INTRODUCTION
Due to their central importance to, and survival in the archaeological record, accurate direct radiocarbon (14C) dating of pottery vessels has been one of the “Holy Grails” of archaeology. Compound-specific 14C dating of lipids preserved within the clay matrix of archaeological potsherds is technically extremely challenging, with previous attempts failing to achieve the accuracy and precision required (e.g. Hedges et al. Reference Hedges, Tiemei and Housley1992; Stott et al. Reference Stott, Berstan, Evershed, Hedges, Bronk Ramsey and Humm2001, Reference Stott, Berstan, Evershed, Bronk-Ramsey, Hedges and Humm2003; Berstan et al. Reference Berstan, Stott, Minnitt, Bronk Ramsey, Hedges and Evershed2008). Recently, however, we have reported the first accurate dates achieved for such residues based on compound-specific 14C analyses of C16:0 and C18:0 fatty acids (FAs) isolated from the clay walls of Neolithic pottery vessels (Casanova et al. Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Van De Velde, van Wijk, Cotton, Daykin and Evershedin press). The preparative-capillary gas chromatography (pcGC) isolation technique required two major advances, namely a new trap design allowing the solvent-less recovery of the trapped analytes and a heat-based cleaning method to prevent cross-contamination (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2018). These methodological improvements have enabled reliable and accurate dating of the two FAs characteristic of degraded animal fats. Furthermore, the two independent 14C dates obtained provide an important internal quality control; the 14C age of the FAs should agree at the 2-σ error level (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2018). The samples used thus far in the validation of the compound-specific pot lipid dating method, outlined in Casanova et al. (Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Van De Velde, van Wijk, Cotton, Daykin and Evershedin press) have originated from archaeological sites located inland where human dietary subsistence was dominated by domesticated terrestrial animals, such that the target FAs derived from dairy or carcass fats of ruminant and non-ruminant animals.
None of the pottery dated thus far has originated from coastal areas where the exploitation of marine products may have occurred. At such locations, FAs preserved in pottery vessels would likely be affected by a reservoir effect (Heron and Craig Reference Heron and Craig2015), requiring marine reservoir correction in order to obtain reliable calibrated dates (Cook et al. Reference Cook, Ascough, Bonsall, Hamilton, Russell, Sayle, Scott and Bownes2015). Particularly problematic would be potsherds containing mixed marine- and terrestrial-derived FAs (Cramp and Evershed Reference Cramp and Evershed2014; Cramp et al. Reference Cramp, Evershed, Lavento, Halinen, Mannermaa, Oinonen, Kettunen, Perola, Onkamo and Heyd2014a), as this would increase the complexity of marine reservoir corrections.
Marine product processing in pots can be identified by the presence of specific aquatic biomarkers alongside the C16:0 and C18:0 FAs, namely: (i) long-chain dihydroxy fatty acids (DHYAs), (ii) isoprenoid fatty acids (IFAs) and (iii) long-chain ω-(o-alkylphenyl)alkanoic acids (APAAs); (Hansel et al. Reference Hansel, Copley, Madureira and Evershed2004; Evershed et al. Reference Evershed, Copley, Dickson and Hansel2008; Hansel and Evershed Reference Hansel and Evershed2009; Cramp and Evershed Reference Cramp and Evershed2014). Furthermore, δ13C values determined for the C16:0 and C18:0 FAs can reveal the mixing of both terrestrial and marine commodities in the same vessel (Copley et al. Reference Copley, Hansel, Sadr and Evershed2004, Cramp et al. Reference Cramp, Evershed, Lavento, Halinen, Mannermaa, Oinonen, Kettunen, Perola, Onkamo and Heyd2014a, Reference Cramp, Jones, Sheridan, Smyth, Whelton, Mulville, Sharples and Evershed2014b). It is known, however, that the relative abundances of C16:0 and C18:0 FAs differ between terrestrial and marine organisms and the relationship between their mixing proportions and the resulting δ13C values is not necessarily linear (Mukherjee et al. Reference Mukherjee, Copley, Berstan, Clark and Evershed2005). It is unclear whether this effect will adversely affect the validity of the internal quality control criteria, such that the 14C dates obtained for C16:0 and C18:0 FAs in a potsherd are no longer consistent within 2-σ (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2018, Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Van De Velde, van Wijk, Cotton, Daykin and Evershedin press). It is certainly possible that C16:0 and C18:0 FAs in sherds arising from mixtures of terrestrial- and marine-derived food residues may yield different apparent 14C ages.
Generally, MRE corrections require generation of terrestrial/marine mixing curves using dedicated software (e.g. OxCal, CALIB). This requires an understanding of the local deviation (ΔR) from the global marine calibration curve for a specific time period as well as the percentage of marine-derived C (% marine ) present (Cook et al. Reference Cook, Ascough, Bonsall, Hamilton, Russell, Sayle, Scott and Bownes2015). The ΔR values can be obtained by 14C dating historical marine specimens (of known date of collection), pairing 14C measurements on terrestrial and marine organisms from secure contexts at the site of interest or by both dating tephra layers deposited at sea and on land (Ascough et al. Reference Ascough, Cook and Dugmore2005). The evaluation of the % marine , however, is more challenging. Such considerations are often applied to bone collagen from omnivores which can feed on both terrestrial and marine resources (Cook et al. Reference Cook, Ascough, Bonsall, Hamilton, Russell, Sayle, Scott and Bownes2015). Typically, δ13C and δ15N values are recorded on bulk collagen to understand the local diet and the percentage of marine resources consumed. Preferably, endmembers for pure terrestrial and pure marine organisms are recorded for samples local to the site, but in the majority of cases, more general (non-local) reference values for endmembers are used (Cook et al. Reference Cook, Ascough, Bonsall, Hamilton, Russell, Sayle, Scott and Bownes2015).
Herein, we evaluate whether the approach commonly applied to bone collagen to estimate the contribution of aquatic resources could be applied to FAs extracted from pottery vessels for MRE correction of pot lipids 14C dates. The approach was to undertake 14C dating in order to determine the influence of aquatic resources on CSRA of lipids from potsherds and establish appropriate methods to correct for the MRE. We focused on lipids preserved in pottery vessels with a clearly mixed marine/terrestrial signal from the site of Bornais (South Uist, UK). Our approach involved: (i) lipid residue analyses of pottery vessels including compound-specific δ13C determinations on FAs, (ii) calculation of the local deviation from the global marine calibration curve at the site using paired marine and terrestrial animal remains, (iii) 14C dating of FAs from a range of pottery vessels, (iv) a multiproxy investigation (i.e. biomarkers, stable isotopes and 14C analyses) to evaluate the proportion of mixing of marine and terrestrial lipids, and (v) application of relevant marine reservoir corrections to the 14C dates obtained from pot lipids.
METHODS
Site Description
The site of Bornais is located on the island of South Uist, in the Outer Hebrides, UK (Supplementary material S1). The site comprises four mounds with a long duration of occupation defined by 109 14C dates on seeds and bone collagen, from the late Iron Age (LIA 1 and LIA 2; 5th–6th century AD) to the Early, Middle and Late Norse (EN, MN, LN, respectively; mid-9th–14th century AD) period (Marshall Reference Marshall2005, Reference Marshall, Bronk Ramsey and Cook2016, Reference Marshall, Bronk Ramsey and Cookforthcoming; Sharples Reference Sharplesforthcoming). The recovery of plant macrofossils indicates the cultivation of rye and barley, while the faunal assemblage displays a particularly rich diversity of terrestrial animals (ca.18,000 bones, including those of small vertebrates, birds, fish and mollusks; Sharples and Davis Reference Sharples and Davisforthcoming, Sharples et al. Reference Sharples, Ingrem, Marshall, Mulville, Powell, Reed, Barrett and Gibbon2016). Domesticated animals dominate (~ 95%) the terrestrial faunal assemblage, which comprised cattle (ca 40%), sheep (ca. 45%) and pigs (ca. 10%). The mortality profiles derived from the cattle suggest they were exploited for their milk (Sharples et al. Reference Sharples, Ingrem, Marshall, Mulville, Powell, Reed, Barrett and Gibbon2016). Fish bones (eel, saithe, cod, haddock, ray, turbot, mackerel etc.) and mollusk shells (limpets and winkles) were extremely abundant at the site (ca. 17,000 identified specimens), while marine mammal bones, e.g. seal, were rare (Sharples et al. Reference Sharples, Ingrem, Marshall, Mulville, Powell, Reed, Barrett and Gibbon2016).
Lipid Residue Analysis
Lipid residue analyses of samples of pottery were performed using a methanolic sulphuric acid extraction procedure (Correa-Ascencio and Evershed Reference Correa-Ascencio and Evershed2014). The total lipid extracts (TLEs) were analyzed by gas chromatography (GC) and GC-mass spectrometry (GC-MS) for the identification and quantification of biomarkers, including aquatic biomarkers following established procedures (Evershed et al. Reference Evershed, Heron and Goad1990; Cramp and Evershed Reference Cramp and Evershed2014). Compound-specific δ13C values of FAs were determined by GC-Combusted-Isotope ratio MS (GC-C-IRMS; supplementary material S2).
Pretreatment Methods for 14C Analyses
Approximately 300 mg of coarse bone powder were weighed into a culture tube and pretreated using a modified Login procedure (Longin Reference Longin1971) as described in Knowles et al. (Reference Knowles, Monaghan and Evershed2019). Briefly, bone powder was demineralized in HCl (0.5 M, 10 mL, ~18 hr, room temperature [RT]) followed by a wash with NaOH (0.1 M, 10 mL, 30 min, RT) and a second acid wash with HCl (0.5 M, 10 mL, 30 min, RT). The extracted collagen was rinsed with ultrapure MilliQ-water (MQ-water; 3 × 10 mL) in between each acid and base wash and centrifuged (3000 rpm, 5 min). The collagen was then gelatinized at pH 3 with HCl (0.001 M, 10 mL, 75 °C, 20 hr) and filtered through precombusted glass fiber before freeze drying (Knowles et al. Reference Knowles, Monaghan and Evershed2019).
Surface cleaned shells were ultrasonically agitated in MQ-water (5 mL, 5 min) before drying at 60 °C. When dried, the shells (~30 mg) were crushed roughly before the surface was acid etched (~20%) with HCl (0.2 M, 10 mL). Samples were rinsed with MQ-water (3 × 10 mL) and dried at 60 °C in a drying cabinet (Knowles et al. Reference Knowles, Monaghan and Evershed2019).
Sherds containing lipid concentrations, typically above 500 µg.g–1, were selected for 14C determinations. Pieces of 2–10 g of the potsherd was sampled, depending on the lipid concentrations and size of the potsherds. The lipids were extracted in culture tubes using H2SO4/MeOH (4% v/v, 3 x 8 mL, 70°C, 1 hr). Samples were centrifuged after each extraction (2500 rpm, 10 min) and the three supernatants (methanolic fractions) combined into a second culture tube containing double-distilled water (5 mL). The lipids, including fatty acid methyl esters (FAMEs) formed from the reaction of methanol with the FAs during the first step, were extracted from the methanolic solution with n-hexane (4 × 5 mL) and blown down to dryness at room temperature under a gentle nitrogen stream. The TLEs were derivatized with BSTFA (20 µL, 70°C, 1 hr). Excess BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide) was removed under a nitrogen stream, then ~180 µL of n-hexane was added to obtain a solution containing C16:0 and C18:0 FAMEs at a concentration at ca. 5 µg.µL–1 of carbon. The solution was transferred to an autosampler vial for isolation of C16:0 and C18:0 into individual traps using a preparative capillary GC (pcGC) instrument following the methods described in Casanova et al. (Reference Casanova, Knowles, Williams, Crump and Evershed2017, Reference Casanova, Knowles, Williams, Crump and Evershed2018, Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Van De Velde, van Wijk, Cotton, Daykin and Evershedin press).
14C Determinations
Organic materials (FAMEs and collagen) were combusted to CO2 using a Vario Microcube Elemental Analyser (EA, Elementar). The shells (carbonate-based) were digested in H3PO4 (1 mL, 85% v/v, 70°C) under a He headspace using a Carbonate Handling System (CHS, Ionplus; Wacker et al. Reference Wacker, Fülöp, Hajdas, Molnár and Rethemeyer2013; Knowles et al. Reference Knowles, Monaghan and Evershed2019) to generate CO2. Resulting CO2 was transferred to the Automated Graphitisation Equipment (AGE 3, Ionplus; Wacker et al. Reference Wacker, Němec and Bourquin2010; Knowles et al. Reference Knowles, Monaghan and Evershed2019) under a He stream and adsorbed on Zeolite traps before being released into reaction tubes. The CO2 was reduced to graphite under H2 (580oC, 2 hr, 420 mbar) on a preconditioned iron catalyst. A Pneumatic Sample Press (PSP, Ionplus) was used to press the graphitized samples into Al targets.
All 14C determinations were performed at the BRAMS (Bristol Radiocarbon Accelerator Mass Spectrometer) facility which is equipped with a mini 14C dating system (BRIS-MICADAS) instrument (ETH Zurich, Zurich, Switzerland; Synal et al. Reference Synal, Stocker and Suter2007). Samples were analyzed alongside size-matched processing standards and blanks (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2018; Knowles et al. Reference Knowles, Monaghan and Evershed2019).
Corrections and Calibration of 14C Measurements
14C measurements on FAs from single pottery vessels were corrected for the presence of the methyl derivative C (Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2017, Reference Casanova, Knowles, Williams, Crump and Evershed2018) and subjected to a 2-σ equivalency test and, if successful, combined as described in Casanova et al. (Reference Casanova, Knowles, Bayliss, Dunne, Barański, Denaire, Lefranc, di Lernia, Roffet-Salque, Smyth, Barclay, Gillard, Claßen, Coles, Illet, Jeunesse, Krueger, Marciniak, Minnitt, Van De Velde, van Wijk, Cotton, Daykin and Evershedin press) before testing the validity of calibration on mixed marine/terrestrial resources. Reservoir correction and calibration of the mixed resources was performed in OxCal v4.3 (Bronk Ramsey Reference Bronk Ramsey2009) using the “Marine/mixed curve” tool using the IntCal13 and Marine13 curves (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). This incorporates the percentage of marine derived resources present in the TLEs and the ΔR value for the site (see Results section).
The local reservoir effect was calculated for every pair combination of terrestrial/marine organisms in each context using the online ΔR calculation tool (Reimer and Reimer Reference Reimer and Reimer2016). These individual ΔR values were subjected to a χ2 test at the 5% level (both for each context and all together) to detect potential outliers before calculation of their weighted average, with error calculation as recommended by Russell et al. (Reference Russell, Cook, Ascough and Dugmore2010).
The mixing of marine/terrestrial commodities was quantified in each potsherd using two independent methods: 14C dates and δ13C values of C16:0 and C18:0 FAs (Figure 1). By comparing the mixing ratios obtained by the two methods, it is possible to evaluate whether the FA δ13C values (determined by GC-C-IRMS) can be used to estimate the proportion of marine-derived C in the FAs for use in MRE corrections of their 14C dates. This is an important consideration, especially for sites where terrestrial and marine remains are absent from the archaeological record and so cannot be used to provide reference 14C ages.

Figure 1 Flowchart showing the methods used to assess the validity of the δ13C values method for the estimation of the % marine products and correction of the CSRA dates on the FAs.
The first method of quantifying the % marine is based on the weighted average of 14C determinations on the short-lived terrestrial and marine organisms, from the same context/phase as the potsherds dated, as endmembers using Equation (1).
Where % marine is the percentage of aquatic C in the lipid residue and Age pot , Age terr , Age marine are the combined 14C ages on the individual FAs, for terrestrial animals and marine organisms, respectively.
The second method uses the δ13C values of the individual FAs of UK reference animals (cattle and sheep raised on a pure C3 diet; Copley et al. Reference Copley, Berstan, Dudd, Docherty, Mukherjee, Straker, Payne and Evershed2003) as the terrestrial endmembers (pigs were hypothesized not to have been processed in potsherds; Sharples et al. Reference Sharples, Ingrem, Marshall, Mulville, Powell, Reed, Barrett and Gibbon2016), and fish, winkles and limpets captured from UK waters (corrected for the Suess effect; Cramp and Evershed Reference Cramp and Evershed2014) to serve as the marine endmembers. The terrestrial endmembers correspond to the average values for both C16:0 and C18:0 FAs and were found to be δ13C16:0 = –30.0 ± 0.6‰ and δ13C18:0 = –32.2 ± 0.6‰ for adipose fats and δ13C16:0 = –29.2 ± 1.0‰ and δ13C18:0 = –34.0 ± 0.9‰ for dairy fats. Both ruminant adipose and dairy values were used as endmembers to evaluate whether one should be used over the other. The marine endmembers were δ13C16:0 = –22.7 ± 2.2‰ and δ13C18:0 = –21.7 ± 2.5‰. The relationship between the relative proportions of marine and terrestrial fats and the δ13C values is theoretically non-linear, due to the differing relative abundances of the of FAs in the different foodstuffs (Mukherjee et al. Reference Mukherjee, Copley, Berstan, Clark and Evershed2005), however, the success of the internal quality control on the CSRA dates (see Results section) suggests a linear relationship within analytical uncertainty. A linear mixing curve could therefore be employed to estimate the % marine contribution and associated uncertainty using the propagation of analytical errors. The % marine values obtained on both FAs were then combined as a weighted average with uncertainties calculated according to Russell et al. (Reference Russell, Cook, Ascough and Dugmore2010). Such a model is a conservative approach and probably overestimates the uncertainties. Furthermore, it cannot take into account the fact that the true % marine values must be constrained between 0 and 100%.
As a comparison, the % marine values of the TLEs were also estimated (using the same endmembers) using the software package FRUITS (v2.1). This software employs a Bayesian approach to quantify the contribution of different food sources using isotopic data (Fernandes et al. Reference Fernandes, Millard, Brabec, Nadeau and Grootes2014). The output of this software is given both as means and standard deviations (represented by box-and whiskers plots) or as probability distributions constrained to between 0 and 100%. The full range of data points for the probability distributions of the % marine after Bayesian modeling was exported and implemented as a prior information file into the mixing marine/terrestrial tool in OxCal.
RESULTS AND DISCUSSION
Characterization of Lipid Residues in Pottery Vessels
Forty-nine pottery vessels from layer BCC, Mound 2, MN period were subjected to lipid residue analyses (S2). TLEs with concentrations >5 µg.g–1 were recovered from 96% (n = 47) of the potsherds, at an average lipid concentration of 1.2 mg.g–1 (supplementary material S3). A total of 80% of the TLEs with residues (n = 39) were dominated by the C16:0 and C18:0 FAs characteristic of degraded animal fats (Figure 2a). Many of the TLEs (47% of the sherds with residues; n = 22) exhibited marine biomarkers. The long-chain DHYAs (C18, C20 and C22) were detectable in 30% (n = 14; Figure 2b), long-chain APAAs (C18, C20 and C22) in 23% (n = 11; Figure 2c) and the IFAs (phytanic acid and 4,8,12-trimethyltridecanoic acid [TMTD]) in 30% (n = 14) of the sherds with residues. In total, only 4% (n = 2; BN-140, BN-173) of the potsherds with lipid residues contained all three classes of aquatic biomarkers, 21% (n = 10) contained two aquatic biomarkers and 26% (n = 12) showed one aquatic biomarker. No aquatic biomarkers were detected in the remainder of the TLEs (n = 25, 53% of the sherds with organic residues). The δ13C values of the palmitic and stearic acids were determined by GC-C-IRMS (Figure 2d). Significantly, the C16:0 and C18:0 FAs displayed δ13C values characteristic of mixtures between ruminant and marine or porcine products (Cramp et al. Reference Cramp, Evershed, Lavento, Halinen, Mannermaa, Oinonen, Kettunen, Perola, Onkamo and Heyd2014a, Reference Cramp, Jones, Sheridan, Smyth, Whelton, Mulville, Sharples and Evershed2014b). The extracts yielding the most enriched stable carbon isotope values also contained aquatic biomarkers, strongly suggesting the processing of marine products rather than porcine. Several TLEs were relatively more enriched in 13C, but show no detectable aquatic biomarkers suggesting they did not survive or could denote the processing of marine commodities under conditions not conductive to the formation of thermally produced aquatic biomarkers (APAAs). The use of Δ13C (= δ13C18:0 – δ13C16:0) values allows the identification of sherds where FAs are predominantly of dairy product origin (< –3.1‰; Copley et al. Reference Copley, Berstan, Dudd, Docherty, Mukherjee, Straker, Payne and Evershed2003). A total of 22 sherds yielded Δ13C values below –3.1‰, with aquatic biomarker identification for half of them supports the hypothesis of some mixing of dairy products with marine products. The other sherds with higher Δ13C values of >–3.1‰ could result from the mixing of ruminant carcass and marine products.

Figure 2 Partial gas chromatogram of the TLE (a), and GC/MS SIM mass chromatograms showing detection of DHYAs (b) and APAAs (c), for potsherd BN-173. Scatter plots of δ13C16:0 plotted against δ13C18:0 from lipid residues characteristic of animal fats at Bornais for all the TLEs (Cramp et al. Reference Cramp, Jones, Sheridan, Smyth, Whelton, Mulville, Sharples and Evershed2014b, forthcoming and this study), (d) for the 22 potsherd extracts selected for 14C dating by CSRA and position of the average reference values (crosses) (e), and the theoretical mixing lines of terrestrial and marine end-members with the approximate percentage of marine fat/oil marked on the lines (f). Stars denote the detection of aquatic biomarkers. Shaded areas indicate the reference ellipses for δ13C values of modern animals and the crosses are the values used as endmembers. The dashed lines correspond to the areas where lipid residues are hypothesized to be affected to varying degrees by the MRE.
Additionally, 131 potsherds from all the phases and mounds at the site were previously analyzed by lipid residue analysis (Cramp et al. Reference Cramp, Jones, Sheridan, Smyth, Whelton, Mulville, Sharples and Evershed2014b, Reference Cramp, Casanova and Evershedforthcoming). The results suggested a dominance of dairy and ruminant carcass product processing, as well as some mixing of non-ruminant and marine fat/oil (Figure 1d). Only the pottery from the LIA1 phase lacked aquatic biomarkers.
A total of 21 potsherds (from all phases) with sufficient lipid concentration and containing either none or at least one aquatic biomarker, were subjected to CSRA (Figure 1e).
ΔR Value Determination
In order to determine the age of the structures associated with the pots, and the local reservoir effect at the site of Bornais a range of fish bones (n = 13), marine mollusk shells (n = 14) and terrestrial animal bones (n = 8) were 14C dated (Table 1). These were assessed together with other available 14C measurements on terrestrial animal bones and grains (layer BCC, n = 7; Marshall et al. forthcoming-b). All these materials derive from the LIA2, EN, MN and LN settlement structures. On a context-by-context basis, marine and terrestrial organisms were subjected to χ2 statistical testing to detect outliers for exclusion (Table 1). Two marine samples from context BAF and two terrestrial animal bones from context BCC were, therefore, excluded from ΔR determination. The ΔR values calculated using all the pairs of terrestrial/marine organism (80 in total) per context are reported in Table 1. No ΔR was calculated for contexts BBA and BBD as they were dated based on only one material type, and for AG, the two marine organisms from this context failed the χ2 test.
Table 1 14C determinations of terrestrial and marine organisms from Bornais and ΔRs calculated for the diverse contexts based on the multiple paired terrestrial/marine organisms. *refers to statistical outliers that have been excluded from ΔR calculation.

With the exception of context BCC, all the contexts demonstrated a negative ΔR, varying from –214 ± 26 to −45 ± 21. Interestingly, layer BCC (MN phase) shows a ΔR of 28 ± 150; the large uncertainty associated with this value results from high variability in the 14C ages of the marine organisms, which could be classified into three distinct groups: Group (a) gave a ΔR of –107 ± 54, Group (b) 242 ± 55, and Group (c) –31 ± 56. The MRE of Group (c), comprised only fish bones and likely reflects the mobility of the fish species (Russell et al. Reference Russell, Cook, Ascough, Barrett and Dugmore2011). Groups (a) and (b) comprise both winkles and limpets and their MREs do not appear to be species dependent. The grouping could, therefore, either correspond to two different collection points of the mollusk shells (likely collection points nearby are either completely coastal, or sea lochs with the potential for substantial terrestrial runoff) or simply the introduction of older material into a later context (although, this offset was only observed for some limpet and winkle shells, but not for fish bones).
The MREs calculated from Groups (a) and (c) gave statistically indistinguishable 14C determinations and are in good agreement with ΔR values calculated for the other contexts. Only shells from Group (b) were excluded from the overall ΔR determination due to uncertainty in the security of the context in light of its high ΔR value. The remaining 56 ΔRs failed the statistical identicality test, therefore layer BAG (ΔR = –214 ± 26), showing the lowest ΔR and the pairs BN-F-14/SUERC-2684, BN-F-14/OxA15420 which showed the highest ΔR values were excluded from the calculation. With the removal of the organisms and terrestrial/marine pairs identified as outliers the remaining 53 ΔR values are statistically identical (T’ = 69.3, T’(5%) = 71.0, υ = 53) and average to –65 ± 46. These data suggest there is no significant difference in the reservoir effect from the LIA2 to LN period at the site. This ΔR value of –65 ± 46 is also consistent with the previously reported ΔR values for the North Atlantic, including the (Inner, and Outer) Hebridian Islands of –47 ± 52 for the period 3500 BC-1450 cal AD (Reimer et al. Reference Reimer, McCormac, Moore, McCormick and Murray2002; Ascough et al. Reference Ascough, Dugmore, Cook, Higney, Barber and Scott2004, Reference Ascough, Cook and Dugmore2005, Reference Ascough, Cook, Church, Dugmore, Arge and McGovern2006, Reference Ascough, Cook, Dugmore and Scott2007, Reference Ascough, Cook and Dugmore2009, Reference Ascough, Church and Cook2017; Russell et al. Reference Russell, Cook, Ascough and Dugmore2010, Reference Russell, Cook, Ascough and Scott2015; see supplementary material S4).
14C Dating of Pottery Vessels
C16:0 and C18:0 FAs from 21 sherds were dated, of which six were dated in duplicate. This includes potsherds from all phases present at Bornais, both with and without aquatic biomarkers present. Of these, 17 sherds successfully passed the internal quality control criterion, whereby the 14C dates of the C16:0 and C18:0 FAs must agree within 95% confidence. Three failed, and seven did not yield sufficient C for both FAs to be dated independently. These last ten sherds were therefore not further considered as no internal control on the C16:0 and C18:0 FAs was present to ensure the security of the dates (supplementary materials S5).
Two of the pottery vessels dated in duplicate failed the internal quality control the first time, but either passed it the second time (BN-35) or yielded insufficient C for two targets (BN-101). Three pot dates that were duplicated gave indistinguishable dates for both extracts. The duplicate analysis of potsherd BN-74 produced statistically non-identical results between the two extractions. The CSRA dates successfully passed the internal criterion for both extractions and as the C16:0 and C18:0 dates are essentially independent, it is unlikely that both FAs in one extraction could be contaminated to the same degree (giving rise to identical, but inaccurate, dates; Casanova et al. Reference Casanova, Knowles, Williams, Crump and Evershed2018). This difference could, therefore, reflect an inhomogeneous partitioning of the marine and terrestrial products in the same potsherd (due to different filling levels during cooking for example), and could potentially be monitored and corrected for in the future by recording δ13C values on the two different TLEs (not performed in this case). Table 2 reports the combined measurements on the potsherds which passed the internal control.
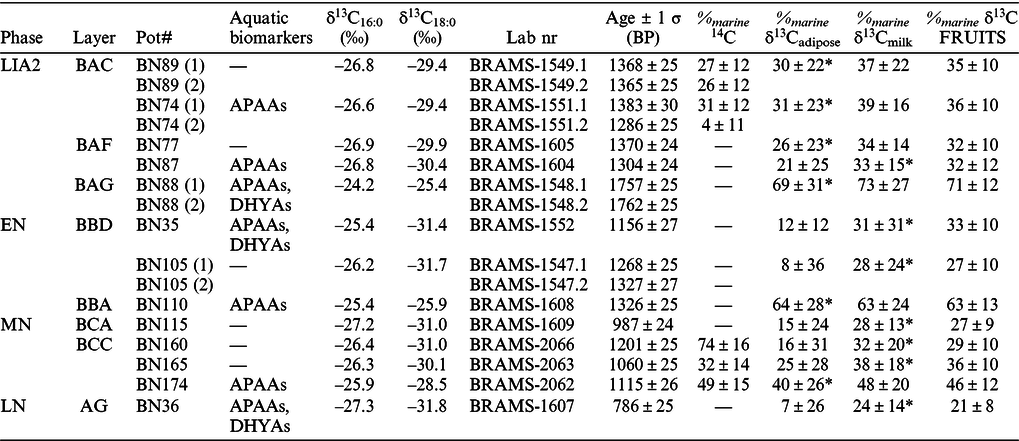
Table 2 Summary of 14C dated potsherds from Bornais, including the presence of aquatic biomarkers, δ13C values of individual FAs, combined 14C determinations of C16:0 and C18:0 FAs (which passed the internal quality control criterion) and the percentage of marine fat/oil within the TLEs. The % marine were calculated using reference 14C measurements on marine-terrestrial samples (% marine 14C), using a linear mixing with δ13C values on ruminant adipose products (% marine δ13Cadipose) and δ13C values on ruminant dairy products (% marine δ13Cmilk) as endmembers and finally using δ13C values implemented in FRUITS (% marine δ13C FRUITS). *refers to the preferred endmembers for the terrestrial fats based on the Δ13C value (i.e. milk if Δ13C < –3.1‰, ruminant adipose otherwise) and used for the % marine calculation using FRUITS (v2.1; here the mean and standard deviation are presented and the full probability distributions are in supplementary material S6).

These results suggest that the internal quality control is valid in this case of mixed marine/terrestrial resources and can be used as evidence for the reliability of the CSRA measurements. The error introduced by mixing the FAs of different abundances is likely below the AMS error and the internal quality control criterion is still applicable.
The four sherds from the BAC and BAF contexts of phase LIA2, the three from the EN phase and the three from the BCC context of the MN phase were shown to have 14C ages between the age of the terrestrial organisms and their contemporaneous marine analogues (Tables 1 and 2). These include the five sherds (BN-89, BN-77, BN-105, BN160 and BN-165) which did not exhibit aquatic biomarkers. These dates suggest, therefore, mixing of terrestrial and marine resources in all the pots from which the sherds derived.
The sherd BN-88 from the BAG context LIA2 phase exhibited not only the most enriched δ13C values but also the oldest age obtained in this investigation. This date is older than the marine reference fish bone from this context and, indeed the reference fish bones from other LIA2 contexts. The second dating of the potsherd confirmed the accuracy of the compound-specific 14C measurement, suggesting the FAs likely derived from a pure marine fat/oil residue and that the MRE (based on only one pair) was underestimated in this case, unless the potsherd was residual and corresponds to the LIA1 phase, although no aquatic biomarkers were detected in potsherd extracts from this particular phase.
The potsherd BN-115 (987 ± 24 BP) from context BCA (not dated) of the MN phase, which lacked aquatic biomarkers exhibited an age consistent with the MN phase, and thus is likely to be entirely composed of terrestrial animal fats.
For the LN phase, the FA date on pot BN-36 (786 ± 25 BP) is younger than that of the terrestrial organism (BN-MB-7: 930 ± 25 BP). Based on the δ13C values, the sherd plots close to the reference dairy fat ellipses despite containing aquatic biomarkers. This result is surprising and suggests that the dating of this phase, based on only one terrestrial organism could be erroneous. Younger ages from other LN contexts from Bornais were obtained in the range 900 to 650 14C years BP (uncalibrated), which would support this hypothesis (Marshall et al. Reference Marshall, Bronk Ramsey and Cook2016, Reference Marshall, Bronk Ramsey and Cookforthcoming).
These measurements clearly confirm that lipid dates can be affected by the marine reservoir effect and such dates will therefore require calibration using relevant ΔR values and proportionately mixed terrestrial/marine curves. The mixing of marine and terrestrial products influences the determined δ13C values and 14C dates of FAs and this does not appear to have an adverse effect on the internal quality control criterion. Interestingly, MREs are evident in TLEs from potsherds lacking detectable aquatic biomarkers. The results therefore suggest that 14C dates could be used to detect a (low-)level of marine organism processing in pots where aquatic biomarkers are undetectable. This would be especially relevant for sites where potential exists for processing of non-ruminant products or where aridity effects are possible, shifting the δ13C values away from the ruminant products ellipses.
Correction of the MRE and Calibration
The % marine in the lipid residues was quantified for the sherds which passed the internal quality control criterion (Table 2; supplementary material S5, S6). To ensure a fair evaluation of the use of FA δ13C values for determination of the degree of marine/terrestrial product mixing, only potsherds from contexts which were securely dated using more than one marine/terrestrial organism were used for validating the correction and calibration (BAC and BCC). The validity of using δ13C values of FAs for the quantification of marine-derived C was evaluated by comparison with reference values obtained by 14C dates.
Overall, no significant differences in % marine were noted in the use of δ13C values from ruminant adipose or milk fats as terrestrial end members in a simple linear mixing model (Table 2, Figure 2f). Therefore, only the one most representative of the terrestrial endmembers was used for MRE corrections (i.e. milk if Δ13C < –3.1‰, ruminant adipose if Δ13C > –3.1‰).
The range of calibrated terrestrial dates on mammals for BRAMS-1710, BRAMS-1711, BRAMS-1713, in the LIA2 phase, BAC context, were 672–773 cal AD, 662–769 cal AD, and 685–772 cal AD, respectively (95% probability, Figure 3a). The % marine within the FAs in pot BN-89 was determined to be 27 ± 12% using 14C dates, 30 ± 22% using δ13C values of adipose endmembers in the simple linear mixing and 35 ± 10% (mean and standard deviation) when implemented in FRUITS (Table 2). All these estimates are statistically indistinguishable and the calibrated ages after MRE correction agrees with the reference age of the terrestrial organisms (Figure 3a).

Figure 3 Corrections and calibrations for potsherds of the (a) LIA phase, (b) MN phase in OxCal v4.3 against the IntCal13 calibration curve (Bronk Ramsey Reference Bronk Ramsey2009; Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). The distributions plotted in dark grey correspond to the reference age of terrestrial animal bones, in light grey the uncorrected determinations on pot lipids and in black the corrected determinations on pot lipids using either the 14C or δ13C methods using adipose or milk as endmembers.
Turning to potsherd BN-74, the first extract yielded estimates of 31 ± 12% marine fat/oil using 14C dates, and 31 ± 23% and 36 ± 10% using δ13C values of adipose FAs as endmembers for the mixing lines and FRUITS, respectively. The calibrated age from pot BN-74 (first extract) agrees with the age of terrestrial organisms (Figure 3a). Nonetheless, the second extract of the pot BN-74, which yielded results statistically different to the first extract, showed a % marine of 4 ± 11% using 14C as end-members, suggesting an underestimation of the proportion of marine products in the TLE based on the δ13C values in this case. As mentioned previously, this potsherd is likely affected by inhomogeneous deposition of the marine fats in certain areas of the vessel, implying that determination of δ13C values and 14C dates on the same TLE is required for satisfactory quantification of the % marine using δ13C values.
The range of calibrated terrestrial dates (excluding outliers, Table 1) varies from 993–1052 cal AD (55% probability) and 1081–1152 cal AD (OxA-15522; 41% probability) to 1039–1206 cal AD (OxA-1540, 95% probability) for the MN phase, BCC context (Figure 3b). The results for potsherds BN-165 using δ13C values of milk FAs and BN174 using δ13C values of adipose FAs showed, similarly to BN-89 and BN-74 (first extract), a good agreement with the age of reference terrestrial animals using the different methods (Figure 3b).
The % marine in the pot BN-160 is, however, estimated to be 74 ± 16% based on 14C dates, 26 ± 34% and 29 ± 10% based on δ13C values milk endmembers in the linear mixing curve and FRUITS, respectively (Table 2). These results are not identical within a 1-σ error but are within 2-σ. The potsherd BN-160 was calibrated to 908–1212 cal AD by 14C estimates, 730–1044 cal AD and to 780–1016 cal AD (95% probability for all) using the dairy endmember in the linear mixing model and FRUITS, respectively. The end of the last two distributions overlap only at the start of the calibration of the reference terrestrial organisms (Figure 3b). It should be noted that for potsherd BN-160, the 14C dates suggest that marine fat/oil are dominant in the TLE whereas the δ13C values suggest a dominance of dairy products. Unless the CSRA date is inaccurate, this implies that this potsherd, like BN-74, could be affected by a differential partitioning of the marine products and that δ13C values recorded on the initial TLE are not representative of the second TLE used for 14C dating.
Overall, MRE corrections of lipid residues, using δ13C calculations using the simple linear mixing model showed a wider probability distribution than those obtained using 14C dates and δ13C values used in the FRUITS software. However, the calibrated range of the corrected CSRA determinations on pot lipids using both methods clearly overlaps with the calibrated range of the reference terrestrial organisms. The precision of the calibrated ages depends almost entirely on the uncertainties associated with the calculated % marine , as illustrated with reduced errors obtained using the FRUITS software instead of the simple linear mixing curve. The results demonstrate no significant difference in the use of ruminant adipose or dairy δ13C values as end members for the quantification of the % marine . In practice, however, one should be chosen over the other based on the Δ13C values to ensure that the terrestrial endmember is representative of the animal products processed in the vessels at the time (i.e. dairy if Δ13C < –3.1‰ or adipose if Δ13C > –3.1‰). On the other hand, the 14C dates provide an accurate estimate of the % marine present in the FAs and could be used for quantification of marine products in TLEs instead of a calendar age. The % marine in potsherds BN-74 (second extraction) and BN-160 were underestimated, leading to inappropriate corrections. However, this could be accounted for in the future if the 14C measurements and δ13Cvalues are recorded on the same lipid extract to avoid potential inconsistencies associated with inhomogeneous deposition of the lipids within vessels and use more reliable δ13C values for the quantification of marine products.
One limitation of the δ13C approach is the estimation of % marine due to the wide range of reference values (from ca. –26‰ to –20‰) observed in modern marine organisms. The reference ellipses commonly plotted comprise only 68% of the reference values (i.e. 1-σ). The average values used to generate an endmember here are not centred in the ellipses (Figure 2f). Therefore, potsherds with individual FAs δ13C values plotting at the edge of the reference marine ellipse can be purely marine but, the % marine deposited in the sherd can be underestimated using the linear mixing curves (Table 2, Figure 2f). This phenomenon is illustrated in the case of potsherd BN-88 which is likely to contain predominantly marine fats based on the CSRA dates. The δ13C values plotted just outside the marine reference ellipse and marine-derived C was quantified to be 69 ± 31% with the adipose endmember, and the % marine appeared to be underestimated in this case. Potsherd BN-110 could also contain a dominance of marine products based on FA δ13C values (i.e. 63 ± 28% with adipose FAs used as endmembers; Table 2, Figure 2f). This suggests that the linear mixing does not account particularly well for the dominance of marine products at the boundaries. Therefore, the use of the mean and standard deviation for the reported % marine products would lead to some underestimation when applying MRE corrections in the case of a dominance of marine products. This would be overcome using the full probability distribution calculated in FRUITS as prior information on the percentage marine.
We suggest that a linear mixing curve can give valid corrections if marine products are not dominant in the TLE, however, the FRUITS software would deal more adequately with the boundaries (if probability distribution are used) and should be used preferably to access the % marine in the TLEs.
CONCLUSION
The processing of mixed terrestrial/marine fats in pottery vessels at the site of Bornais was revealed through lipid biomarker and CSRA analyses. CSRA and comparison with the 14C dates of associated marine and terrestrial samples also enabled the detection of marine product processing in cases were no aquatic biomarkers were detected. We therefore suggest that in such circumstances, 14C measurements could be used as a tracer for the detection and quantification of marine product processing in pots. Compound-specific dates from potsherds from Bornais were successfully subjected to MRE correction, and assessed against independent ages determined for contemporaneous terrestrial organisms using:
-
(i) An appropriate ΔR (-65 ± 45) for the site and time period.
-
(ii) An estimate of the proportion of marine resource processed in the pots calculated using δ13C values of individual pot FAs and from a modern reference database (linear mixing or implementation in FRUITS).
-
(iii) A mixed calibration approach in OxCal software.
These corrected ages agreed well with the calibrated age of terrestrial reference materials which confirmed the efficacy of using FA δ13C values to estimate the % marine , meaning that an approach similar to that commonly adopted for bone collagen can be used to correct for MRE present in lipids. For future MRE corrections and calibrations of lipids dates, we recommend:
-
(i) Calculating a ΔR for the site using a paired terrestrial/marine reference materials approach or using a previously published ΔR relevant for the spatiotemporal area.
-
(ii) Recording δ13C16:0 and δ13C18:0 values from the same TLE as that used for 14C dating to determine the % marine , avoiding the negative impact of potential inhomogeneity of lipid distribution in vessels.
-
(iii) Using mixing model endmembers calculated from modern reference values valid for the location of interest, by using either those of the database for UK animals (excluding the species not present at the site; Copley et al. Reference Copley, Berstan, Dudd, Docherty, Mukherjee, Straker, Payne and Evershed2003; Cramp and Evershed Reference Cramp and Evershed2014) or δ13C values recorded from reference animals, representative of other locations and environmental conditions (e.g. arid environments, Dunne et al. Reference Dunne, Evershed, Salque, Cramp, Bruni, Ryan, Biagetti and di Lernia2012).
-
(iv) Using endmembers from dairy reference fats in the case of potsherds with Δ13C < –3.1‰ or using endmembers from the reference ruminant adipose fats values for potsherds with Δ13C > –3.1‰, to determine % marine in the TLEs.
-
(v) Employing FRUITS or other Bayesian approaches (if available) to quantify % marine in the TLEs using a probability density function.
-
(vi) Correcting CSRA dates for the MRE using mixed atmospheric and marine calibration curves (e.g. in OxCal).
Acknowledgments
This work was undertaken as part of a project ERC funded Advanced Grant (NeoMilk) and a Proof of Concept grant (LipDat) to RPE (FP7-IDEAS-ERC/324202; H2020 ERC-2018-PoC/812917) and supporting the doctoral and post-doctoral contract of EC, respectively. We thank the NERC, BBSRC and the University of Bristol for funding the BRAMS facility. We thank Kirsty Harding for the selection of archaeological pottery materials from the site of Bornais. We acknowledge Alex Bayliss for help with Bayesian modeling. Adrian Timpson is thanked for advice in mixing model statistics.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/RDC.2020.11