INTRODUCTION
Legumes have proved to be a promising option in small-scale farming systems of sub-Saharan Africa by combining benefits for the farmer, soil and environment. The protein rich grains, for example, are an important component in the diet of the mainly subsistence small-scale farmers. Furthermore, the ability to fix atmospheric nitrogen is a valued feature for soil improvement and with potential for sustainable intensification of agricultural systems (Vadez et al., Reference Vadez, Berger, Warkentin, Asseng, Ratnakumar, Rao, Gaur, Munier-Jolain, Larmure, Voisin, Sharma, Pande, Sharma, Krishnamurthy and Zaman2012). Lablab purpureus (L.) Sweet is one of the most diverse domesticated legumes, cultivated mainly in parts of Asia and Africa, and offers many opportunities to improve food and forage production in semi-arid areas (Maass et al., Reference Maass, Knox, Venkatesha, Angessa, Ramme and Pengelly2010). Besides being better adapted to drought than cowpeas (Vigna unguiculata (L.) Walp.) and common beans (Phaseolus vulgaris L.), which are widely cultivated in semi-arid areas of the tropics (Hendricksen and Minson, Reference Hendricksen and Minson1985; Maundu et al., Reference Maundu, Ngugi and Kabuye1999), lablab is highly valued because of its multi-purpose uses that include protein rich grains, healthy vegetable leaf and pod products, high quality forage and green manure. The high agro-morphological and physiological diversity of lablab, in particular, the short-season types, offer additional options for coping with frequent droughts and reductions in rainfall and rainfall reliability, sustaining soil fertility and stabilizing on-farm production (Maass et al., Reference Maass, Knox, Venkatesha, Angessa, Ramme and Pengelly2010). However, to increase the potential adoption by farmers and improve agricultural extension and advisory services in semi-arid areas, the phenological responses of promising short-season lablab types need to be better understood.
Matching crop phenology to environmental and climatic conditions is a key concept to be optimized for efficient resource use in (sub)-tropical farming systems (Imaizumi and Kay, Reference Imaizumi and Kay2006; Lawn and James, Reference Lawn and James2011). In particular, triggering the switch from vegetative to reproductive growth is critically important, since the timing of the transition to flowering and the environmental conditions experienced during this growth phase directly influence yield (Putterill et al., Reference Putterill, Laurie and Macknight2004; Zhang et al., Reference Zhang, Wang and Hesketh2000). Consequently, physiological research is considered to be a fundamental part of crop selection and breeding programmes and can be exploited in cropping system improvement (James and Lawn, Reference James and Lawn2011). Finally, understanding and quantifying the effects and interactions of photoperiod and temperature on flowering control directly helps to predict and model the time of flowering and maturity under different environmental conditions (Zhang et al., Reference Zhang, Wang and Hesketh2000), as long as other important factors like water and nutrient availability are constant.
In summary, photoperiod is considered to be one of the most significant environmental factors influencing flowering time in legumes and the variation in photoperiod sensitivity amongst and within legume species is high (Nelson et al., Reference Nelson, Berger and Erskine2010; Roberts and Summerfield, Reference Roberts, Summerfield and Atherton1987). Three main measures have been developed to describe photoperiod sensitivity. First, the optimum photoperiod where flowering is observed soonest; second, the critical photoperiod – the daylength above or below which flowering is delayed (quantitative response) or inhibited (qualitative response) and third, the photoperiod sensitivity expressed as the delay of flowering per unit change in photoperiod. Legumes from temperate regions are quantitative long-day plants (LDP), whereas most legumes originating from the tropics are quantitative short-day plants (SDP). Flowering of LDP is triggered when the photoperiod exceeds a critical threshold and consequently days get longer. Contrary, SDP flower only when the day length falls below a certain critical photoperiod. However, photo-insensitive or day-neutral plants (DNP) exist within all legume species (Nelson et al., Reference Nelson, Berger and Erskine2010; Roberts and Summerfield, Reference Roberts, Summerfield and Atherton1987). In plant science, photoperiod sensitivity is usually analysed as photothermal response, where both photoperiod and temperature effects are considered simultaneously. Quantitative models to predict flowering time are simplified additive linear models with temperature and photoperiod as possible predictors and flowering time as response variable (Keatinge et al., Reference Keatinge, Qi, Wheeler, Ellis and Summerfield1998; Summerfield et al., Reference Summerfield, Roberts, Ellis and Lawn1991).
Extensive research on photoperiod response of soybean, in particular, has been undertaken because of its economic importance. The findings from Zhang et al. (Reference Zhang, Wang and Hesketh2000) show that the period from emergence to flowering in soybean decreases dramatically when daylength is reduced during late growing season. Further, the authors demonstrate that the degree of reduction in flowering time with photoperiod sensitivity, varies amongst varieties. The authors show that flowering time in late-maturing varieties is stronger controlled by photoperiod than in early-maturing types. For some early-maturing varieties, photoperiod sensitivity could not be detected clearly in field experiments (Zhang et al., Reference Zhang, Wang and Hesketh2000). In growth chamber experiments, the authors demonstrate that long-day photoperiods delay (photoperiod ≥ 14 h) or even inhibit (photoperiod ≥ 16 h) flowering in soybean. However, flowering is influenced by both photoperiod and temperature at the same time, adding to the complexity in understanding photoperiodism. The critical daylength, for example, increases as inverse functions of both increasing photoperiod and decreasing temperature and, consequently, the critical daylength becomes longer with higher mean temperatures (Hadley et al., Reference Hadley, Roberts, Summerfield and Minchin1984). Similar observations are made for cowpea (Vigna unguiculata (L.) Walp.) (Hadley et al., Reference Hadley, Roberts, Summerfield and Minchin1983). Ellis et al. (Reference Ellis, Summerfield, Omanga, Qi and Roberts1998) studied photoperiod and temperature effects on pigeonpea (Cajanus cajan (L.) Millsp.) in Kenya and the authors observed a delay in the progress towards flowering under long-day conditions as well. These researchers further demonstrated that supra-optimal temperature conditions during the photosensitive floral initiation prolonged the vegetative phase of pigeonpea even under short-day conditions (Ellis et al., Reference Ellis, Summerfield, Omanga, Qi and Roberts1998; Omanga et al., Reference Omanga, Summerfield and Qi1995). For chickpea (Cicer arietinum L.), Roberts et al. (Reference Roberts, Hadley and Summerfield1985) made different observations. The authors determined that time to flowering decreases under long day conditions of 15 h in comparison to 12 h photoperiod. However, from the genotypes included in the analysis, early-maturing ones were less sensitive to photoperiod than late-maturing chickpea varieties. Chickpea is, therefore, assigned to the long-day grain legumes, with a linear function of the mean temperature describing the progress towards flowering (Roberts et al., Reference Roberts, Hadley and Summerfield1985; Summerfield et al., Reference Summerfield, Roberts and Hadley1987). Consequently, many legumes including lablab, are physiologically plastic with both daylength and temperature influencing their growth habit (Kim and Okubo, Reference Kim and Okubo1995). Within the lablab landraces, short-day and long-day photoperiod types exist (Kim et al., Reference Kim, Okubo and Kodama1992). Kim and Okubo (Reference Kim and Okubo1995) also reported for a lablab dwarf variety from India that photoperiod and temperature control the shift from indeterminate to determinate growth; the critical daylength shortens as temperature rises. They concluded that 13 h is the critical daylength at 25 °C, whilst at 30 °C, a daylength between 10 and 11 h is required for determinate growth. This agrees with the findings of Keatinge et al. (Reference Keatinge, Qi, Wheeler, Ellis and Summerfield1998) who concluded that time to flowering in lablab (forage type from Honduras) would become excessively long at higher latitudes and greater photoperiod fluctuations and elevations with lower potential of reproduction success. In commercial production systems, where photoperiod sensitivity can be an undesirable trait, Maass et al. (Reference Maass, Knox, Venkatesha, Angessa, Ramme and Pengelly2010) reported that photoperiod-insensitive lines have been bred and released as year-round cultivars in India and Bangladesh.
The objective of our study was to examine the photothermal response of early-flowering lablab genotypes selected by Whitbread et al. (Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011) using a combination of field and growth chamber experimentation to impose varying daylength and temperature regimes. This enhanced physiological understanding is important for identifying the potential adaptation of early-flowering lablab accessions to (sub)-tropical environments as a climate smart farming practice.
MATERIAL AND METHODS
Three datasets were used to investigate the response of daylength and temperature on flowering time of short-season lablab types. The first one (field trial 1) derived from data reported in Whitbread et al. (Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011). The dataset was reworked and used to compare thermal time to flowering of lablab accessions tested at three locations in Limpopo province of South Africa: Dalmada (23°87´S, 29°53´E), Tompi Seleka (24°47´S, 29°27´E) and Venda (22°58´S; 30°26´E) planted on different dates (10/12/2002, 13/02/2008, 13/02/2006, respectively). The second dataset (Field trial 2) was from a planting date experiment undertaken at Venda in 2012/2013 using 10 of the lablab accessions identified by Whitbread et al. (Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011) as short-season grain types. The third dataset generated on growth chamber studies undertaken at Georg-August University of Göttingen, Germany, where seven accessions were grown under controlled conditions with various temperature and daylength regimes.
Germplasm
The original germplasm was obtained from the Australian Tropical Forages Genetic Resources Centre (ATFGRC) in Biloela, Australia (http://www.daff.qld.gov.au/services/plant-industries-services/australian-tropical-crops-and-forages-collection). Based on the findings of Whitbread et al. (Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011), nine consistently early-flowering lablab accessions were selected as well as the cultivars ‘Highworth’ (CPI 30212) and ‘Rongai’ (CPI 17883) serving as controls, to further quantify photoperiod sensitivity. Origin, morphological and agronomic characteristics of the selected germplasm is summarized according Pengelly and Maass (Reference Pengelly and Maass2001) and Whitbread et al. (Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011) in Table 1.
Table 1. Origin, morphological and agronomic characteristics of nine lablab accessions and two cultivars included in photoperiod analysis study (Adapted from Pengelly and Maass, Reference Pengelly and Maass2001; Whitbread et al., Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011).

*accession included in evaluation trial from Whitbread et al. (Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011).
†accession included in the sowing date trial.
‡accession included in the growth chamber experiment.
CPI, Commonwealth (of Australia) Plant Introduction.
CQ, Commonwealth Scientific and Industrial Research Organisation (CSIRO) Queensland number.
Q, Queensland number.
DAP, days after planting.
DM, dry matter.
n.a., not available.
Field experimentation
Photoperiod sensitivity was not considered in Whitbread et al. (Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011). To investigate this aspect, data were analysed for the effect of planting time on flowering in combination with the daily maximum and minimum temperature observations collected from the field sites described in that study (Dalmada, Tompi Seleka and Venda). Site and crop management details are summarized in Table 2.
Table 2. Site information and crop management details for an evaluation trial of lablab accessions in Limpopo Province of South Africa.

A sowing date trial was conducted during the 2012/2013 growing season at University of Venda experimental farm, about 2 km west from Thohoyandou town in Vhembe district – this is close to the Venda site described in field trial 1. The area receives about 780 mm annual rainfall and it is highly seasonal, with 85% occurring between October and March (climatic summer) predominantly falling during February and March (Figure 1). The amount of incoming solar radiation reaches peaks of about 35 MJ m−2 d−1 with a light intensity of about 300 µmol m−2 s−1 The trial was located on a deep well-drained clay, Hutton form (Soil Classification Working Group, 1991), Ferrasol according to the classification of the Food and Agriculture Organization (FAO) belonging to Land Type Ab179 (Mzezewa and van Rensburg, Reference Mzezewa and van Rensburg2011) with soil pH neutral to slightly acid, adequate K and very low plant-available P (Mabapa et al., Reference Mabapa, Ogola, Odhiambo, Whitbread and Hargreaves2010).

Figure 1. Daily minimum and maximum temperatures and rainfall at University of Venda, Thohoyandou, Limpopo Province, South Africa from December 2012 until September 2013.
Daily and average daylength during the sowing date experiments for Venda were calculated based on geographic coordinates using R package ‘RAtmosphere’ (Figure 2) (Teets, Reference Teets2003).

Figure 2. Daylength and mean daily temperature at University of Venda, Thohoyandou; Limpopo Province, South Africa throughout the year. With indications for daylength at different sowing dates included in the sowing date trial.
The sowing date field trial was implemented as a randomized complete block design with sowing date as main plots and the different lablab accessions as sub plots, replicated three times. Sowing was done at 1-month intervals from 11/12/2012 and four subsequent sowings on 11/01/2013, 11/02/2013, 11/03/2013 and 13/04/2013, resulting in daylength decreasing from 13.56 h at the first sowing to 11.67 h by the final date of sowing (Figure 2). The temperatures ranged from high mean daily temperatures at the December, January and February sowing dates (24.3, 24.7 and 25.4 °C, respectively) with mean maximum temperatures of above 28 °C to comparatively low mean temperatures of 20.5 and 18.4 °C, respectively, at the March and April sowings with very low mean minimum temperatures of below 15 °C from April onwards (Figure 1). Each plot was 10 m × 2 m and consisted of 10 rows with an inter-row spacing of 1 m and 20 cm between plants (50 000 plants ha−1). All seeds were inoculated with Bradyrhizobium strain CB756 (XS21) prior to sowing. Superphosphate was applied during sowing at a rate equivalent to 20 kg P ha−1. The seeds were sown by hand at 4–6 cm depth and thinned two weeks after emergence to the desired spacing. Weeds were controlled manually and pests with Chlorpyriphos as required. Additional irrigation was not applied. The data collected included time to 50% flowering (50% of plants flowering) in days after planting (DAP). Additional to agronomic data, daily rainfall, as well as minimum and maximum temperatures were recorded throughout the experiment on a daily basis. Unfortunately, there was a complete crop failure observed due to animal feeding for accession CPI 52552 after sowing at the 11/03/2013.
Growth chamber experiments
Based on the availability of seed, six of the nine lablab accessions included in the sowing date trial plus accession CPI 52535 from the evaluation experiment by Whitbread et al. (Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011) were chosen for further evaluation under controlled conditions in a growth chamber (CLF Plant Climatics, Model: PGC-205). The selected accessions showed consistently early flowering and high-yielding characteristics even in water-limited environments (Pengelly and Maass, Reference Pengelly and Maass2001; Whitbread et al., Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011). These accessions were grown at four daylength regimes (10, 12, 14, 16 h of full light intensity) at a constant day/night temperature of 28 °C and relative humidity of 75%, replicated three times. The same experiment was repeated using a constant day/night temperature of 20 °C. Both experiments lasted 110 DAP and were terminated, regardless whether all treatments started flowering. Average light intensity in the growth chambers was set to the maximum of 350 µmol m−² s−1. Three seeds were sown per pot (height: 13 cm, diameter: 16.5 cm) and thinned to two plants per pot 7 days after emergence. The potting mix was a 6:2:2 ratio of humus, sand and loam (vol./vol.). Pots were transferred to the growth chambers seven DAP and placed in separate growth chambers (two growth chambers in two floors with individual light adjustment possibilities; length: 2.15 m, width: 0.7 m, height: 0.6 m) for each daylength regime. A completely randomized design was applied and pots rotated once a week. Watering was realized three times a week to avoid water shortage. From 1 month after planting, a complete fertilizer solution (Hakaphos® rot) was applied at 10-day intervals. The parameters measured included time to flowering of each individual plant in DAP. Flowering time was recorded when 50% of the buds on one plant fully flowered.
Data analysis
For the field trials, site-specific daylength was computed using R package ‘RAtmosphere’ (Teets, Reference Teets2003). Mean photoperiod as well as mean temperatures were calculated for the phenological phase from planting to flowering for each site, sowing date and accession individually for the field trial datasets. To evaluate photoperiod sensitivity, time to 50% flowering was determined with respect to DAP and thermal time (Tt , °Cd). Thermal time, expressed in degree days (°Cd), was computed using the algorithms in CERES-Maize, which divides each day into eight 3-h time periods on the basis of daily inputs of maximum and minimum temperatures (Jones et al., Reference Jones and Kiniry1986). Base, optimal and maximal temperatures (Tb, T optimal, T max, °C) were assumed to be 10, 30 and 40 °C, respectively, as suggested by Hill et al. (Reference Hill, Robertson, Pengelly, Whitbread and Hall2006).
Further, the development towards flowering was expressed as development rate – the reciprocal of the duration from sowing to flowering ((1/f) = D, d−1). The thermal and photothermal response of flowering were described using the triple-plane rate model (Summerfield et al., Reference Summerfield, Roberts, Ellis and Lawn1991).
First, for photoperiod-insensitive plants, the development rate is expressed as a function of mean daily temperature (T, °C) only from sowing to flowering as
The same formula can be applied for daylength shorter than the critical photoperiod (Pc ) in photoperiod-sensitive SDP (or longer than the critical photoperiod in photoperiod-sensitive LDP).
Second, after adding mean daily photoperiod (P, h d−1) as variable to the additive linear response model, the development rate is described as
for daylength between the critical photoperiod (Pc ) and ceiling photoperiod (Pce ), where a', b' and c' are genotypic coefficients (Iannucci et al., Reference Iannucci, Terribile and Martiniello2008; Summerfield et al., Reference Summerfield, Roberts, Ellis and Lawn1991).
Third, the maximum delay in flowering is reached when the daylength exceeds the ceiling photoperiod (Pce ) in SDP (for daylength below Pce in LDPs) and the development is expressed as
independent of variations in P or T.
From the photothermal model, the critical photoperiod (Pc ) can be predicted for photo-sensitive plants:
(Keatinge et al., Reference Keatinge, Qi, Wheeler, Ellis and Summerfield1998; Summerfield et al., Reference Summerfield, Roberts, Ellis and Lawn1991).
Additionally, a mixed model was used to further describe photoperiod response of the tested lablab accessions. In a first step, flowering response was scored as a simple yes/no event for the different temperature and daylength regimes. Second, the critical photoperiod (Pc ) above which flowering was accelerated in SDP was quantified by piecewise regression analysis for photoperiod-sensitive accessions using the R package ‘segmented’ (Muggeo, Reference Muggeo2003, Reference Muggeo2008). All statistical analyses were computed using R 2.15.1 (R 2008).
RESULTS
Field trial 1
In contrast to the well-studied forage-type lablab cv. Rongai, the lablab accessions included in this study are short-season with flowering times of 70 days or less. Time to flowering remained relatively stable across a range of sites and planting dates under field conditions in South Africa and the variation in flowering time in DAP or thermal time were limited (Table 3). Whereas average temperatures from planting to flowering were comparatively similar at all three sites ranging from 21 to 24 °C, mean daylength was about 13.50 h at Dalmada during the period from planting to flowering (December to March) and about 12.20 h at Venda and Tompi Seleka from February to May. In Dalmada, accession CPI 52513 flowered earliest at 52 DAP, whilst in Venda and Tompi Selaka, Q 6880B flowered earliest at 45 and 43 DAP, respectively (<605 °Cd). Cultivar Highworth flowered consistently early (63 to 68 DAP/800 – 862 °Cd) compared to cv. Rongai an indeterminate cultivar (157 DAP/1728 °Cd).
Table 3. Summary of flowering time data in days after planting (DAP) and thermal time (°Cd) for 11 different lablab accessions from three sites and planting dates in Limpopo Province of South Africa.

n.a., not available.
(Source: recalculated from raw data used in Whitbread et al., Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011).
Field experiment – sowing date trial
The variation in flowering time was considerably high, when expressed in DAP, and ranged from 50 to above 100 DAP for the majority of the tested accessions and from 50 to 80 DAP for sowing dates after December (CPI 52513, CPI 525233, CPI 52552, CPI 52554, CPI 60795, CPI 81364 and CQ 3620) (for details, see Figure S1). However, if expressed in °Cd the sowing dates after December had little impact on time to flowering, which was consistently at around 800 °Cd for the same accessions (Figure S1). For the December sowing date, though, flowering was delayed. The extent of delay in flowering time was relatively low for CPI 52513, CPI 52533 and CPI 52554, with Tt requirements of about 1000 °Cd in the December sowing. However, for accessions CPI 81364 and CQ 3620, the thermal time period increased to about 1100 °Cd. The greatest increase was observed for CPI 52552 where thermal time to flowering was greater than 1200 °Cd in the December sowing. The opposite was true for accession Q 6880B where time to flowering was observed later after sowing in April (1000 °Cd) than after sowing in December (800 °Cd). Only for accession CPI 60795 (800 °Cd), thermal time to flowering remained constant across all planting dates. Flowering of cultivars Highworth and Rongai, was significantly delayed in the December and January sowings when compared to later dates. The thermal time requirement to flowering was almost doubled with Tt > 1500°Cd for cv. Highworth and Tt of almost 1800 °Cd for cv. Rongai in the December sowing. Since daylength decreased from the December (13.56 h) to the April (11.67 h) sowing date, cvs. Highworth and Rongai showed a strongly quantitative SDP response and are, therefore, considered photoperiod-sensitive. In comparison to cvs. Highworth and Rongai, the response of accessions CPI 52513, CPI 525233, CPI 52552, CPI 52554, CPI 81364 and CQ 3620 to increasing daylength can be regarded as weak. From the sowing date field experiment, only CPI 60795 can be categorized as consistently early flowering and independent of photoperiod. Interpretation of photoperiod sensitivity is, however, limited if analysed irrespective of temperature (in DAP) as illustrated in Figure S1. Variation in flowering time of the studied short-season accessions appears great if expressed in DAP, but relatively constant if expressed in thermal time units for sowing dates from January till April.
Growth chamber experiment
In general, lower temperature resulted in time to flowering being longer. At 20 °C, all accessions flowered within 110 DAP (Figure 3a). At 28 °C, however, only accessions CPI 81364 and Q 6880B flowered at all daylengths from 10 to 16 h (Figure 3b). Accessions CPI 52554 and CPI 60795 flowered only at daylength regimes from 10 to 14 h at 28 °C, whilst CPI 52513 and CPI 52535 only at daylength of 10 and 12 h. At 20 °C mean temperature, an increase of time to flowering in DAP was observed with increased daylength for all accessions, except cv. Highworth, from about 60 to 80 days at a daylength of 10 h up to 85 to 110 days at a daylength of 16 h. Cultivar Highworth only flowered under short-day conditions of 10 h at 28 °C and at daylength of 10 and 12 h at 20 °C. Within accessions, variation in flowering response to daylength was rather low for the majority of the tested accessions. On average flowering was delayed by 4 days with a 2 h increase in daylength. Only accession Q 6880B was highly responsive to changes in daylength at temperatures of 20 °C, and flowering was accelerated significantly with decreasing daylength. Under temperatures of 28 °C and at all daylengths, Q 6880B however, flowered within a very short time of about 50 days.

Figure 3. Time to 50% flowering in days after planting (DAP) and thermal time (°Cd) for different daylengths and lablab accessions under controlled environment in a growth chamber: (a) at 20 °C and (b) at 28 °C. (*indeterminate growth up to 110 DAP).
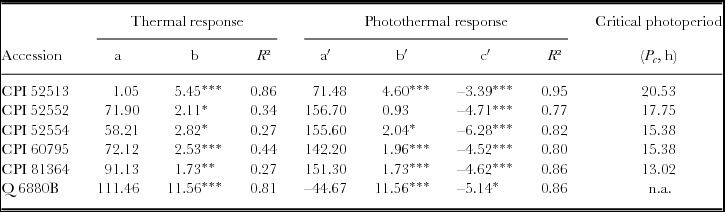
Applying the triple-plane-rate model to analyse the photothermal response, temperature alone was not enough to explain phenological development towards flowering. The coefficient of determination was low (R² < 0.5) for the tested accessions except for CPI 52513 and Q 6880B, with R² of 0.86 and 0.81, respectively (expressed by b, Table 4). However, for all accessions, the interval from planting to 50% flowering, expressed as inverse of the duration, was highly correlated (R² ≥ 0.77) when both mean temperature and mean photoperiod were considered.
Table 4. Estimated relations derived from the triple-plane rate model of flowering response of the rate of progress from sowing to 50% flowering for different lablab accessions under controlled environment in a growth chamber. Values of constants a, a′, b, b′ and c′ (all ×10−4) derived from regressing the rate of progress to flowering (1/f = D) against mean air temperature and mean photoperiod.

*P < 0.05; **P < 0.01; ***P < 0.001; a and a′: day−1; b and b′: °C−1; c′: h−1; n.a., not available.
The effect of photoperiod was significant and negative for all tested accessions (expressed by c', Table 4), meaning that, with increasing daylength, the development rate decreased significantly. Cultivars Highworth and Rongai were excluded from the triple-plane-rate model analysis because of their strongly qualitative photoperiod response in this experiment as it was terminated 110 DAP independent of the flowering success.
Finally, the use of piecewise regression analysis to estimate the ‘changepoint’ (Pc ) in thermal time as a function of daylength from all three datasets confirmed the results from the triple-plane rate model analysis: except Q 6880B, all accessions can be classified as photoperiod-sensitive, but with varying degree. For accession Q 6880B, neither a significant change in thermal time requirement to 50% flowering nor a significant effect of daylength on flowering time was found. The same was true for accession CPI 60795 under temperatures of <28 °C, whereas with temperatures ≥28 °C, no flowering was determined within 110 DAP at daylength of ≥16 h. For accessions CPI 52513 and CPI 52535, no significant effect of photoperiod on thermal time requirements could be found for mean daily temperatures of ≤24 °C, but under temperatures of 28 °C, no flowering within 110 DAP was observed at 14 and 16 h daylength. Accession CPI 81364 flowered throughout all tested temperature and daylength regimes, but thermal time requirements to 50% flowering significantly increased from 800 to 1100 °Cd at daylength from ≤14 h onwards. The same was true for accession CPI 52554, whereas indeterminate growth up to 110 DAP was observed at 28 °C with a daylength of 16 h and significant acceleration of the flowering response at 14 h (Figure 3). Thermal time requirements to flowering for cv. Highworth showed a high variation from 600 to 1200 °Cd at daylength of ≤14 h, but continuous vegetative growth up to 110 DAP was observed at 14 and 16 h as well as 12, 14 and 16 h at 20 and 28 °C, respectively, under controlled conditions.
DISCUSSION
Lablab purpureus – short season grain types
A major finding of this study, in particular, the analysis of the triple-plane rate model, is that the tested short-season lablab accessions are photoperiod-responsive SDP and that both, temperature and photoperiod trigger the flowering response. These findings are in agreement with those of Kim and Okubo (Reference Kim and Okubo1995) and support evidence that the switch from the vegetative to reproductive phase in lablab is strongly determined by the interaction between temperature and photoperiod. Observed flowering times were highly variable in terms of DAP at different temperature regimes (Figure 3, Figure S1). In particular, data derived from the sowing date field experiment in Venda, South Africa including different photoperiod and temperature conditions, revealed a high variation in observed flowering times in DAP, ranging from about 60 to 120 DAP for the different short-season lablab accessions (Figure S1). These observations are similar to results from Keatinge et al. (Reference Keatinge, Qi, Wheeler, Ellis and Summerfield1998), where the flowering time of lablab originating from Honduras ranged from 69 DAP at 26.9 °C and 11.5 h daylength to 172 DAP at 16.9 °C and 14.5 h daylength under controlled conditions. The strong dependency of development time on temperature make the interpretation of flowering time in DAP across a range of sites and sowing dates rather difficult. The presentation of development in thermal time instead makes it easier to compare results of different experiments or studies (Trudgill et al., Reference Trudgill, Honek, Li and Straalen2005). Figure 4 summarizes the results from the different data sets included in the analysis (field trial and controlled environment experiment). The cultivars Highworth and Rongai are clearly photoperiod-responsive SDP, as their flowering time increases continuously with increasing daylength. Flowering times for these cultivars were below 1000 °Cd under daylength conditions of ≤ 12 h; however, flowering times increased to about 1500 °Cd for Hightworth and 2000 °C for Rongai at daylength of ≥ 13.5 h. Accessions CPI 52513, CPI 52554, CPI 60796 and CPI 81364 instead showed a comparatively weak photoperiod response as flowering was only delayed by 100 to 300 Cd at daylength above 13.5 h and, in general, much lower in comparison to the cultivars Highworth and Rongai. Only at higher temperatures in the growth chamber experiment was flowering significantly delayed for the accessions CPI 60796 and CPI 81364 or not observed within 110 DAP for the accessions CPI 52513 and CPI 52554 at daylength above 13.5 h. These observations indicate that 28 °C is above the optimal temperature range for most of the short-season lablab accessions included in the growth chamber experiment (CPI 52513, CPI 52535, CPI 52554, CPI 60796, CPI 81364, Highworth), as their development was clearly delayed if measured in °Cd except for accession Q 6880B. Regardless of conditions, accession Q 6880B showed no significantly delayed development, indicating no photoperiod sensitivity even at higher temperatures (Figure 4). Temperatures of about 28 °C should, however, still be within the optimal range, as most of the accessions originate from tropical countries and are successfully cultivated in India with similarly high temperatures (Maass et al., Reference Maass, Jamnadass, Hanson and Pengelly2005). Nevertheless, the species–specific selection of cardinal temperatures might not be exact enough to quantify the development of lablab accessions from all over the world. However, implementing cultivar-specific cardinal temperatures would add to the complexity and increase the difficulty of applying such concepts. At the same time, Pc seemed to be influenced by temperature itself, as no flowering was observed within 110 DAP at higher temperatures of 28 °C in the growth chamber experiment at daylength of ≥14 h for CPI 52513 and CPI 52535, and daylength of ≥16 h for CPI 52554 and CPI 60795 in comparison to 20 °C where all short-season lablab accessions flowered within 110 DAP (Figure 3). This is in agreement with observations of Kim and Okubo (Reference Kim and Okubo1995), highlighting that the shorter the critical daylength, the higher the temperatures are. Therefore, it is not always suitable to define only one value for Pc , as this parameter seems to be temperature dependent. The results further proved that below the critical daylength (Pc ) or as long as photoperiod requirements are met, the development is dominated by temperature only – within the optimal range, reproductive development is accelerated as temperatures increase.

Figure 4. Summary of field trial and controlled environment experiment data representing flowering time in °Cd in response to daylength for lablab accessions grouped according to their photoperiod sensitivity.
Consequently, the key findings from this study are that the short-season lablab accessions are SDP and that Pc above which flowering is delayed, decreases with increasing temperatures except for Q 6880B, where no influence of temperature on Pc was found. At temperatures above 20 °C, flowering was significantly delayed at daylength of 13.5 h and higher for CPI 52513, CPI 52533, CPI 52535, CPI 52552, CPI 52554, CPI 81364 and CQ 3620. This is in accordance with Keatinge et al. (Reference Keatinge, Qi, Wheeler, Ellis and Summerfield1998), who determine Pc to be 13.9 h for lablab. They further concluded that time to flowering in lablab would become excessively long with lower potential reproduction success at higher latitude and elevation (Keatinge et al., Reference Keatinge, Qi, Wheeler, Ellis and Summerfield1998).
Nevertheless, the analysis of the different data sets derived from field and controlled environment experiments showed some inconsistency, which made it difficult to extract clear relations and dependencies in respect to photoperiod sensitivity for all accessions. This is because the development of plants does not only respond to environmental factors like temperature and photoperiod, but is further strongly influenced by water and nutrient availability, for instance (Subbarao et al., Reference Subbarao, Johansen, Slinkard, Nageswara, Saxena, Chauhan and Lawn1995). However, the actual experiments were designed to exclude the effects of water or nutrient stress on flowering response. Another critical aspect are the differences in light intensity reached in studies using the controlled environment of growth chambers which are usually much lower in comparison to light intensity under field conditions. However, there is little evidence in the scientific literature whether such differences are significant for flowering response. Furthermore, some of the inconsistencies within the data, in particular, the field observations, can be attributed to the developmental plasticity of short-season lablab accessions. The accelerated development (663.9 °Cd) of accession CPI 81364 at Venda under short-day conditions (12.4 h) and warm temperatures (23.1 °C) in comparison to the comparatively long flowering time (774.0 °C) under almost similar conditions in Tompi Seleka (Table 3) can be ascribed to developmental plasticity. In comparison to the other lablab accessions, the determined photoperiod-sensitivity of CPI 81364 was rather weak under controlled conditions (Figure 3). In general, variability in flowering time observed in the field was relatively high as it is usually difficult to control for all environmental factors which influence development under field conditions. Only flowering time of accession CPI 52513 in the evaluation of Whitbread et al. (Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011) was contradictory to the observations of the sowing date and growth chamber experiments, where the development was accelerated under longer day conditions (13.5 h) in Dalmada in comparison to the other sites with daylength below 13 h (Table 3). However, in Dalmada the seasonal rainfall was below 300 mm and supplementary irrigation was applied (Whitbread et al., Reference Whitbread, Ayisi, Mabapa, Odhiambo, Maluleke and Pengelly2011). Therefore, the higher drought sensitivity of CPI 52513 noted by Grotelüschen (Reference Grotelüschen2014) and soil moisture deficit may have resulted in accelerated development under field conditions in this Dalmada trial. In general, the phenological plasticity of legumes adds to the complexity of interpreting genotype x environment interactions. High variation in flowering response determined by diverse environmental triggers apart from photoperiod and temperature is a widespread phenomenon in legumes (Subbarao et al., Reference Subbarao, Johansen, Slinkard, Nageswara, Saxena, Chauhan and Lawn1995). Nevertheless, the observed flexibility of development is an advantageous feature to better respond to soil moisture availability in semi-arid environments, for instance.
Limitations of the estimation of photoperiod sensitivity
Many photoperiod analyses (e.g., Gaynor et al., Reference Gaynor, Lawn and James2011; Iannucci et al., Reference Iannucci, Terribile and Martiniello2008; Keatinge et al., Reference Keatinge, Qi, Wheeler, Ellis and Summerfield1998; Papastylianou and Bilalis, Reference Papastylianou and Bilalis2011) use the triple-plane rate model of flowering (Summerfield et al., Reference Summerfield, Roberts, Ellis and Lawn1991) to study photoperiod sensitivity of annual crops. However, the model is strictly additive and ignores interaction effects of temperature and photoperiod (Folliard et al., Reference Folliard, Traoré, Vaksmann and Kouressy2004; Wallace and Yan, Reference Wallace and Yan1998). Furthermore, the present study highlights some restrictions in applying the simple regression model on data derived from field studies, where mean daily temperatures are correlated to sunshine hours per day, as observed for the study site at the University of Venda, Thohoyandou; Limpopo Province, South Africa (Figure 2). Consequently, the variation in critical daylength together with temperature, as shown by current results, has a hyperbolic characteristic itself, adding to the complexity of quantifying photothermal response (Roberts and Summerfield, Reference Roberts, Summerfield and Atherton1987; Wallace and Yan, Reference Wallace and Yan1998). Due to the correlation of temperature and photoperiod in the data, the observations from the field experiment in South Africa needed to be excluded from the triple-plane rate analysis in this study. Otherwise, development time would have increased (if expressed in DAP) with decreased daylength and mean daily temperatures would have led to misinterpretation of photoperiodic response (Figure S1). In this case, the analysis of flowering time in thermal time units makes the quantification of the impact of photoperiod clearer (Figure S1).
In terms of thermal time requirement, development is consistent and synchronized for the tested lablab accessions up to a daylength of 13 h. Nevertheless, development, expressed as duration in DAP, can vary highly even under a daylength of 13 h (Figure S1) as a result of varying temperatures. It is, therefore, recommended to consider the three-dimensional character of photoperiodic response.
Moreover, daylength is never static in natural environments and directly influences changes in the mean day temperatures over the year. To set suitable photoperiod references for the analysis of field observations is, therefore, complex. Some studies use photoperiod at sowing, others photoperiod at flowering or the mean photoperiod from sowing to flowering. Calculating means may not be representative, as it is difficult to determine the actual photoperiod that has triggered or inhibited the switch from vegetative to reproductive development. In fact, the changing character of photoperiod within the year or cropping period is neglected in the model by Summerfield et al. (Reference Summerfield, Roberts, Ellis and Lawn1991). Moreover, the effect of decreasing or increasing daylength itself, or the impact of strictly constant daylength in controlled environment experiments has rarely been studied in annual crops.
Finally, the linear regression model applied is unable to describe a qualitative photoperiod response and phenomena such as a reversion in the development from vegetative to reproductive back to vegetative (Carberry et al., Reference Carberry, Ranganathan, Reddy, Chauhan and Robertson2001). Observations of no flowering as recorded at temperatures of 28 °C in the growth chamber experiment (Figure 3b), for instance cannot be appropriately considered in this analysis. This makes the interpretation of the results from the triple-plane rate model (Table 4) problematic. On the other hand, many authors (e.g. Iannucci et al., Reference Iannucci, Terribile and Martiniello2008) confirm the usefulness of evaluating photothermal response of flowering time with linear models that permit the estimation of base temperature and thermal time requirement. Such models simplify the complexity of photoperiod response and are, in general, very effective in describing genotype, environment and genotype × environment effects (Lawn and James, Reference Lawn and James2011). It is of great importance to manage the complexity of genotypic diversity in flowering behaviour as it is risky to extrapolate individual photothermal responses and computed coefficients without precaution (Iannucci et al., Reference Iannucci, Terribile and Martiniello2008).
Plasticity in photoperiod response – chance and challenges for agricultural systems
In general, grain legumes have high intraspecific diversity in terms of flowering time, as observed in the studies on lablab accessions and cultivars, which can be exploited for developing plant types that are well adapted to specific environments (Lawn and James, Reference Lawn and James2011; Nelson et al., Reference Nelson, Berger and Erskine2010). As daylength has an effect on crop phenology and morphology, potential productivity is directly influenced (Craufurd and Wheeler, Reference Craufurd and Wheeler2009; Bhattacharya and Vijaylaxmi, Reference Bhattacharya, Vijaylaxmi, Yadav, McNeil and Redden2010). The cultivation of potential short-season lablab accessions under optimal daylength conditions in the tropics and subtropics increases the synchrony of flowering and, consequently, pod setting and maturity. An increased synchrony of flowering and maturity facilitates crop management and harvest, which is of great interest for labour-restricted small-scale farming systems (Bhattacharya and Vijaylaxmi, Reference Bhattacharya, Vijaylaxmi, Yadav, McNeil and Redden2010; Nelson et al., Reference Nelson, Berger and Erskine2010).
Furthermore, shortened growing periods make the studied short-season lablab types interesting for farming in unstable environments, as short-season early-maturing types may be able to escape from external drought at grain filling or shortened growing windows (Blum, Reference Blum2005, Reference Blum2009). Therefore, the estimation of flowering time is increasingly important for agronomists and breeders, for whom the right timing of resource use is crucial for production success (Bhattacharya and Vijaylaxmi, Reference Bhattacharya, Vijaylaxmi, Yadav, McNeil and Redden2010; Craufurd and Wheeler, Reference Craufurd and Wheeler2009). Flowering within optimal environmental conditions secures production success, making short-season lablab types increasingly interesting for the design of resilient farming systems. The significance of predicted temperature increases in line with climate variation on the phenology of photoperiod-sensitive crops has not yet been fully examined (Craufurd and Wheeler, Reference Craufurd and Wheeler2009; Nelson et al., Reference Nelson, Berger and Erskine2010). Present results indicate that higher temperatures can increase the magnitude of photoperiod sensitivity and influence the threshold of the critical photoperiod (Figure 3) (Roberts and Summerfield, Reference Roberts, Summerfield and Atherton1987; Wallace and Yan, Reference Wallace and Yan1998). The predicted temperature increase for potential cropping areas in Sub-Saharan Africa might, therefore, lead to a delay in the development of photosensitive lablab types. This highlights the importance of breeding efforts from India and Bangladesh, for example, that aim to release photo-insensitive short-season lablab genotypes, which increase independence of customary growing periods (Maass et al., Reference Maass, Knox, Venkatesha, Angessa, Ramme and Pengelly2010). Moreover, the pronounced phenological plasticity of legumes adds to the complexity of determining G × E effects and is complicated to be captured well within crop growth models. However, magnitude of flexibility in growth and development of legumes in response to resource availability holds promising potential for farming with increasing climate uncertainties.
CONCLUSION
To integrate new germplasm into new environments, quantifying photothermal response information is critical to understand the timing of phenological events, such as flowering and maturity. The analysis proved that both temperature and photoperiod influence the development of the studied legume accessions and cultivars showing that photoperiod sensitivity should be interpreted as a photothermal response. This study has revealed considerable intraspecific physiological variation in flowering time amongst the lablab accessions and cultivars tested. In comparison to the forage types, cvs. Highworth and Rongai, the remainder can be classified as consistently early-flowering SDP, with a thermal time requirement of about 800 °Cd to flower under daylength conditions of ≤13.5 h and within their optimal temperature regime. The results proved that below the critical daylength (Pc ) or as long as photoperiod requirements are met, the development is dominated by temperature only – within the optimal range, reproductive development is accelerated as temperature increases. The critical photoperiod, Pc above which flowering is delayed, however, decreases with increasing temperatures. Since daylength does not exceed 13 h between latitude 30 N and 30 S covering the semi-arid tropical regions, these lablab accessions can be further evaluated for their potential contribution to productivity and sustainability of the farming systems of such regions.
Acknowledgements
We thank the Department of Plant Ecology and Ecosystem Research, Georg-August University of Göttingen, Germany, in particular Dr. Hertel and Dr. Coners, for the provision of the growth chamber facilities. Further, we are very thankful for the technical support throughout the growth chamber experiments by Ms. Bode from the Department of Crop Production Systems in the Tropics, Georg-August University of Göttingen, Germany. Furthermore, we also thank Mr. Masia, the student research assistant from the Department of Soil Science, University of Venda, Thohoyandou, South Africa for assisting with field data collection and the general fieldwork activities. The project was funded by the German Federal Ministry of Education and Research via the ‘Limpopo Living Landscapes’ project within the SPACES program (grant number 01LL1304A).
SUPPLEMENTARY MATERIALS
For supplementary material for this article, please visit http://dx.doi.org/10.1017/S0014479716000429