Introduction
Seed dormancy mechanisms in plants have evolved to optimize the time of germination, avoiding germination in periods that are only ephemerally favourable and, therefore, increasing seedling survival (Fenner and Thompson, Reference Fenner and Thompson2005; Baskin and Baskin, Reference Baskin and Baskin2014; Willis et al., Reference Willis, Baskin, Baskin, Auld, Venable, Cavender-Bares, Donohue and Rubio de Casas2014). Recently, it has also been suggested that physical dormancy (hereafter referred to as PY) has evolved to allow seeds to escape seed predation, preventing the production and release of volatile compounds detectable by post-dispersal seed predators (Paulsen et al., Reference Paulsen, Colville, Kranner, Daws, Högstedt, Vandvik and Thompson2013). PY is caused by one or more layers of tightly packed palisade cells in the seed or fruit coat; such layers are impermeable to water and, once the seed or fruit coat becomes permeable, dormancy cannot be reversed (Baskin et al., Reference Baskin, Baskin and Li2000; Baskin and Baskin, Reference Baskin and Baskin2014). It has been suggested that seed hardness protects against microbial attack and extends seed longevity and persistence in the soil seed bank (Dalling et al., Reference Dalling, Davis, Schutte and Arnold2011; Jayasuriya et al., Reference Jayasuriya, Athugala, Wijayasinghe, Baskin, Baskin and Mahadevan2015). Therefore, PY has a great ecological importance (Allen and Meyer, Reference Allen and Meyer1998).
Hard seeds can be softened artificially by different mechanisms such as mechanical or acid scarification (Baskin and Baskin, Reference Baskin and Baskin2014). However, the mechanisms involved in breaking physical dormancy under field conditions are scarcely known and seem to be highly variable among species (Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003; Van Assche and Vandelook, Reference Van Assche and Vandelook2006; Gama-Arachchige et al., Reference Gama-Arachchige, Baskin, Geneve and Baskin2012; Jaganathan et al., Reference Jaganathan, Wu, Han and Liu2017). Seed softening might occur in response to fire (Moreira and Pausas, Reference Moreira and Pausas2012; Jayasuriya et al., Reference Jayasuriya, Athugala, Wijayasinghe, Baskin, Baskin and Mahadevan2015; Liyanage and Ooi, Reference Liyanage and Ooi2015; Cochrane, Reference Cochrane2017), daily fluctuating temperatures (Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003; Baskin and Baskin, Reference Baskin and Baskin2014), moisture changes (Baskin and Baskin, Reference Baskin and Baskin2014), animal ingestion of seeds or fruits (Venier et al., Reference Venier, García, Cabido and Funes2012a; Jaganathan et al., Reference Jaganathan, Yule and Liu2016), the action of soil microorganisms (Soriano et al., Reference Soriano, Huante, Gamboa-deBuen and Orozco-Segovia2014), or a set of environmental conditions acting during seed storage in the soil (Jaganathan et al., Reference Jaganathan, Wu, Han and Liu2017; Liu et al., Reference Liu, Abudureheman, Zhang, Baskin, Baskin and Zhang2017). Moreover, it has been suggested that differences in timing of PY break in field conditions may be related to differences in the seed coat structure, especially to epidermis thickness of the lens, a potential water-gap in the seed coat consisting of a point of weakness of elongated palisade epidermal cells that eventually split apart, allowing water entry to the seed in the final stage of seed softening (Baskin, Reference Baskin2003; Taylor, Reference Taylor2005; Venier et al., Reference Venier, Funes and García2012b; Jaganathan et al., Reference Jaganathan, Wu, Han and Liu2017). Water gaps open in response to an appropriate environmental signal. Thus, understanding how timing of germination of seeds with PY is controlled in field conditions is necessary to determine the environmental conditions that the water gap needs to open (Baskin, Reference Baskin2003).
Some species exhibit differences in fruit morphology; those different fruits may be found in a single individual plant, a phenomenon known as heterocarpy or heteromorphism (Lu et al., Reference Lu, Tan, Baskin and Baskin2010; Baskin et al., Reference Baskin, Lu, Baskin, Tan and Wang2014; Lu et al., Reference Lu, Tan, Baskin and Baskin2015) or, although much less common, individual plants with a single morph, which may constitute different varieties of the same species (Pometti et al., Reference Pometti, Vilardi, Cialdella and Saidman2010). The production of seeds/fruits of different morphologies could be an adaptation of species to spatio-temporal variability of habitats (Imbert, Reference Imbert2002; Lu et al., Reference Lu, Tan, Baskin and Baskin2010, Baskin and Baskin, Reference Baskin and Baskin2014; Baskin et al., Reference Baskin, Lu, Baskin, Tan and Wang2014; Lu et al., Reference Lu, Tan, Baskin and Baskin2015). The fruits or seeds within a species may vary in size, colour or morphology, as well as in dispersal, dormancy or germination characteristics (Lu et al., Reference Lu, Tan, Baskin and Baskin2010; Baskin and Baskin, Reference Baskin and Baskin2014; Baskin et al., Reference Baskin, Lu, Baskin, Tan and Wang2014; Zhang et al., Reference Zhang, Wang, Baskin, Baskin, Luo and Hu2016). Different trade-offs among species that differ in fruit features have been described (Lu et al., Reference Lu, Tan, Baskin and Baskin2010, Reference Lu, Tan, Baskin and Baskin2013; Baskin et al., Reference Baskin, Lu, Baskin, Tan and Wang2014; Lu et al., Reference Lu, Tan, Baskin and Baskin2015). For example, diaspore dimorphism was found to be associated with the diversification of the degree of dormancy and the spread of offspring in time and space (Baskin et al., Reference Baskin, Lu, Baskin, Tan and Wang2014; Zhang et al., Reference Zhang, Wang, Baskin, Baskin, Luo and Hu2016 and references therein). Studies about the relationship of fruits with different morphology and their type of dormancy were performed mostly in seeds with physiological dormancy (Baskin et al., Reference Baskin, Lu, Baskin, Tan and Wang2014; Zhang et al., Reference Zhang, Wang, Baskin, Baskin, Luo and Hu2016), whereas studies on seeds with PY are scarce.
Vachellia caven (Gillies ex Hook. & Arn.) Seigler & Ebinger (ex Acacia caven Gillies ex Hook. & Arn.; Seigler and Ebinger, Reference Seigler and Ebinger2005) is a tree native to Argentina. The species has PY (Funes and Venier, Reference Funes and Venier2006) and previous studies have shown that neither high temperatures that simulate a fire nor cattle ingestion may break seed dormancy in this species (Venier et al., Reference Venier, Cabido and Funes2017; Venier et al., Reference Venier, García, Cabido and Funes2012a). Previous work in some Fabaceae species reported a seasonal pattern in dormancy loss, suggesting that fluctuating temperatures may be a key factor to break dormancy and trigger germination (Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003). Vachellia caven exhibits five types of fruit morphs (Pometti et al., Reference Pometti, Vilardi, Cialdella and Saidman2010). Two of them are highly represented in Córdoba forests, Argentina; they differ in their mechanisms for releasing the seeds, with one being dehiscent and the other indehiscent. Those morphs may be found in different individuals (Pometti et al., Reference Pometti, Vilardi, Cialdella and Saidman2010). The presence of individuals with different fruit morphologies has led some authors to consider the existence of two varieties of this species, i.e. V. caven var. caven (indehiscent fruits) and V. caven var. dehicents (dehiscent fruits) (Aronson, Reference Aronson1992; Pometti et al., Reference Pometti, Vilardi, Cialdella and Saidman2010). However, this species can also be considered heteromorphic because its different fruit morphs can be found in a single individual (Baranelli et al., Reference Baranelli, Cocucci and Anton1995).
The presence of the two fruit morphs (indehiscent and dehiscent) in V. caven may have ecological significance. For example, seeds from the two fruit morphs might differ in their PY breaking behaviour. Accordingly, we expect that the morph which exposes the seeds earlier (dehiscent) would break seed dormancy as fast as possible in order to exploit the favourable conditions to germinate as soon as they are dispersed and to reduce their exposure to post-dispersal seed predators (Chambers and McMahon, Reference Chambers and MacMahon1994; Crawley, Reference Crawley and Fenner2000). Furthermore, those fruits that retain the seeds within the fruit for a longer time (indehiscent fruit morph) could break seed dormancy later, since it would be favourable for them to keep dormancy until the disperser has brought them to a new place or fruit dehiscence has occurred. Therefore, we performed a burial experiment to explore the patterns of dormancy break in field conditions in seeds with PY from two different fruit morphs of V. caven. Moreover, we related these patterns to (1) environmental conditions that could influence the loss of PY, and (2) histological features of the lens that could differ between seeds of both fruit morphs, and could explain different mechanism of dormancy break in field conditions.
Materials and methods
Study species
Vachellia caven is one of the most widespread shrub or tree species of subtropical South America; in Argentina it occurs mainly in lowland and mountain arid and semi-arid forests from central to northern regions (Aronson, Reference Aronson1992; Zuloaga and Morrone, Reference Zuloaga and Morrone1999). This species shows remarkable climatic tolerance and ecological adaptability and is able to colonize areas degraded by anthropogenic activities, such as intense agriculture, grazing or fire (Aronson, Reference Aronson1992; Cabido et al., Reference Cabido, Manzur, Carranza and González-Albarracin1994). Most individuals of this species are usually shrubs of 1–3 m in height, with some individuals attaining 8–10 m in height (Aronson, Reference Aronson1992). Flowering occurs in August–September (Cialdella, Reference Cialdella1984), and fruits ripen from January to February and are dispersed from the tree from February/March to April. The fruits are dispersed by cattle (observed in the indehiscent morph; Venier et al., Reference Venier, García, Cabido and Funes2012a) and by medium-size birds (Aronson, Reference Aronson1992), and seeds could be removed by ants (authors’ personal observations). Without the intervention of dispersers, the indehiscent fruit morph would release the seeds before the new reproductive season (authors’ personal observations). Seeds of V. caven have PY conferred by lignified epidermal palisade-like cells and the presence of callose in the cuticle that would make the seed coat impermeable to water (Venier et al., Reference Venier, Funes and García2012b). Previous studies observed that this species may germinate at different temperatures, with optimal temperatures being 25/15°C and 35/20°C (Funes and Venier, Reference Funes and Venier2006).
Study area
The study area is located in the Chaco Serrano in Córdoba province, central Argentina, at the southern end of the Gran Chaco. The vegetation is a mosaic of a seasonally subtropical forest dominated by Lithraea molleoides, Zantoxylum coco and Prosopis spp. (Giorgis et al., Reference Giorgis, Cingolani, Chiarini, Chiapella, Barboza, Ariza Espinar, Morero, Gurvich, Tecco, Subils and Cabido2011). The climate is monsoonal; mean annual temperature is 19°C and mean annual rainfall is 700 mm (which mainly falls in summer, between November and March), with a long dry season in winter, from April to October (Capitanelli, Reference Capitanelli, Vazquez, Miatello and Roqué1979).
Seed collection and initial characterization
In January and February 2014 we collected fruits from the two fruit morphs belonging to V. caven. In the study area a single individual may have either both fruit morphs or only one morph. The fruits were collected from at least 20 different individuals from the Chaco Serrano of Córdoba, Argentina. For each fruit morph we mixed the seeds from all the individuals and performed a pool of seeds for each morph. From this pool, we randomly chose 100 seeds of each morph and weighed each of them with a precision balance (±0.1 mg). In addition, in order to obtain the initial degree of PY in seeds of each morph before the burial experiment, we conducted a germination experiment in the laboratory. Three replicates (20 seeds each) of each of the two morphs were put to germinate for 30 days. After that period, the seeds were scarified individually with sandpaper and placed again to germinate for 30 days. Seeds were placed in 9-cm diameter Petri dishes on filter paper moistened with distilled water and incubated in a germination chamber at 25 ± 1°C (12 h/12 h daily photoperiod, light density of about 30 μmol m–2 s–1).
Burial experiment
To study patterns of dormancy break in field conditions, in April 2014 we buried fresh seeds of V. caven in 0.3-mm mesh nylon bags in the field (Van Staden et al., Reference Van Staden, Kelly and Bell1994). We closed the bags with sealing tape of different colours in order to distinguish the morphs at the time of exhumation. The date for seed burial corresponds to the end of the primary dispersal of both morphs in field. The burial area was located in La Bolsa (31°44′16.46′′S, 64°25′31′′W), Córdoba, Argentina, in the Chaco Serrano region where the species occurs naturally. The burial area was 2.5 × 5 m and the bags were buried 5 cm deep in the soil and protected against large seed removers with a wire cloth. In order to cover potential local heterogeneity within the burial area, bags were randomly distributed but ensuring that always, at each point of the burial area, bags of both morphs were presented. Each nylon bag contained 100 fresh seeds. Every 3 months from the start of the experiment, five nylon bags per morph were extracted from the soil. Thus, we buried 55 nylon bags per morph with a total of 5500 seeds of each morph. However, the experiment ended when 25 nylon bags were extracted because by that time all seeds of both morphs within the extracted bags had died or germinated, and therefore there were no dormant seeds. The experiment lasted 14 months.
Environmental conditions were measured in the burial area. Daily maximum, minimum and average temperatures were recorded using two sensors (Thermochron iButton DS1921G) which obtained data every 3 h during the whole experiment. In addition, a rain gauge was placed next to the burial area and the amount of rain (mm) was recorded after every rainy event.
After each exhumation, we spread the contents of each bag and counted: (a) the number of clearly germinated seeds, i.e. seeds with easily distinguishable roots (hereafter referred to as emerged seedlings); (b) the number of hard seeds; (c) the number of dead seeds, i.e. seeds that exhibited a high degree of fungal infection or that were rotted, and (d) the number of missing seeds, i.e. even though the bags were not damaged we detected lack of some seeds, probably due to a high seed decomposition or germination events a long time before bag extraction. The seeds that were classified as hard seeds (item b) were allowed to germinate in Petri dishes (with a maximum of 25 seeds each) for 30 days, and those that had not germinated after that period were scarified with sandpaper and placed to germinate for 30 days. This procedure was followed in order to reclassify the hard seeds into the following categories: (b1) germinable (seeds that germinated in the laboratory without scarification); (b2) dormant (hard seeds that germinated in the laboratory after mechanical scarification); and (b3) dead (seeds that were detected as dead in the laboratory experiment and seeds that died in the burial experiment (item c)). Finally, we obtained five categories of seeds: (1) emerged (germinated under field conditions), (2) germinable (germinated in the laboratory without scarification), (3) dormant (germinated in the laboratory after scarification), (4) dead (rotted or showing fungal infection) and (5) missing (Holmes and Moll, Reference Holmes and Moll1990).
In addition, in order to know if the buried seeds effectively need an environmental signal for breaking dormancy, other seeds of both fruit morphs were maintained in the laboratory under dark and dry conditions during the whole burial experiment. From this laboratory pool, on each exhumation date, seeds of each morph (three Petri dishes per morph, 15 seeds each) were also allowed to germinate in a germination chamber for 30 days (control experiment). After that period, seeds that had not germinated were scarified and placed again in the germination chamber for 30 days. These seeds were classified as germinable, dormant or dead. For all the experiments the germination chamber was set at 25 ± 1°C (12 h/12 h daily photoperiod, light density of about 30 μmol m–2 s–1). Seeds were checked for germination twice a week and germinated seeds were recorded and removed. The Petri dishes were moistened when necessary.
Histological measurements of the seed coat
In order to relate the patterns of dormancy break in the field in seeds with PY to histological features of the lens that could differ between morphs, the structure of the epidermis in the lens zone was studied in seeds of both fruit morphs of V. caven. Five seeds obtained from mature fruits of each morph were used for histological characterization of the palisade cell layer in the lens zone. To soften the seeds, they were scarified with sandpaper at the opposite side of the hilar zone and placed to imbibe water in Petri dishes on filter paper moistened with distilled water. The seeds were cut into thick portions near the hilar zone with a razor blade, fixed in 2.5% glutaraldehyde–2% paraformaldehyde, and embedded in Spurr low viscosity resin (embedding media kit, data sheet #217, Polysciences, Inc.). Semi-thin sections (0.3–0.4 µm thick) were made using a diamond knife of an ultramicrotome (Leica Ultracut R). The sections were stained with 1% Aniline Toluidine as a general stain to distinguish lignified and pectic cell walls, and observed under a light microscope (Nikon Eclipse Ti). A digital photograph of each seed was taken to measure the length of the epidermis using the imaging software Nis Elements-BR. Measurements were made in radial lines through epidermis, from the cuticle to the parenchyma layer. In V. caven, the lens zone is a depression in the seed coat and might correspond to a thin structure of the epidermis that is different from the rest of the seed coat epidermis (Venier et al., Reference Venier, Funes and García2012b). Thus, in each seed, height of the epidermis was measured at three points: in the centre of the lens and to the left and right sides of the lens.
Data analysis
Differences in seed mass between seeds of both fruit morphs were evaluated using generalized linear models (GLM) with a Gaussian error structure. Differences between fruit morphs and dates (five exhumation dates) in seed proportion in each of the five classes of seeds were analysed with a GLM with binomial error structure with a logit link function or quasi-binomial error structure when data were overdispersed. For missing and dormant seeds, dates with zero seeds in those categories were not included in the analysis. When differences among treatments were significant, a ‘Di Rienzo, Guzmán and Casanoves’ (DCG) a posteriori test was performed for all the variables (Di Rienzo et al., Reference Di Rienzo, Guzmán and Casanoves2002). Histological measurements of seed coats were analysed with GLMs with a Gaussian error structure correcting for the heteroscedasticity in the factor morph using the function varIdent. All analyses were performed using the software InfoStat (Di Rienzo et al., Reference Di Rienzo, Casanoves, Balzarini, Gonzalez, Tablada and Robledo2009) and its interface in R (R 2.15.0, R Development Core Team, 2012).
Results
Initial seed characterization
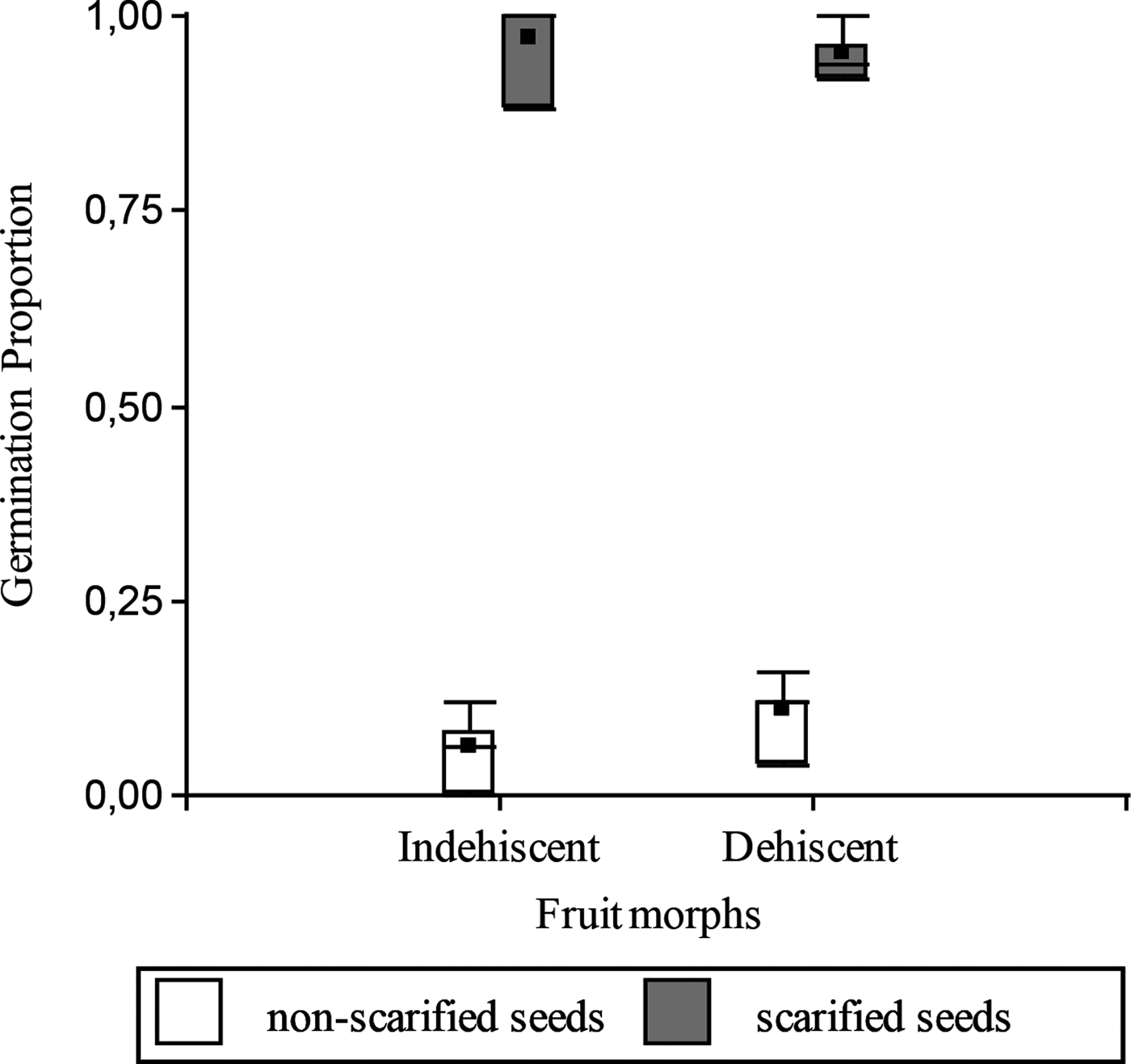
Seed mass was not statistically different between morphs (mean ± SE for both morphs: 0.1 ± 1.3 × 10–3 g; F = 0.13; P = 0.714). In the laboratory, before the burial experiment (April 2014), the proportion of germinable seeds (without scarification) was low (less than 0.20) and did not differ between fruit morphs (t = 1.31; P = 0.2390; Fig. 1); hence both fruit morphs had a similar degree of initial PY. Moreover, scarified seeds of both fruit morphs showed high germination proportion, without significant differences between them (t= –0.51; P = 0.6258; Fig. 1).
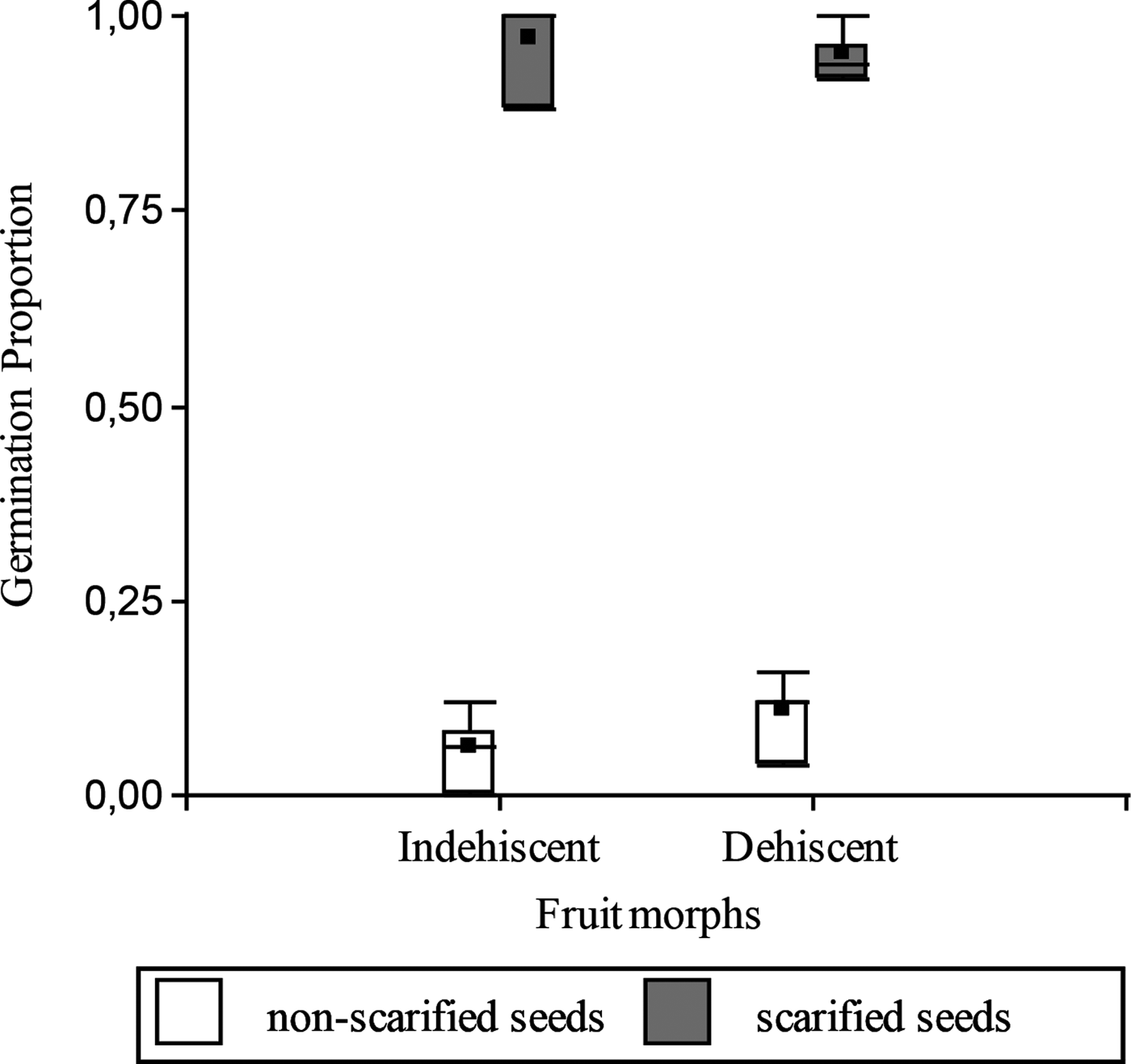
Figure 1. Seed germination proportion of scarified (grey) and non-scarified (white) seeds of the dehiscent and indehiscent morph of Vachellia caven, in the laboratory before the burial experiment (April 2014).
Burial experiment
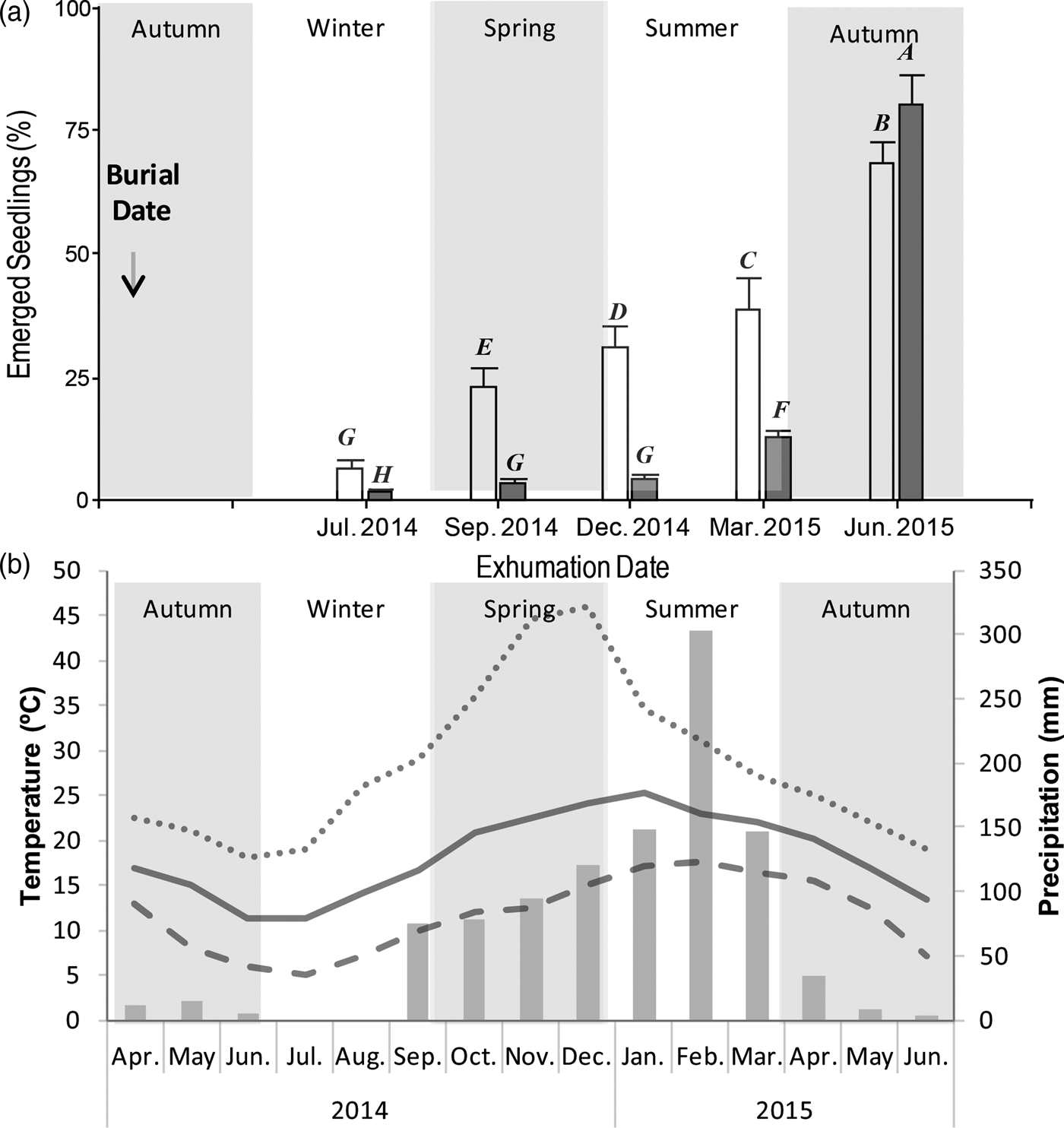
Seeds of the two morphs of V. caven showed different temporal patterns of PY break under field conditions (Fig. 2). For the indehiscent fruit morph, a gradual increase in the number of emerged seedlings was observed during the experiment, whereas the dehiscent fruit morph showed a remarkable increase of emerged seedlings at the end of the experiment. The number of emerged seedlings was different between fruit morphs on most of the exhumation dates, with a higher percentage of emerged seedlings for the indehiscent morph on all the dates except in June 2015, when the pattern was reversed and a higher germination percentage was observed in the dehiscent morph (Fig. 2a, Table 1).
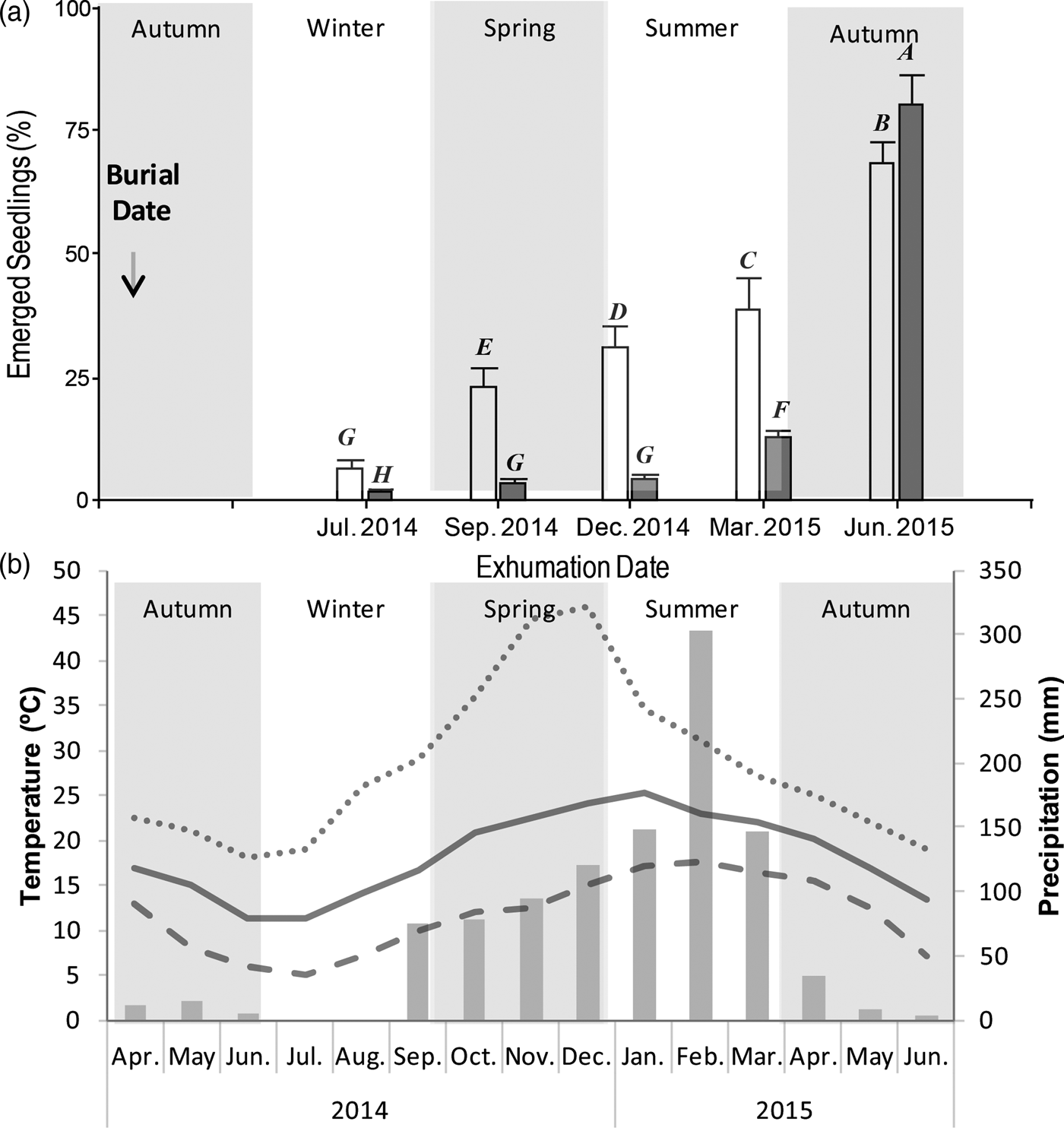
Figure 2. Proportion of emerged seedlings from two morphs of Vachellia caven. Seeds were buried under field conditions and exhumed on five dates. (a) Germination proportion of seeds from indehiscent (white) and dehiscent (grey) morphs (mean ± SE). Different letters over each bar indicate significant differences in the post hoc test for the interaction term (morph×date). (b) Temperatures and precipitation during the burial experiment. Lines represent absolute month maximun (dotted), minimum (dashed) and mean (continuous) temperatures (°C); barrs represent total monthly precipitation (mm).
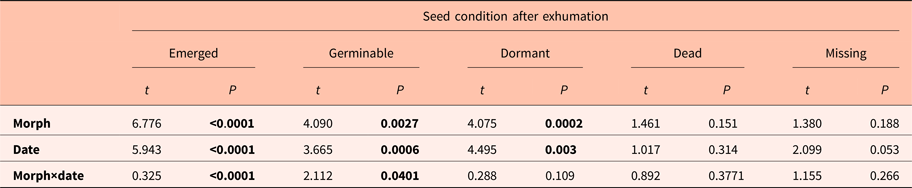
Table 1. Statistics (t values) and P values for the proportion of seeds under different conditions in relation to morphs (dehiscent and indehiscent), exhumation date (five dates) and their interaction

Statistics were obtained from GLMs (see supplementary Table S1 for means and standard error of each seed category on each date). Significant P values (P ≤ 0.05) are indicated in bold.
Temperature and precipitation decreased from the first autumn until the middle of the winter was observed (Fig. 2b). After that, an increase in temperature and precipitation was recorded until the beginning of summer. During spring and early in summer maximum soil temperatures were reached, and the highest thermal amplitude was recorded. The maximum amount of precipitation occurred in February. No freezing event occurred throughout the year.
The different proportions of non-emerged seedlings (germinable, dormant, dead and missing seeds) from exhumed bags per fruit morph and date are summarized in Fig. 3a,b. Significant differences were observed in the proportion of germinable and dormant seeds between fruit morphs and among dates (Table 1). The highest germinable seeds values were observed on the first, second and fourth exhumation dates in the indehiscent morph, whereas very few germinable seeds were observed in the dehiscent fruit morph on all dates. The highest proportions of dormant seeds were observed on the first three dates for the dehiscent fruit morph, being lowest on the fourth date for both morphs; neither morph had dormant seeds on the last date. Dead and missing seed proportions were not different between fruit morphs and among exhumation dates (Table 1; Fig. 3a,b).

Figure 3. Mean ± SE of the cumulative proportion of dormant (white), germinable (grey), dead (light grey) and missing (black) seeds, inside exhumed bags (a,b) and control seeds (maintained under laboratory conditions) (c,d) of the two morphs of Vachellia caven on five dates.
The pool of seeds that were mantained in the laboratory (control) showed no differences in the proportion of germinable, dormant or dead seeds between fruit morphs or dates (Fig. 3c,d; supplementary material Table S2). The proportion of dormant seeds was high for both fruit morphs during the whole experiment in the laboratory.
Histological measurements of seed coat
The mean height of the epidermis in the centre of the lens did not showed significant differences between seeds from both fruit morphs (mean ± SE: 71.22 ± 3.84 and 61.73 ± 5.09 µm for seeds of the indehiscent and dehiscent fruit morph, respectively; F = 2.22, P = 0.1749). Also, no differences were found for the length of the epidermis at the other two measured points (left of the lens: indehiscent morph mean ± SE = 82.89 ± 2.75 µm; dehiscent morph mean ± SE = 81.28 ± 7.76 µm; F = 0.03; P = 0.8782; right of the lens: indehiscent morph mean ± SE = 84.99 ± 3.58 µm; dehiscent morph mean ± SE = 79.4 ± 7.96 µm; F = 0.41; P = 0.5397).
Discussion
Species that show different fruit morphologies might also differ in other regenerative traits, such as dormancy and germination (Lu et al., Reference Lu, Tan, Baskin and Baskin2010, Reference Lu, Tan, Baskin and Baskin2013; Baskin et al., Reference Baskin, Lu, Baskin, Tan and Wang2014; Lu et al., Reference Lu, Tan, Baskin and Baskin2015; Zhang et al., Reference Zhang, Wang, Baskin, Baskin, Luo and Hu2016). In this study, we analysed PY break in field conditions of two different fruit morphs of V. caven. Consistent differences in the temporal patterns of dormancy break were observed between seeds of both fruit morphs. Contrary to our predictions, the seeds of the indehiscent morph started germination earlier and showed a gradual increase in the percentage of emerged seedlings throughout the experiment. Furthermore, the dehiscent morph showed a marked seedling emergence at the end of the experiment. All in all, we observed that in seeds of both fruit morphs different signals might be involved in breaking PY (Van Assche and Vanderlook, Reference Van Assche and Vandelook2006). Therefore, the existence of both fruit morphs might confer the species with different strategies (a) to cope with environmental heterogeneity and (b) to reduce attack by seed predators through staggered seed germination (Dalling et al., Reference Dalling, Davis, Schutte and Arnold2011) or by preventing the attack by rodents, which cannot easily detect buried impermeable seeds (Paulsen et al., Reference Paulsen, Colville, Kranner, Daws, Högstedt, Vandvik and Thompson2013).
Most seeds of the dehiscent morph germinated abruptly by the end of the experiment after being in the soil for one year, showing that even though their seeds are released early from the fruit, they may persist dormant in the soil until germination. However, before germination occurred we did not observe a single environmental signal, such as frost; certainly, temperatures at 5 cm soil depth during winter were 5°C only on two or three dates. If those temperatures had boosted dormancy break, a high number of seedlings would have emerged during spring or summer (2014–2015; Fig. 2 a,b). Therefore, one possible explanation for the high percentage of emerged seedlings observed in the dehiscent fruit morph in June 2015 is that seeds from this fruit morph requires a period of high temperatures and great thermal amplitude – December 2014 to March 2015 (Fig. 2b) – followed by a decrease in temperature and thermal amplitude – from April 2015 – in order to break seed dormancy. Taylor (Reference Taylor2005) proposed a two-stage softening model for other Fabaceae species. This model includes a first preconditioning stage produced by constant or fluctuating temperatures, and is accelerated by increasing temperatures and humidity typical of summer. The seeds remain impermeable during this step; then there is the second stage of softening, which is achieved by fluctuating temperatures of summer or autumn. Seeds that achieve softening in autumn generally need lower temperatures during the final stage, as with our dehiscent morph. A similar mechanism was more recently described by Gama-Arachchige et al. (Reference Gama-Arachchige, Baskin, Geneve and Baskin2012) through the stepwise PY-breaking behavior of Geranium carolinianum. Also, a sensitivity cycling, of alternating temperatures or alterations of wet–dry conditions to dormancy break in seeds with PY has been proposed in previous studies (Baskin, Reference Baskin2003; Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003; Van Assche and Vanderlook, Reference Van Assche and Vandelook2006; Jayasuriya et al., Reference Jayasuriya, Baskin and Baskin2009).
The indehiscent fruit morph exhibited a gradual increase in the percentage of emerged seedlings from July 2014 (3 months after the start of the experiment). This pattern seems to be related to a gradual increase in thermal amplitudes, at least on the first three exhumation dates (Fig. 2a,b). However, the environmental variables recorded during our burial experiment do not clearly support why seed germination still increased when thermal amplitude decreased. Although there is no strong evidence to support this phenomenon, it has been suggested that dormancy break could be mediated by microorganisms, particularly when a low germination percentage with no clear seasonal pattern is observed, similar to our indehiscent morph (Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003). The presence of a permanent number of non-dormant seeds might be a strategy to explore novel habitats, with seeds ready to germinate in the new environment (Willis et al., Reference Willis, Baskin, Baskin, Auld, Venable, Cavender-Bares, Donohue and Rubio de Casas2014). The fact that this morph has a fruit as a dispersal unit, which is prepared for being dispersed longer distances (Aronson, Reference Aronson1992), might be complemented with their capacity to reach novel environments with a number of seeds able to establish.
It is striking that most of the seeds of both morphs germinated at the beginning of winter, i.e. the highest percentage of emerged seedlings of the indehiscent morph and almost all the emerged seedlings of the dehiscent morph, when precipitation start to decrease until spring. However, a previous study observed that this species has a high germination at 20/10°C (Venier et al., Reference Venier, Cabido and Funes2017) and a high survival of seedlings under water stress (Venier et al., Reference Venier, Cabido, Mangeaud and Funes2013), suggesting that seeds could survive with low water availability in the environment and remain in the seedling stage until the rainy season. This may be a strategy to ensure their establishment, capitalizing on the resources mobilized by the first rains of spring, when much of the annual vegetation has still not established, i.e. establishing V. caven seedlings at a time of low competition.
The seed softening process in species with PY may take place over periods ranging from a few weeks to many years (Taylor, Reference Taylor2005). The species V. caven as a whole showed dormancy break of its seeds in 14 months. This period is brief compared with other species with PY (Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003; Cuneo et al., Reference Cuneo, Offord and Leishman2010; Marchante et al., Reference Marchante, Freitas and Hoffmann2010) but there are species that showed a similar amount of time for breaking PY (Holmes and Moll, Reference Holmes and Moll1990) or even lower (Gama-Arachchige et al., Reference Gama-Arachchige, Baskin, Geneve and Baskin2012). The high field germination of V. caven suggests that, after a disturbance, this species might recolonize new areas from the seeds available in the soil only in the year after the disturbance occurs. However, studies using different burial depths and climatic conditions are necessary to have a broader view of the regeneration behaviour of this species.
The proportion of germinable seeds (i.e. seeds that germinated in the laboratory without scarification) of both morphs was low on the five exhumation dates, suggesting that most of the seeds that break dormancy in the soil obtained a sufficient amount of water to germinate under field conditions. In other Fabaceae (Delonix regia) the number of germinable seeds after being buried was higher in a dry year than in a humid year (Jaganathan et al., Reference Jaganathan, Wu, Han and Liu2017). Therefore, considering that our experiment was performed in a humid year, the amount of germinable seeds could increase in drier years (precipitation from April 2014 to April 2015 in our experiment was 1033.5 mm, whereas the historical precipitation for the study area is 725.5 mm (CIMA-CONICET-UBA)). In addition, during the burial experiment the proportion of dormant seeds was higher in the dehiscent than in the indehiscent fruit morph, with no dormant seeds being recorded on the last exhumation date for either morph. When the dormant seeds were scarified and set to germinate, we observed that most of the seeds were viable. Additionally, in both morphs, in most of the bags exhumed, a proportion of the seeds were dead. Probably, some of these dead seeds had germinated and decomposed after germination long before the end of the experiment, so we were not able to discriminate if they had germinated or simply died due to the action of microorganisms (Dalling et al., Reference Dalling, Davis, Schutte and Arnold2011). All in all, the amount of dead seeds was not different between morphs and did not modify the patterns observed in emerged seedlings. Finally, control seeds that were maintained in the laboratory while the burial experiment was underway did not show an increase in the proportion of germinable seeds, which also suggests the need for an environmental signal for breaking PY.
The different patterns of PY loss shown by seeds of both fruit morphs of V. caven would not be related to differences in the histological structure of the lens, as the results of the measurements in the lens area showed no significant differences between them. Nevertheless, other aspects of the epidermis, such as lignin concentration and how tightly packed the cells are in the palisade layer, are important in determining whether passage of water into the seed is blocked (Kelly et al., Reference Kelly, Van Staden and Bell1992; Baskin et al., Reference Baskin, Baskin and Li2000), and should be incorporated in future studies.
In summary, in this study we observed two different strategies for breaking PY in seeds associated with two different fruit morphs (dehiscent and indehiscent) in V. caven. The presence of these two different PY break seed behaviour, even within the same plant individual, could be considered a bet-hedging strategy and therefore could confer the species with higher possibilities of establishing and coping with environmental heterogeneity (Venable, Reference Venable2007; Jaganathan, Reference Jaganathan2016). Those characteristics may contribute to the understanding of the success of this species in open and disturbed environments where V. caven mainly occurs (Zak and Cabido, Reference Zak and Cabido2002), showing that PY has ecological significance for species in plant communities.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S096025851800003X
Table S1. Mean and standard error for the proportion of seeds of the different categories in relation to morphs and exhumation dates.
Table S2. Statistics and P values for the control experiment separated by type of seeds (germinable, dormant, dead).
Acknowledgements
We thank the Universidad Nacional de Córdoba (UNC) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). We are thankful to Leandro Ortega, Claudia Nome and Valeria Quevedo from CIAP-INTA for technical support in the process of obtaining histological sections of seeds, CIMA/CONICET-UBA for providing historical precipitation data from the 3rd National Communication on Climate Change and Jorgelina Brasca for revising the English style of the manuscript.
Financial support
Funding for this work was provided by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-PIP 0456 CO).