Introduction
The Eriocaulaceae are perennial or, rarely, annual plants that vary in height from 0.5 cm to 2.0 m (Andrade, Reference Andrade2007); they have pantropical distributions but are concentrated in the Neotropical region (Giulietti and Hensold, Reference Giulietti and Hensold1991). The Cadeia do Espinhaço mountain range in Minas Gerais State, Brazil, is considered to be the principal centre of diversity for this family, and more than 90% of its species are endemic (Giulietti et al., Reference Giulietti, Harley, Queiróz, Wanderley and Van den Berg2005). The family is characterized by its rosette habit, from which scapes emerge bearing inflorescences of the capitulate type. The genus Syngonanthus Ruhland is represented by approximately 200 species (Mabberley, Reference Mabberley1987), and was divided by Ruhland (Reference Ruhland and Engler1903) into five sections based on floral characteristics: Syngonanthus Ruhland, Carphocephalus (Koern.) Ruhland, Chalarocaulon Ruhland, Eulepis (Bong. ex Koern.) Ruhland and Thysanocephalus (Koern.) Ruhland. A number of species of this genus are popularly known as ‘sempre-vivas’ (ever-living plants) and have significant commercial value (Giulietti et al., Reference Giulietti, Giulietti, Pirani and Menezes1988). Endemism and the intensive harvesting of these plants for commercial purposes have resulted in some species being classified as threatened with extinction (Giulietti et al., Reference Giulietti, Giulietti, Pirani and Menezes1988). The different species occur in regions of campos rupestres of the Cadeia do Espinhaço Range with distinct soil water conditions, such as sandy soils, bogs and/or swampy areas, and at altitudes that vary between 800 and 2000 m (Giulietti et al., Reference Giulietti, Harley, Queiróz, Wanderley, Pirani, Cavalcnti and Walter2000). The occurrence of these plants across various habitats is reflected in physiological, anatomical and morphological adaptations that vary from species to species, allowing them to survive under even very adverse environmental conditions (Angosto and Matilla, Reference Angosto and Matilla1993; Menezes and Giulietti, Reference Menezes and Giulietti2000). As such, the chances of a non-dormant seed developing into a healthy seedling will depend on the environmental conditions it can tolerate during germination (Sheldon, Reference Sheldon1974).
Germination is one of the most critical stages in the life cycle of any plant (Kigel, Reference Kigel, Kigel and Galili1995; Villalobos and Peláez, Reference Villalobos and Peláez2001), and two of the most important environmental factors influencing (or controlling) this process are light and temperature (Baskin and Baskin, Reference Baskin and Baskin1988; Bewley and Black, Reference Bewley and Black1994; Benech-Arnold and Sánchez, Reference Benech-Arnold, Sánchez, Kigel and Galili1995). Germination patterns reflect the habitat, life strategy, phylogenetic relationships and the geographical distribution of a given species (Schütz and Rave, Reference Schütz and Rave1999; Ellison, Reference Ellison2001). Strategies and life cycles are generally similar among congeneric species and differences in their germination behaviour may reflect adaptations to the habitats in which they are found (Specht and Keller, Reference Specht and Keller1997; Van Assche et al., Reference Van Assche, Van Nerum and Darius2002).
Considering their phylogenetic proximity and the occurrence of species in different microhabitats throughout the region of campos rupestres vegetation, the germination characteristics and seed size of seven species of Syngonanthus were evaluated in the present study under laboratory conditions to test if (1) the germination responses of these different species allow us to characterize a particular germination pattern for the genus; and (2) there is a correlation between the germination characteristics and the geographic distribution as well as the microhabitat of the different species.
Materials and methods
Study area
The Cadeia do Espinhaço Range has been declared a Biosphere Reserve by Unesco (Unesco, 2005). It covers an area approximately 1000 km long (generally N–S) by 50–100 km wide through the states of Minas Gerais and Bahia (Brazil) (Menezes and Giulietti, Reference Menezes and Giulietti2000). The regional climate is considered tropical altitudinal mesothermic (Cwb in the classification of Köppen). The dry season occurs during the austral winter and lasts 6–7 months, followed by the summer rainy season, lasting 5–6 months. The average annual precipitation rate is approximately 1600 mm (Marques et al., Reference Marques, Garcia, Resende and Fernandes2000). The classification of the habitats of species as seasonally xeric, mesic and wetland, was solely based on the soil water status during the year. In the seasonally xeric environments the soil is sandy and allows for more water drainage and therefore the species are subject to water deficit during the dry season. The mesic habitat remains moist throughout the year, with humidity close to saturation of the soil during the rainy season, and the wetland habitat is permanently flooded.
Species studied
Capitula of seven species of Syngonanthus – S. aciphyllus (Bong.) Ruhland, S. anthemidiflorus (Bong.) Ruhland, S. bisulcatus (Korn.) Ruhland, S. caulescens (Poir.) Ruhland, S. gracilis (Bong.) Ruhland, S. verticilatus (Bong.) Ruhland and S. vernonioides – were collected in areas of campos rupestres vegetation in the Cadeia do Espinhaço Range in Minas Gerais State, south-eastern Brazil. Information concerning the geographic distribution of the species, as well as additional collection data is provided in Table 1. Collections of capitula were undertaken during the months of July/August (with the exception of S. vernonioides, collected in November) when these structures were mature and their seeds were dispersed. The capitula were subsequently triturated in a blender and sieved to separate the seeds (Oliveira and Garcia, Reference Oliveira and Garcia2005).
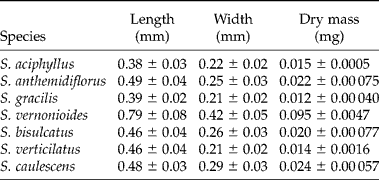
Table 1 List of species, their geographical distributions and data concerning the location of collections of the studied populations

Seed size
Data concerning the length (mm) and width (mm) of the seeds was based on a sampling of 100 individuals, and data of dry mass (mg) was based on a sampling of 400 seeds distributed over four lots of 100 seeds. As the seeds of S. gracilis were very light, however, four lots of 150 seeds were used to determine their average dry mass. The seeds were dried to a constant weight at 105°C and then weighed using an analytical balance. Fresh seeds were digitally photographed and their length and width measurements were calculated using Quantikov Image Analyzer software (Pinto, Reference Pinto1996).
Germination
Germination tests were performed in germination chambers under a 12-h photoperiod (30 μmol m− 2 s− 1) and continuous darkness at constant temperatures from 10 to 40°C at intervals of 5°C. S. vernonioides was not tested at 10°C due to its very low germinability at 15°C. For the germination tests, the seeds were placed in Petri dishes lined with a double layer of filter paper dampened with a nystatin solution (100,000 UI ml− 1) (Oliveira and Garcia, Reference Oliveira and Garcia2005) that maintained a high humidity in the dishes for the entire duration of the experiments. The dark treatment was obtained by wrapping the Petri plates in aluminium foil and black polyethylene bags. The germination of these seeds was evaluated under green light. A total of 200 seeds distributed over four replicates of 50 seeds was used in all light and temperature treatments. Germination was defined as radicle emergence and was verified on a daily basis using a stereomicroscope. The samples were observed until the germination response became constant.
Statistical analyses
All of the data were submitted to non-parametric statistical analyses as they did not demonstrate normality using the Shapiro–Wilk test nor homogeneity using the Brown–Forsythe test (JMP software package, 2002; SAS Institute Inc., Cary, North Carolina, USA). Germination rate was calculated (V = 1/MT, where MT = Σ(Dn)/Σn); where n is the number of seeds that germinate on day D, and D the number of days from the beginning of the germination experiment (Labouriau, Reference Labouriau1983). The optimal germination temperature was defined as that temperature showing the greatest germinability associated with the greatest germination velocity (Labouriau, Reference Labouriau1983). The germinability and average velocity data were compared using the Kruskal–Wallis test, followed by pair comparisons using the Conover test at a 5% significance level (Conover, Reference Conover1999). The statistical analyses were performed using BrightStat software (Stricker, Reference Stricker2008).
Results
Seed size
The different species of Syngonanthus produced very small elliptical seeds that were reddish brown when mature. Sizes varied significantly among the various species, ranging from 0.38 to 0.79 mm in length, and from 0.21 to 0.42 mm in width, and had dry weights between 0.014 and 0.095 mg (Table 2). The seeds of S. vernonioides had the largest values for all three parameters; the seeds of S. gracilis and S. aciphyllus had the shortest lengths; the species S. verticilatus and S. gracilis had the smallest widths; and the seeds of S. gracilis the lowest dry weights.
Table 2 Seed sizes: length, width and dry weight (average±standard error) of seven species of Syngonanthus

Germination characteristics
The seeds of six of the species studied germinated exclusively in the presence of light and within a relatively wide temperature range (Fig. 1); only the seeds of S. gracilis demonstrated low germinability in continuous darkness (maximum of 22% at 35°C). In general, the seeds of species from seasonally xeric habitats germinated within the range of 10–40°C, albeit to only low percentages at the highest temperatures (Fig. 1). The germinabilities of S. gracilis and S. aciphyllus were significantly lower at 10°C. S. vernonioides had low germinability in the range of 20–30°C, and at 15°C its germinability was less than 10% (Fig. 1).

Figure 1 Final germination percentages of the seeds of seven species of Syngonanthus; 12-h photoperiod, at constant temperatures of 10–40°C. Error bars indicate standard error; shaded bars indicate optimal temperature. SX, seasonally xeric; M, mesic; W, wetland habitat.
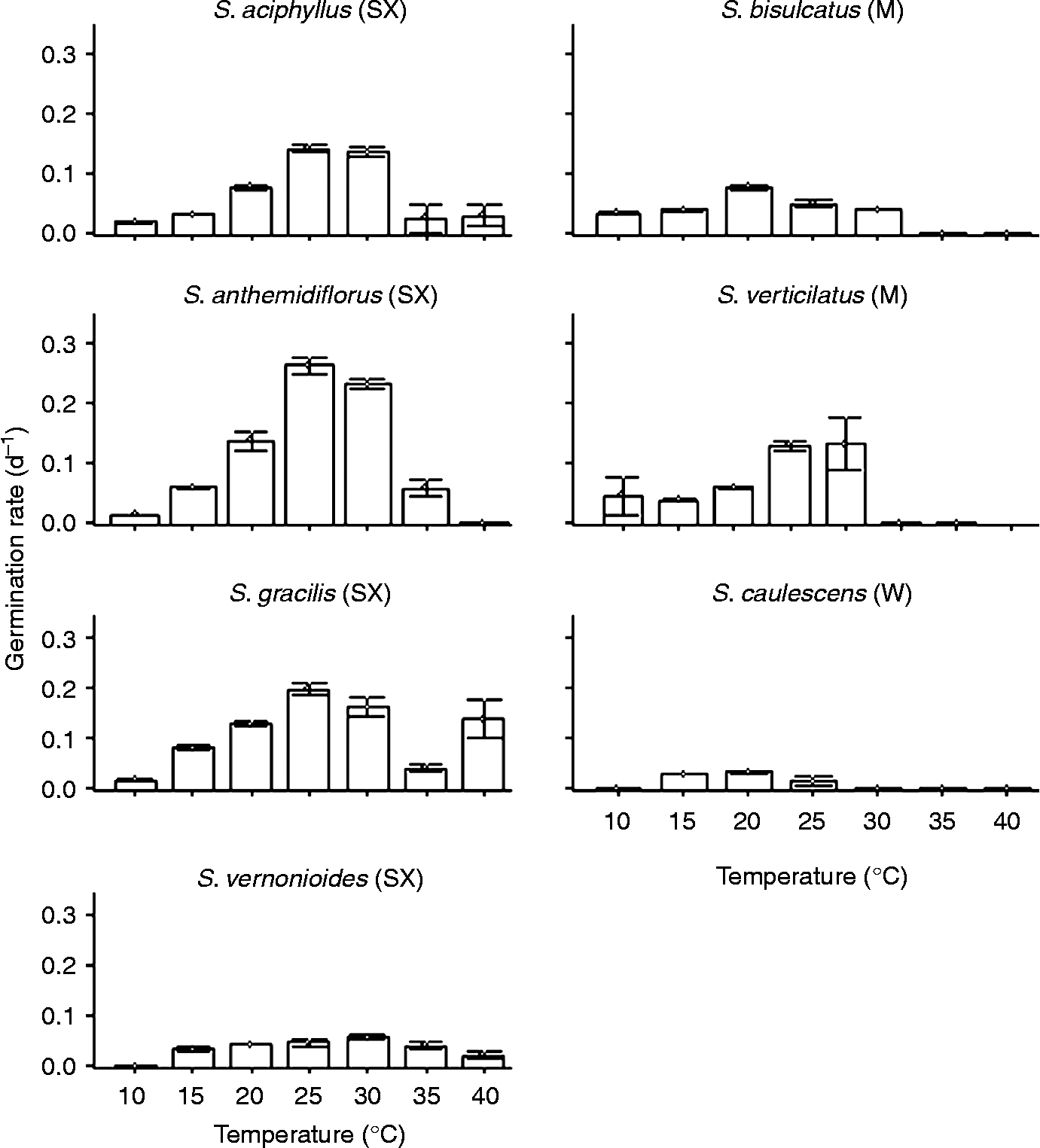
The seeds of species from mesic habitats germinated between 10 and 30°C and germination was completely inhibited at temperatures of 35 and 40°C (Fig. 1). S. caulescens, the only species from a wetlands environment, germinated only within the temperature range of 15–25°C, and even then with very low germinability (maximum of 19%) (Fig. 1). The seeds of S. aciphyllus, S. gracilis and S. verticilatus displayed highest germination velocity in the temperature range from 25 to 30°C; the seeds of S. anthemidiflorus germinated most rapidly at 25°C and the germination velocity of S. bisulcatus was greatest at 20°C (Fig. 2). The germination velocity of these five species was lowest at both the lowest (10 and 15°C) and highest (35 and 40°C) temperatures. The germination velocity of S. caulescens was constant in the range from 15 to 25°C; it was likewise constant for S. vernonioides in the range of 20–30°C, but reduced at extreme temperatures (Fig. 2). The optimal temperature for germination was also habitat dependent, being between 25 and 30°C for species from seasonally xeric habitats, and 15 and 20°C for species from mesic and wetland habitats.

Figure 2 Germination rate (d− 1) of the seeds of seven species of Syngonanthus; 12-h photoperiod, at constant temperatures of 10–40°C. Error bars indicate standard error. SX, seasonally xeric; M, mesic; W, wetland habitat.
Discussion
The seeds of the seven species of Syngonanthus investigated were all very small (sensu Hughes et al., Reference Hughes, Dunlop, French, Leishman, Rice, Rodgerson and Westoby1994; Ekstan et al., Reference Ekstan, Johannessson and Milberg1999), with dry mass and lengths less than or equal to 0.1 mg and 1 mm, respectively. Generally, very small seeds do not contain sufficient nutritional reserves to complete embryonic development and germinate independently, as occurs in species of Orchidaceae and Orobanchaceae (Hilhorst, 2008). However, in spite of their very small size, the seeds of Syngonanthus have sufficient nutritional reserves to complete germination.
The seeds showed a light requirement for germination, a characteristic generally associated with small seeds (Thompson and Grime, Reference Thompson and Grime1983; Bewley and Black, Reference Bewley and Black1994; Milberg et al., Reference Milberg, Anderson and Thompson2000) and open habitats (Leishman and Westoby, Reference Leishman and Westoby1994; Milberg et al., Reference Milberg, Anderson and Thompson2000). Small and positively photoblastic seeds have been reported in other studies with species of Eriocaulaceae, Xyridaceae and Velloziaceae – families typical of campos rupestres vegetation and open environments exposed to high-intensity solar illumination (Mercier and Guerreiro-Filho, Reference Mercier and Guerreiro-Filho1989; Sá e Carvalho and Ribeiro, Reference Sá e Carvalho and Ribeiro1994a, b; Scatena et al., Reference Scatena, Lemos and Lima1996; Garcia and Diniz, Reference Garcia and Diniz2003; Abreu and Garcia, Reference Abreu and Garcia2005; Oliveira and Garcia, Reference Oliveira and Garcia2005; Garcia et al., Reference Garcia, Jacobi and Ribeiro2007; Schmidt et al., Reference Schmidt, Figueiredo, Borghett and Scariot2008).
Temperature is another important environmental factor that controls germination (Baskin and Baskin, Reference Baskin and Baskin1988; Bewley and Black, Reference Bewley and Black1994; Benech-Arnold and Sánchez, Reference Benech-Arnold, Sánchez, Kigel and Galili1995), and seeds germinate within certain temperature ranges that are characteristic of each species (Bewley and Black, Reference Bewley and Black1994). The permissive temperature range for germination can determine the geographic distribution of plants (Thompson, Reference Thompson and Heydecker1973; Labouriau, Reference Labouriau1983; Probert, Reference Probert and Fenner1992) and reflects the adaptation of the species to the environments in which they occur naturally (Schütz and Rave, Reference Schütz and Rave1999).
S. gracilis and S. caulescens are widely distributed species in South America. S. aciphyllus, S. anthemidiflorus, S. bisulcatus and S. verticilatus are found in many regions of the Cadeia do Espinhaço Range, while S. vernonioides is only encountered in a small part of that mountain range. S. caulescens, the only species that occurs in a wetland environment, is the most widely distributed taxon, but had the most restricted temperature range for germination among the species examined here. Other species of Eriocaulaceae (Oliveira and Garcia, Reference Oliveira and Garcia2005), Xyridaceae (Abreu and Garcia, Reference Abreu and Garcia2005) and Velloziaceae (Garcia and Diniz, Reference Garcia and Diniz2003; Garcia et al., Reference Garcia, Jacobi and Ribeiro2007) that are widely distributed (or not) within the Cadeia do Espinhaço Range germinate under a wide range of temperatures. Baskin and Baskin (Reference Baskin and Baskin1988) noted that only the germination characteristics of a species could be eliminated as determinant factors in endemism because these are usually quite similar within a given genus even though it may contain both endemic and widely distributed species. Likewise, the data presented here indicates that the temperature ranges for germination cannot explain the geographic distribution of the species in the present study.
The temperature limits for germination and the optimal temperatures that were observed among the species examined here demonstrated a relationship between water resource availability in the microhabitats that they naturally occupy and their germination responses. S. bisulcatus, S. verticilatus and S. caulescens grow in mesic/wetland environments and did not germinate at the highest temperatures (35 and 40°C; optimal temperatures 15 and 20°C), while S. aciphyllus, S. vernonioides, S. anthemidiflorus and S. gracilis are found in seasonally xeric environments and germinated even under the highest temperatures tested (though at low percentages; optimal temperature 25 and 30°C). Similar results have been observed in other studies with campos rupestres species. Oliveira and Garcia (Reference Oliveira and Garcia2005), for example, noted that high temperatures favoured the germination of S. elegans (a species from seasonally xeric environments) but inhibited germination of S. elegantulus and S. venustus, both from swamp habitats. In addition, Abreu and Garcia (Reference Abreu and Garcia2005) demonstrated that four species of Xyris, typical of boggy soils in campos rupestres vegetation (Wanderley, Reference Wanderley1992), did not germinate at high temperatures. Species of Syngonanthus (Oliveira and Garcia, Reference Oliveira and Garcia2005; and the present study) and Xyris (Abreu and Garcia, Reference Abreu and Garcia2005) generally occur in sandy quartzite soils where high soil humidity dampens the amplitudes of thermal fluctuations and helps maintain relatively low ground temperatures, which is reflected in the low germinability of the seeds of species from mesic/wetland environments at temperatures above 30°C.
The results obtained in the present study demonstrate that, in spite of the variability observed in terms of seed size, all were considered to be very small and positively photoblastic, indicating a pattern for the seeds of the genus Syngonanthus in relation to their size and their light requirements for germination. The geographic distribution of different species of the genus cannot be explained by the responses of seed germination at different temperatures, but suggests some relationship to their edaphic microhabitats, and therefore germination characteristics of the populations studied may have been selected to colonize specific environments at different soil water conditions.
Acknowledgements
The authors thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for providing a grant and financial support to the first author; the Fundação o Boticário de Proteção à Natureza (FBPN) (Process 0662-20051) and FAPEMIG (Process CRAAPQ 4654-5.04/07) for financing the project; the Instituto de Ciências Biológicas (UFMG) for its support; Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) for the collecting licence and the employees of the Parque Nacional da Serra do Cipó for their logistic support. Q.S.G. receives a research productivity scholarship from Conselho Nacional de Pesquisa (CNPq).