INTRODUCTION
Crustaceans constitute an important food source in the world. They are represented in 10 subclasses and eight of these are found in Turkey's seas (Demirsoy, Reference Demirsoy2005).
The maximum length of this species is 20 cm, average length is 12–18 cm. Living habitat ranges from 0 to 200 m from the coast; they live in U-shaped holes that they dig in the muddy and sandy bottoms. Squilla mantis lives in temperate climates and prefers temperatures between 14–24°C, and salinities between 35–40‰ (Maynou et al., Reference Maynou, Abello and Sartor2004). It is similar to the terrestrial praying mantis due to having arms like a lance which enable them to be good predators. While they hide during the day in their burrows, they come out to hunt at night. They have excellent vision for night predation (Schiff et al., Reference Schiff, Abbott and Manning1985; Maynou et al., Reference Maynou, Abello and Sartor2004).
Hunting consists of grabbing their catch very quickly while waiting in their burrows. They usually feed on small shrimps or small crustaceans (Maynou et al., Reference Maynou, Abello and Sartor2004). The life cycle of S. mantis consists of four stages: egg stage, free swimming stage, larval stage and adult stage (Maynou et al., Reference Maynou, Abello and Sartor2004). Sex organs are found in the abdomen between the gut and the heart. Males have accessory glands. A sperm channel opens out from the bottom of the last couple of feet by chitin penis. Fertilization occurs following copulation (Demirsoy, Reference Demirsoy2005). It is defined that S. mantis is a good source of omega-3 (Mili et al., Reference Mili, Bouriga, Missaoui and Jarboui2011).
It is widely distributed in the eastern Atlantic and Mediterranean Sea, and is commercially important in the Mediterranean. Total annual production is about 5810 tons, 85% of this provided in Italy (4970 tons) and followed by Spain (805 tons), France (33 tons) and Croatia (2 tons) (FAO, 2013). An average price of mantis shrimp among European countries ranges from 10 to 20 € kg−1 (FAO, 2011).
There are many studies on Squilla mantis. These studies are not directly related to this species in Turkey, but its existence in the discards in various fishing gears. Başusta et al. (Reference Başusta, Kumlu, Gökçe and Göçer2002) pointed out the number/weight (g) distribution of S. mantis according to the seasons. Several studies were carried out on fishing methods, for example: the quality and quantity of catch with bottom trawler in Izmir Bay (Akyol & Kara, Reference Akyol and Kara2003); catch composition obtained by shrimp gillnets (Metin & Gökçe, Reference Metin and Gökçe2004); performance and composition of catch with shrimp beam trawls and the amount of discards (Yazıcı et al., Reference Yazıcı, İşmen, Altınağaç and Ayaz2006); an examination of catch composition of beam trawls found in the gear catch groups and determination of its impacts in the fishing environment (Aydın et al., Reference Aydın, Gurbet and Ulaş2005); an investigation of catch performance of worn shrimp set nets used in calamari (Loligo vulgaris) fisheries (Gökçe et al., Reference Gökçe, Metin, Aydın and Bayramiç2005); an investigation of beach seine fishery samples (Ertosluk, Reference Ertosluk2006); the determination of non-target catch amount in shrimp trawls (Soykan et al., Reference Soykan, Kınacıgil and Tosunoğlu2006); species composition of demersal trammel set nets (Beğburs & Kebapçıoğlu, Reference Beğburs and Kebapçıoğlu2007); the evaluation of catch data collected by bottom trawl (Ünlüoğlu et al., Reference Ünlüoğlu, Akalın and Türker Çakır2008); catch performance of different coloured trammel set nets (Beğburs & Kebapçıoğlu, Reference Beğburs and Kebapçıoğlu2009); and 444 crustacean species were identified in İzmir Bay (Bakır & Çevirgen, Reference Bakır and Çevirgen2010). Eryaşar (Reference Eryaşar2011) did a study to determine the composition of catch and discard in the bottom trawl fisheries in the Gulf of Mersin.
Abello & Martin (Reference Abello and Martin1993) investigated population dynamics; Righini & Baino (Reference Righini and Baino1996) identified population parameters; Froglia (Reference Froglia1996) worked on population behaviour and the significance in demersal fisheries of S. mantis; Mannini & Massa (Reference Mannini and Massa2000) determined catch amounts of target species including S. mantis; Çobani (Reference Çobani2003) and Fabi & Grati (Reference Fabi and Grati2003) evaluated targeted and untargeted catch in small-scale fisheries; Kevrekidis & Galil (Reference Kevrekidis and Galil2003) determined at which depths they are found; Maynou (Reference Maynou2005) stated an importance in Mediterranean fishery and catch amount; Placenti (Reference Placenti2005) defined catch amount and annual income; Mili et al. (Reference Mili, Jaroui and Missaoui2008, Reference Mili, Bouriga, Missaoui and Jarboui2011, Reference Mili, Ennouri, Jarboui and Missaoui2013) detected morphometrics, fertility and fatty acid composition according to months, and studied abundance, biological features and distribution; Ragonese et al. (Reference Ragonese, Morara, Canali, Pagliarino and Bianchini2012) defined abundance and biological features; and Vila et al. (Reference Vila, Sobrino and Jimenez2013) investigated fishery and life cycle.
Squilla mantis has an economic value for Mediterranean countries. It is fished with shrimp nets in Turkey as a discard species and its economic value is unknown by fishermen; and there are no publications related to population parameters of S. mantis in Turkey. Therefore, in this study we aimed to present some biological data related to the spot-tail mantis shrimp resources from the Aegean Sea in Turkey.
MATERIALS AND METHODS
This study was carried out using samples collected from discards of shrimp gillnets during the 2013 fishing season. A total of 936 Squilla mantis specimens were collected on a monthly basis from April to October 2013 (April, 16; May, 193; June, 225; July, 260; August, 181; September, 45; October, 16) from the catch of commercial fishermen after harvesting by gillnet (40 mm mesh size, 4 m high). These types of fishing gear were employed at 25–40 m depths in an area within Izmir province. Specimens were transported to the laboratory (Ege University Faculty of Fisheries) in iced styrofoam boxes.
Sex of S. mantis is easily identified by the presence of a pair of copulatory organs arising from the base of the third pair of pereiopods corresponding to the 8th thoracic segment in the male and by the presence of the genital plate on the 6th thoracic segment sternite in the female (Abello & Martin, Reference Abello and Martin1993).
The age of specimens was determined using length-frequency data analyses using the Bhattacharya method in FISAT software (Bhattacharya, Reference Bhattacharya1967). Length–weight, width–weight and length–width relationships were derived as W = a × TLb, W = a × CLb and CL = b × TL-a, respectively, in which the parameters of ‘a’ and ‘b’ were calculated by the least squares method (Schaeperclaus, Reference Schaeperclaus1967; Lagler, Reference Lagler1969; Ricker, Reference Ricker1975). W is total weight, TL is total length and CL is carapace length.
The Fulton's Condition Factor (K) was calculated from the equation given by Fulton (Reference Fulton1902):
To estimate the spawning period, a gonadosomatic index was used, where GW is the gonad weight and W is the total weight (King, Reference King1995).
The growths in length and weight were computed using the von Bertalanffy equation (VBGF) (Beverton & Holt, Reference Beverton and Holt1957; Ricker, Reference Ricker1975):
where L t is the total length at age t, L ∞ is asymptotic length (cm), k is the growth rate (year−1), t is the age (year), and t 0 is the hypothetical age at zero length (year) (Sparre & Venema, Reference Sparre and Venema1992; King, Reference King1995).
The growth performance in length (Ø′) and weight (Ø) was estimated using the equations given by Moreau et al. (Reference Moreau, Bambino, Pauly, Maclean, Dizon and Hosillos1986):
The instantaneous total mortality coefficient (Z) was calculated by the catch curve given by Ricker (Reference Ricker1975), where Z equals the slope of the descending portion of the curve. The natural mortality (M) was estimated using the empirical equation of Pauly (Reference Pauly1983):
where T is the water temperature (°C). Fishing mortality (F) was deduced from the formula F = Z–M (Gulland, Reference Gulland1971). The rate of exploitation (E) was estimated as E = F/Z (Gulland, Reference Gulland1971).
Statistical analyses were performed to test the significance difference between calculated and observed values and the number of individuals in each sex using χ2 and Student's t-tests, respectively. All descriptive statistics and graphs were calculated and prepared by Microsoft Excel®.
RESULTS
The mean total length (TL), carapace length (CL) and weight (W) of 936 specimens of Squilla mantis were calculated as 12.57 ± 0.07 cm, 3.02 ± 0.02 cm and 22.14 ± 0.37 g, respectively (Table 1). Females have slightly higher values than males but the difference is statistically insignificant (P > 0.05).
Table 1. Sex, mean total length (cm), carapace length (cm) and weight (g) of Squilla mantis from the Aegean Sea.

Total Length–Weight relationship was derived as W = 0.0098 × TL3.02 for both sexes (R 2 = 0.94). The relationship between Total Carapace–Weight was found as W = 0.8046 × CL2.91 (R 2 = 0.90). According to the statistical analyses there is no difference between the regression coefficient of males and females for Total Length–Weight and Total Carapace– Weight relationships. There is also a strong correlation between the Total Length and Total Carapace of Squilla mantis as R 2 = 0.93 in the equation of CL = 0.2373TL–0.0378 (Table 2).
Table 2. Length–weight relationship of Squilla mantis.

The sex ratio found was (M:F) 1:1.42. Chi square analyses show that the difference between males (41.3%) and females (58.7%) is not statistically significant. Males and females were equally represented in the S. mantis population in the Aegean Sea. The rates of males to females in the sampling period were derived as 1:4.33 in April, 1:1.27 in May, 1:1.32 in June, 1:1.39 in July, 1:1.48 in August, 1:2.21 in November and 1:1.67 in October (Figure 1). These monthly figures showed that the number of males is also less than females in monthly samplings, differences were found to be statistically insignificant in May, June, July and August (P > 0.05) but it was statistically significant in April, September and October (P < 0.05).

Fig. 1. Frequency distribution of mantis shrimp by sexes and months in 2013.
Mean values of Fulton's condition factor (K) at different total lengths for S. mantis in the Aegean Sea are shown in Figure 2. Mean values of the condition factors were 1.00 for males, 1.04 for females and 1.02 for both sexes. The analysis of variance indicates an insignificant difference in the mean values of the condition factor between the two sexes of this species (P > 0.05). The monthly variation of Fulton's condition factors indicates higher values in April (1.22) and August (0.98) (Figure 2).
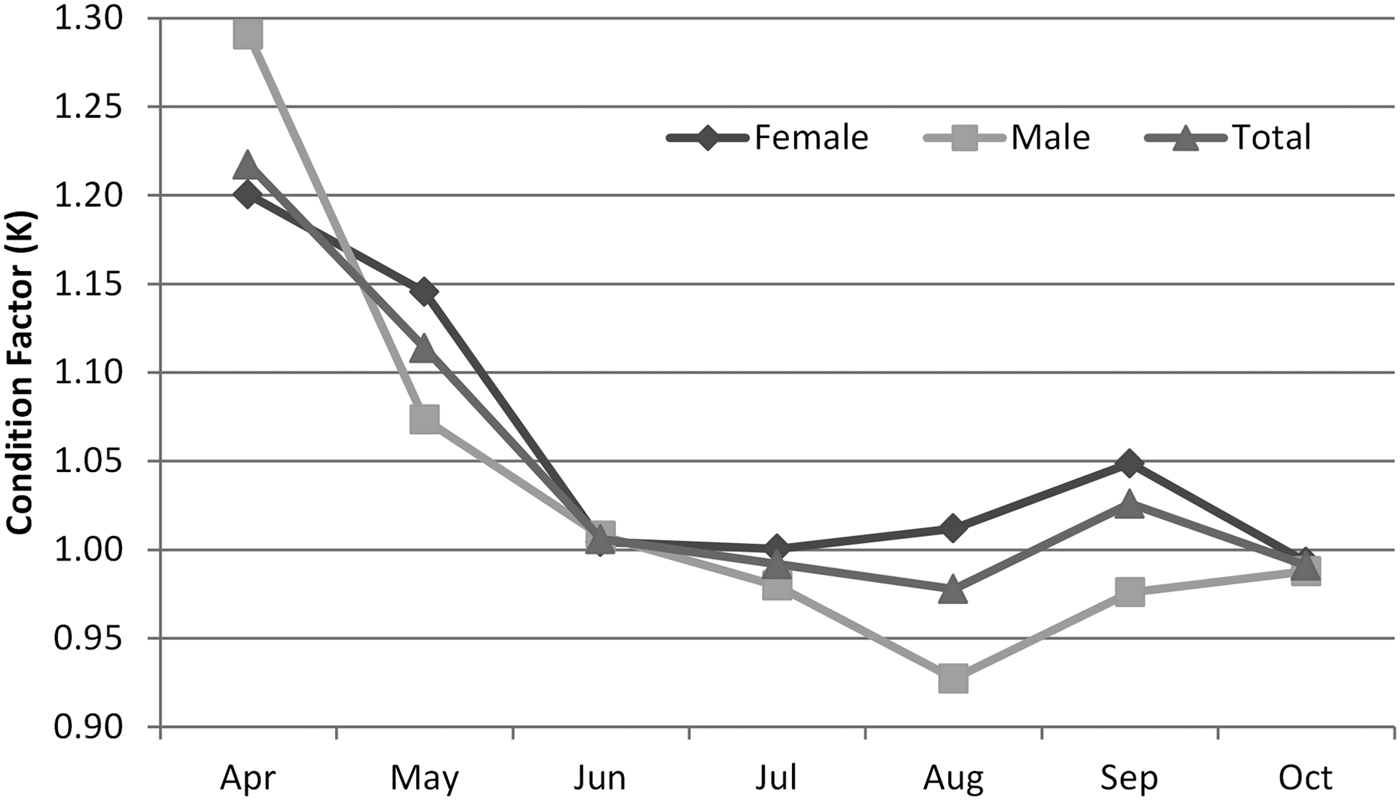
Fig. 2. Seasonal variations in Fulton's condition coefficient (K) of mantis shrimp from Aegean Sea in 2013.
The variation of GSI values show that spawning activity took place in spring to summer (April to August) (Figure 3).

Fig. 3. Variation in gonadosomatic index values of mantis shrimp from Aegean Sea in 2013.
The age of S. mantis was determined by the Bhattacharya method based on length–frequency distribution of the samples. Table 3 shows four age groups derived by this method.
Table 3. Mean length (cm) of mantis shrimp computed using the Bhattacharya method and Von Bertalanffy growth equation (VBGE) at corresponding ages.

These data were also used in order to estimate Von Bertalanffy growth parameters; L ∞, k and t 0 were used to compute calculated lengths. L ∞ and k estimated are respectively 19.69 cm and 0.51. Then t 0 computed from the length converted catch curve was −0.36 (Pauly & Gashutz, Reference Pauly and Gashutz1979) (Table 4). The differences between lengths derived by the two methods were statistically insignificant.
Table 4. Von Bertalanffy growth parameters and growth performances of mantis shrimp from the Aegean Sea.

Asymptotic weight, total length and carapace length are 79.41 g, 19.69 cm and 4.74 cm respectively. According to total lengths, growth performances were 2.278 for female, 2.154 for male and 2.287 for both sexes; the analysis of variance indicates an insignificant difference in the growth performances between the two sexes of this species (P > 0.05) (Table 4).
The instantaneous total mortality coefficient (Z) for S. mantis was 1.90 year−1. According to Pauly (Reference Pauly1983), the natural mortality (M) was computed as 1.16 using L ∞ = 19.69 cm and K = 0.51 year−1 and water temperature (T) of 22.3°C (Kamel, Reference Kamel1993). Fishing mortality (F) was 0.74 year−1 (Figure 4).
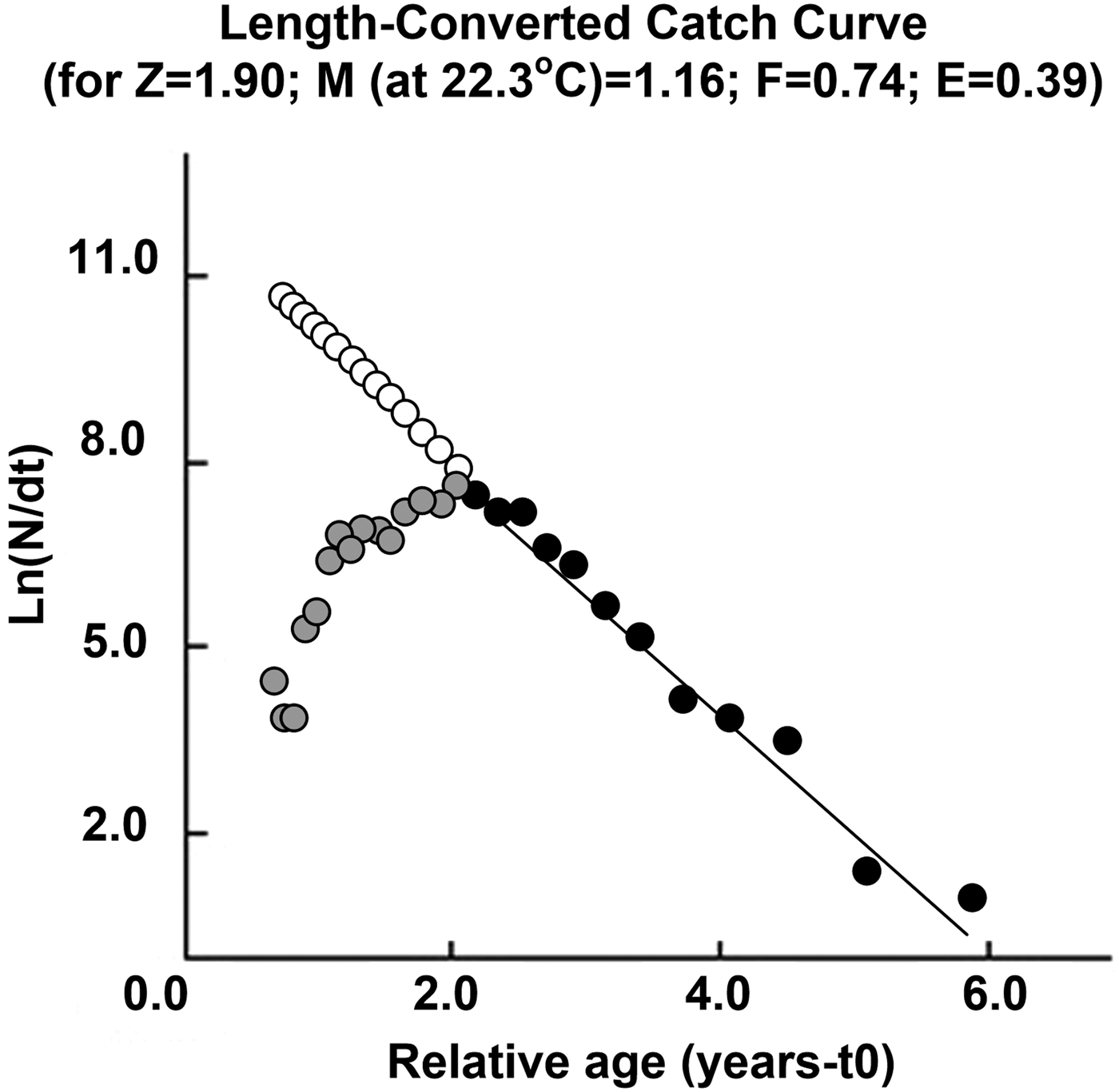
Fig. 4. Length-converted catch curve of mantis shrimp from the Aegean Sea.
The exploitation ratio (E) gives an indication of whether a stock is over-fished based on the assumption that the optimal value of E is approximately equal to 0.5 (Gulland, Reference Gulland1971). The E value obtained in the present study (0.39) was under the optimal value.
DISCUSSION
Mean length values are reported as 141.45 mm in females and 142.02 mm in males of Squilla mantis in Tunisian waters (Mili et al., Reference Mili, Bouriga, Missaoui and Jarboui2011). Ragonese et al. (Reference Ragonese, Morara, Canali, Pagliarino and Bianchini2012) observed that carapace length is 135.00 mm for females and 137.00 mm for males. In contrast to our results, in these two studies, mean length values for males are higher than for females.
In this study male:female ratio was found as 1:1.42. However, male:female ratios were defined as 1:1.12 by Mili et al. (Reference Mili, Bouriga, Missaoui and Jarboui2011) and 1:1.33 by Ragonese et al. (Reference Ragonese, Morara, Canali, Pagliarino and Bianchini2012). In these studies, no statistically significant difference was found between sex-ratios.
Total length-weight relationship are strong (R 2 = 0.93), while for females (b = 3.02) and males (b = 2.95) we record an isometric growth. Froglia (Reference Froglia1996) found positive allometric growth for females (b = 3.04) and males (b = 3.04). Mili et al. (Reference Mili, Jaroui and Missaoui2008) studied off the Tunisian coasts (Gulf of Tunis, Gulf of Hammamet and Gulf of Gabes) and calculated total length-weight relationship (R 2 = 0.95) and for both sexes (b = 3.16), positive allometric growth for female (b = 3.11) and male (b = 3.20) from Gulf of Tunis, total length-weight relationship (R 2 = 0.96) and for both sexes (b = 3.14), positive allometric growth for female (b = 3.07) and male (b = 3.21) from Gulf of Gabes and total length-weight relationship (R 2 = 0.93) and for both sexes (b = 2.99), isometric growth for female (b = 2.97) and male (b = 3.04) from Gulf of Hammamet; these data are similar to this study. Mili et al. (Reference Mili, Bouriga, Missaoui and Jarboui2011) defined strong total length-weight relationship (R 2 = 0.96) and for both sexes (b = 3.14), positive allometric growth for female (b = 3.06) and male (b = 3.21). Ragonese et al. (Reference Ragonese, Morara, Canali, Pagliarino and Bianchini2012) presented total length-weight relationship in females (R 2 = 0.97) and in males (R 2 = 0.95) and found positive allometric growth in females (b = 3.01) and in males (b = 3.03) samples.
Condition factor values are high in April according to spawning period of females and males, and the lowest level is observed in September and October. When gonadosomatic index values (GSI) are considered, reproduction occurs between April and June. In the Gulf of Gabes (South of Tunisia), the highest GSI values are in February and April, and the lowest values are in September (Mili et al., Reference Mili, Bouriga, Missaoui and Jarboui2011). In this study, GSI values were investigated in a 7-month period. Mili et al. (Reference Mili, Bouriga, Missaoui and Jarboui2011) studied GSI values in a 12-month period. This study results are in concordance with GSI values obtained by Mili et al. (Reference Mili, Bouriga, Missaoui and Jarboui2011). According to this result, an intensive spawning period of S. mantis was detected between April and July.
According to growth performances, obtained asymptotic carapace length (CL∞) values are higher in the seas of Turkey than in the Adriatic Sea (Froglia, Reference Froglia1996). Asymptotic total length (TL∞) values are lower in the seas of Turkey than values in the Ligurian Sea (north-western coast of Italy) and Ebro Delta (Spain) (Table 5).
Table 5. Some population parameters of Squilla mantis in the Mediterranean Sea.

The comparison of the Brody growth coefficient (k) shows that the coefficient obtained in this study is higher than the coefficient in southern coasts of Sicily and the Adriatic Sea. The first age values (t 0) have lower values for the Adriatic Sea, but, they are higher in the Gulf of Tunis. In this study, it is observed that asymptotic total length and carapace length values are higher than other studies made in the Mediterranean Sea. This difference can be explained by the variability of environmental conditions (temperature, salinity, food supply) and/or fishing pressure.
In the Bhattacharya analysis, 0–3 age groups were detected. These results indicate that S. mantis can be considered as a fast growing species. The fastest growth occurs especially at 0–1 age interval. The studies made in Cadiz Bay (north-eastern Atlantic Ocean), based on carapace length, determined 1 and 2 age group in females and males (Vila et al., Reference Vila, Sobrino and Jimenez2013). In the southern coasts of Sicily, 1–3 age groups were found for female and male samples (Ragonese et al., Reference Ragonese, Morara, Canali, Pagliarino and Bianchini2012). In Tunisian coasts (Gulf of Tunis, Gulf of Hammamet and Gulf of Gabes), 0–2 age groups were found for female and male samples (Mili et al., Reference Mili, Ennouri, Jarboui and Missaoui2013). According to mortality ratio, an instantaneous mortality ratio for combined sex was 1.90. Froglia (Reference Froglia1996) found an instantaneous mortality ratio higher for both male and female samples (Z = 2.89) in the Adriatic Sea. Ragonese et al. (Reference Ragonese, Morara, Canali, Pagliarino and Bianchini2012) indicated that instantaneous mortality ratio was Z = (0.98) in the southern coasts of Sicily – this level of mortality is lower than values obtained in this study.
Fishing (F) and natural mortality rate (M) were 1.89 and 1.00, respectively in the Central Adriatic Sea (Froglia, Reference Froglia1996). Fishing mortality rate is higher in the Adriatic region than in Turkey because S. mantis is considered a target species in this area and it undergoes severe catch pressure (STECF, 2012).
Exploitation rate obtained in this study is relatively low (0.34) indicating that the fishing pressure has not yet reached the optimum value for catch amount, E = 0.50.
This study was carried out in a 7-month shrimp fishing season. The data obtained during this period show that S. mantis has a significant commercial potential in Turkey compared with other countries where intensive fishing is carried out. Nevertheless, the fishing pressure remaining limited to a 7-month period in Turkey can be considered to be an important issue to protect populations of S. mantis. In this study, it is seen that fishing is done by shrimp set nets, but it is discarded back to the sea. In our country, discard of this in-demand species is an important loss for both the national economy and regional fishing.
In other countries such as Italy, Spain, France and Slovenia, this species is valued economically. As a result of this study, S. mantis population parameters are similar to the European countries that are economically fishing this species. It could be exported to European countries or it could be fished as a target species instead of being discarded in Turkey.











