Introduction
Western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae), are a difficult pest to control in protected crops (Tommasini & Maini, Reference Tommasini and Maini1995). Due to a lack of specialist predators, generalist polyphagous predators are used for biological control, with the most common predators being phytoseiid mites of the genus Neoseiulus and anthocorid flower bugs of the genus Orius (Ramakers, Reference Ramakers, Parker, Skinner and Lewis1995; Riudavets, Reference Riudavets1995; Sabelis & Van Rijn, Reference Sabelis, Van Rijn and Lewis1997). In the UK, Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) and Orius laevigatus Fieber (Hemiptera: Anthocoridae) have been used extensively to control thrips in protected edible crops (Jacobson, Reference Jacobson and Lewis1997).
There has been a lot of interest in using N. cucumeris and O. laevigatus for biological control in ornamental crops (Brødsgaard, Reference Brødsgaard, Parker, Skinner and Lewis1995; Murphy & Broadbent, Reference Murphy and Broadbent1996). Neoseiulus cucumeris is the most widely available natural enemy for control of F. occidentalis, but it is only able to attack first instar thrips (Bakker & Sabelis, Reference Bakker and Sabelis1989). Gilkeson et al. (Reference Gilkeson, Morewood and Elliot1990) and Gillespie & Quiring (Reference Gillespie and Quiring1992) showed that Orius species were good predators of F. occidentalis and suggested that the flower bugs could be used to complement the control obtained using N. cucumeris. Van der Meiracker & Ramakers (Reference Van den Meiracker and Ramakers1991) observed that in sweet pepper, naturally occurring Orius species boosted control of thrips by predatory mites.
Because of the low tolerance to pest damage in ornamental crops, there is a need to prevent pests from establishing (Wardlow, Reference Wardlow1990), rather than controlling their numbers once they are present. However, to prevent pests establishing in ornamental crops, predators may have to be released before the pest is present. This presents a problem, as without food, natural enemies are likely to emigrate from the crop or die. The results of recent research suggest that pollen can be used to retain predators within the crop (Van Rijn & Sabelis, Reference Van Rijn and Sabelis1990a,Reference Van Rijn and Sabelisb, Reference Van Rijn and Sabelis1993; Van Rijn et al., Reference Van Rijn, Van Houten and Sabelis2002; Hulshof et al., Reference Hulshof, Ketoja and Vänninen2003). However, Hulshof et al. (Reference Hulshof, Ketoja and Vänninen2003) showed that pollen acts also as a food for F. occidentalis, boosting both its growth rate and fecundity and this could limit the control achieved by the predators. In contrast, simulation models used by Van Rijn et al. (Reference Van Rijn, Van Houten and Sabelis2002) suggested that limiting the availability of pollen benefited the predators more than the thrips.
Previous research (Skirvin et al., Reference Skirvin, Kravar-Garde, Reynolds, Jones and de Courcy Williams2006) showed that O. laevigatus nymphs were good predators of thrips both in the absence and presence of pollen as a supplemental food, and that there was no interaction between O. laevigatus and N. cucumeris when used together. However, the recovery of the predators was low, despite the presence of pollen as a supplemental food. In order to understand why this observation occurred, more information about the effect of pollen on the predation rates and movement of the two natural enemy species was required.
The research presented in this paper aims to understand how the presence of pollen affected the predation and movement of the two major natural enemies of western flower thrips, O. laevigatus and N. cucumeris.
Because pollen has been shown to increase the population of thrips (Hulshof et al., Reference Hulshof, Ketoja and Vänninen2003; Skirvin et al., Reference Skirvin, Kravar-Garde, Reynolds, Jones and de Courcy Williams2006) other potential supplemental food sources need to be investigated. Fungal hyphae have been suggested as a potential food source, based on observations of survival of mites (de Courcy Williams et al., Reference de Courcy Williams, Kravar-Garde, Fenlon and Sunderland2004). Therefore, in the research presented here, we also examine the effect of Trichoderma viride, a fungus commonly found in glasshouse and other horticultural crops.
Materials and methods
Western flower thrips culturing
The western flower thrips, F. occidentalis, were reared on chrysanthemum plants (mixed varieties) in a perspex insect rearing cage (46 cm by 80 cm by 46 cm), ventilated using a mesh gauze (17 micron mesh size) at the rear. The cage was maintained at ambient temperature and humidity, with a 16:8 light:dark photoperiod.
Predator culturing
All of the predators used in the trials were reared in the laboratory. The original stock was obtained from Syngenta Bioline, UK.
Neoseiulus cucumeris was reared on mite rearing platforms (Overmeer, Reference Overmeer, Helle and Sabelis1985) and supplied with bran mites (Tyrophagus spp.) as a food source. The mites were kept in an incubator at 20–25°C, ambient humidity, and with a 16:8 L:D photoperiod.
Orius laevigatus adults were reared in ventilated plastic containers (20 cm diameter, 14 cm deep) containing chrysanthemum stems as an oviposition substrate and a combination of Myzus persicae (Sulzer) and Ephestia eggs as food. The chrysanthemum stems were examined for the presence of O. laevigatus eggs twice a week. Stems containing eggs were removed and put into separate smaller containers (13 cm diameter, 10 cm deep). Newly hatched nymphs were fed as described for the adults and used in the experiment once they reached the second instar.
Plants used in the experiment
Chrysanthemum (cv. Dark Splendid Regan) cuttings (supplied by Yoder, Toddington, UK) were potted up (1 stem per pot) and placed in a standard glasshouse compartment (20–22°C, 16:8 L:D, ambient humidity) for two weeks before being used in the experiment.
Pollen collection
All pollen used in the experiment was collected from the castor oil plant, Ricinus communis. The castor oil plants were maintained in a glasshouse compartment at ambient temperature and humidity, with a 16:8 L:D photoperiod. The pollen was collected from the flowers by shaking the flowers over a Petri dish, and stored in a fridge overnight before use in the experiments.
Experimental design
All experiments were done on cut stems of chrysanthemum containing eight leaves. The cut stems were placed in a conical flask filled with water and sealed around the base of the stem using cotton wool and parafilm. This was to prevent the thrips and predators moving into the conical flask.
Predator and pest release procedure
For both experiments, the same predator and pest release procedure was used. Ten first instar thrips larvae were transferred onto the cut stems using a fine paintbrush and placed in equal numbers on the first and second fully expanded leaves (five larvae on each leaf). Ten larvae were used as this was the maximum number of larvae per stem that could be reared without over-depleting the thrips culture, as a minimum of 120 first instar thrips larvae were required per set of treatments. Pollen (0.01 g) or fungus (0.05 g) was placed on the first fully expanded leaf using a fine paintbrush in the treatments where pollen or fungus was required. For the predator treatments, one or two third instar O. laevigatus larvae or one or ten adult female N. cucumeris were transferred from the culture onto the meristem of the plant using a fine paintbrush.
Predation rate experiment
The following treatments were used in the predation rate experiment:
● 10 thrips
● 10 thrips+pollen
● 10 thrips+fungus
● 10 thrips+1 N. cucumeris (2 replicates)
● 10 thrips+pollen+1 N. cucumeris (2 replicates)
● 10 thrips+fungus+1 N. cucumeris (2 replicates)
● 10 thrips+1 O. laevigatus
● 10 thrips+pollen+1 O. laevigatus
● 10 thrips+fungus+1 O. laevigatus
All treatments were run at the same time, with the plants placed individually in a Petri dish containing water plus detergent inside a cylindrical cage once the pest and predators had been released. The Petri dish acted as trap for any thrips or predators that had walked off the plant. These cages were then placed in a controlled environment room at 20 (±1)°C, ambient humidity and 16:8 light:dark photoperiod for 24 h. After 24 h, the cages were removed from the controlled environment room. Each leaf, with its accompanying portion of stem, and the meristem were placed individually into a pot of alcohol. The pots were examined under a microscope, and the number of thrips on each leaf and the meristem were recorded. The number of thrips and predators in the Petri dish was also counted. The number of thrips eaten was then calculated by subtracting the number found from the number placed on the plant. The experiment was repeated ten times.
Movement experiment
The following treatments were used in the movement experiment:
● Control (no thrips or pollen or fungus)+10 N. cucumeris
● Pollen+10 N. cucumeris
● Fungus+10 N. cucumeris
● 10 thrips+10 N. cucumeris
● 10 thrips+pollen+10 N. cucumeris
● 10 thrips+fungus+10 N. cucumeris
● Control (no thrips or pollen or fungus)+2 O. laevigatus
● Pollen+2 O. laevigatus
● Fungus+2 O. laevigatus
● 10 thrips+2 O. laevigatus
● 10 thrips+pollen+2 O. laevigatus
● 10 thrips+fungus+2 O. laevigatus
All treatments were examined at the same time. As with the predation rate experiment, the plants were placed individually in a Petri dish containing detergent and water inside a cylindrical cage after introduction of the pests and predators. The cages were then placed on two shelves in a controlled environment room for either 2, 5 or 24 h to determine whether there was an effect of time-dependent prey reduction on movement. A total of eight replicates of each of the time treatments were done, with the time treatments randomized by date of experiment and shelf to enable comparisons between all treatments. After the appropriate time period, the plants were sampled using the same procedure as for the predation rate experiment, and the number of thrips eaten was calculated. The position of each of the predators on the plant was recorded.
Statistical analysis
The counts of thrips eaten by predators per plant in the predation rate experiment were analysed using a log-linear model (a generalized linear model (GLM) assuming a Poisson error distribution and natural logarithm link function). The overall effects of the supplemental food and predator treatments, and of the interaction between these factors, were assessed from the accumulated analyses of deviance (comparison with critical values from the appropriate Chi-square distribution). Individual treatment comparisons were made based on the fitted parameters on the loge(count) scale. Predicted mean counts for each treatment combination were calculated to aid interpretation.
For the movement experiment, the counts of predators and thrips in different locations were separately analysed also using a log-linear model (Poisson error distribution, natural logarithm link function) within the GLM framework. In addition, the proportions of predators or thrips moving to specific locations within the plant were analysed using a logit model (a generalized linear model (GLM) assuming a binomial error distribution and logit link function). Separate analyses were performed for each predator species. The overall effects of supplemental food and the time from release, and of the interaction of these factors, on either the spatial distribution of predators or thrips (log-linear model), or the proportion of predators or thrips at specific locations (logit model), were assessed from the accumulated analyses of deviance as indicated above. Further treatment contrasts were included to extract information about the relative effects of individual treatment combinations (e.g. comparison of the different food supplements; comparison of the shorter time periods with 24 h). Individual treatment comparisons were made based on the fitted parameters (on either the natural logarithm or logit scales), with predicted mean counts or proportions calculated for each treatment combination, to aid interpretation.
Results
Predation rate experiment
The numbers of thrips eaten by O. laevigatus and N. cucumeris under the different supplemental food treatments are summarized in table 1. The results suggest that pollen reduces the number of thrips eaten by both predators, but that the fungus does not affect the number of thrips eaten by the predators. This is supported by the analysis of deviance which showed a significantly lower number of thrips eaten when pollen was present (P<0.001, d.f.=2, χ2=12.82). There was also a significant effect of predator, with N. cucumeris eating fewer thrips than O. laevigatus (P<0.001, d.f.=2, χ2=136.91).
Table 1. The mean number of thrips (±standard error) eaten by a single Orius laevigatus or Neoseiulus cucumeris in the presence and absence of supplemental food sources (pollen and the fungus Trichoderma viride), precited by the GLM.
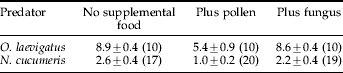
The numbers of observations are given in brackets.
Movement experiments
The mean numbers of thrips eaten by O. laevigatus or N. cucumeris under the different supplemental food treatments are summarized in table 2. The results from this experiment and the analysis of deviance support the findings of the predation rate experiment, with pollen leading to a significant reduction in the number of thrips eaten by both predators (χ2=9.28, d.f.=2, P<0.001). Also, the analysis confirmed that O. laevigatus ate significantly more thrips than N. cucumeris (χ2=40.91, d.f.=1, P<0.001).
Table 2. The mean number of thrips (±standard error) eaten by two Orius laevigatus or ten Neoseiulus cucumeris in the presence and absence of supplemental food sources (pollen and the fungus Trichoderma viride) after 2, 5 and 24 h, predicted by the GLM.
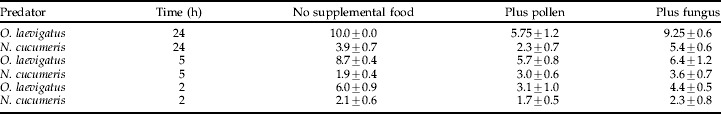
The number of observations for the 24 h runs was 8, but only 7 for the 2 and 5 h runs, due to replicates where the predator was not found being omitted from the calculation of the mean.
The analysis of the movement of N. cucumeris indicates that there are significant effects of both time (χ2=31.76, d.f.=8, P<0.001) and supplemental food (χ2=3.06, d.f.=8, P=0.002) and the interaction between these two factors (χ2=2.13, d.f.=16, P=0.005), but that there was no significant effect of the presence of prey. The analysis showed that the proportion of mites remaining on the meristem decreased as the assessment time increased (χ2=75.42, d.f.=2, P<0.001), but was unaffected by supplemental food type, though the proportion of mites moving to leaves 1 and 2 (where the supplemental food was placed) was significantly affected by the supplemental food type (χ2=10.05, d.f.=1, P=0.002), with a greater proportion found on these leaves in the presence of pollen and fungus. However, after 24 h, the mites were more likely to be retained on the plants containing pollen compared to the fungus-containing and control plants with 48.6, 27.8 and 22.9% of the mites remaining on the plants, respectively (averaged over presence and absence of prey). The presence of prey had little impact on the movement of N. cucumeris after 2 h (table 3), but after 5 h the presence of thrips increased the proportions of predators on the leaves in the absence of any supplement and reduced the proportion in the presence of either supplement. After 24 h, the only impact of the presence of thrips is to increase the proportion of predators on the leaves for the pollen supplement treatment.
Table 3. The proportion of Neoseiulus cucumeris and Orius laevigatus (±standard error) remaining on the plants after 2, 5 and 24 h for the supplemental food treatments in the presence and absence of thrips prey, as predicted by the GLM.

For O. laevigatus movement, there were significant effects of assessment time (χ2=4.92, d.f.=8, P<0.001), supplemental food (χ2=7.62, d.f.=8, P<0.001) and the presence or absence of thrips (χ2=5.29, d.f.=4, P<0.001) as well as a significant interaction between assessment time and supplemental food (χ2=1.79, d.f.=16, P=0.026). The interaction terms were caused by differences in the distributions of the predators for the fungus and pollen treatments between either 2 and 5 h or 5 and 24 h (table 3). In the pollen treatment, the predators were retained on the plant to a greater extent than for the fungus treatment and the control (averaged over all time periods), with the predators being concentrated towards the top of the plant (meristem plus leaves 1 and 2). The fungus treatment also led to more predators being retained on the plants, particularly around leaves 1 and 2, when averaged over all time periods.
Discussion
Effect of pollen on predator movement and thrips predation
The results confirm previous work that showed that N. cucumeris is not as good a predator of western flower thrips as O. laevigatus (Skirvin et al., Reference Skirvin, Kravar-Garde, Reynolds, Jones and de Courcy Williams2006). This is due to the fact that the mites are only able to attack first instar western flower thrips (Bakker & Sabelis, Reference Bakker and Sabelis1989), whilst O. laevigatus is able to attack a wide range of thrips instars (Gilkeson et al., Reference Gilkeson, Morewood and Elliot1990; Gillespie & Quiring, Reference Gillespie and Quiring1992). Although less than 24-h-old first instar thrips were used in the experiments, it is possible that within 24 h, the larvae became too large for the N. cucumeris to eat, reducing the measured predation rate. However, the consistently low predation rates suggest that the prey becoming too large was not the major cause of the reduced predation rates for N. cucumeris.
The results of the present study also confirm previous studies that have shown that pollen reduces the predation rate of the predators (Skirvin et al., Reference Skirvin, Kravar-Garde, Reynolds, Jones and de Courcy Williams2006). Pollen acted to reduce the movement of both predators, with a much greater proportion of the predators being retained on the plant in the presence of pollen, especially after 24 h, confirming the suitability of pollen as an alternative food source for both of the predators, as well as its ability to retain predators on plants (Van Rijn & Sabelis, Reference Van Rijn and Sabelis1990a,Reference Van Rijn and Sabelisb; Van Rijn & Van Houten, Reference Van Rijn and Van Houten1991; Jacobson, Reference Jacobson1993; Van Delden et al., Reference Van Delden, Diederik, Mols, Rossing and Van der Werf1995). However, the lack of an effect of thrips presence on the movement of N. cucumeris highlights the limited ability of this predator to synchronize with its thrips prey, and perhaps indicates that this species prefers to feed on pollen as opposed to thrips. The attraction of both predators to the leaves containing pollen may be the reason for the lower levels of predation on the plants containing pollen. Despite the increased coincidence of predators and prey, the pollen may be more nutritionally advantageous for the predators than thrips, and it is certainly easier for the predators to eat pollen than to capture thrips prey. This may lead to a ‘preference’ for the pollen.
The reduced predation in the presence of pollen contrasts with simulation modelling studies (Van Rijn et al., Reference Van Rijn, Van Houten and Sabelis2002), where it was predicted that in the presence of pollen, predators would achieve a greater control of thrips. However, pollens vary in their nutritional suitability to predators (Hulshof et al., Reference Hulshof, Ketoja and Vänninen2003), and the pollen used in our experiments may have been more attractive to predators than the one simulated in the modelling studies. Also, the amount of pollen will affect the predation rate, as if pollen is not limiting, then the predators will be more likely to encounter pollen than thrips, leading to a reduced predation pressure on the thrips.
The results of the predation rate experiment suggest that greater information is required about the use of pollen as a supplemental food in glasshouse crops, especially if it can reduce predation on pests by up to 40%, and that it will be essential to determine the minimum amount of pollen required to ensure predators survive, but not to limit their movement and searching behaviour.
The results of the work on movement over different time periods show that in both the presence and absence of supplemental food, the majority of thrips are eaten within the first 5 h of the experiment. In the absence of supplemental food, but with thrips available, the proportion of both predators remaining on the plant decreases markedly after 5 h, showing that both predators are responding to the availability of food.
For O. laevigatus, it can be seen that in the presence of pollen only, the rate of leaving the plant remains constant irrespective of the time period, suggesting that there was sufficient pollen to retain the predators on the plant. When both pollen and thrips are present, the rate of leaving still remains constant, which suggests that the predators are supplementing their diet with pollen as thrips availability declines.
For N. cucumeris, more predators are retained on the plant after 24 h with both pollen and thrips available, than with pollen or thrips alone. The reasons for this are not clear; it may be an artefact of the experimental design, where the predators that have left the plant are unable to return over the short time periods, but do so over the 24-h period, leading to a greater retention of predators on the longer time scale. However, it could be that at the short time scales, the predators have been unable to search the plant sufficiently well to locate the available food sources and further investigation of the searching behaviour of the predators will be necessary to determine if this is the case.
The retention of predators on plants with pollen confirms the results of Skirvin et al. (Reference Skirvin, Kravar-Garde, Reynolds, Jones and de Courcy Williams2006), where greater numbers of predators were recovered on plants containing pollen than those without. Further research is required to determine whether the retention of predators on plants containing pollen will be detrimental to biological control, especially at low pest densities where there is a need for the predators to be actively searching over a large area to locate the sparsely distributed prey. It will be particularly important to understand how the amount of pollen provided and the spatial arrangement of pollen affects the distribution and predation rates of predators on thrips, as these factors will be important in determining the success of using pollen to enhance biological control.
Effect of the fungus, Trichoderma viride, on predator movement and thrips predation
The results with fungus as a supplementary food source are less clear cut. No evidence of a reduction in predation on western flower thrips was observed in the presence of the fungus, Trichoderma viride. The fungus also had little impact on the movement of N. cucumeris, suggesting that it does not function as an alternative food source for this predator. This contrasts with previous work (de Courcy Williams et al., Reference de Courcy Williams, Kravar-Garde, Fenlon and Sunderland2004), which suggested that fungal hyphae formed a food source for N. cucumeris, but the fungal species was not identified. The movement experiments for O. laevigatus indicate that the fungus led to a greater proportion of predators being retained on plants compared to control plants with no fungus, but only in the presence of thrips. This may be due to the attraction of the predators to an aggregation of thrips, as analysis of the movement of thrips suggests that they were more likely to remain on the leaves containing the fungus for at least the first 5 h. Further experimental work is necessary to determine whether the thrips and predators can use T. viride as a supplemental food and to unravel the interactions between fungal supplemental food and the presence of thrips on predator predation and movement.
Implications for biological control
The results of this study have shown that pollen is a suitable supplemental food for both N. cucumeris and O. laevigatus but that it leads to a reduction in predation ability of these predators. In the experiments presented here, the pollen was supplied in a moderate quantity, and it may be that pollen needs to be applied in much lower quantities, as suggested by Van Rijn et al. (Reference Van Rijn, Van Houten and Sabelis2002). This would ensure that the predators actively forage for prey, but with the benefits of an alternative food source were still available from the pollen in the absence of prey. Further research is necessary to determine the most effective method for applying pollen to plants as a supplemental food so that the predatory ability of the predators is not compromised by a preference for the alternative food source.
The present results confirm the previous conclusions of Skirvin et al. (Reference Skirvin, Kravar-Garde, Reynolds, Jones and de Courcy Williams2006) that N. cucumeris is not a particularly effective predator of Frankliniella occidentalis, which is surprising since it is one of the most commonly used predators for the commercial control of F. occidentalis (Bakker & Sabelis, Reference Bakker and Sabelis1989). The results presented here suggest that the nymphs of O. laevigatus are much more effective, and should prove to be a very effective biological control agent for thrips. However, further research is now required to determine whether it is possible to produce the nymphs as a commercial product, the normal practice being to purchase adults.
If the increased retention of predators on plants with fungus up to 5 h was due to the use of the fungus as a food source, this shows a high potential for it to be used as a supplemental food source, particularly as it did not have a negative impact on the predation of thrips by the predators. However, the equivocal nature of our results mean that further research is necessary to understand whether Trichoderma viride or other fungal species can be utilized as supplemental food sources by predators.
Supplemental food sources are a useful tool for biological control since they allow greater survival, and potentially reproduction of predators when food sources are scarce. However, they need to be used in a way that does not reduce the predatory ability or searching ability of the predators, and the complexity of the effects means that more research is required to determine the most effective methods for using supplemental food sources in an integrated biological crop protection programme.
Acknowledgements
The authors would like to thank the UK Department for Environment, Food and Rural Affairs for funding the research, James Bell for his comments on the manuscript and John Whipps for his useful discussions on the choice of fungal species.





