’s syndrome. Surgical closure of the atrial septal defect resolved cyanosis, but the child deceased due to severe valvar dysfunction related to his underlying syndrome. Bubble studies from both the upper and lower half of the body may help to determine the mechanisms of unexplained cyanosis.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
Not applicable.