INTRODUCTION
In rivers and estuaries, the run-off plays an important role in governing the physico-chemical environment of the system. This is true particularly in the tropics, where although climatic variation is less, the region experiences a major hydroclimatic variation during the monsoon period characterized by a major riverine runoff. The complex interaction between the runoff and the physico-chemical environment plays an important role in structuring the biological community. Moreover, the changes in the sedimentary environment are greater in the tropics compared to the higher altitudes resulting in a wide variation in the macrobenthic species diversity (Alongi, Reference Alongi1990).
The west coast of India receives the heaviest rainfall in the Indian subcontinent due to the orography of the region (Suprit et al., Reference Suprit, Shankar, Venugopal and Bhatkar2012). Further, due to the geography of the region, the major portion of the heavy summer-monsoon rainfall flows into the Arabian Sea through the rivers (Suprit et al., Reference Suprit, Shankar, Venugopal and Bhatkar2012). The Mandovi river is a typical west coast river with a length of 87 km and basin area of 2032 km2 (Rao, Reference Rao1975). Since the river is rain-fed, the discharge shows strong seasonality with major discharge (~100 hm3) during the SW monsoon period (Suprit et al., Reference Suprit, Shankar, Venugopal and Bhatkar2012).
Although the Mandovi river is one of the well-studied estuaries along the west coast (Shetye et al., Reference Shetye, Shankar, Neetu, Suprit, Michael, Chandramohan, Shetye, Shankar and Dileep Kumar2007), little is known of the effect of run-off on the benthic community of the system. Moreover, the Mandovi–Zuari estuarine system is considered the life-line of the state of Goa. Fringed with mangrove vegetation, the backwater system of both the rivers is an important fishing ground, especially during the fishing ban period in the monsoon. It is also the major route for the transportation of minerals mined upstream to the Mormugao port in the Zuari river. In an ecologically and economically important river like Mandovi, it is important to know the natural variation in the benthic recruitment so as to elucidate the patterns induced by anthropogenic changes. According to the IPCC 2007 report (Solomon et al., Reference Solomon, Qin, Manning, Chen, Marquis, Averyt, Tignor and Miller2007), monsoon and river discharge will also be affected by climate change and in turn influence the regional climate system. Therefore, such studies can form the bases for measuring the ecosystem process and for the management of fisheries and other important resources. Thus we aimed at investigating the effect of temporal variation in riverine run-off and associated environmental changes in structuring the macrobenthic community of the Mandovi estuary.
MATERIALS AND METHODS
Study area
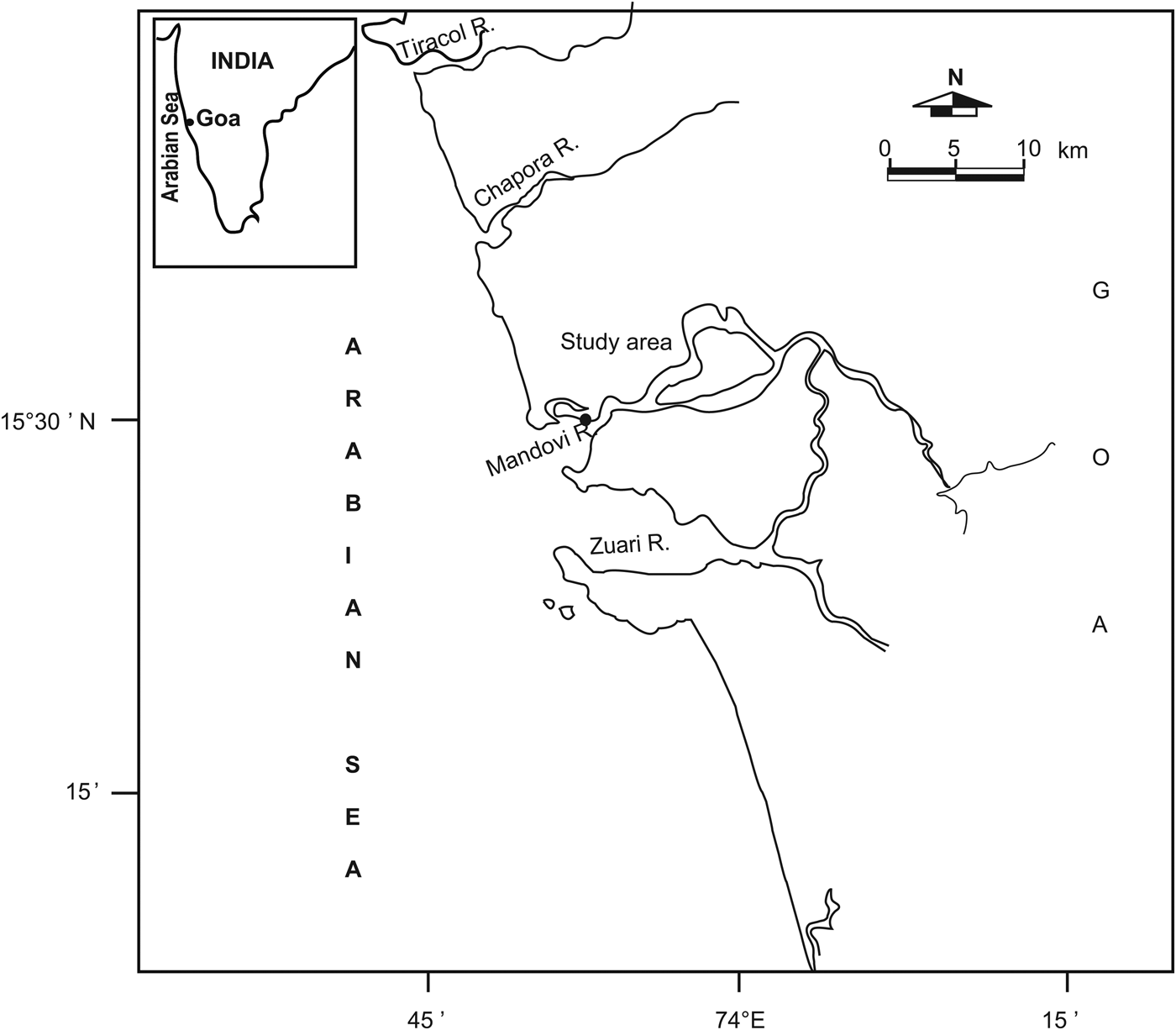
The study was conducted at a fixed location off Chorao Island (15°30′N and 73°50′E; Figure 1) in the Mandovi river. Five distinct temporal regimes are identified in the Mandovi based on the rainfall pattern by Suprit et al. (Reference Suprit, Shankar, Venugopal and Bhatkar2012): the lean-season regime (LSR; January–May) characterized by a very dry regime and discharge is primarily baseflow; monsoon-onset regime (MOR; June) is wet and, since the soil is unsaturated, discharge does not respond to rainfall; peak-monsoon regime (PMR), marked by peak monsoon period and saturated soil, indicating that the basin responds to rainfall; end-monsoon regime (EMR; September–October) and post-monsoon regime (PMonR; November–December).

Fig. 1. Location of the study area.
Sampling was conducted for a period of two years from June 2007 to June 2008 and from January 2009 to June 2009. Ten replicate samples were collected fortnightly, using a Van Veen grab (0.04 m2 area). Faunal samples were preserved in 10% buffered formalin rose Bengal solution. In the laboratory, the samples were sieved on 0.5 mm mesh size sieve and the materials retained were preserved in 5% formalin. All the organisms were carefully washed again, sorted and preserved in 5% buffered formalin. The fauna were then counted (ind m−2) and identified up to the lowest possible taxa under a stereo-microscope. Biomass (g m−2) was estimated by wet weight method.
Monthly rainfall data and total sunshine was obtained from the Meteorological Station, Goa. Temperature, salinity and chlorophyll-a (Chl a) data were obtained from the Data Centre of National Institute of Oceanography (CSIR), Goa, while total suspended solid (TSS) data is from Shynu et al. (Reference Shynu, Purnachandra Rao, Kessarkar and Rao2012).
Data analysis
One-way analysis of variance (ANOVA) analysis was performed to find out the significant temporal variation of biological and environmental parameters. For significant ANOVA results, Tukey's post-hoc tests were used to examine the difference in the means. The univariate measures such as Shannon–Wiener diversity index (H′), Margalef's species richness (d) and evenness (J′) were calculated. The temporal pattern of macrofaunal community was analysed by hierarchical agglomerative clustering with group-average linking on the Bray–Curtis coefficient (Clarke & Warwick, Reference Clarke and Warwick2001) on the log transformed abundance data of species. A similarity profile (SIMPROF) test was carried out for detecting statistically significant cluster (Clarke & Gorley, Reference Clarke and Gorley2006; Clarke et al., Reference Clarke, Somerfield and Gorley2008). Similarity percentage programme (SIMPER) was then used to identify the species contributing to intra-group similarity and those species responsible for dissimilarity between groups. LINKTREE analysis was performed to find the environmental parameters that contributed to the clustering (Clarke & Warwick, Reference Clarke and Warwick2001). To relate macrofaunal assemblages to environmental parameters, the canonical correspondence analysis (CCA) (ter Braak, Reference ter Braak1986) was performed. Only the important species based on the SIMPER analyses were selected for the analyses. The percentage average similarity/standard deviation (![]() /SD) ratio was used to find the species that contributed to the similarity within the cluster and dissimilarity between groups. A species with large
/SD) ratio was used to find the species that contributed to the similarity within the cluster and dissimilarity between groups. A species with large ![]() and small SD value will have a large
and small SD value will have a large ![]() /SD ratio and hence the species will not only contribute to the dissimilarity between groups but it will also consistently contribute to the similarity within the group. Therefore, a species with high
/SD ratio and hence the species will not only contribute to the dissimilarity between groups but it will also consistently contribute to the similarity within the group. Therefore, a species with high ![]() /SD ratio and percentage similarity contribution are considered good discriminating species (Clarke & Warwick, Reference Clarke and Warwick2001). In the present study
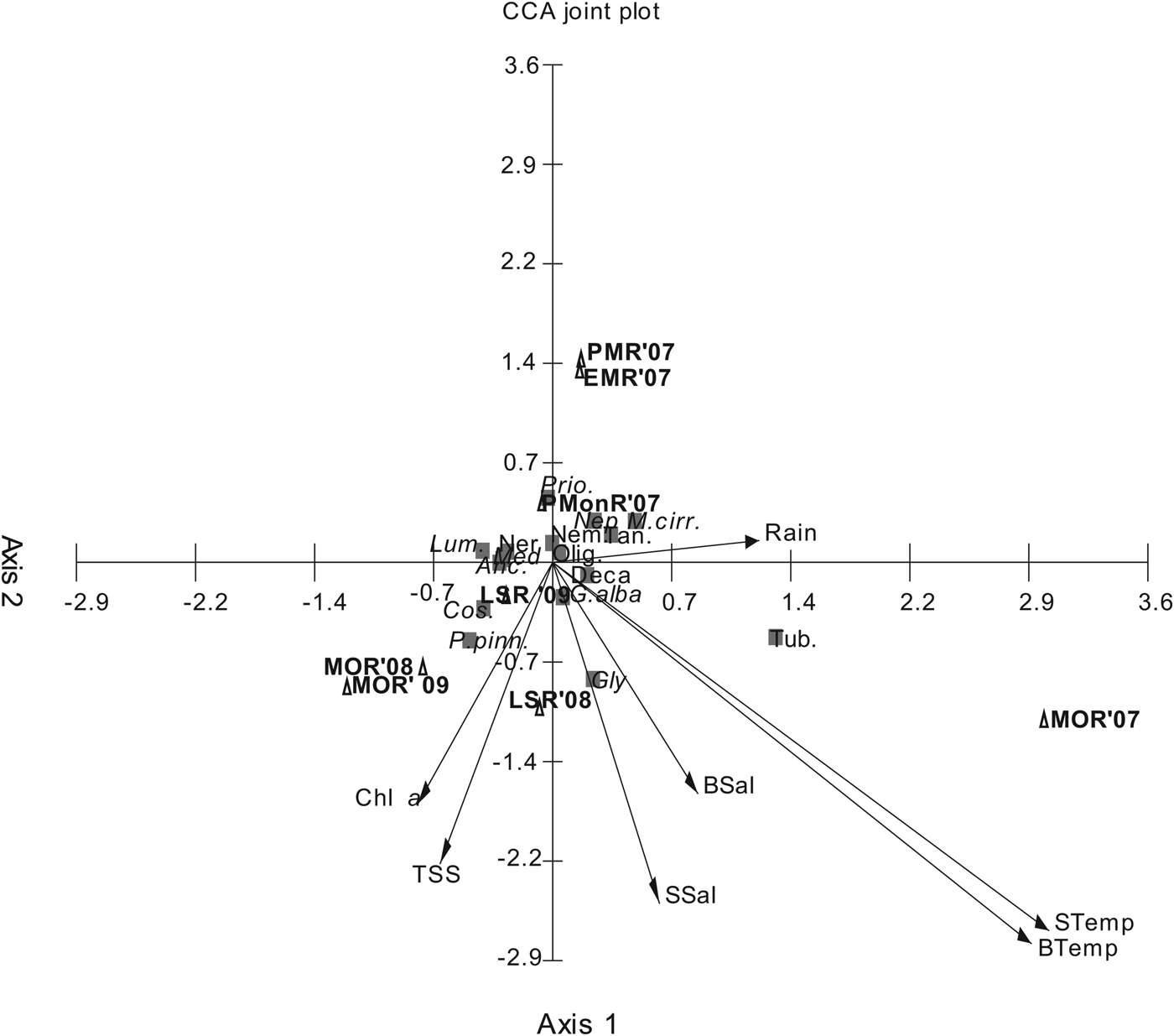
/SD ratio and percentage similarity contribution are considered good discriminating species (Clarke & Warwick, Reference Clarke and Warwick2001). In the present study ![]() /SD ratio >2 was arbitrarily considered as the cut off point. CCA is displayed in an ordination diagram with sites and benthic variables represented by points and environmental parameters by arrows (ter Braak, Reference ter Braak1986). The benthic variables and site points jointly represent the dominant patterns in community while benthic group point and the arrow of environmental variables jointly reflect the distribution of benthos along each of the variables. The analyses were carried out using Primer 6, Multivariate statistical package program (MVstep) 3.1 (Kovach, Reference Kovach1998) and Statistica 10 software packages.
/SD ratio >2 was arbitrarily considered as the cut off point. CCA is displayed in an ordination diagram with sites and benthic variables represented by points and environmental parameters by arrows (ter Braak, Reference ter Braak1986). The benthic variables and site points jointly represent the dominant patterns in community while benthic group point and the arrow of environmental variables jointly reflect the distribution of benthos along each of the variables. The analyses were carried out using Primer 6, Multivariate statistical package program (MVstep) 3.1 (Kovach, Reference Kovach1998) and Statistica 10 software packages.
RESULTS
Environmental characteristics
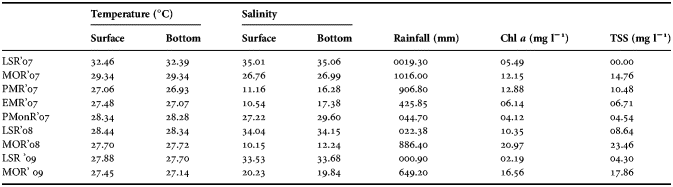
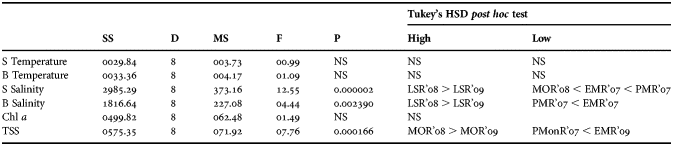
The rainfall showed variation with maximum precipitation recorded during MOR'07 (1016 mm) and minimum during LS regimes (0.9–22.38 mm; Table 1). Surface water temperature varied from 27°C to 32°C, with the highest value recorded during LSR'07 and lowest during PMR'07. Bottom water temperature followed a similar trend. Surface and bottom water temperature did not show significant temporal variation (P > 0.05; Table 2). Surface and bottom water salinity varied from 10 to 35 and from 12 to 35, respectively. The highest salinity was in surface and lowest value was observed in bottom water during LSR'07. Surface and bottom water salinity showed significant temporal variation (Table 2). The significant effect was further examined by Tukey's HSD post hoc test, which showed appreciably high values of salinity during the LSR'08 and LSR'09 (Table 2). Total suspended solids (TSS) were highest during the MOR'08 (23.46 mg l−1) and low during LSR (0–8.64 mg l−1). One-way ANOVA and Tukey's HSD post hoc test detected significant temporal variation in TSS with highest values during MOR'08 and MOR'09 (Table 2). Chl a ranged from 1.9 to 23.4 mg l−1 and there was no significant temporal variation (Table 2).
Table 1. Environmental parameters in the study area.

Table 2. One-way ANOVA results of environmental variables. S, surface; B, bottom; TSS, total suspended solid; NS, not significant. Only the variables that were significantly high/low are represented.
Macrofaunal community
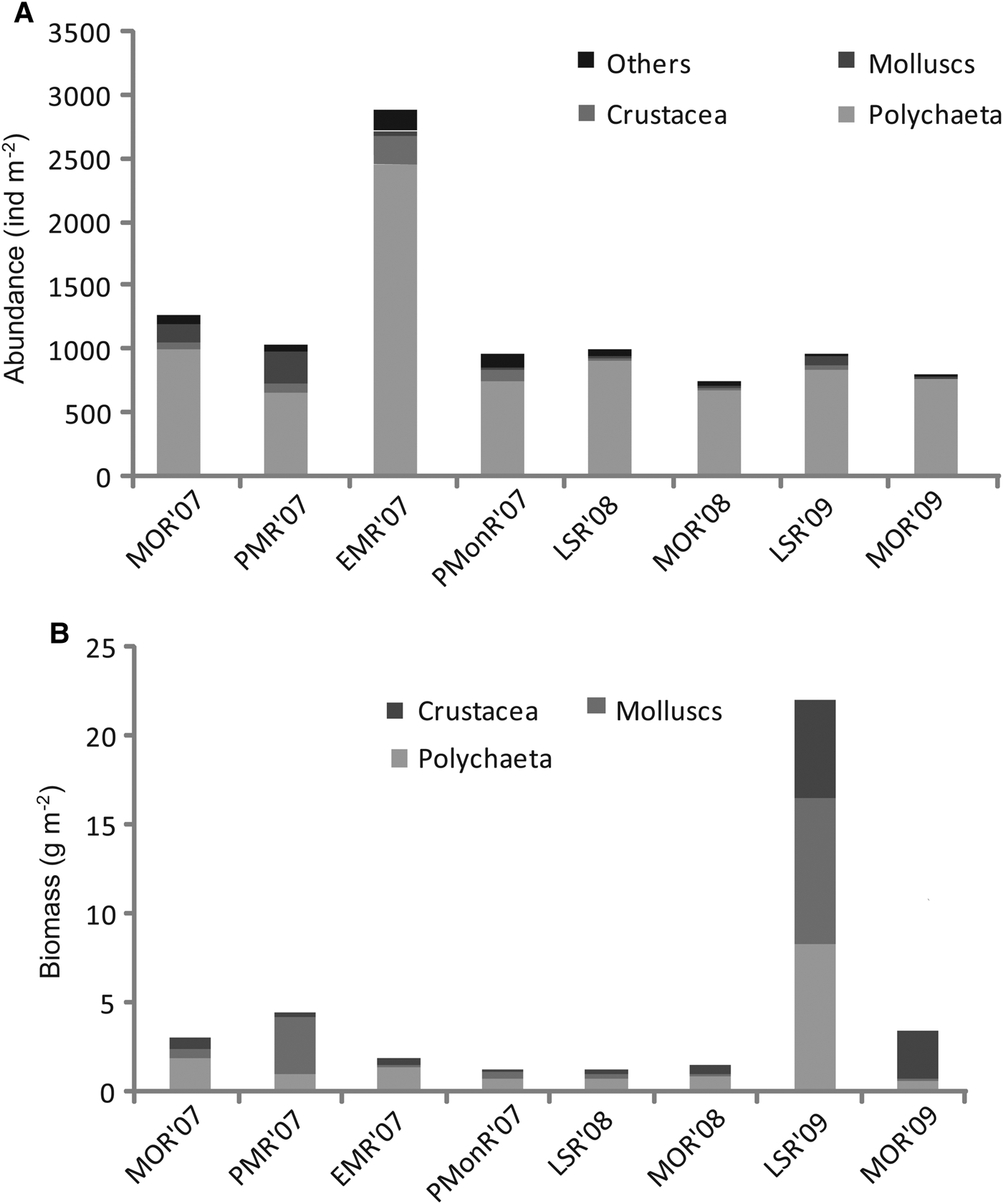
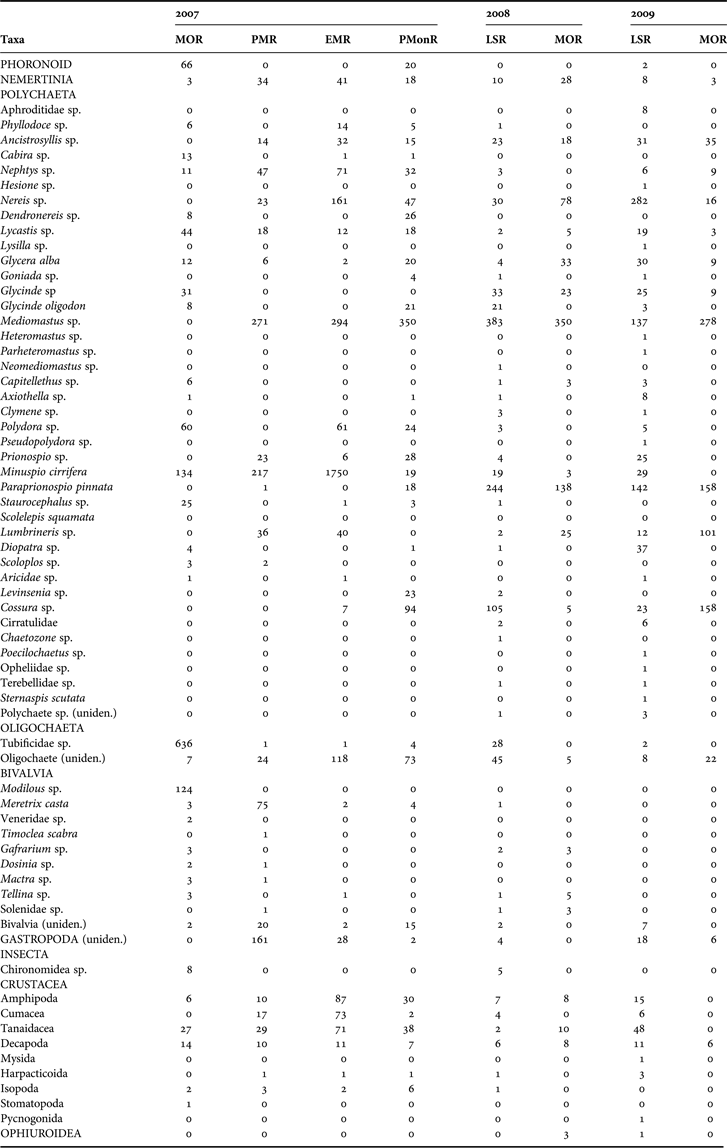
Sixty-six taxa belonging to 17 macrofaunal groups were identified during the study (Table 3). In general, a total of 13009 macrobenthic forms were collected, dominated by Polychaeta (76%). Oligochaeta (10%), crustacea (6%), bivalvia (3%), gastropoda (2%) and other minor faunal groups contributed (3%). The average faunal abundance varied from 748 to 2892 ind m−2 recorded during MOR'08 and EMR'07 regimes (Figure 2A). ANOVA detected a significant temporal variation in abundance (F = 3.71; P < 0.01) and Tukey's post hoc test detected significantly high values during EMR'07.

Fig. 2. (A) Temporal variation in macrofaunal abundance; (B) biomass.
Table 3. Macrofaunal abundance in the Mandovi estuary during the study period.

Uniden., unidentified forms.
Abundance of polychaetes ranged from 659 to 2453 ind m−2 during the PMR'07 and EMR'07 regimes, respectively. Among the polychaetes, Minuspio cirrifera was the most dominant and density ranged from 0 to 1750 ind m−2 with highest during the EMR'07 regime. Mediomastus sp. was next in dominance and abundance ranged from 0 to 383 ind m−2 recorded during the MOR'07 and PMonR, respectively. Tubificidae Oligochaeta dominated during the MOR'07 (636 ind m−2). Abundance of Paraprionospio pinnata ranged from 1 to 244 ind m−2 (PMR'07 and LSR'08). Crustacean abundance ranged from 20 to 245 ind m−2 with highest abundance during the EMR'07 regime (Figure 2A).
The average macrofaunal biomass ranged from 1.27 to 22.74 g m−2 recorded during LSR'08 and LSR'09 respectively (Figure 2B), but did not vary significantly over time (P > 0.05). Group-wise, polychaetes constituted 60% of the total biomass, followed by crustaceans (18%), bivalves (14.7%) and gastropods (7.2%).
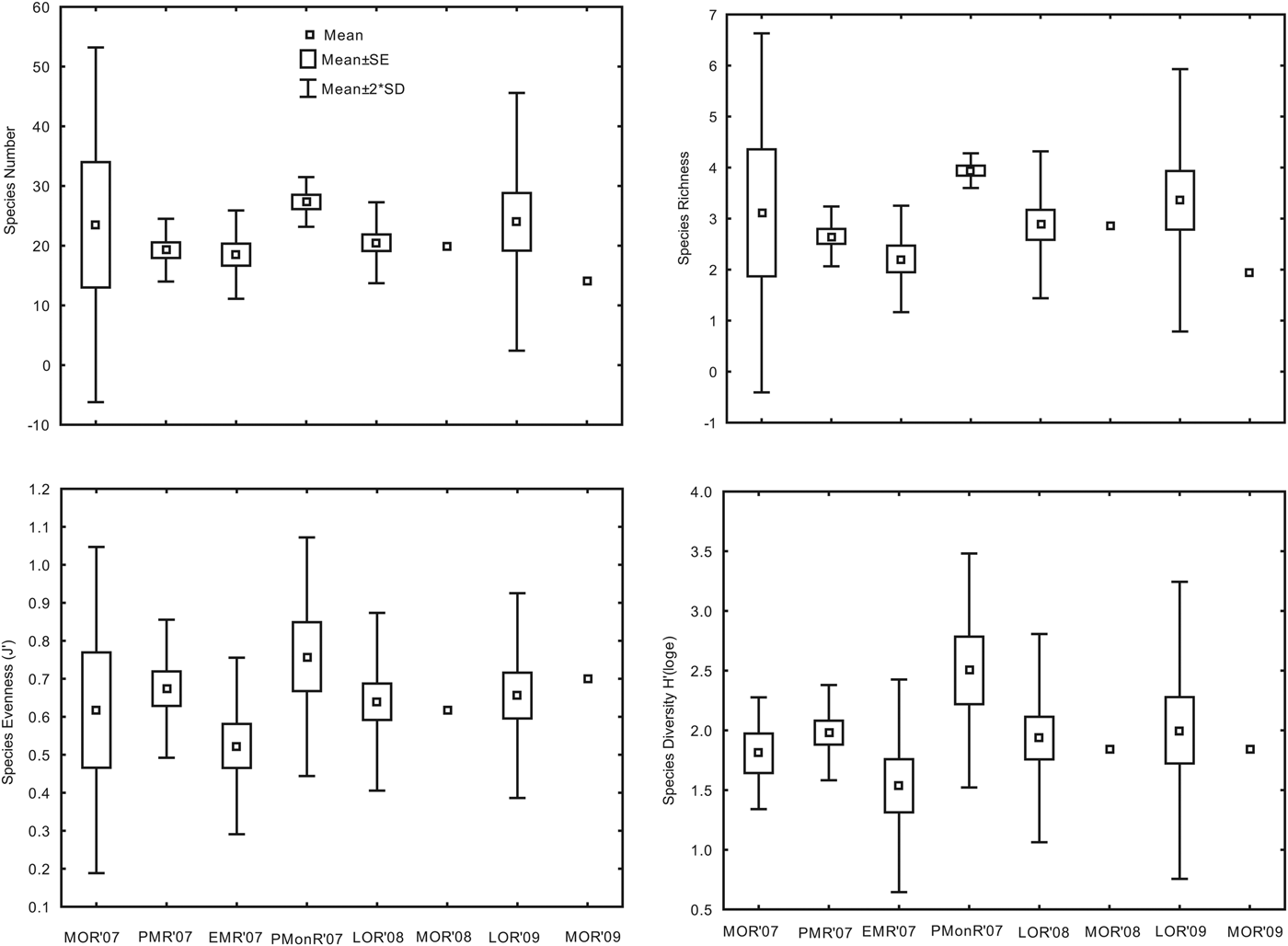
The number of macrofaunal taxa recorded in each sample ranged from 14 (MOR'09) to 27 (PMonR'07; Figure 3). Similarly, species richness (d) ranged from 1.94 (MOR'09) to 3.94 (PMonR'07). The Margalef's species evenness (J′) ranged from 0.52 (EMR'07) to 0.76 (PMonR'07). Shannon–Wiener diversity (H′) ranged from 1.54 to 2.50 recorded at EMR'07 and PMonR'07. None of the diversity indices showed statistically significant variation (P > 0.05).

Fig. 3. Temporal variation in species diversity indices in the study area.
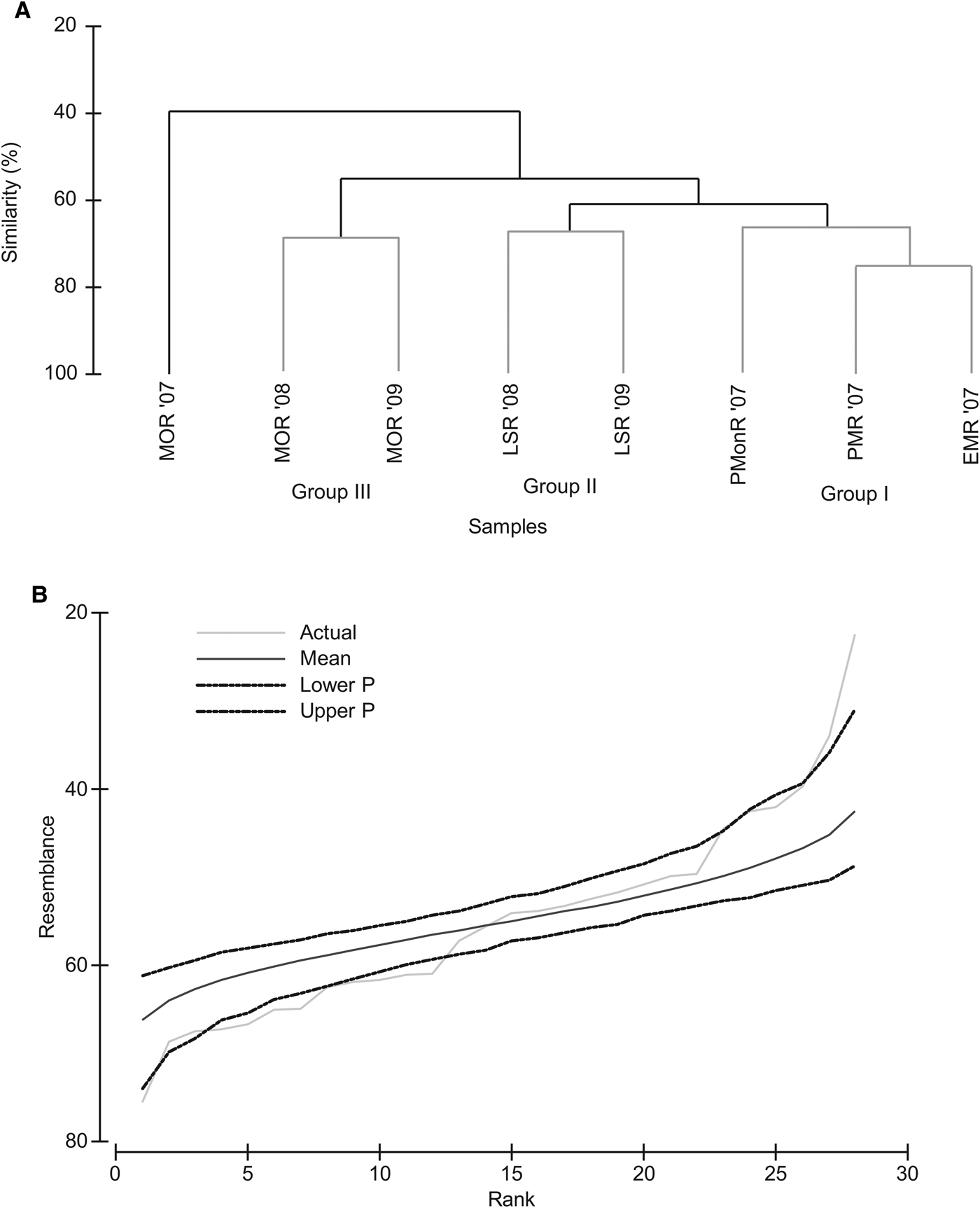
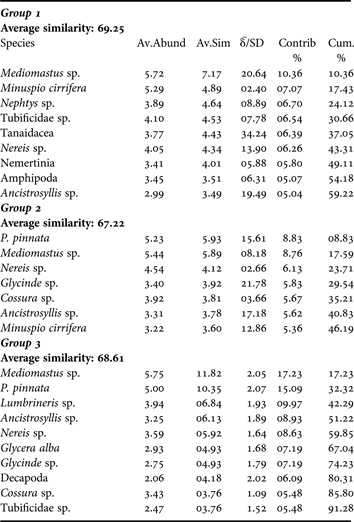
Percentage similarity (Bray–Curtis) cluster analyses based on the faunal abundance showed variation between the sampling periods. The SIMPROF test showed that clustering observed were statistically significant rejecting the null hypothesis (Figure 4). Group I consisted of sampling period of EMR, PMR and PMonR of 2007 and was dominated by Mediomastus sp., Minuspio cirrifera, Nephtys sp. (Table 4). However, Tanaid, Nereis sp. and Ancistrosyllis sp. with high ![]() /SD ratio (Table 4) contributed significantly to characterise Group I. The LSR period of 2008 and 2009 pooled to form Group II. Mediomastus sp. and Paraprionospio pinnata contributed to the similarity of the group, but Glycinde sp. Ancistrosyllis sp. and Minuspio cirrifera were significantly better discriminator (
/SD ratio (Table 4) contributed significantly to characterise Group I. The LSR period of 2008 and 2009 pooled to form Group II. Mediomastus sp. and Paraprionospio pinnata contributed to the similarity of the group, but Glycinde sp. Ancistrosyllis sp. and Minuspio cirrifera were significantly better discriminator (![]() /SD >2). The third group (MOR'08 and MOR'09) were dominated by Mediomastus sp., Paraprionospio pinnata, Lumbrineris sp. The Group I separated from Group II at significant level of π = 1.76; P = 3.38%. Group I and II significantly differed from Group III (π = 2.14; P = 1.2%), while Group IV (MOR'07) separated significantly from the rest of the cluster (π = 4.39; P = 0.1%). The grey lines of the dendrogram indicate that the group is homogeneous and hence the null hypothesis is retained. As seen in the Figure 4B the actual similarity profile departs significantly from the permuted mean (π = 4.47; P = 0.1%). The species that contributed to the clustering were analysed using SIMPER and data is presented in Table 4.
/SD >2). The third group (MOR'08 and MOR'09) were dominated by Mediomastus sp., Paraprionospio pinnata, Lumbrineris sp. The Group I separated from Group II at significant level of π = 1.76; P = 3.38%. Group I and II significantly differed from Group III (π = 2.14; P = 1.2%), while Group IV (MOR'07) separated significantly from the rest of the cluster (π = 4.39; P = 0.1%). The grey lines of the dendrogram indicate that the group is homogeneous and hence the null hypothesis is retained. As seen in the Figure 4B the actual similarity profile departs significantly from the permuted mean (π = 4.47; P = 0.1%). The species that contributed to the clustering were analysed using SIMPER and data is presented in Table 4.

Fig. 4. (A) SIMPROF based on log transformed species abundance data. Black lines indicate significant clustering (P < 0.05); (B) shows the ordered similarities between season plotted against their ranks.
Table 4. SIMPER analysis based on groups obtained from Bray–Curtis cluster and SIMPROF showing the species that contributed to the similarity in the groups.

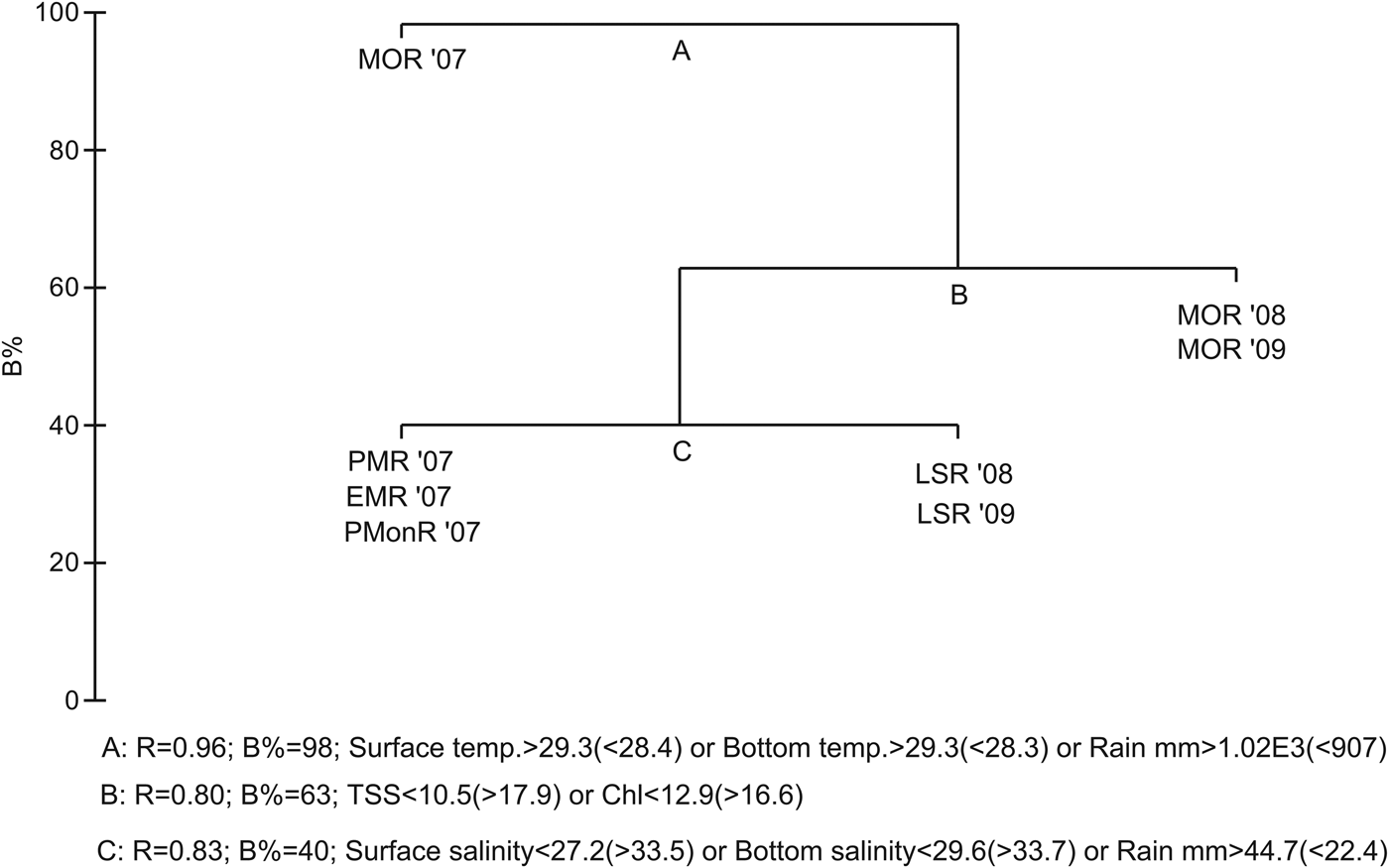
The LINKTREE analyses (Figure 5) showed a similar splitting of samples as observed in SIMPROF (Figure 4A). The first split (A) separates MOR'07 from the rest of the samples and is characterized by surface and bottom water temperature >29.3 and rainfall >1.02E3 (R = 0.96; B%= 98). The next split separates Group III from Group I and II due to TSS <10.5 and Chl a <12.9 (R = 0.80; B% = 63). Group I split from Group II due to salinity <27.2 and rainfall <44.7 mm (R = 0.83; B%= 40). The global BEST test showed a significant (R = 0.61; P = 4%) link between the macrofaunal community and three of the studied variables, i.e. surface and bottom temperature and TSS. The CCA further confirmed the results of LINKTREE. The first two ordination axes of CCA, accounted for 77% of explained total variance in the dominant species abundance by the measured environmental variance (Figure 6). The first canonical axis accounted for 48% and reflected gradient to surface and bottom water temperature, rainfall and surface water salinity. The second axis was associated with Chl a, and TSS. The CCA also reflected the temporal variability in the occurrence of macrofaunal species as observed in cluster analyses (Figure 4, SIMPROF).

Fig. 5. Linkage tree analysis (LINKTREE) showing binary clustering of regimes based on species composition and the environmental variables.

Fig. 6. CCA ordination diagram for macrobenthic species in relation to environmental parameters and sampling period. Environmental parameters: STemp- surface water temperature; BTemp, bottom water temperature; SSal, surface water salinity; BSal, bottom water salinity; Chl a, chlorophyll a; TSS, total suspended solid; macrobenthic species: P.pinn, Paraprionospio pinnata; Med, Mediomastus sp.; Anc, Ancistrosyllis sp.; Nep, Nephtys sp.; Ner, Nereis sp.; G.alba, Glycera alba; Glyc, Glycinde sp.; Prio, Prionospio sp.; M.cirr, Minuspio cirrifera; Lum, Lumbrineris sp.; Cos, Cossura sp.; Nem, Nemertinia; Tub, Tubificidae Oligochaeta; Olig, Oligochaeta; Tan, Tanaidacea; Deca, Decapoda.
DISCUSSION
The environmental parameters showed significant temporal variation (one-way ANOVA). The variation observed in the environmental parameter was a result of the changes brought about by the SW monsoon. The surface and bottom water salinity showed temporal variation, typical of tropical estuaries. The low salinity during monsoon is attributed to the riverine runoff which completely flushes the estuary, turning the estuarine water fresh (Shetye et al., Reference Shetye, Shankar, Neetu, Suprit, Michael, Chandramohan, Shetye, Shankar and Dileep Kumar2007). While surface and bottom water salinity was similar during PMonR and LSR indicating that the area is well mixed during the non-monsoon period. The high TSS during the monsoon regime is the result of increase land runoff during this period. The variation in the environmental variables was also evident from the clustering of the sampling period (Figures 5 and 6).
The variation in the environment due to the effect of the annual monsoon also influenced the macrofaunal community of Mandovi estuary. The macrofaunal community structure is determined by a number of factors such as salinity, temperature, food availability, recruitment and hydrographic conditions (Henning & Kröncke, Reference Henning and Kröncke2005). That the variation in the environment affected the macrofaunal community is evident from the SIMPROF clustering (Figure 4) and confirmed by the LINKTREE and CCA analysis (Figures 5 and 6). The observed changes in the environmental variables, influenced by rainfall and the estuarine condition during the non-monsoon period resulted in a community dominated by a few species. A low species community in the soft sediments of estuaries is a general trend in the estuaries world over (Giménez et al., Reference Giménez, Borthagaray, Rodríguez, Brazeiro and Dimitriadis2005).
Further, the observed variability was basically due to the difference in the abundance of dominant species indicating that few species play an important role in structuring the macrofaunal community of the study site. The MOR'07 separated from MOR'08 and MOR'09 since, during 2007 there was unusually high rainfall (1016 mm), which was much higher than the PMR regime. The macrofaunal community of MOR'08 and MOR'09 clustered due to overall low macrofaunal abundance. Low macrofaunal abundance with onset of the monsoon is a common phenomenon in tropical regions (Alongi, Reference Alongi1990). The MOR is characterized by sudden changes in the environment (drop in salinity, increased TSS) which cause the defaunation. Discussing the seasonality in estuarine macrofauna, Alongi (Reference Alongi1990) suggested defaunation either due to mortality or displacement of adult and larval population resulting in the low abundance and species number. Further, decrease in salinity can also trigger the gonadal release in the macrofaunal species with planktonic larval stage (Kinne, Reference Kinne and Kinne1977; Broom, Reference Broom1982). This period is also characterized by high primary productivity, stimulated by the nutrients brought by the riverine run-off and directly by the organic matter input. Thus, the planktonic recruiting larvae are ensured of abundant food. Further, the water turbulence during the monsoon helps in the wide dispersal of both, larvae and the adults of the sedentary benthic species (Dobbs & Vozarik, Reference Dobbs and Vozarik1983; Hernández-Arana et al., Reference Hernández-Arana, Rowden, Attrill, Warwick and Gold-Bouchot2003). The MOR'07 was completely dominated by Tubificidae Oligochaeta which is known to increase in disturbed areas (Caspers, Reference Caspers, Brinkhurst and Cook1971). They are tolerant to high organic enrichment and reduced oxygen level. Their response to disturbance results in rapid increase in population within a short period of time and is facilitated by the intense asexual reproduction (Giere & Pfannukuche, Reference Giere and Pfannukuche1982).
The regime of PMR'07 to PMonR'07 clustered, as they were represented by diverse species resulting from recruitment and transport of adults (Table 3). The sedimentation of suspended solids, organic matter brought from the riverine runoff, influences the community by changes in the sediment texture, increase in organic matter which will favour the colonization of juveniles as well as the re-colonization of suspended adult fauna. The onset of faunal succession in the Mandovi estuary was largely due to the first stage of recruitment (Harkantra & Rodrigues, Reference Harkantra and Rodrigues2004). The LSR period has a more stable community, represented by a few species tolerant to the estuarine conditions. The importance of climatic changes in structuring the macrofaunal community has also been reported by Hernández-Arana et al. (Reference Hernández-Arana, Rowden, Attrill, Warwick and Gold-Bouchot2003), Grilo et al. (Reference Grilo, Cardoso, Dolbeth, Bordalo and Pardal2011) and others.
Some of the dominant benthic species in the region were Minuspio cirrifera, Paraprionospio pinnata, Mediomastus sp., Tubificidae Oligochaeta, Nephtys sp., Nereis sp., Lumbrineris sp., Ancistrosyllis sp. and Oligochaeta. Most of the spionidae (Minuspio cirrifera and Paraprionospio pinnata) species and capitellidae (Mediomastus sp.) and Tubificidae Oligochaeta are small-sized and have a fast growth rate. The family Spionidae has opportunistic life histories (Rouse & Pleijel, Reference Rouse and Pleijel2001), that is, they are capable of increasing their population size above normal levels after a disturbance, and they show adaptations in their life history that allow them to rapidly colonize disturbed areas (Ansari et al., Reference Ansari, Ingole and Parulekar1986; Sivadas et al., Reference Sivadas, Ingole and Nanajkar2011). These organisms produce several broods per year and provide a continuous supply of larvae, which can settle at the favourable conditions (Grassle & Grassle, Reference Grassle and Grassle1974; Hannan, Reference Hannan1981). Once established, features such as fast growth, early maturity, offspring protection and lecithotrophy help to rapidly increase their population (Grassle & Grassle, Reference Grassle and Grassle1974; McCall, Reference McCall1977). Mediomastus sp. is a common inhabitant of muddy sediments and also an opportunistic species (Grassle & Grassle, Reference Grassle and Grassle1974). This organism rapidly colonizes newly disturbed or defaunated areas (Hyland et al., Reference Hyland, Baptiste, Campbell, Kennedy, Kropp and Williams1991). Recruitment of Mediomastus sp. has been reported to occur during late summer followed by high mortality in winter (Sanders et al., Reference Sanders, Grassle, Hampson, Morse, Garner- Price and Jones1980; Grassle, Reference Grassle, Fischer and Pfannenstiel1984). Generally the representative of Mediomastus sp. lives within the top 2 cm of sediment in thin-walled, semi-permanent tubes that protrude several mm above the sediment surface. Juveniles of Mediomastus sp. were observed throughout the period with peak during PMonR and LSR. Therefore the presence of juveniles throughout the year indicates that Mediomastus sp. in the Mandovi estuary showed more or less continuous recruitment. Another capitellid, Heteromastus similis, also showed presence all year round (Leno & Bastida, Reference Leno and Bastida1998) and continuous reproduction is especially found in small-sized species. This strategy is present in most of the Spionidae and Capitellidae which are among the families showing the highest variability in egg size, developmental mode and reproductive strategies.
Minuspio cirrifera was dominant during the EMR in the area when all the other fauna were in low numbers or were absent. Increase in their abundance in response to disturbance has been related to factors such as decreased competition and predation, change in sediment texture, unstable bottom sediment and fluctuation in food (Sousa, Reference Sousa, Bertness, Gaines and Hay2001). The run-off from the land can cause sediment enrichment which forms additional food for the deposit feeding species like Minuspio cirrifera. As a result Minuspio cirrifera is the first species to colonize the benthic habitat in the Mandovi estuary, especially after the monsoonal disturbance, as the species responds rapidly to the defaunation and increased sedimentary organic matter. In marine benthic annelids three intermingled recruitment strategies and two survival patterns have been described. The range was from the classic opportunistic life style (Grassle & Grassle, Reference Grassle and Grassle1974) of large recruitment over a very short period of time followed by mass mortality, as shown by Minuspio cirrifera to prolonged recruitment with lower mortality, as shown by Mediomastus sp.
The climate of India has two major meteorological events: (1) the south-west monsoon which accounts for 75% of the annual rainfall; and (2) tropical cyclones (Ramesh Kumar, Reference RameshKumar2010). An alteration in these meteorological events can have disastrous effects on the ecosystem functioning. Human-induced global climate change has resulted in sea-level rise, ocean acidification and changes in ocean circulation (Solomon et al., Reference Solomon, Qin, Manning, Chen, Marquis, Averyt, Tignor and Miller2007). Climate-related changes have been evident in the Indian sub-continent from the increased occurrence of extreme weather events during the last few decades (Prasanna Kumar et al., Reference Prasanna Kumar, Roshin, Narvekar, Dinesh Kumar and Vivekanandan2009; Ramesh Kumar et al., Reference RameshKumar, Krishna, Sankar, Unnikrishnan and Pai2009; Unnikrishnan et al., Reference Unnikrishnan, Ramesh Kumar and Sindhu2011). The changes in the monsoonal circulation along with vertically integrated moisture transport and elevated sea surface temperature has weakened the SW monsoon and increased the incidence of prolonged monsoonal-break during the peak monsoon period (Ramesh Kumar et al., Reference RameshKumar, Krishna, Sankar, Unnikrishnan and Pai2009; Ramesh Kumar Reference RameshKumar2010; Unnikrishnan et al., Reference Unnikrishnan, Ramesh Kumar and Sindhu2011). Further, the increase in occurrence of intense tropical cyclones in the Arabian Sea is related to the disruption of the natural decadal sea surface temperature cycles (Prasanna Kumar et al., 2009).
The life cycle of tropical coastal fauna are so synchronized with monsoonal rain (Alongi, Reference Alongi1990), that changes in the rainfall pattern that are not in harmony with the natural temporal variation may have a negative impact on the benthic community (Przeslawski, Reference Przeslawski, Ahyong, Byrne, Wörheide and Hutchings2008; Grilo et al., Reference Grilo, Cardoso, Dolbeth, Bordalo and Pardal2011; Sivadas et al., Reference Sivadas, Ingole and Sen2012). For example, estuarine tropical organisms spawn during the monsoon season followed by recruitment during the relatively calm period, i.e. EMR and PMonR. The calm conditions are cues for the fauna to settle in the benthic system. Therefore, a sudden break period during the peak monsoon period may result in the untimely settling of the planktonic benthic larvae. However, once the monsoon resume, the sudden physical disturbances (riverine run-off, sediment instability and low salinity) will be deleterious to the newly settled benthos. The juveniles and sedentary fauna will be more susceptible to such physical stress compared to the mobile forms which are able to shift to suitable habitats. The constant physical disturbance during the most vulnerable phase of the benthic life may therefore have a detrimental impact on the community. Further, such changes will have a greater effect on the species with short planktonic stages with specific habitat preference compared to those with long planktrophic larvae which can prolong their settlement (Elkin & Marshall, Reference Elkin and Marshall2007). Moreover, changes in the ocean circulation pattern can also drift the larvae to unsuitable habitats, reducing their survival rate or causing recruitment failure. Therefore, although the tropical estuarine communities are adapted to the annual monsoonal disturbance, changes in the natural monsoon pattern along with extreme events (intense rainfall events, cyclones, flooding, turbidity and coastal run-off) may affect the recruiting population and overall distribution of the macrobenthos (Solomon et al., Reference Solomon, Qin, Manning, Chen, Marquis, Averyt, Tignor and Miller2007; Przeslawski, Reference Przeslawski, Ahyong, Byrne, Wörheide and Hutchings2008; Grilo et al., Reference Grilo, Cardoso, Dolbeth, Bordalo and Pardal2011).
CONCLUSION
The macrofaunal community in the Mandovi estuary showed temporal variation related to the environmental changes brought about by the SW monsoon. The monsoon regimes increase the food input in the system which favours the recruitment of benthic species. Further, it also ensures the wide dispersal of the sedentary benthic species. The sediment erosion has both negative (mortality) and positive (transport) effects on the faunal community. Thus, it can be concluded that MOR characterized by sudden changes in environment cause defaunation, migration and spawning of the macrofauna. During the PMR to PMonR, food is abundant in the form of organic matter, increased primary productivity and the calm conditions favour the recruitment process. The LSR is a more stable period and the community is represented by species typical of estuarine conditions. Therefore, the annual rainfall influences the macrobenthic assemblage structuring of the Mandovi estuary. Hence, climate-induced changes in the rainfall pattern may have a negative impact on the benthic fauna. Mandovi is an ecologically and economically important river for the state of Goa and understanding the natural variation of the system is necessary for delineating changes caused by human activities. Therefore, the results of such studies can help in the conservation and management of the biological resources of the important estuarine system.
ACKNOWLEDGEMENTS
The authors are grateful to the Director, NIO, for his encouragement. We are grateful to our colleagues Mr Sunder, Mr Michael, Dr M. Gauns, Dr P. Kessarkar and Indian Meteorological Department Goa for permission to use their data and the entire SIP project (SIP 1301 and 1308) team for all the help rendered during the field study. We also would like to thank Mrs Ramola Antao, English Professor, Goa University for language correction. The MS has benefited from the discussion with Dr M.R. Ramesh Kumar and useful suggestions of anonymous referees.
FINANCIAL SUPPORT
U.G. was supported by research grants from Department of Science, Environment and Technology (Government of Goa) and S.K.S. was supported by Council of Scientific and Industrial Research, (CSIR) Government of India through fellowship grants. This is contribution number 5328 of CSIR-NIO, Goa.