Noonan syndrome is a genetic disorder with a constellation of malformations involving a variety of systems, including distinctive facial features, developmental delay, renal anomalies, lymphatic malformations and congenital heart disease. Reference Roberts, Allanson, Tartaglia and Gelb1–Reference Turner3 With an estimated prevalence of 1 in 1000–2500 live births, Reference Roberts, Allanson, Tartaglia and Gelb1 Noonan syndrome is either inherited in an autosomal dominant fashion or occurs sporadically due to a de novo mutation caused by a variety of gene mutations affecting the RAS-MAPK pathway, Reference Liao and Mehta4,Reference El Bouchikhi, Belhassan and Moufid5 placing Noonan syndrome within a larger family of genetic syndromes referred to as “RASopathies.” Reference Tidyman and Rauen6
While Noonan syndrome affects many organ systems, the cardiac anomalies are among its more dramatic manifestations and contribute to mortality and disability among Noonan patients. The prevalence of cardiovascular disease among Noonan patients is approximately 80–90%. Reference Karnik and Geiger7 The most common cardiac manifestations are pulmonary valve stenosis (60–70%), hypertrophic cardiomyopathy (20–30%) and atrial septal defect (10–30%), with lower prevalence of atrioventricular canal defect, mitral valve anomalies, coarctation of the aorta, tetralogy of Fallot, ventricular septal defect and aortic root dilation. Reference Pierpont and Digilio8,Reference Prendiville, Gauvreau and Tworog-Dube9 There are some loose genotype-phenotype associations between the specific abnormal gene and the likelihood of a particular cardiac anomaly. Reference Kouz, Lissewski and Spranger10 Patients are at risk for arrhythmia, decreased function or even sudden death; thus, it is important that they are identified and addressed on an individual basis. Intervention and treatment are tailored to the structural issue.
Advances in genetic technology and increased survival of Noonan syndrome patients have led to the identification of an increasingly large population of adults with Noonan syndrome. As fertility does not seem to be affected by Noonan’s syndrome, affected individuals can become pregnant. Several case studies have collectively described safe pregnancy outcomes for women with Noonan syndrome. Reference Chase, Holak and Pagel11–Reference McLure and Yentis18 However, there is a dearth of literature available to counsel patients regarding potential pregnancy risks in the setting of the known medical complications of Noonan syndrome, or how the physiological changes of pregnancy may affect the patient and the developing fetus. Reference Adam19–Reference Sanghavi and Rutherford21
Our study aimed to identify pregnancy-associated risks in the setting of maternal Noonan syndrome during the antepartum, intrapartum and early postpartum periods and how the patient’s cardiovascular history may impact these risks.
Materials and methods
Approval for this study was granted by the Yale University Institutional Review Board. We performed a retrospective chart review of patients at a single centre, Yale-New Haven Hospital, from 2012 to 2020. We utilised the services of the Yale Joint Data Analytics Team to compile a database including all female patients in the electronic medical record with a diagnosis of Noonan syndrome. Inclusion criteria were women who had been pregnant at any time and with a genetic or unambiguous clinical diagnosis of Noonan syndrome. A genetic diagnosis was made by genetic testing which revealed one of the genetic mutations commonly associated with Noonan syndrome. Reference Liao and Mehta4 An unambiguous clinical diagnosis was made by a phenotype consistent with Noonan syndrome and a first-degree relative who had undergone genetic testing (in some cases the offspring of the patient).
Chart review was conducted to obtain medical history, obstetrical history and outcomes of the offspring. Medical history collected included genetic testing, medical conditions, medications and surgical history. Cardiovascular risk was evaluated using Modified World Health Organization Classification of Maternal Cardiovascular Risk and CARPREG II risk index. Reference Suwanrath, Thongphanang, Pinjaroen and Suwanugsorn22,Reference Silversides, Grewal and Mason23 Obstetrical history obtained included pregnancy course (medications, abnormal findings on prenatal screening, pregnancy-related medical conditions), gestational age at delivery, method of delivery, postpartum course (maternal and fetal) and any genetic testing performed. Cardiac phenotype and history of intervention to date for the offspring were also recorded.
Echocardiography images were analysed to obtain quantitative cardiac information for patients. When possible, the most recent echocardiogram prior to first pregnancy was selected for analysis. Alternatively, a recent echocardiogram performed after first pregnancy was accepted. All images were analysed by a cardiologist (RWE) experienced in congenital heart imaging. Pulmonary stenosis was graded as mild, moderate or severe based on the peak gradient across the valve as measured by Doppler ultrasound: mild pulmonary stenosis less than 36 mmHg, moderate between 36 and 64 mmHg, and severe greater than 64 mmHg. Reference Cuypers, Witsenburg, van der Linde and Roos-Hesselink24 Ventricular septal thickening consistent with hypertrophic cardiomyopathy was confirmed if the septum was measured to be greater than 13 mm thick in any view. Reference Hensley, Dietrich, Nyhan, Mitter, Yee and Brady25 Presence or absence of an atrial septal defect was also specifically evaluated in these images.
Results
Five women with Noonan syndrome met inclusion criteria with a combined 10 total pregnancies. Nine out of 10 pregnancies occurred within the 9-year period of record review, with one pregnancy occurring prior to that period.
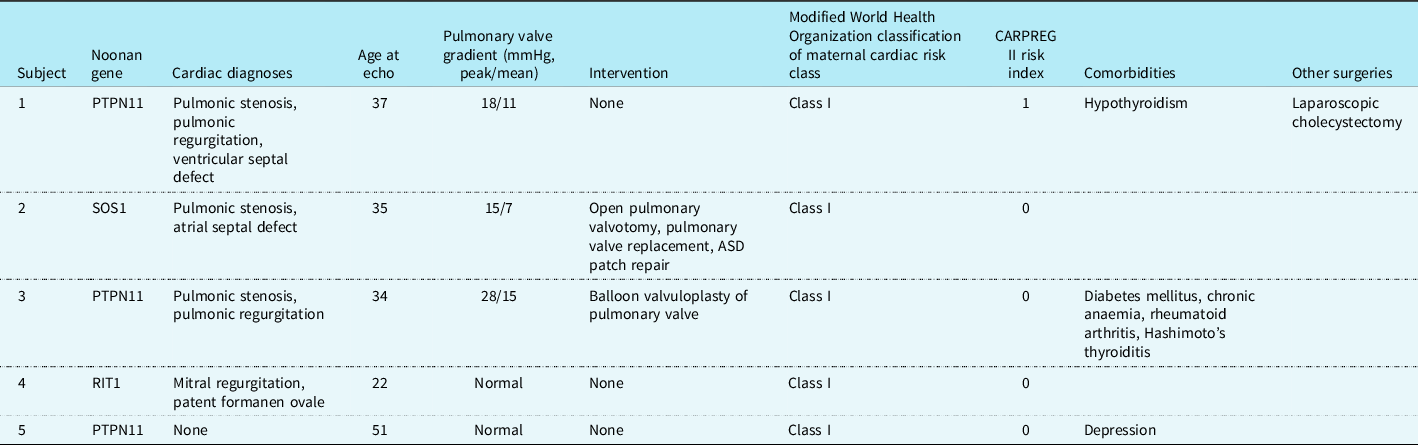
Three women carried a diagnosis of pulmonary valve stenosis (Table 1). One patient had undergone repair by balloon valvuloplasty and one had undergone open pulmonary valvotomy with closure of an ASD as a child followed by pulmonary valve replacement as an adult to optimise haemodynamics prior to pregnancy. At the echocardiogram closest to the time of first pregnancy, all three women with pulmonary valve involvement had mild pulmonary stenosis (range: 15–28 mmHg, mean: 20, SD: 7). None of the patients had hypertrophic cardiomyopathy. One out of five patients (20%) had a repaired atrial septal defect, with an intact atrial septum at the time of most recent echocardiogram. One out of five patients (20%) had no history of cardiac abnormality. All five women were classified as WHO Cardiovascular Risk Class I at the time of pregnancy, predictive of no increased risk or maternal mortality and no or mild increase in morbidity, and had a CARPREG II score of 0–1, indicating a 5% risk of primary cardiac events. None of the women had any other known serious medical comorbidity directly or commonly associated with Noonan syndrome such as renal disease or bleeding diathesis.
Table 1. Maternal medical comorbidities
Of 10 total pregnancies studied, nine (90%) resulted in the birth of at least one living infant, while one pregnancy (10%) resulted in vaginal delivery of a stillborn infant after intrauterine fetal demise (Table 2). Because one delivery was a set of twins, the total number of living infants delivered was 10.
Table 2. Pregnancy outcomes

LOS, length of stay; NICU = neonatal ICU; PPROM, pre-term premature rupture of membranes.
No patient experienced cardiac or other non-obstetric complication during the antepartum period. The single intrauterine fetal demise at 36 weeks of gestation had significant lymphatic abnormalities, conferring a high likelihood of fetal Noonan syndrome. One pregnancy was significant for a self-resolving fetal cystic hygroma, a pre-natal finding that increases the likelihood of fetal Noonan syndrome. One pregnancy was affected by gestational hypertension. No cases of pre-eclampsia or gestational diabetes were identified.
The average gestational age at delivery among the 10 pregnancies was 36 weeks (range: 29–40, SD: 3.5). Five out of 10 pregnancies (50%) resulted in pre-term birth, with an average gestational age among pre-term deliveries of 33 weeks (range: 29–36, SD: 2.2). Of these, four out of five had pre-term premature rupture of membranes, while one had an indicated pre-term birth in the setting of a bleeding placenta previa. Eight pregnancies (80%) required caesarean delivery for various reasons including abnormal fetal testing and failure to progress in labour. Three pregnancies (30%) involved maternal complications at the time of delivery – one bleeding placenta previa required emergent caesarean delivery, one intra-partum haemorrhage during caesarean delivery, and one case of intrapartum chorioamnionitis. These three events resolved without further sequelae.
Six neonates (60%) required care in the neonatal intensive care unit for respiratory difficulties and/or prematurity with length-of-stay ranging from 3 days to 3 months. One neonate, delivered at 32 weeks, died in the neonatal intensive care unit at 5 weeks of age in the setting of severe lymphatic complications of chylous effusions; the remaining neonates were discharged from the hospital. Seven out of 11 neonates (64%), including the one with neonatal demise, received the diagnosis of Noonan syndrome after genetic testing; three were not felt to have Noonan syndrome and the one stillbirth had findings highly suspicious for Noonan syndrome (bilateral pleural effusions and structural heart disease) but was not formally tested.
Discussion
We identified five women with Noonan syndrome with 10 total pregnancies. The majority of mothers had pre-existing, though mild, heart disease. Although many of the pregnancies resulted in living infants, this sample experienced high rates of prematurity, conversion to caesarean section, and neonatal intensive care unit stays for the neonate. No maternal complications resulted in long-term morbidity.
This study is one of the largest groups of pregnant patients with Noonan syndrome described in a single series. Maternal medical complications were low and no maternal morbidity occurred during the antepartum period. Noticeably, there were no cardiac complications, despite the presence of complex congenital heart disease. This was consistent with the low risk of cardiovascular events as predicted for all patients by the modified World Health Organization criteria and the CARPREG II risk index. However, a high rate of obstetrical complications was observed, including pre-term premature rupture of membranes and pre-term birth, need for caesarean delivery, chorioamnionitis and obstetrical haemorrhage. No significant short-term maternal morbidity occurred as a result of these obstetrical complications. These results are consistent with prior studies showing that women with Noonan syndrome can have a safe pregnancy and delivery course.
In contrast, outcomes for the offspring were more varied and are directly associated with the presence or absence of fetal/neonatal Noonan syndrome and/or neonatal prematurity. Aside from the known risk of fetal/neonatal Noonan syndrome and associated complications, the rates of prematurity and the associated sequelae were higher than expected as compared to the general population. Because three cases of pre-term premature rupture of membranes occurred in a single individual, we are unable to determine if the maternal Noonan’s syndrome conferred a direct risk of pre-term premature rupture of membranes or if the individual had other intrinsic risk factors for pre-term premature rupture of membranes. We did not observe an association between maternal congenital heart disease and adverse fetal/neonatal outcomes.
Our risk stratification appeared to appropriately identify patients as low risk for cardiovascular morbidity during pregnancy, and our study reinforces the importance for patients with high risk of cardiac disease to seek multidisciplinary preconception counselling. In fact, one of the patients in our study (patient 2) presented pre-pregnancy for a cardiac evaluation and at the time was found to have severe pulmonary insufficiency and significant right ventricular dilation. She was advised and ultimately went for surgical pulmonary valve replacement with appropriate recovery and right ventricular remodelling prior to her pregnancy, which likely reduced her risk of cardiovascular complications in pregnancy. Thus, all women with Noonan syndrome who wish to become pregnant should undergo preconception counselling, particularly if there has been a prior cardiac history or procedure. While current recommendations for adults with Noonan syndrome suggest regular cardiac monitoring, the frequency of such monitoring might reasonably be increased in the event that a patient wishes to become pregnant.
We observed in our study population that members of the same family demonstrated significantly different phenotypes despite carrying the same gene mutation. Affected mothers should be counselled and monitored closely for every pregnancy, regardless of her own phenotype or the presence of a previous successful pregnancy. They should be advised explicitly on the meaning of an autosomal dominant pattern of inheritance, and their attitudes regarding heritability of a phenotypically diverse syndrome should be explored in depth.
The strength of our study is the use of retrospective chart review to aggregate a sample of patients previously undescribed outside of isolated case reports. The greatest limitation of our study is the small sample size. Some potential subjects could not be included because of a lack of genetic testing, even if there was strong suspicion that the person likely had Noonan syndrome. In future studies, expanding our population to multiple centres might clarify the results of our study, particularly the high rate of pre-term premature rupture of membranes and subsequent fetal and neonatal complications of prematurity. Another limitation of our study is its retrospective nature. While we were able to obtain relevant obstetrical, cardiac and medical data from reviewing patient charts, this information is not always complete or as detailed as desired. There may also be a selective bias for inclusion, in that individuals with more severe manifestations of Noonan syndrome may be unable or unwilling to attempt pregnancy.
Women with Noonan syndrome and low-risk, well-managed cardiac lesions are able to achieve successful pregnancies. When paired with close monitoring and pre-pregnancy optimisation, maternal cardiovascular complications were rare. Obstetrical complications were encountered more often than expected, including increased rates of caesarean delivery and pre-term premature rupture of membranes. Fetal Noonan syndrome and prematurity contributed to worse neonatal outcomes, including stillbirth, prolonged neonatal intensive care unit stays and neonatal demise. This study provides insight to the maternal and fetal risks associated with pregnancy in Noonan syndrome. Future multi-institutional studies of this condition are needed to confirm these findings.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
Our research does not involve human and/or animal experimentation. All research protocols were approved by the Yale University Institutional Review Board.





