Acute limb ischaemia in children is rare but may lead to life-long complications and limb loss. Its incidence in neonatal population is low, 2.4/1000 newborns admitted to ICUs will experience a thromboembolic event.
Acute limb ischaemia is usually post-traumatic or iatrogenic secondary to catheterisation but rarely secondary to arterial occlusive disease as in adult. No consensus management guidelines exist for acute limb ischaemia in the paediatric population and surgical management of infants is challenging because of the small vessel size and the arterial spasms. So, more conservative approaches as thrombolytic agents or systemic heparin anticoagulation appear to be best supportive care.
Unlike most cases of iatrogenic vascular injuries defined as consecutive to catheterisation procedures, the occlusive artery dissection we present here was related to surgical vascular access devices.
Case study
The patient was a 5-month-old infant with a history of trisomy 21 and failure to thrive, referred to our hospital for perimembranous interventricular communication closure. As femoral arterial puncture failed, vascular access was achieved by a surgical superficial femoral artery denudation. At the fourth post-operative day, he suffered with subacute ischaemia: pain, cold, and pulseless extremity. Doppler ultrasound demonstrated a superficial femoral artery dissection and a popliteal artery thrombosis.
Given the ischaemia worsening in spite of heparin infusion, a lower limb angiography by contralateral femoral puncture was performed and confirmed the arterial injuries (Fig 1). Many percutaneous balloon dilatations were attempted close to the dissection area. Finally, the right iliac dissection was treated by two ABSORB® bioresorbable stents. Angiographic result showed an immediate revascularisation of the superficial femoral artery (Fig 2) and a clinical improvement (palpable pedal pulses, warm foot, and mobility recovery). Surgery was successful and post-operative outcomes were simple, no post-cardiotomy syndrome was reported.
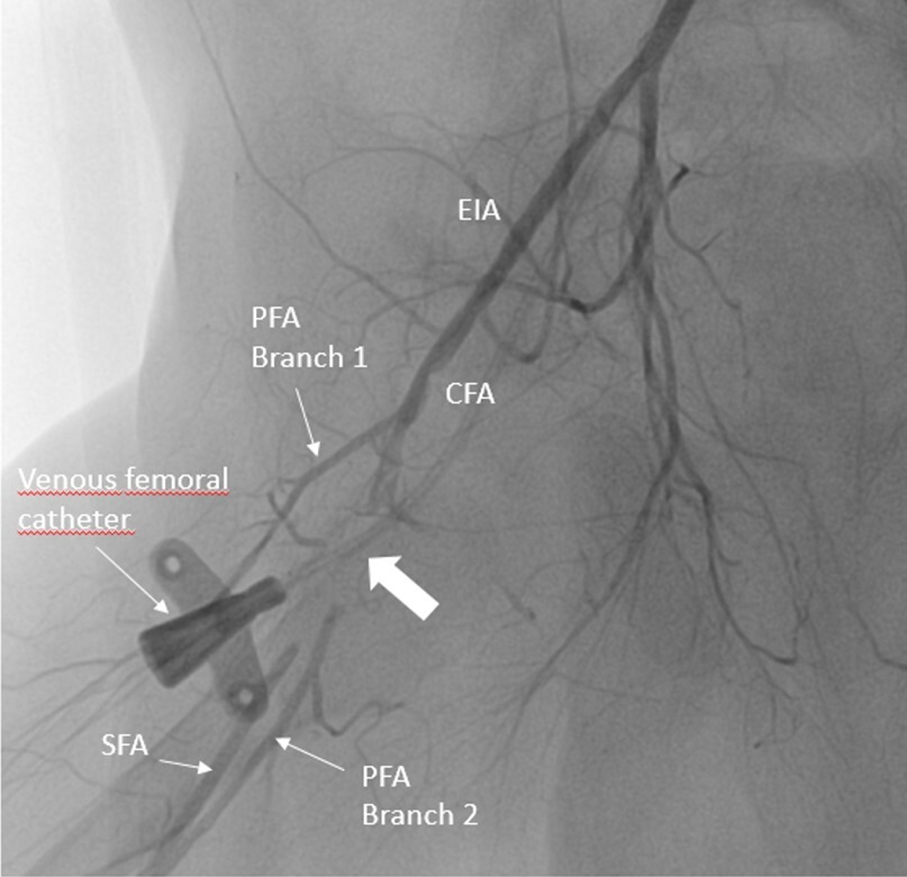
Figure 1. Femoral angiography showing a local occlusive dissection of the right superficial femoral artery. The thick white arrow shows the site of occlusion on the PFA. On the picture, we can see the catheter for femoral central access. CFA = common femoral artery; EIA = external iliac artery; PFA = profunda femoral artery; SFA = superficial femoral artery.

Figure 2. Post-angioplasty angiography of the femoral artery with normalised distal flow and reperfusion of the superficial femoral artery, showing a distal blood flow recovery at the right superficial femoral artery. EIA = external iliac artery; SFA = superficial femoral artery.
At 15 months and at 4 years follow-up, a steady clinical status was achieved: no adverse event was reported, no cardiac shunt was found, and the right leg was properly revascularised.
Discussion
Aetiology
Arterial injuries in children could be congenital or traumatic, but mainly iatrogenic. Catheterisation is responsible for approximatively 90% cases of neonatal limb ischaemia, arterial thrombosis usually occurring at the site of insertion of catheter.
Lesions due to arterial cannulation for cardiac surgery are less common. Very few cases of paediatric acute limb ischaemia as a result of recent surgery were already mentioned, but the type of surgery was not specified. These children were later managed by either surgery or anticoagulation.
So, it seems that we are the first to report a case of acute limb ischaemia following a congenital cardiac surgery in an infant treated by percutaneous angioplasty.
Treatment
Unlike adults, the management of acute limb ischaemia in children is poorly defined, because children differ in physiology and pharmacologic responses. Non-operative treatment methods are generally successful in children, likely because of the early angiogenesis of collateral vessels.
Taub in 2006 was the first to suggest a protocol for management of distal lower extremity ischaemia related to femoral artery catheterisation. Reference Taub, O’Connell and Singh1 Matos et al. in 2012 Reference Matos, Fajardo, Dalsing, Motaganahalli, Akingba and Murphy2 encouraged anticoagulation and surgery only for cases with tissue loss. Finally, guidelines from the American College of Chest Physicians recommended initial therapeutic anticoagulation, preferably unfractionated heparin, for 5–7 days. Reference Monagle, Chan and Goldenberg3 Recently, was proposed a prompt diagnosis with ultrasound followed by anticoagulation and surveillance ultrasound at 2-week intervals. Anticoagulation treatment allows a resolution within 2 weeks in one-third of patients, with few requiring surgical or thrombolytic intervention.
Open surgical revascularisation is rather useful for traumatic vascular injuries in old children with large vessels, when surgery is challenging in younger children according to the vessel diameter and the vasospasm.
Amputation should be delayed for as long as possible since the eventual line of demarcation may be some way distal to the original line of ischaemia. It should be undertaken with full consideration given to future prosthetic limb application and prevention of joint contracture.
In this report, none of these approaches to treat the right leg ischaemia was taken. Revascularisation was achieved through femoral angioplasty with Absorb® Bioresorbable Vascular Scaffold. Control angiography showed immediate and good results as well as a partial collateral circulation (Fig 2).
The first-generation Absorb® Bioresorbable Vascular Scaffold indicated for ischemic heart disease due to de novo native coronary artery lesions was promising, but withdrawn from clinical use in 2017 because of several limitations compared to the metallic stents: delivery issues, stent recoil, limited ability to over-expand, need for very precise sizing, high cost, and poor outcomes in small coronary vessels (<2.5 mm). Randomised trials demonstrated major adverse cardiac events and higher frequency of stent thrombosis compared to second-generation metallic everolimus drug-eluting stents. Reference Smits, Chang and Chevalier4,Reference Serruys, Chevalier and Sotomi5 Despite lack of superior outcomes in the coronary arteries, some trials suggest that the use of Absorb® scaffold in the periphery arteries is safe, technically feasible, and associated with improved clinical status. Drug-eluting stents in peripheral arteries principal drawback are the exposition to elevated mechanical forces and consequently a high risk of stent fracture. Their lasting durability in the vessel impairs vasomotor tone, remodelling, and autoregulation. Short-term safety of an Absorb Bioresorbable Vascular Scaffold was demonstrated in simple lesions below the knee, reporting excellent 6, 12, and 36-month vessel patency rates. Reference Varcoe, Schouten, Thomas and Lennox6–Reference Varcoe, Thomas and Lennox8 The treatment of advanced, infrapopliteal disease, with complex lesions, is successful, with low rates of driven revascularisation and amputations at 12 months. Reference Dia, Venturini and Kalathiya9 Same results were obtained in an Asian adult cohort with chronic limb-threatening ischaemia. Reference Kum, Ipema and Chun-Yin10
In conclusion, we report originally the case of an acute limb ischaemia occurring after a paediatric cardiac surgery and treated by angioplasty. Our work supports the evidence that Absorb® Bioresorbable Vascular Scaffold is safe and efficient and provides short-term vascular support before degrading to allow restoration of vessel vasomotor tone and endothelial function.
Acknowledgements
We thank Dr. Rene Pretre for its contribution to surgical interventricular communication closure, Dr. Nicolas Durand for anesthetic management, and Dr. Claude Mialhe for its expertise, advices, and suggestions on the manuscript. We thank the surgical team for its outstanding work.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the national guidelines and local regulations (Monegasque Law n°1.265 of 23 December 2002 on the protection of individuals with regard to the biomedical research) and with the Helsinki Declaration of 1975 as revised in 2008.





