Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most prevalent psychiatric disorders with a childhood onset (Faraone et al. Reference Faraone, Biederman, Spencer, Wilens, Seidman, Mick and Doyle2000). Approximately 4–66% of cases persist into adulthood and affect an estimated 2–4% of the adult population (Simon et al. Reference Simon, Czobor, Balint, Meszaros and Bitter2009). Adult ADHD is mainly characterized by the symptoms of inattention and impulsivity, while the level of hyperactivity diminishes with increasing age. It has a major impact on social and academic functioning and on psychiatric co-morbidity (Barkley, Reference Barkley2002).
ADHD is thought to be a neurodevelopmental disorder; however, its exact neuroanatomy and pathophysiology, and its longitudinal course have not been fully revealed. The causes of changes in clinical symptom presentation as a function of age are also unknown. Structural and functional imaging studies in adults with ADHD, similarly to those in children and adolescents (Bush et al. Reference Bush, Valera and Seidman2005; Valera et al. Reference Valera, Faraone, Murray and Seidman2007), have suggested smaller volume and dysfunction of dorsal and ventral prefrontal, dorsal anterior cingular and inferior parietal gray matter; and alterations in white matter connections subserving working memory, attention and decision making (Makris et al. Reference Makris, Biederman, Valera, Bush, Kaiser, Kennedy, Caviness, Faraone and Seidman2007, Reference Makris, Buka, Biederman, Papadimitriou, Hodge, Valera, Brown, Bush, Monuteaux, Caviness, Kennedy and Seidman2008; Castellanos et al. Reference Castellanos, Margulies, Kelly, Uddin, Ghaffari, Kirsch, Shaw, Shehzad, Di, Biswal, Sonuga-Barke, Rotrosen, Adler and Milham2008; Ehlis et al. Reference Ehlis, Bahne, Jacob, Herrmann and Fallgatter2008). A 10-year longitudinal structural imaging study recruiting subjects with ADHD (age range: 5–19 years) showed, besides significantly smaller volumes, growth trajectories for all brain structures parallel for patients and controls (Castellanos et al. Reference Castellanos, Lee, Sharp, Jeffries, Greenstein, Clasen, Blumenthal, James, Ebens, Walter, Zijdenbos, Evans, Giedd and Rapoport2002), which is congruent with the developmental lag model of ADHD.
In contrast to imaging studies, the use of electrophysiological techniques, especially of event-related potentials (ERP) is quite rare in the literature when investigating the psychophysiological functioning of adults with ADHD. Electrophysiological techniques play an important role in exploring neural underpinnings of cognitive functions due to their functional relevance and high time resolution (Banaschewski & Brandeis, Reference Banaschewski and Brandeis2007). P300 (P3), the widely examined late ERP component, has been incriminated in various psychiatric disorders and has been supposed to reflect executive and attentional functions, including updating of working memory, event categorization, attentional resource allocation as well as attentional reorientation (Donchin & Coles, Reference Donchin and Coles1988; Polich, Reference Polich2007). These are all important cognitive domains in ADHD.
P3 has some variants which have been supposed to underlie different cognitive mechanisms and show different scalp distributions. The classic P3 as recorded in the traditional two-stimulus oddball paradigm (also called the go/nogo paradigm) corresponds to P3b that accompanies target detection in the three-stimulus oddball. This component shows a parietal scalp distribution and is thought to have neuroelectric generators in inferior parietal, temporal and right prefrontal regions (Polich, Reference Polich2007). P3a or novelty P3, another variant of P3, can be recorded after detection of a distractor (rare, unattended or salient stimuli) with a frontocentral maximum of amplitude, peaks 60–80 ms earlier than the P3b, and is hypothesized to have generators in the prefrontal, insular and superior parietal regions (Kiss et al. Reference Kiss, Dashieff and Lordeon1989; Smith et al. Reference Smith, Halgren, Sokolik, Baudena, Musolino, Liegeois-Chauvel and Chauvel1990; Kiehl et al. Reference Kiehl, Laurens, Duty, Forster and Liddle2001; Downar et al. Reference Downar, Crawley, Mikulis and Davis2002; Horovitz et al. Reference Horovitz, Skudlarski and Gore2002; Bledowski et al. Reference Bledowski, Prvulovic, Goebel, Zanella and Linden2004a, Reference Bledowski, Prvulovic, Hoechstetter, Scherg, Wibral, Goebel and Lindenb). P3 components, different from those previously mentioned, may be detected in tasks that require response inhibition or response control. Such components, Stop P3 (van Boxtel et al. Reference van Boxtel, van der Molen, Jennings and Brunia2001; Kok et al. Reference Kok, Ramautar, De Ruiter, Band and Ridderinkhof2004) and Nogo P3 (Fallgatter et al. Reference Fallgatter, Brandeis and Strik1997; Strik et al. Reference Strik, Fallgatter, Brandeis and Pascual-Marqui1998), have a fronto-central scalp distribution and generators, in part, in the anterior cingular cortex. The magnitude of classic P3 amplitude reflects the effort of attention allocation and correlates with the volume of gray matter contributing to wave generation (Ford et al. Reference Ford, Sullivan, Marsh, White, Lim and Pfefferbaum1994), while the latency of P3 indexes the processing speed of stimulus evaluation (Polich, Reference Polich2007). According to a general consensus, stimulus modality has no significant effect on P3 amplitude and latency or scalp topography, while shorter interstimulus intervals (ISI) produce smaller amplitudes than longer intervals (Key et al. Reference Key, Dove and Maguire2005; Polich, Reference Polich2007). However, it appears likely that ISI per se does not independently affect P3 amplitude, but this relationship may be attributable to the target-to-target interval (Gonsalvez et al. Reference Gonsalvez, Gordon, Grayson, Barry, Lazzaro and Bahramali1999).
The most robust findings in the extended P3 literature regarding children with ADHD show a decrease of amplitude and increase of latency (Barry et al. Reference Barry, Johnstone and Clarke2003). Despite the fact that P3 seems to be a promising endophenotype for ADHD (Doyle et al. Reference Doyle, Willcutt, Seidman, Biederman, Chouinard, Silva and Faraone2005), there is a scarcity of P3 studies in adult ADHD. Moreover, to date, the synthesis of these data has not been carried out. For this purpose, in our meta-analysis we investigated target-related P3 (or P3b) characteristics in adult ADHD patients as compared with control subjects, and examined whether P3 varies as a function of age and gender.
Method
Search strategy
We searched the Ovid Medline and PsycINFO databases applying the time limit from January 1994 to December 2009 as a search window for the relevant publications. Keywords were ‘ADHD’, ‘adult’, ‘adulthood’, ‘EEG’, ‘electroencephalography’, ‘ERP’, ‘evoked potential’, ‘event related potential’, ‘electrophysiology’, ‘psychophysiology’ and ‘neurophysiology’. Reference lists of identified papers were also reviewed. To be included, studies must have been written in English, contained both adult ADHD and matched healthy control groups, used DSM-IV criteria for ADHD diagnosis and must have presented target-related P3 data assessed in both groups. Two studies using the Stop Signal Task for obtaining Stop P3 component were excluded (Bekker et al. Reference Bekker, Overtoom, Kooij, Buitelaar, Verbaten and Kenemans2005; MacLaren et al. Reference MacLaren, Taukulis and Best2007), as this component fell outside the range of interest of the meta-analysis. Based on these inclusion and exclusion criteria, six studies were identified for the purpose of our meta-analysis.
Data extraction
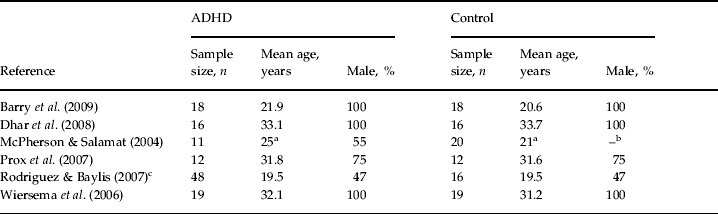
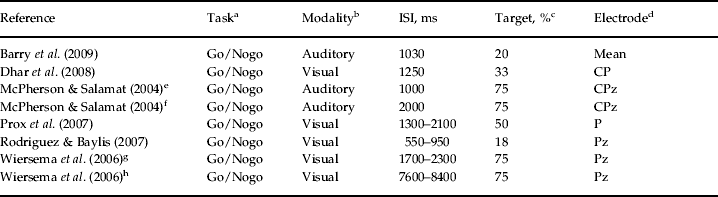
These six studies were heterogeneous regarding their methodology. They reported data that were acquired in different task conditions with regard to the neuropsychological paradigm that they used as well as to their stimulus modality, ISI or the ERP component. For the purpose of the current meta-analysis, an attempt was made to include those data from the individual publications that were deemed comparable across studies. With regard to ISI, the individual studies applied a broad time range (i.e. 550–8400 ms), with more than one ISI condition in two studies (McPherson & Salamat, Reference McPherson and Salamat2004; Wiersema et al. Reference Wiersema, van der Meere, Antrop and Roeyers2006). Though the length of ISI may have an impact on P3 characteristics, considering the small number of studies we included the data of two different ISI conditions from both studies (see below). Demographic and methodological data for each of the six studies are presented in Table 1 and Table 2.
Table 1. Patient demographics for subjects in studies included in the meta-analysis
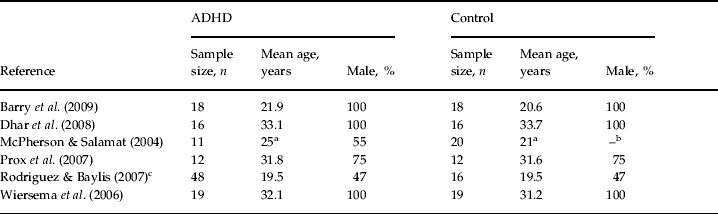
ADHD, Attention deficit hyperactivity disorder; Male, proportion of males in the sample.
a Modal age in an age range (19–31 years and 17–25 years, respectively).
b Data missing.
c Rodriguez & Baylis (Reference Rodriguez and Baylis2007)presented only data of the total sample for mean age and gender ratio.
Table 2. Electrophysiological and methodological data for studies included in the meta-analysis
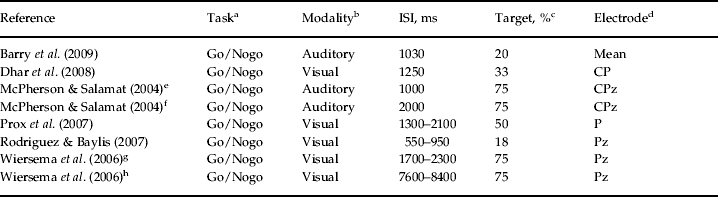
ISI, Interstimulus interval; Go/Nogo, a type of two-stimulus oddball paradigm; Mean, across-sites mean from nine frontal, central and parietal electrode sites; CP, CPz, P, Pz, different central–parietal and parietal electrode sites; P3, P300.
a Neuropsychological task used in the study for eliciting P3.
b Stimulus modality in the task.
c Probability of the target stimulus in the task.
d Electrode site on the scalp for the registration of P3 activity.
e,f Different task conditions with two different interstimulus intervals of 1000 and 2000 ms in the study of McPherson & Salamat (Reference McPherson and Salamat2004).
g,h Different task conditions with two different interstimulus intervals (fast: 1700–2300 ms; slow: 7600–8400 ms) in the study of Wiersema et al. (Reference Wiersema, van der Meere, Antrop and Roeyers2006).
In particular, Barry et al. (Reference Barry, Clarke, McCarthy, Selikowitz, Brown and Heaven2009) obtained several auditory target- and visual non-target-related ERP components in an inter-modal go/nogo paradigm. We included the target-related P3 data. Dhar et al. (Reference Dhar, Been, Minderaa and Althaus2008) used a visual continuous performance task which was cued on two-thirds of the trials and they analysed valid and invalid cued target-related N2 and P3 components. Since they did not report numerical data, we estimated the mean amplitude of the invalid cued target-related P3 components, which was included for the meta-analysis. For this purpose, we made area-based measurements manually in the interval from 300 to 500 ms on the grand averages for P3 at CP3 and CP4 electrode sites in left and right invalid conditions presented in the paper of Dhar et al. (Reference Dhar, Been, Minderaa and Althaus2008) . McPherson & Salamat (Reference McPherson and Salamat2004) reported both P3a and P3b data acquired in an auditory go/nogo paradigm with three different ISI conditions (1 s, 2 s and 4 s). We included the P3b data with both ISI1 and ISI2 conditions (i.e. ISI 1 s and 2 s). Prox et al. (Reference Prox, Dietrich, Zhang, Emrich and Ohlmeier2007) obtained, among others, P3 components in a visual go/nogo paradigm, but did not report numerical data. Upon our request, for the purpose of the current meta-analysis, they kindly provided the α error (‘p value’) for P3/P4 electrodes (i.e. p=0.267), which we used to derive an estimate for effect size for the meta-analysis. Rodriguez & Baylis (Reference Rodriguez and Baylis2007) reported P3a and P3b data acquired in a visual go/nogo paradigm with respond-to-target and suppress-to-target conditions. We included P3b data from the respond-to-target condition. Wiersema et al. (Reference Wiersema, van der Meere, Antrop and Roeyers2006) reported P3 data acquired in a visual go/nogo paradigm with two different ISI conditions (1700–2300 ms and 7600–8400 ms). We included P3 data with both the fast and slow conditions.
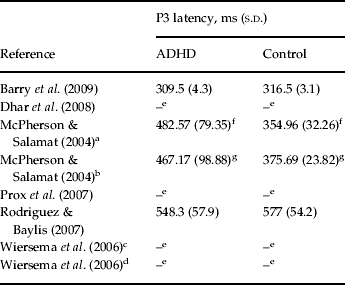
We should note that due to lack of sufficient data, we were not able to conduct the analysis of performance and P3 latency data of studies included in the meta-analysis. For a review of available data, see Tables 3 and 4.
Table 3. Performance data for studies included in the meta-analysis
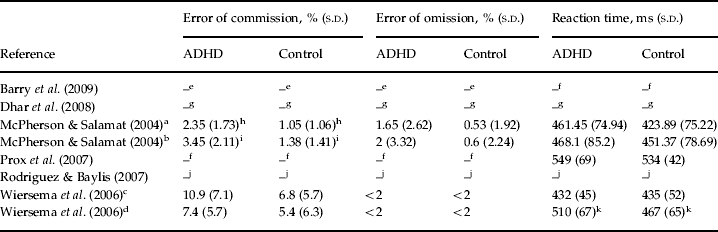
ADHD, Attention deficit hyperactivity disorder; s.d., standard deviation.
a,b Different task conditions with two different interstimulus intervals of 1000 and 2000 ms in the study of McPherson & Salamat (Reference McPherson and Salamat2004).
c,d Different task conditions with two different interstimulus intervals (fast: 1700–2300 ms; slow: 7600–8400 ms) in the study of Wiersema et al. (Reference Wiersema, van der Meere, Antrop and Roeyers2006).
e Barry et al. (Reference Barry, Clarke, McCarthy, Selikowitz, Brown and Heaven2009)did not report numerical data, but described high performance accuracy for both ADHD and control groups without significant difference between groups.
f Data missing.
g Dhar et al. (Reference Dhar, Been, Minderaa and Althaus2008)did not report numerical data. They found a significant effect for the factor ADHD with regard to percentage of errors (p=0.047, i.e. more inaccurate), reaction time (p=0.038, i.e. slower) and variability of reaction time (p=0.004, i.e. more variable) using analysis of variance.
h Patients with ADHD had significantly more errors of commission (p=0.001).
i Patients with ADHD had significantly more errors of commission (p<0.001).
j Rodriguez & Baylis (Reference Rodriguez and Baylis2007)calculated d' as a measure of signal sensitivity using the theory of signal detection. They found that the control group had significantly more signal sensitivity than the ADHD group (p=0.0005). Regarding reaction time, no significant difference was found.
k Patients with ADHD had significantly longer reaction time (p<0.008).
Table 4. P3 latency data for studies included in the meta-analysis
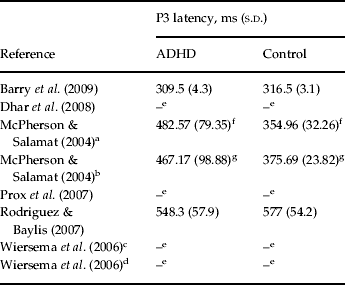
P3, P300; s.d., standard deviation; ADHD, attention deficit hyperactivity disorder.
a,b Different task conditions with two different interstimulus intervals of 1000 and 2000 ms in the study of McPherson & Salamat (Reference McPherson and Salamat2004).
c,d Different task conditions with two different interstimulus intervals (fast: 1700–2300 ms; slow: 7600–8400 ms) in the study of Wiersema et al. (Reference Wiersema, van der Meere, Antrop and Roeyers2006).
e Data missing.
f Patients with ADHD had significantly longer P3 latency (p<0.001).
g Patients with ADHD had significantly longer P3 latency (p<0.001).
Statistical analyses
Pooled effect size (Cohen's d) was calculated across the studies to define the differences between the adult ADHD and the control groups. Cohen's d was defined as the difference between two means divided by the pooled within-group standard deviation of both groups. The closer the Cohen's d approaches zero, the smaller the difference between the two groups. We consider absolute values of Cohen's d between 0.20 and 0.39 as small, between 0.40 and 0.69 as medium, and from 0.70 as large effect sizes.
A random effect meta-regression analysis was applied to estimate the pooled effect size of the difference between the ADHD and control groups across various studies and in order to investigate the association between the effect size in relation to age and gender. The meta-regression analysis was based on van Houwelingen et al.'s (Reference van Houwelingen, Arends and Stijnen2002) general linear mixed-model technique using the approximate likelihood approach. In particular, the effect size from each study was regressed on an intercept and on study-level demographic covariates, which included mean age (years) and gender composition (% of males) in each of the individual studies. A common weighted statistical effect-size estimate for the ADHD group versus control group difference was calculated using the DerSimonian–Laird estimator (DerSimonian & Laird, Reference DerSimonian and Laird1986), based on the random effect component of the mixed model that incorporated both fixed and random effects.
Results
Across the six studies (two of them with two arms) included in the meta-analysis there were a total of 154 ADHD and 140 control subjects matched according to age and gender. The mean age and age range were 27.6 (s.d.=5.3) and 17–57 years for the ADHD and 26.2 (s.d.=6.2) and 17–57 years for the control subjects, respectively. The mean percentages of males in the ADHD and control groups were 78.9% and 87%, respectively.
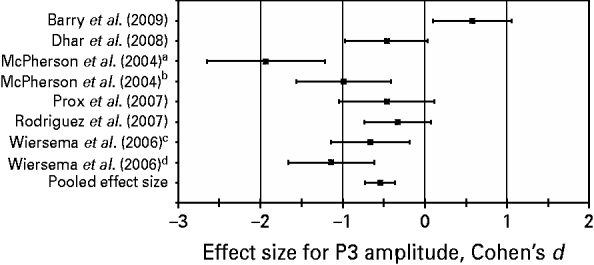
Results of the meta-regression analysis of the P3 amplitude across studies indicated that the medium pooled effect size in terms of Cohen's d was −0.55 [p=0.0006, 95% confidence interval (CI)=−0.76 to −0.33]. The negative value of the estimate of the effect size (with a 95% CI that excludes zero) indicates that overall compared with the control subjects, the adult ADHD patients show a statistically significant decrease in P3 amplitude during target detection. Effect sizes for individual studies, ranging from d=−1.93 to d=0.58, and the pooled effect size are presented in Fig. 1. We note that a positive effect size was found in only one of the studies.
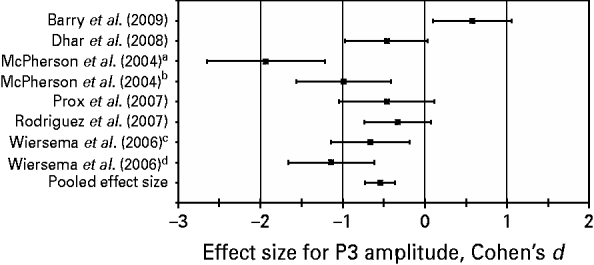
Fig. 1. Effect sizes (Cohen's d, 95% confidence intervals) for P300 (P3) amplitude in individual studies and pooled effect size for P3 amplitude estimated across studies. A Cohen's d below zero stands for a decreased mean P3 amplitude in the attention deficit hyperactivity disorder group as compared with the control group. a,b,c,d Regarding the studies of McPherson et al. (Reference McPherson and Salamat2004) and Wiersema et al. (Reference Wiersema, van der Meere, Antrop and Roeyers2006), different effect sizes are depicted for different interstimulus interval conditions of each study [1000 ms (a), 2000 ms (b), 1700–2300 ms (c), and 7600–8400 ms (d), respectively].
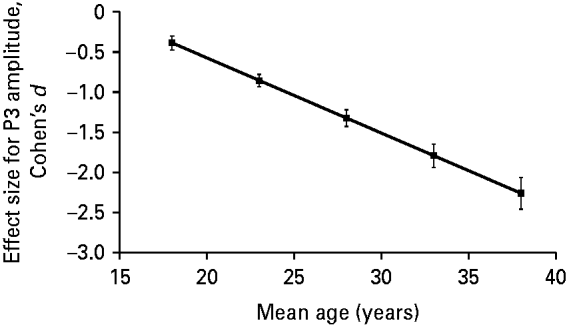
In addition to the estimation of the effect size across studies, we investigated whether the effect size for the group difference in terms of P3 amplitude varied across studies as a function of age and gender. Results of the meta-regression analysis revealed a significant association (β=−0.094, 95% CI=−0.151 to −0.036, t=−4.18, p=0.0087) between the effect size for P3 amplitude and the mean age of ADHD patients in an individual study. We present this association in Fig. 2 after adjusting data for an equal proportion of genders in the sample using meta-regression analysis. As indicated, the higher the mean age of adult ADHD patients, the more negative (i.e. the higher in absolute value) the effect size for P3 amplitude.
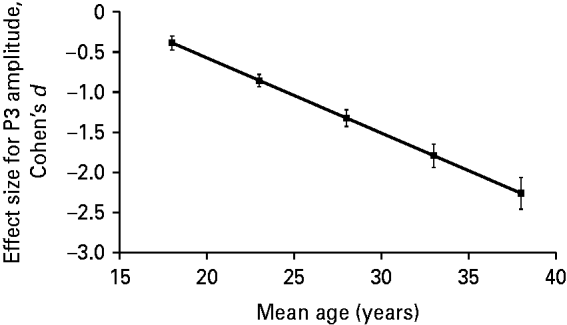
Fig. 2. Profile plot for the relationship between effect size (Cohen's d) for P300 (P3) amplitude and the mean age of attention deficit hyperactivity disorder (ADHD) patients adjusted for an equal proportion of genders in the sample using meta-regression analysis. Meta-regression analysis showed a significant negative relationship between the effect size for P3 amplitude and the mean age of ADHD patients in a given study (β=−0.094, t=−4.18, p=0.0087). The Pearson correlation coefficient for the above association, weighted for study size and adjusted for difference in gender composition across studies, was −0.67.
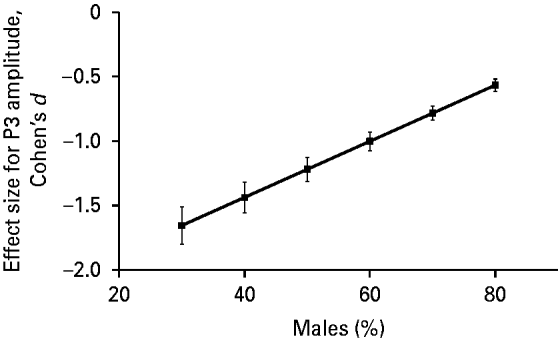
The association between the effect size for P3 amplitude and gender composition (percentage of males) of the ADHD group in a particular study was also statistically significant (β=0.022, 95% CI=0.008–0.036, t=4.02, p=0.01). We present this association in Fig. 3 after adjusting the data for the mean age of the sample using meta-regression analysis. Fig. 3 indicates that the higher the percentage of males in the ADHD group in a particular study, the less negative (i.e. the smaller in absolute value) the effect size for P3 amplitude.
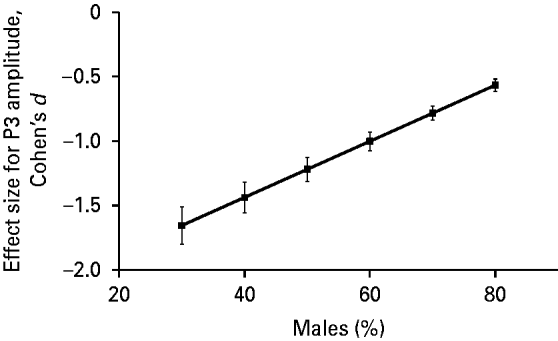
Fig. 3. Profile plot for the relationship between effect size (Cohen's d) for P300 (P3) amplitude and gender composition (% male) of attention deficit hyperactivity disorder (ADHD) group adjusted for mean age of the sample using meta-regression analysis. Meta-regression showed a significant association between the effect size for P3 amplitude and gender composition of the ADHD group in a given study (β=0.022, t=4.02, p=0.01). The Pearson correlation coefficient for the above association, weighted for study size and adjusted for difference in gender composition across studies, was 0.60.
Discussion
To our knowledge, this is the first meta-analysis of P3 characteristics in adults with ADHD. The principal finding of the investigation is that adult patients with ADHD had significantly reduced P3 amplitude across studies as compared with controls. Accordingly, our findings are similar to those reported for patients in childhood, which consistently showed that patients with ADHD have significantly reduced P3 amplitude during target detection. Additionally, as our meta-regression analysis revealed, such a reduction in P3 amplitude becomes more and more expressed with increasing age. A significant association between a lower percentage of males in the sample and the degree of abnormality in P3 amplitude was also identified.
Our main finding may be explained, at least in part, by a dysfunction of the ventral attention network in adults with ADHD. Specifically, as mentioned above, target-related P3 is thought to reflect the activation of cortical areas, particularly that of the temporoparietal junction, which can be associated with the functioning of the ventral attention network (Corbetta et al. Reference Corbetta, Patel and Shulman2008). There is evidence that the sources of input signals into this network are the prefrontal and anterior cingular cortical areas, directly or indirectly through their projection via the locus coeruleus–norepinephrine system (LC-NE) (Shulman et al. Reference Shulman, McAvoy, Cowan, Astafiev, Tansy, d'Avossa and Corbetta2003; Nieuwenhuis et al. Reference Nieuwenhuis, Aston-Jones and Cohen2005). Therefore, the activation of the ventral attention network can be conceptualized as a later phase of event processing. It follows, and may be triggered by, early sensory and decision-making processes in prefrontal areas, and in turn results in an interaction with and facilitation of the dorsal attention network when attention is switched or reoriented to behaviorally relevant stimuli. This interpretation is consistent with recent theories of the role of phasic LC-NE activity, which is proposed to facilitate behavior ensuing a task-related decision and to help optimize task performance (Aston-Jones & Cohen, Reference Aston-Jones and Cohen2005; Bouret & Sara, Reference Bouret and Sara2005; Dayan & Yu, Reference Dayan and Yu2006).
Hence, it is conceivable that the reduced P3 amplitude during target detection in adults with ADHD may reflect a dysfunction of the ventral attention network. Currently it is not clear whether such a dysfunction would represent a consequence of a frontal lobe abnormality or occur primarily from an impairment in the temporal and/or parietal cortices, which have so far received very little attention in the pathogenesis of ADHD (Cherkasova & Hechtman, Reference Cherkasova and Hechtman2009). However, our finding is consistent with the frontal and temporoparietal cortical gray and white matter abnormalities detailed above, and may in turn underlie the aforementioned abnormalities in working memory, attention, and decision-making functions detected in adult patients with ADHD. Deficits in these domains of cognition may play a particularly important role in the clinical symptoms of inattention, distractibility and impulsivity.
Nevertheless, besides a dysfunction of the ventral attention network per se, a decreased target-related P3 amplitude may also reflect altered neural mechanisms underlying task performance of adults with ADHD. Fundamental research shows that task difficulty strongly affects P3 amplitude (Kok, Reference Kok2001). Increased task requirements increase memory load, which may interfere with attentional processes underlying P3 generation and result in decreased P3 amplitude in normal subjects. Regarding adults with ADHD, it is conceivable that due to attentional dysfunction, they exhibit an increased effort to successfully perform a task not only with increased task difficulty but under default conditions. This increased effort and probably the recruitment of additional brain areas may be reflected in decreased target-related P3 amplitude. Consistent with this hypothesis and similar to other studies, there was no close relationship between the physical measures of task performance and P3 characteristics in the studies included. Despite the fact that five of six studies reported a significantly decreased P3 amplitude for ADHD patients, only three identified significantly more errors or less signal sensitivity (McPherson & Salamat, Reference McPherson and Salamat2004; Rodriguez & Baylis, Reference Rodriguez and Baylis2007; Dhar et al. Reference Dhar, Been, Minderaa and Althaus2008) and two (Wiersema et al. Reference Wiersema, van der Meere, Antrop and Roeyers2006; Dhar et al. Reference Dhar, Been, Minderaa and Althaus2008) described larger reaction time for the ADHD group. While due to the small number of studies with published data, we could not conduct a formal meta-analysis of performance data (see Method section), the aforementioned findings might support the idea that reduced P3 amplitude is an independent psychophysiological indicator of attentional disturbances of adults with ADHD.
We found a significant association between age in adults with ADHD and the degree of abnormality of P3 amplitude. In healthy individuals, following a typical increase in childhood (Taylor, Reference Taylor1988; Johnstone et al. Reference Johnstone, Barry, Anderson and Coyle1996), P3 amplitude to target stimuli decreases with age (van der Stelt et al. Reference van der Stelt, Kok, Smulders, Snel and Boudewijn1998; Schiff et al. Reference Schiff, Valenti, Andrea, Lot, Bisiacchi, Gatta and Amodio2008). Results of our meta-analysis suggest that this decrease in P3 amplitude is more pronounced in adult ADHD patients. While the full functional significance of this finding is unclear, we think that this result may be interpreted in a neurodevelopmental context.
In particular, brain maturation in healthy children and adolescents is accompanied by myelinization and synaptic pruning. It has been shown that the time-course of these parallel processes varies by brain region and leads to cortical thinning (Toga et al. Reference Toga, Thompson and Sowell2006). Some data suggest that these changes continue through puberty into young adulthood (Sowell et al. Reference Sowell, Peterson, Thompson, Welcome, Henkenius and Toga2003). In children and adolescents with ADHD, longitudinal studies found a developmental delay in brain maturation. In a 10-year longitudinal structural imaging study, Castellanos et al. (Reference Castellanos, Lee, Sharp, Jeffries, Greenstein, Clasen, Blumenthal, James, Ebens, Walter, Zijdenbos, Evans, Giedd and Rapoport2002) showed that besides smaller volumes in all regions, growth trajectories for all brain structures were parallel for patients and controls. Additionally, a normalization of caudate volume by mid-adolescence occurred, which can be associated with the decrease of hyperactive symptoms with increasing age. Examining cortical thickness, Shaw et al. (Reference Shaw, Eckstrand, Sharp, Blumenthal, Lerch, Greenstein, Clasen, Evans, Giedd and Rapoport2007) reported a delay of 3–5 years in regional cortical maturation of children with ADHD compared with controls. Stanley et al. (Reference Stanley, Kipp, Greisenegger, MacMaster, Panchalingam, Keshavan, Bukstein and Pettegrew2008) found increased level of phosphomonoesters in the right parietal lobe in 8- to 10-year-old children with ADHD, which has been considered as a sign of decreased synaptic pruning. In addition, in a 5-year follow-up study, children with ADHD in full remission showed normalization of cortical thickness in the right parietal cortex, but not in any other region (Shaw et al. Reference Shaw, Lerch, Greenstein, Sharp, Clasen, Evans, Giedd, Castellanos and Rapoport2006). Whilst these data suggest a possible association between regional brain volumes and the severity and time-course of ADHD, we note that these longitudinal studies recruited patients with a narrow range of age in childhood and adolescence.
Thus, due to lack of longitudinal imaging studies of subjects with ADHD that continue into adulthood, the pattern of developmental trajectories for brain regions of those in their 20s and 30s is unclear. Considering Sowell et al.'s (Reference Sowell, Peterson, Thompson, Welcome, Henkenius and Toga2003) finding of developmental changes that continue into young adulthood, it can be hypothesized that, similar to certain childhood cases ‘outgrowing’ ADHD, abnormal brain regions of adults with ADHD, particularly the temporoparietal junction, show improvement and delayed normalization with age. Nonetheless, our finding that the effect size for P3 increases with age is not in line with the idea of delayed normalization. It is conceivable that patients with ADHD that persists into adulthood may neurodevelopmentally differ from those with full remission in childhood or adolescence. Therefore, our finding of the association between effect size for P3 amplitude and increasing age of ADHD patients underlines the need for longitudinal studies. Overall, the use of the ERP approach that we focused on in this investigation has the advantage of measuring the dynamics of the brain with high time resolution. In particular, this technique adds unique information about brain functionality that cannot be captured by other methods, including structural or functional MRI (Banaschewski & Brandeis, Reference Banaschewski and Brandeis2007).
Our meta-analysis revealed a significant association between female gender and a more pronounced reduction in target-related P3 amplitude in adults with ADHD as compared with controls. This finding may be due to different associations between P3 characteristics and female gender in healthy versus ADHD subjects. Specifically, most of the studies dealing with the gender effect on P3 amplitude in healthy subjects report higher amplitudes for women, as compared with men (Hoffman & Polich, Reference Hoffman and Polich1999; Steffensen et al. Reference Steffensen, Ohran, Shipp, Hales, Stobbs and Fleming2008). Therefore, an increased proportion of females in the sample could result in an increase of P3 amplitude in healthy controls, and would underlie the increased effect size if such an association with increasing age is not manifested in ADHD subjects. Further studies are needed to investigate this possibility. Alternatively, our finding may be attributable to the fact that some of the female ADHD subjects who were not referred to treatment in childhood due to the lack of disruptive behavior (which is typical of boys) refer themselves to treatment in adulthood, in many cases due to co-morbid psychiatric diseases (Biederman et al. Reference Biederman, Mick, Faraone, Braaten, Doyle, Spencer, Wilens, Frazier and Johnson2002; Gershon, Reference Gershon2002). These adult females without prior treatment may compose a group of patients with poorer prognosis and persisting structural brain pathology. Therefore, the involvement of such female ADHD patients in the original studies may increase the group difference in terms of P3 amplitude. Clearly, further studies are needed for the better understanding of this possible association between gender and P3 characteristics in adult ADHD.
Certain limitations of our meta-analysis have to be mentioned. Principal limitations include the small number of studies, the relatively small number of subjects enrolled in the individual studies and the heterogeneous methodology, especially the fact that the P3 paradigms were distributed across the visual and auditory modalities (which, due to the small number of studies, precluded a formal analysis by modality). Whereas these limitations are expected to reduce statistical power for the analyses, it is noteworthy that our investigation yielded a statistically significant effect size with regard to the main question of the effect size for P3 amplitude between subjects with ADHD and healthy controls. Furthermore, we identified significant associations for potentially important demographic variables such as age and gender. Since the cross-sectional design used in the individual studies may be a source of bias, we think that longitudinal investigations are warranted to confirm the above associations. Finally, due to lack of sufficient data, we were not able to conduct the analysis of performance and P3 latency data, which could have provided further information on attentional functioning in adults with ADHD. Such analysis in future studies will be instrumental in resolving fundamental issues, including whether reduction of P3 amplitude is related to an increased latency variability in ADHD (Kok, Reference Kok2001), and whether in general it can be conceived as an independent psychophysiological indicator of attentional disturbances in adults.
Nonetheless, our meta-analytic results underline the relevance of the decreased target-related P3 amplitude in the understanding of the pathophysiology of adult ADHD. Our findings support the idea that the dysfunction of attentional processes related to target detection, fundamental in children with ADHD, persists in adult patients and may be associated with age. As P3 has also been related to other psychiatric disorders (Bramon et al. Reference Bramon, Rabe-Hesketh, Sham, Murray and Frangou2004; Schulze et al. Reference Schulze, Hall, McDonald, Marshall, Walshe, Murray and Bramon2008) and may be associated with externalizing psychopathology in general (Gilmore et al. Reference Gilmore, Malone and Iacono2010), it does not seem to be specific for ADHD. However, it is supposed to be a marker of the dysfunction of target-related attentional processes that are secondary in other disorders, but primary in ADHD. Additionally, it may provide unique information about attention-related brain dynamics that cannot be captured by other structural or functional imaging techniques. Hence, the P3 ERP component may be a promising target in ADHD research in order to reveal the more exact nature of the deficit of underlying attentional networks and can play an important role in understanding ADHD from a life span perspective.
Declaration of Interest
None.