Introduction
Growth rates of individuals and populations are often closely connected to the spatial distribution of individuals, as a consequence both of variability in resource and habitat quality and of density-dependent feedbacks (Turchin, Reference Turchin1989). In insect herbivore systems, individuals are often strongly aggregated on specific plant individuals creating large variabilities in population density (Morris et al., Reference Morris, Grevstad, Herzig, Jolivet and Cox1996; Hunter, Reference Hunter2000; Prokopy & Roitberg, Reference Prokopy and Roitberg2001; Raffa, Reference Raffa2001). These aggregations are not always connected to an apparent variation in site characteristics but are often due to aggregative behaviour. From an individual perspective, this might seem maladaptive as higher densities often reduce individual growth rates and reproduction. Theoretical studies suggest, however, that the enhanced detectability of damaged plants may compensate for fitness losses due to a lower resource availability (Monkkonen et al., Reference Monkkonen, Hardling, Forsman and Tuomi1999; Stamps & Krishnan, Reference Stamps and Krishnan2005). Damage-related cues have the dual advantage that they indicate the proper host plant species and that host plants are of satisfactory quality for feeding. The down sides are that damaged plants are already used by potential competitors and also that damage-related cues may be used by other competitors, or natural enemies, to locate resource concentrations (Blossey, Reference Blossey1995; Zilkowski & Bartelt, Reference Zilkowski and Bartelt1999; Ayres et al., Reference Ayres, Aures, Abrahamson and Teale2001).
In several systems, high larval densities are directly beneficial to individuals by increasing larval growth rates (Morris et al., Reference Morris, Grevstad, Herzig, Jolivet and Cox1996; Clark & Faeth, Reference Clark and Faeth1997; Ayres et al., Reference Ayres, Aures, Abrahamson and Teale2001; Fordyce, Reference Fordyce2003; Wise et al., Reference Wise, Kieffer and Abrahamson2006). This is well-known in several tree pests, where high densities may be necessary to break host plant defences and where females arriving to undamaged but potentially susceptible trees emit pheromones to attract additional females (Raffa, Reference Raffa2001; Pajares et al., Reference Pajares, Ibeas, Diez and Gallego2004). High densities may also increase larval growth and survival due to group defences (Hunter, Reference Hunter2000). This is, however, mostly observed in species with active defences, such as larval secretions, or in species with aposematic colouration (Hunter, Reference Hunter2000). These beneficial effects may naturally reverse at very high densities when resources become limiting (Raffa, Reference Raffa2001).
This paper examine the causes and consequences of feeding aggregations (Blossey, Reference Blossey1995) in two closely related leaf beetles (Galerucella calmariensis L. and G. pusilla Duftschmidt) feeding on purple loosestrife (Lythrum salicaria L.), a native plant of Eurasia and an invasive in North America (Hultén & Fries, Reference Hultén and Fries1986). Both species may have very strong negative effects on host plant fitness, causing complete defoliation and zero seed set (Blossey, Reference Blossey, Delfosse and Scott1992; Hambäck et al., Reference Hambäck, Ågren and Ericson2000). As a consequence, beetles have been introduced in North America to reduce the invasiveness of purple loosestrife (Hight et al., Reference Hight, Blossey, Laing and Declerck-Floate1995; Blossey et al., Reference Blossey, Skinner and Taylor2001). Recent studies show that male beetles of both species produce pheromones when feeding on purple loosestrife, but not when feeding on other plants, and that these pheromones attract males and females of either species to host plants (Bartelt et al., Reference Bartelt, Cosse, Zilkowski, Weisleder, Grode, Wiedenmann and Post2006, Reference Bartelt, Cosse, Zilkowski, Wiedenmann and Raghu2008). This paper further examines this cross-species attraction, the role of host plant volatiles and the effect on individual fitness. First, attraction was examined within and across species to natural and artificial plant damage. Second, density-dependent effects on pupal mass were examined on potted plants under controlled conditions and on naturally occurring plants. The long-term aim is to understand the role of positive and negative density dependent feedbacks in the population dynamics of this leaf beetle system.
Methods
Olfactometer studies
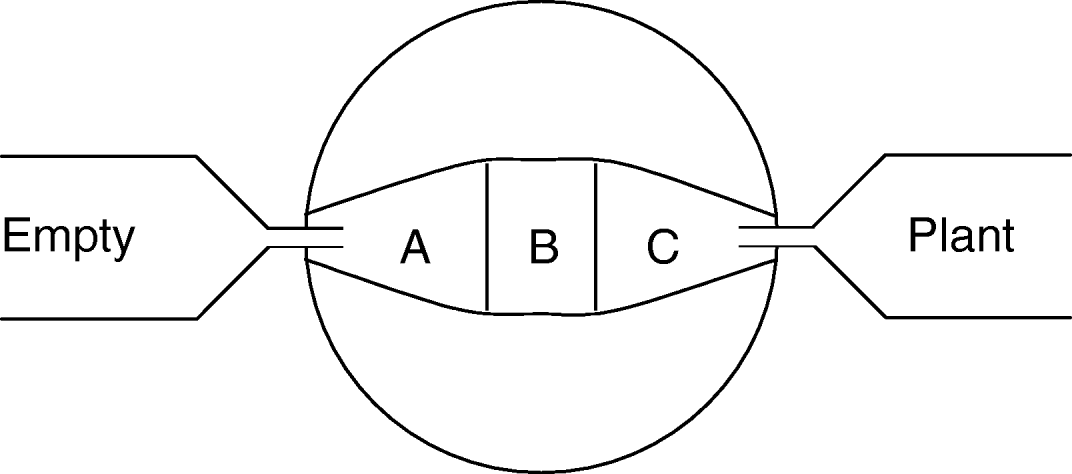
The abilities of G. calmariensis and G. pusilla to locate undamaged and damaged host plants through olfactory cues were examined in two-armed olfactometers during June 2003 (fig. 1, see also Pettersson et al., Reference Pettersson, Karunaratne, Ahmed and Kumar1998). The olfactometers were made of three layers of perspex sandwiched together, with an arena excised in the middle sheet. This arena had a square central zone (2.5×2.5 cm) and two tapered arm zones, each 3.8 cm long and ending with a 0.6 cm broad space where odour sources were connected with teflon tubing via 4 mm holes through the periphery. An air stream over the arena was created by attaching a water pump to the centre of the arena, and the air flow was regulated to approximately 3 ml sec−1, which provided an even cover of the applied odour in the arm zone. For this experiment, mating pairs were collected from the field. Pairs were identified to species based on the shape of the male copulatory organ (Manguin et al., Reference Manguin, White, Blossey and Hight1993).
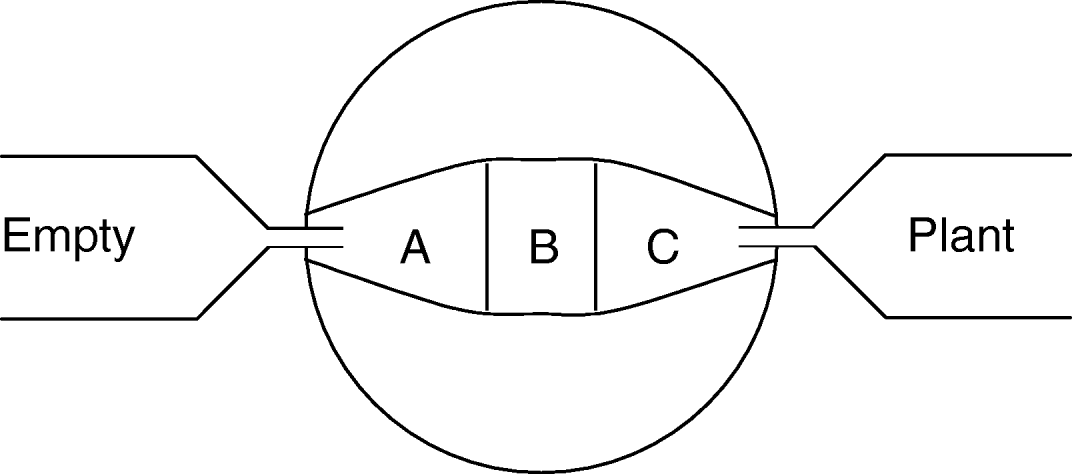
Fig. 1. Two-armed olfactometer used when investigating insect responses to plant odours. The arena for the beetle are areas labeled A, B and C, while plants were placed in a chamber at one or both ends (labeled empty vs. plant). To create an unidirectional odour flow, the center of the olfactometer was connected to an air suction apparatus. At the start of a trial, beetles were released in the arena center (at B) and positions within the arena were then recorded every three minutes. To prevent beetles reaching plants, a fine net covered the tube connecting the behavioural arena with plant chambers.
Each trial was run as follows. At the start of a trial, a single beetle was placed in each olfactometer and allowed five minutes to acclimatize before measurements started. The beetle individual was not starved prior to trials. The beetle position was then recorded every third minute for 60 min (i.e. 20 recordings) by noting if the beetle was in the central square or in either arm (fig. 1, area A, B or C). If an individual moved less than 5 mm over three consecutive periods, it was excluded. At the end of each run, the number of recordings per area was summed. A minimum of 17 individuals, per species, were tested for each stimulus, and the same individual was used only once for each treatment combination. Observed responses were identical for trials with male and female damage, and for male and female test individuals (P>0.4 in all cases), and only pooled data are presented. Beetle responses were tested in a Wilcoxon sign-rank test. The replicate in this test was the individual beetle, and the test was performed on the number of recordings for an individual in either arm (A vs. C). All recordings of an individual in the central part were excluded from the analysis. Between runs, olfactometers were cleaned, first with water and mild detergent, and second with 70% ethanol.
To examine the responses by the two Galerucella species to inter- and intraspecific damage, the following combinations of stimuli were tested in the olfactometer (randomly positioned in relation to the two arms in the olfactometer): (i) undamaged Lythrum – blank control; (ii) Lythrum damaged by standardised punching – blank control; (iii) Lythrum damaged by conspecific – blank control; (iv) Lythrum damaged by the other species – blank control; (v) Lythrum damaged by conspecific – Lythrum damaged by standardised punching; (vi) Lythrum damaged by conspecific – Lythrum damaged by other species; and (vii) conspecific male – blank control. Undamaged plants were carefully handled to minimise unintended volatile emission. Standardised punching was done by making ten holes in plant leaves (diameter=4 mm), similar to larval feeding, shortly before trials. The beetle damage was not standardised, but in each case a single individual fed for a few hours. Even though this caused some variation in damage, visual examination before testing suggested only minor variation among test plants. Beetles were sexed prior to damage and were removed from the leaves before the trial (leaving faeces). All trials were run within a two-week period during June to reduce the phenological variability in plant and insect quality. The trial with conspecific males was only tested on female beetles, and without leaf material. Tests with a humidity control, comparing attraction to wet vs. dry filter paper, showed no response.
Larval density experiment
The effect of larval density on pupal mass was examined in a common garden experiment from mid-June until late July 2003. In this experiment, newly hatched larvae of G. pusilla, at three densities (5, 15 or 30 larvae per plant), were placed on 15–20 cm tall potted plants (N=15). These densities cover the range of larval densities for similar sized plants in the field (Hambäck et al., Reference Hambäck, Ågren and Ericson2000). The larvae used in the experiment originated from eggs that were laid on plants in the laboratory from field-caught adult females (identified as above). The tests also included a control with no larvae, to examine the larval effect on aboveground plant biomass and reproduction. Plants used in the experiment originated from seeds collected on plants in northern Uppland and had been sown earlier in spring (April). The soil was a standard potting soil, with 30% sand mix. To avoid larval movements among plants, pots were placed at least 10–15 cm apart, and there was at least this distance between leaves of different plant individuals. Throughout the study, no colonisation was observed on control plants. Larvae were not protected against predators, but potted plants were placed in an area with limited natural vegetation were predators are very scarce and no predators were observed during the experiment.
The larvae were allowed to complete their development on the plant, and the experiment was terminated when all larvae had moved from the plant down in to the soil. To locate pupae, the soil in the pot was searched thoroughly, and pupae were found either in direct proximity to the plant or along the pot wall. The pupae were immediately frozen and weighed at a later time. On all plants, we also measured plant height weekly, the proportion leaf damage in 5% intervals and flower production at the end of the study. The proportion leaf damage was calculated by scoring 0, 50 or 100% damage on single leaves, and values were then summed over all leaves on the plant individual. The proportion pupae located at the end of the experiment varied between 60–70%; the mortality cause was not apparent. As a consequence, two separate regressions were performed on pupal mass (ln-transformed) with initial and final larval densities, respectively, as the independent variable. In both cases, visual inspection of residuals indicated no deviations from test assumptions.
Field study
To examine density effects on pupal mass in the field, all final stage larvae were collected twice a week from 131 marked plant individuals in three localities in northern Uppland, Sweden (60°N, 18°E) during the main larval period (four weeks in July 2004). Collected larvae were identified to species, using larval colour (Hambäck, Reference Hambäck2004), and were allowed to pupate in individual vials with leaves from the original host plant individual. The colour difference between species is easily detectable (G. calmariensis has a rich yellow colour, G. pusilla has a washed-out yellowish-white colour) with a close to 100% reliability (identified based on the male copulatory organ, N≫1000). All larvae that took longer than six days to complete pupation were discarded. Pupal development in Galerucella spp. usually takes 3–4 days from the end of feeding. The analysis suggested that this approach was appropriate, as the time period between larval collection and pupation did not affect pupal mass (P=0.2). Effects on pupal mass (ln-transformed) were modelled with three explanatory variables in a linear mixed effects model, using plant individual as random factor. To examine species differences, species identity (G. calmariensis and G. pusilla) was included in the model. To examine density-dependent responses, egg density (number of eggs per plant, measured before larval hatching) was included in the model. To examine if the timing of larval development on the plant affects pupal mass, the number of days since the first larvae collected on the plant individual were recorded. If the first larvae on plant A was collected on day 0 and the second larvae on plant A was collected on day 5, then the timing of larval development for these two larvae was 0 and 5 days, respectively. The final analysis excluded one plant individual with an extreme egg density. The model was analysed using the nlme-package in R 2.8.0 (R Development Core Team, 2007).
Results
The olfactometer studies showed a similar pattern for both species. There was no attraction by either species to undamaged purple loosestrife, while both species were seemingly attracted to plants damaged by conspecific adults and to plants damaged by standardised punching (fig. 2, test statistics in figure). However, even though the pattern was quantitatively similar for both species, the attraction by G. calmariensis to artificially damaged plants was marginally non-significant. Both species also showed stronger attraction to plants damaged by conspecific adults compared with artificially damaged plants (fig. 2). In addition, G. pusilla was more strongly attracted to plants damaged by G. calmariensis compared with plants damaged by adult G. pusilla, while G. calmariensis was indifferent to the identity of the damaging insect (fig. 2). Finally, neither G. pusilla females nor G. calmariensis females were significantly attracted to conspecific males, but this test may have been affected by the low sample size.

Fig. 2. Responses by Galerucella calmariensis (Gc) and G. pusilla (Gp) to undamaged and damaged host plants (ns, P>0.05). Trials were performed in a two-armed olfactometer.
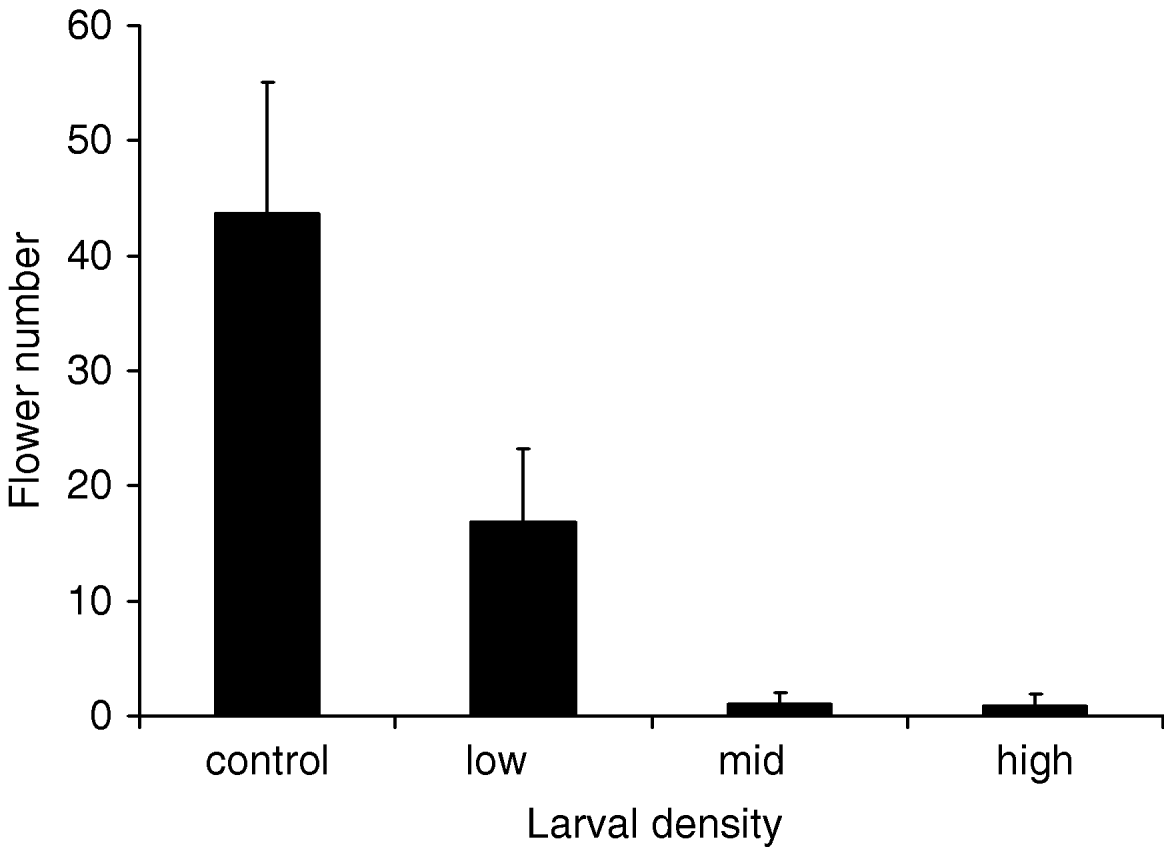
In the controlled experiment with potted plants, total leaf damage increased, final plant size and reproduction decreased and pupal mass increased with larval density. First, total leaf damage increased considerably with larval density and an average of 41% of the leaf area was consumed on plants with 30 larvae (ln(leaf damage)=0.42 (SE=0.20, P=0.04)+0.11×initial larval density (SE=0.01, P<0.0001), N=15). Second, individual plants without larvae had grown on average 48% more than plants with 30 larvae at the end of the study (effect of initial larval density: F=54.3, P<0.0001, N=15; fig. 3). Plants with the highest larval density did not increase in height after two weeks of the experiment. Third, flowering was almost reduced to zero at the two higher larval densities, and the overall effect on floral numbers was non-linearly related to larval density (fig. 4). Fourth, the mean size of pupae in the treatment with 30 larvae was 18% larger than the mean size of pupae in the treatment with only five larvae (fig. 5a). This effect was also reflected in the analysis against final larval density, where pupal mass was 35% higher on plants with 20 larvae compared to plants with single larvae (fig. 5b). There was no effect on initial larval density on larval survival (final/initial larval density).

Fig. 3. Plant height (mean±SE) for treatments with 0, 5, 15 or 30 larvae (initial density, counting from the top).
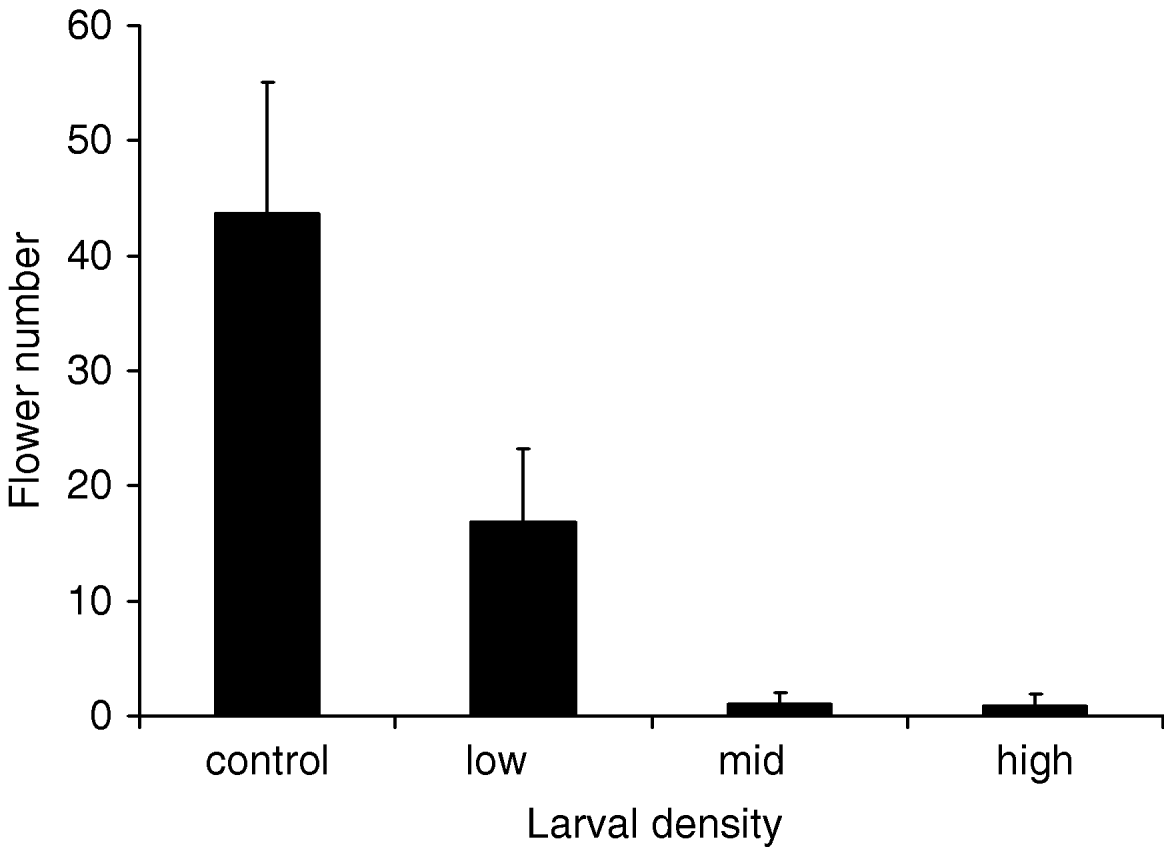
Fig. 4. Flower number (mean±SE) for plants with 0, 5, 15 or 30 larvae (initial density). Logistic regression–flowering probability=0.82 (SE=0.43, P=0.06)−0.15×initial larval density (SE=0.05, P=0.002), N=56.

Fig. 5. Pupal mass for Galerucella pusilla relative to (a) initial larval density (ln(pupal mass)=1.32 (SE=0.05, P<0.0001)+0.0067×initial larval density (SE=0.0029, P<0.03)), N=40) and (b) final larval density (ln(pupal mass)=1.32 (SE=0.05, P<0.0001)+0.016×final larval density (SE=0.0069, P<0.01)), N=40) on potted purple loosestrife plants.
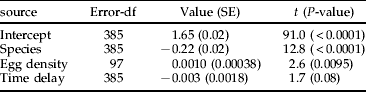
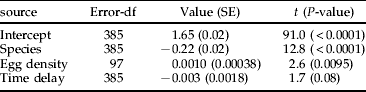
In the field, pupal mass was, as expected, larger for G. calmariensis, and both species had a similar positive response to egg density (table 1 and fig. 6; all interactions between species and other variables were non-significant at P>0.4). There was a non-significant tendency that pupal mass decreased by the timing of larval development.

Fig. 6. Pupal mass of Galerucella calmariensis (filled symbol) and G. pusilla (open symbol) in relation to egg density on naturally occurring plants in the field (lines correspond to predictions based on statistical modelling (table 1)). The right-most data points had an extreme influence on the analysis and were excluded.
Table 1. Linear mixed effects model on pupal mass (ln-transformed, 130 plant individuals). Egg density was estimated as the number of eggs per cm plant height, and time delay was estimated as the number of days between the first larvae collected on the plant individual and the collection of a specific larvae.
Discussion
This study shows that aggregative feeding may be beneficial in two closely related chrysomelid beetles, Galerucella calmariensis and G. pusilla, feeding on purple loosestrife (Lythrum salicaria) (Blossey, Reference Blossey1995). Earlier studies suggested that males of both species produce the same pheromone, dimethylfuran lactone, while feeding on host foliage and that the pheromone attracts individuals of both sexes and both species (Bartelt et al., Reference Bartelt, Cosse, Zilkowski, Weisleder, Grode, Wiedenmann and Post2006, Reference Bartelt, Cosse, Zilkowski, Wiedenmann and Raghu2008). A cross-species attraction to beetle-damaged plants was also seen in this study (fig. 2), although we have no evidence that this attraction was caused by male produced pheromones. A novel observation was that the attraction in olfactometers was not symmetrical among the two species. Individuals of G. pusilla showed stronger attraction to plants damaged by G. calmariensis than to plants damaged by conspecifics. One possible reason for the differential attraction could be that G. calmariensis, being slightly larger, incur larger leaf damage and, therefore, emit more attractants. This hypothesis, however, does not match with the lack of pattern for G. calmariensis, where attraction, if anything, was stronger to plants damaged by G. pusilla. While the mechanistic reason underlying the asymmetric attraction cannot be understood, using heterospecific cues may be adaptive if this improves host search efficiency (Monkkonen et al., Reference Monkkonen, Hardling, Forsman and Tuomi1999; Stamps & Krishnan, Reference Stamps and Krishnan2005). In the study area, G. pusilla has a later activity peak than G. calmariensis and also has a much lower abundance (see also McAvoy & Kok, Reference McAvoy and Kok2004) in contrast to more southern localities (Blossey, Reference Blossey1995). The presence of G. calmariensis, therefore, may be a very useful host cue for both species, and mark-recapture studies suggest that attraction to damaged plants may occur from at least 50 m (Grevstad & Herzig, Reference Grevstad and Herzig1997).
Besides the effect of previous feeding on subsequent attraction, individual beetles were also attracted to mechanically damaged plants, albeit weaker than to beetle-damaged plants (fig. 2). This attraction to mechanical damage suggests that not only beetle pheromones are involved in the aggregation process but also plant induced chemicals. The attraction to artificial damage probably involves green leaf volatiles, which have been shown to attract other species in the genus to their respective host plant. Both G. lineola (Peacock et al., Reference Peacock, Lewis and Powers2001b) and G. vittaticollis (Hori et al., Reference Hori, Ohuchi and Matsuda2006) are attracted by cis-3-hexenyl-acetate, particularly to damaged plants emitting large quantities (for similar data on other chrysomelid beetles see Kalberer et al., Reference Kalberer, Turlings and Rahier2001; Peacock et al., Reference Peacock, Lewis and Herrick2001a). Bartelt et al. (Reference Bartelt, Cosse, Zilkowski, Wiedenmann and Raghu2008) also found that antennae from G. calmariensis respond to at least six different green-leaf volatiles, including cis-3-hexenyl-acetate, even though they found no attraction in the field. Interestingly, the present and previous studies on G. calmariensis and G. pusilla show that individual beetles are not attracted to undamaged plants in olfactometers, suggesting that emissions from undamaged plants may be below the beetles' detection threshhold (Hambäck et al., Reference Hambäck, Pettersson and Ericson2003).
The attraction to damaged plants does not seem to be coincidental, as beetles in this study benefitted from increasing larval densities through an increased pupal mass. Increased pupal mass, and concomitant increases in adult size, translate into a higher egg production (relative egg production=11.2×female length–21.3, R 2=0.35, N=38, Hambäck, unpubl data) and, therefore, could be interpreted as an increased fitness due to aggregation. This type of positive density-dependent feedback is known in several other coleopteran species, both in scolytid beetles and in other chrysomelid beetles (Brown & Weis, Reference Brown and Weis1995; Morris et al., Reference Morris, Grevstad, Herzig, Jolivet and Cox1996; Storer et al., Reference Storer, Wainhouse and Speight1997; Nahrung et al., Reference Nahrung, Dunsta and Allen2001; Raffa, Reference Raffa2001). In scolytid beetles (Raffa, Reference Raffa2001), and possibly also in chrysomelid beetles (Morris et al., Reference Morris, Grevstad, Herzig, Jolivet and Cox1996), positive feedbacks arise because large attacks drain plant defences and indirectly increase plant quality. I am unaware if a similar mechanism could account for the positive feedback from Galerucella feeding, as plant defences in purple loosestrife are not yet understood. An alternative mechanism, however, could be that plant quality is improved through an increased availability of soluble carbohydrates in damage plants. Additional studies are clearly needed to distinguish among these alternatives.
Irrespective of the mechanism, the aggregative feeding and the positive density-dependent feedback may have important consequences for the temporal dynamics in Galerucella beetles on purple loosestrife. Natural Galerucella populations in Sweden show large year-to-year fluctuations, and the observed positive feedback may be involved both in the destabilisation of population densities and in the relative low population growth rates at low population densities (Hambäck, unpubl. data). The observed feedbacks may also affect the establishment of Galerucella populations for the biological control of purple loosestrife in North America. Grevstad (Reference Grevstad1999) found that the probability of population establishment increased with release size, in the range from 20–540 adults (see also Hight et al., Reference Hight, Blossey, Laing and Declerck-Floate1995), and the current study suggests that this density-dependent establishment probability may be caused by Allee-effects. Aggregation pheromones may also reduce the likelihood of population spread to small uncolonized sites, but this situation may be different in North America where beetles are colonizing virgin habitats compared to the more stable spatial distribution in Europe.
At higher Galerucella densities, on the other hand, negative density-dependent feedbacks may increase in importance. As shown in this and other studies (Blossey, Reference Blossey, Delfosse and Scott1992; Katovich et al., Reference Katovich, Becker and Ragsdale1999; Hambäck et al., Reference Hambäck, Ågren and Ericson2000; Landis et al., Reference Landis, Sebolt, Haas and Klepinger2003), high Galerucella densities may cause complete defoliation and limit larval growth, and buds that are important for very small larvae may be depleted even at intermediate densities (fig. 3: Katovich et al., Reference Katovich, Ragsdale, Skinner and Becker2001). This bud depletion may be particularly problematic for late-hatching larvae that easily die from a lack of high quality resources during early development. In the field part of this study, delayed egg laying tended to reduce pupal mass, and this may be due to a lack of both leaf and floral resources. Earlier studies also suggest that the lack of buds may increase mortality from a higher exposure to natural enemies (Sebolt & Landis, Reference Sebolt and Landis2002).
Aggregative feeding, additionally, may affect the spatial variability of feeding (Turchin, Reference Turchin1986; Hambäck & Englund, Reference Hambäck and Englund2005). For instance, Hambäck et al. (Reference Hambäck, Ågren and Ericson2000, see also Hambäck et al., Reference Hambäck, Pettersson and Ericson2003) showed that plants growing in the open may have two magnitudes higher egg loads than plants growing less than two meters away, but inside a shrub. These studies could rule out mechanisms such as predators, repellency or bottom-up effects, underlying such an associational refuge and suggested that the difference arose due to host-finding interference. The observed magnitude, however, could not be easily interpreted as pure interference, but a small initial difference due to host finding interference could initiate a positive density-dependent feedback, through attraction of later arrivals (Turchin, Reference Turchin1986).
Acknowledgements
I thank Jan Pettersson for allowing access to his olfactometers. Markus Brage, Carola Orrmalm and Anna Sandhammar assisted in both field and laboratory studies. Funding was provided by Stiftelsen Oskar och Lilli Lamms Minne and by the Swedish Research Council (Vetenskapsrådet).