In general, two whey waste streams originate from dairy processing with notable compositional differences in terms of lactose, minerals, proteins and lactic acid as well as pH levels. Sweet whey, a by-product of the rennet type cheese making, has a pH of 6–6·5, while acid whey, originating from the production of acid coagulated dairy products such as soft cheese and Greek yogurt, has a pH of 4·2–4·5 and contains higher levels of lactose and minerals, lower levels of proteins and, more importantly, more lactic acid (Fischer & Kleinschmidt, Reference Fischer and Kleinschmidt2015). The sweet whey stream is mainly processed downstream by concentrating and spray drying to obtain a whey powder that can be used as a food ingredient in many food products (de Wit, Reference de Wit2001). However, this process has not been applied successfully for acid whey processing due to failure of lactose to crystallise properly which in turns creates problems downstream such as lumping and caking of whey powders.
Proper crystallisation of lactose depends on a number of factors including degree of supersaturation, solution temperature, viscosity, concentration of lactose, presence of minerals and organic acids, and pH (Herrington, Reference Herrington1934). The presence of minerals in lactose solutions may improve or reduce the crystallisation rate depending on their concentration and type (Jelen & Coulter, Reference Jelen and Coulter1973). Furthermore, the presence of lactic acid (LA) and Ca directly affects the behaviour of lactose, hindering the removal of water surrounding lactose molecules by inducing a formation of a strong hydration layer and thereby inhibiting the overall crystallisation (Wijayasinghe et al. Reference Wijayasinghe, Vasiljevic and Chandrapala2015). Furthermore, pH was found to be an important factor in lactose crystallisation due to acceleration of lactose mutarotation under high alkaline conditions (Herrington, Reference Herrington1934). In contrast, Jelen & Coulter (Reference Jelen and Coulter1973) found that changes in pH have minor contributions in the overall lactose crystallisation process and Bhargava & Jelen (Reference Bhargava and Jelen1996) found that the changes in pH did not correlate with changes in growth rates of lactose crystals. Due to these contradictory statements, it was vital to establish the effects of the presence of LA and Ca on the kinetics of lactose crystallisation as affected by pH. Thus, a greater understanding of this phenomenon may assist in enabling efficient extraction of lactose from the acid whey waste stream and thus improve its valorisation.
Materials and methods
Materials
Commercial food grade lactose was obtained from Murray Goulburn Co-operative Co. Ltd (Brunswick, Australia). An analytical grade 85% w/w lactic acid (LA) solution and calcium chloride (CaCl2) were obtained from Sigma – Aldrich Pty. Ltd. (Castle Hill, NSW, Australia).
Preparation of solutions
Lactose powder was dissolved in Milli-Q water at 55 °C to obtain a clear 45% w/w lactose solution. Four solutions were made up with necessary amounts of LA and Ca with the use of the LA solution and a 2·4% w/w stock CaCl2 solution. The first solution was prepared with the addition of 0·05% w/w LA plus 0·035% w/w Ca to resemble the sweet whey waste stream. Second, third and fourth solutions were prepared with the addition of 1% w/w LA and 0·12% w/w Ca having pH values of 4·8, 3·0 and 6·5, respectively. The pH adjustments were carried out using 0·1 m HCl and NaOH as necessary. Pure lactose solution was treated as the control.
Concentration
Concentration of the lactose solutions was performed using a rotary evaporator to achieve ~50 ± 5% w/w total solids. A laboratory refractometer (Atago abbe, Tokyo, Japan) was used for concentration determinations.
Crystallisation of lactose
Concentrated solutions were subjected to crystallisation using the method adapted by Chandrapala et al. (Reference Chandrapala, Wijayasinghe and Vasiljevic2016). The next day, lactose crystals were separated using 0·45 µm filter papers and were oven dried at 80 °C for 2 h.
 $${\rm \%} \;{\rm Yield} \,=\, \displaystyle{\matrix{{\rm Mass}\;{\rm of}\;{\rm lactose}\;{\rm crystal}\;{\rm obtained} \cr \quad (({\rm g}/100\;{\rm g}){\rm water}) \hfill} \over \matrix {{\rm Maximum\;} \;{\rm theoratical}\;{\rm crystal\;} \;{\rm yield}\; \cr \hskip-3.5pc(({\rm g/}100\;{\rm g}){\rm water})} } \times 100$$
$${\rm \%} \;{\rm Yield} \,=\, \displaystyle{\matrix{{\rm Mass}\;{\rm of}\;{\rm lactose}\;{\rm crystal}\;{\rm obtained} \cr \quad (({\rm g}/100\;{\rm g}){\rm water}) \hfill} \over \matrix {{\rm Maximum\;} \;{\rm theoratical}\;{\rm crystal\;} \;{\rm yield}\; \cr \hskip-3.5pc(({\rm g/}100\;{\rm g}){\rm water})} } \times 100$$
Freeze-drying of lactose solutions
All samples were frozen at −20 °C for 24 h and then freeze-dried (Dynavac FD 300, Dynavac Engineering Pty, Australia) for 72 h.
Differential scanning calorimetry (DSC)
Method by Wijayasinghe et al. (Reference Wijayasinghe, Vasiljevic and Chandrapala2015) was adapted to measure the thermal characteristics of concentrated, freeze dried and lactose crystals using the DSC machine (Mettler Toledo Schwerzenbach, Switzerland).
Fourier transform infrared spectroscopy (FTIR)
The FTIR spectra of concentrated solutions and lactose crystals were obtained in the range of 600–4000 cm−1 using Fourier transform infrared spectroscopy as described by Wijayasinghe et al. (Reference Wijayasinghe, Vasiljevic and Chandrapala2015).
Statistical analysis
The whole experimental design was replicated two times. When necessary, one-way/two-way ANOVA with a 95% confidence interval was used. The ANOVA data with P < 0·05 were considered statistically significant.
Results and discussion
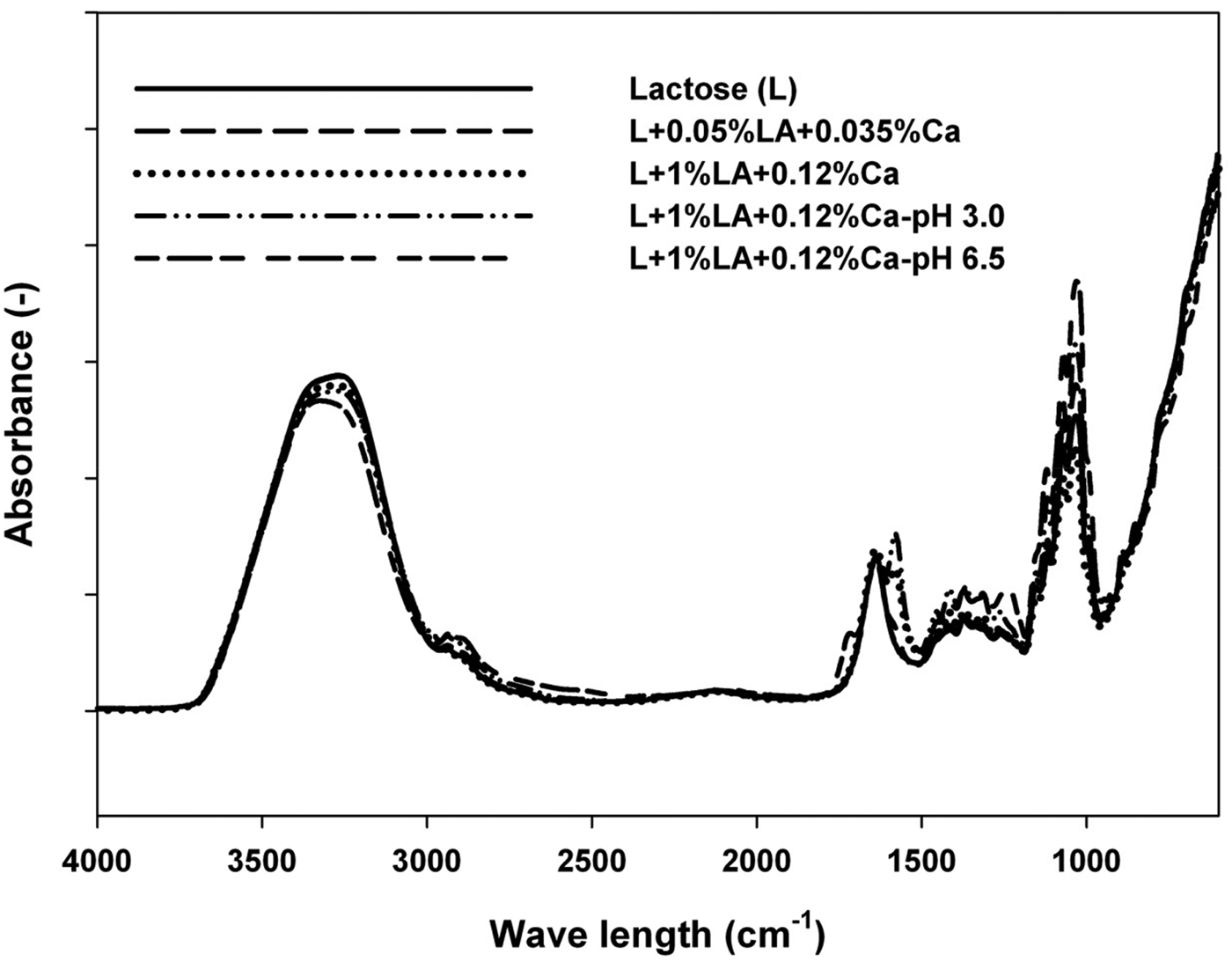
The pure lactose sample resulted in ~84% yield of lactose crystals (Table 1). In contrast, this yield was significantly reduced to ~55% in the presence of 1% w/w LA and 0·12% w/w Ca. This was concomitantly followed with the rise of the crystallisation temperature (T cr), up from ~166 °C, which was determined for the pure lactose solution, to 189 °C in the presence of high concentrations of LA and Ca (Table 1) indicating a delay in crystallisation. In addition, a substantial decline in the enthalpy associated with the loss of water (~163 J/g) in the same sample as compared to that of pure lactose (~1110 J/g) was observed (Table 1). Highly hygroscopic LA strongly interacts with water molecules, leading to a formation of a strong hydration layer consisting lactic acid and H3O+ ions around lactose molecules via strong H bonds (Wijayasinghe et al. Reference Wijayasinghe, Vasiljevic and Chandrapala2015). In addition, Ca2+ has the ability to organise the structure of water (Bhargava & Jelen, Reference Bhargava and Jelen1996). Divalent Ca2+ ion has a strong electric field which acts as a water structure promoter (Von Hippel & Schleich, Reference Von Hippel and Schleich1969). These structural changes of water molecules can directly affect the solubility of lactose and thereby the supersaturation of the lactose solution and thus lead to different behaviour during crystallisation (Mullin, Reference Mullin, Jancic and de Jong1979). Furthermore, Ca2+ ions strongly interact with four to six layers of water molecules via ion-dipole interactions (Reid & Fennema, Reference Reid, Fennema, Damodaran, Parkin and Fennema2008), which again further restricts the mobility of water molecules. Such an impact on the water mobility was confirmed by decreased intensity of the H bonding region between different hydration states (2800–3500 cm−1) (Raut et al. Reference Raut, Allada, Pavan, Deshpande, Patil, Patil, Deshmukh, Sakharkar, Bodkel and Mahajan2011). In addition, bending vibrations of OH groups of water denoted by a peak at ~1630 cm−1 (Nikanishi, Reference Nikanishi1962), frequently observed for the pure lactose (Fig. 1), was accompanied with an appearance of another peak at ~1570 cm−1 in the presence of 1% w/w LA and 0·12% w/w Ca which additionally indicated different water behaviour governed by these two solutes.

Fig. 1. FTIR spectra of concentrated lactose solutions.
Table 1. pH, crystal yield, onset, peak and end set temperatures and enthalpy associated with loss of water in concentrated lactose solutions and the crystallisation temperatures of freeze dried lactose samples as affected by the presence of LA and Ca and pH

All Values are means of 3 independent observations (n = 3); the results are presented as means ± standard deviation (sd).
Reducing the concentration of LA from 1% w/w to 0·05% w/w and Ca from 0·12 w/w to 0·035% w/w decreased the yield of lactose crystals slightly (~76%) along with a modest decline in the enthalpy associated with the loss of water (~992 J/g) (Table 1). The diminished strength of the hydration layer consisting of H3O+ and lactic acid minimised the formation of lactic acid-water complexes, decreased the impact of Ca on the solubility of lactose and enhanced attractions between individual lactose molecules and thus consequently improved water mobility, which all may have sequentially resulted in an improved yield. Similarly, FTIR spectra were characterised by the peaks identical to those of the pure lactose.
When pH reduced down to 3, the yield of lactose crystals was reduced slightly (52%) in the presence of 1% w/w LA and 0·12% w/w Ca compared to the same system (55%) without pH adjustment, which had pH of 4·81. At this low pH and in the presence of high concentrations of LA and Ca, lactose was characterised with the lowest dehydration enthalpy (~151 J/g), indicating retention of an elevated amount of water. FTIR spectra indicated slight changes in the OH bending vibrations of water at pH 3 indicated by increased intensity of a peak at ~1570 cm−1 and decreased intensity of a peak at 1630 cm−1. In addition, under the same conditions FTIR showed changes in C–C/C–H aliphatic, C–CH3 aliphatic stretching vibrations of LA and C–C/C–O/C–H ring stretching vibrations of lactose (Pamula et al. Reference Pamula, Blazewicz, Paluszkiewicz and Dobrzynski2001) denoted by high intensity peaks in a region between 1180 to 1500 cm−1. At this pH, below its pK a, LA is present largely in its undissociated form, which is very hygroscopic and might have influenced the observed changes.
Increasing pH to 6·5 at the same high concentrations of LA and Ca resulted in significantly increased yield (~71%) and dehydration improved immensely (Table 1). These results indicate that lactose molecules moved more freely and were able to position better to form crystals, while at the same time more water was evaporated at these high concentration of impurities. Even T cr has a similar value to that of the pure lactose (Table 1). In the present study, lactose monohydrate was considered in a 100% crystalline form which was measured by subtracting the heat of crystallisation from the heat of fusion (Wijayasinghe et al. Reference Wijayasinghe, Vasiljevic and Chandrapala2015). Following this approach, the freeze dried sample of the pure lactose solution consisted of ~50% crystalline lactose. This crystallinity was substantially reduced in the presence of 1% w/w LA and 0·12% w/w Ca at unadjusted pH as it contained only ~25% crystalline lactose. However, by simply increasing pH of the same solution the crystallinity of the freeze dried sample was substantially improved, up to ~62% crystallinity, greater than that of the pure lactose solution. Complementarily, only one OH bending vibration peak at 1630 cm−1 was evident, which was similar to that of the pure lactose. At high pH, LA appears mostly in the dissociated form of lactate, which has ability to increase the rate of crystallisation (Smart, Reference Smart1988). The FTIR spectra also contained an intense peak at ~1230 cm−1 which was subdued in the other samples. In the past, this peak was attributed to the OH bending vibrations of lactose molecules, which also means that a change in lactose structure could be predicted and observed with alterations in pH. Lactose molecule has a hemiacetal structure which enables the molecule to interchange between α and β anomers by mutarotation. The ratio between α and β form depends on the compositional and processing conditions such as temperature, concentration, pH and presence of foreign substances (Hartel & Shastry, Reference Hartel and Shastry1991). Thus, in this particular case, a pH high above pK a of LA leads to a structural modification of lactose driven by a different rate of mutarotation that results in attainment of a favourable equilibrium of lactose anomeric forms. These changes in anomeric equilibrium of lactose manipulate the water-lactose behaviour and contribute to varying degrees of crystallisation.
Conclusion
Lactose crystallisation behaviour is affected by pH of the system in addition to the concentrations of LA and Ca. Adjusting pH to 6·5 increased lactose crystallinity. This pH adjustment induced structural modifications of lactose changing equilibrium between anomeric forms of lactose and likely manipulated the water–lactose attractions. Consequently mobility of water and lactose molecules was enhanced improving the crystallinity of lactose. Therefore, it appears that pH of acid whey should be increased to alleviate problems associated with processing of this waste stream.