INTRODUCTION
Angiostrongylus cantonensis (common name: the rat lungworm) is a metastrongyloid nematode originally identified in the Guangzhou region of China in the brown rat, Rattus norvegicus (Chen, Reference Chen1935). It was first assigned to the genus Pulmonema which was later synonymized with Angiostrongylus (Dougherty, Reference Dougherty1946) resulting in the current combination, A. cantonensis. It was identified as a human pathogen in 1945 (Beaver and Rosen, Reference Beaver and Rosen1964) and is now recognized as the leading cause of eosinophilic meningitis worldwide (Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008; Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009; Murphy and Johnson, Reference Murphy and Johnson2013). It was the aetiological agent in over 2877 human cases of eosinophilic meningitis and is gaining recognition as an emerging zoonosis (Wang et al. Reference Wang, Wu, Wei, Owen and Lun2012). Dogs and certain wildlife species are important biosentinels for angiostrongyliasis, highlighting the potential risk for humans living in regions where these species are affected (Ma et al. Reference Ma, Dennis, Rose, Spratt and Spielman2013).
While A. cantonensis is the leading cause of eosinophilic meningitis, several other aetiological agents must be considered (Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009). Serological tests for angiostrongyliasis are commercially available, though have not been widely adopted for routine use. Consequently, diagnosis is often intuitive, relying on an accurate patient history and non-specific tests such as computed tomography (CT) or magnetic resonance imaging (MRI). A patients travel history or history of mollusc consumption greatly assist the diagnostic endeavour. Microscopic examination of cerebrospinal fluid (CSF) can be helpful, though the low sensitivity of this technique limits its use (Eamsobhana and Yong, Reference Eamsobhana and Yong2009). Given the potential lethality of angiostrongyliasis, it must be given due consideration in all cases of eosinophilic meningitis.
Traditionally, A. cantonensis is endemic to the temperate and tropical parts of the Far East (York et al. Reference York, Butler and Lord2014), though its current range includes Southeast Asia, the Pacific Islands, parts of South and Central America and the Caribbean (Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008). Relatively new epidemiological data have emerged from Australia and the USA, suggesting that the parasite's geographical range is expanding (Teem et al. Reference Teem, Qvarnstrom, Bishop, da Silva, Carter, White-McLean and Smith2013; Chan et al. Reference Chan, Barratt, Roberts, Lee, Shea, Marriott, Harkness, Malik, Jones, Aghazadeh, Ellis and Stark2015; Iwanowicz et al. Reference Iwanowicz, Sanders, Schill, Xayavong, da Silva, Qvarnstrom and Smith2015). Australian cases of angiostrongyliasis have been reported since the 1970s, possibly as early as 1959 (Prociv and Carlisle, Reference Prociv and Carlisle2001), though interest in this parasite was renewed due to recent reports of lethal cases on Australia's eastern coast.
A review summarizing current knowledge on various aspects of A. cantonensis is provided. This includes its life cycle and transmission, the global epidemiology of angiostrongyliasis, clinical manifestations, current diagnostic approaches, therapy, and measures for control and prevention. The molecular biology of A. cantonensis is also discussed. Particular attention is paid to the significance of angiostrongyliasis in Australia given the increasing reports of angiostrongyliasis in Australian humans and wildlife (Blair et al. Reference Blair, Orr, Delaney and Herkes2013; Ma et al. Reference Ma, Dennis, Rose, Spratt and Spielman2013; Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013; Spratt, Reference Spratt2015). An increased awareness of this emerging zoonosis is paramount to improving the prognosis for affected patients and reducing human, and companion animal suffering as the range of the parasite increases.
CLASSIFICATION
Angiostrongylus cantonensis is placed in the family Angiostrongylidae in the superfamily Metastrongyloidea which includes over 180 species across 45 genera. Angiostrongylus cantonensis was originally described as Pulmonema cantonensis by Chen (Reference Chen1935) and was subsequently placed in the genus Angiostrongylus by Dougherty (Reference Dougherty1946). Later, Ubelaker (Reference Ubelaker1986) split Angiostrongylus into five genera: Angiostrongylus (in carnivores), Parastrongylus (murids), Angiocaulus (mustelids), Gallegostrongylus (gerbils and one murid) and Sterfanskostrongylus (insectivores), though this classification, placing A. cantonensis in Parastrongylus, is now rarely used (Cowie, Reference Cowie2013a ).
Twenty-one species of Angiostrongylus are currently recognized, with the most significant being A. costaricensis and A. cantonensis, given their role as zoonotic pathogens (Spratt, Reference Spratt2015). Other significant species include Angiostrongylus malaysiensis, Angiostrongylus vasorum and Angiostrongylus mackerrasae. These species are considered animal pathogens but their zoonotic potential is yet to be demonstrated (Spratt, Reference Spratt2015).
LIFE CYCLE AND TRANSMISSION
Elucidation of the A. cantonensis life cycle was initially accredited to Mackerras and Sandars (Reference Mackerras and Sandars1955) who studied what they believed to be A. cantonensis in R. norvegicus captured on river banks in Queensland, Australia. These parasites were later identified as A. mackerrasae, although the life cycles of A. cantonensis, A. mackerrasae and A. malaysiensis are extremely similar, differing mostly in their host preferences (Bhaibulaya, Reference Bhaibulaya1975; Spratt, Reference Spratt2015). Angiostrongylus costaricensis also has a similar life cycle, though possesses a tropism for the mesenteric arteries of its definitive host, where egg production takes place (Mota and Lenzi, Reference Mota and Lenzi2005). In contrast, A. cantonensis produces eggs in the pulmonary arteries of its definitive hosts (Thiengo et al. Reference Thiengo, Simoes Rde, Fernandez and Maldonado2013; Spratt, Reference Spratt2015). The life cycle of A. cantonensis (Fig. 1) involves one of many potential intermediate hosts (molluscs) and definitive hosts (various rat species), and a large number of potential paratenic hosts.
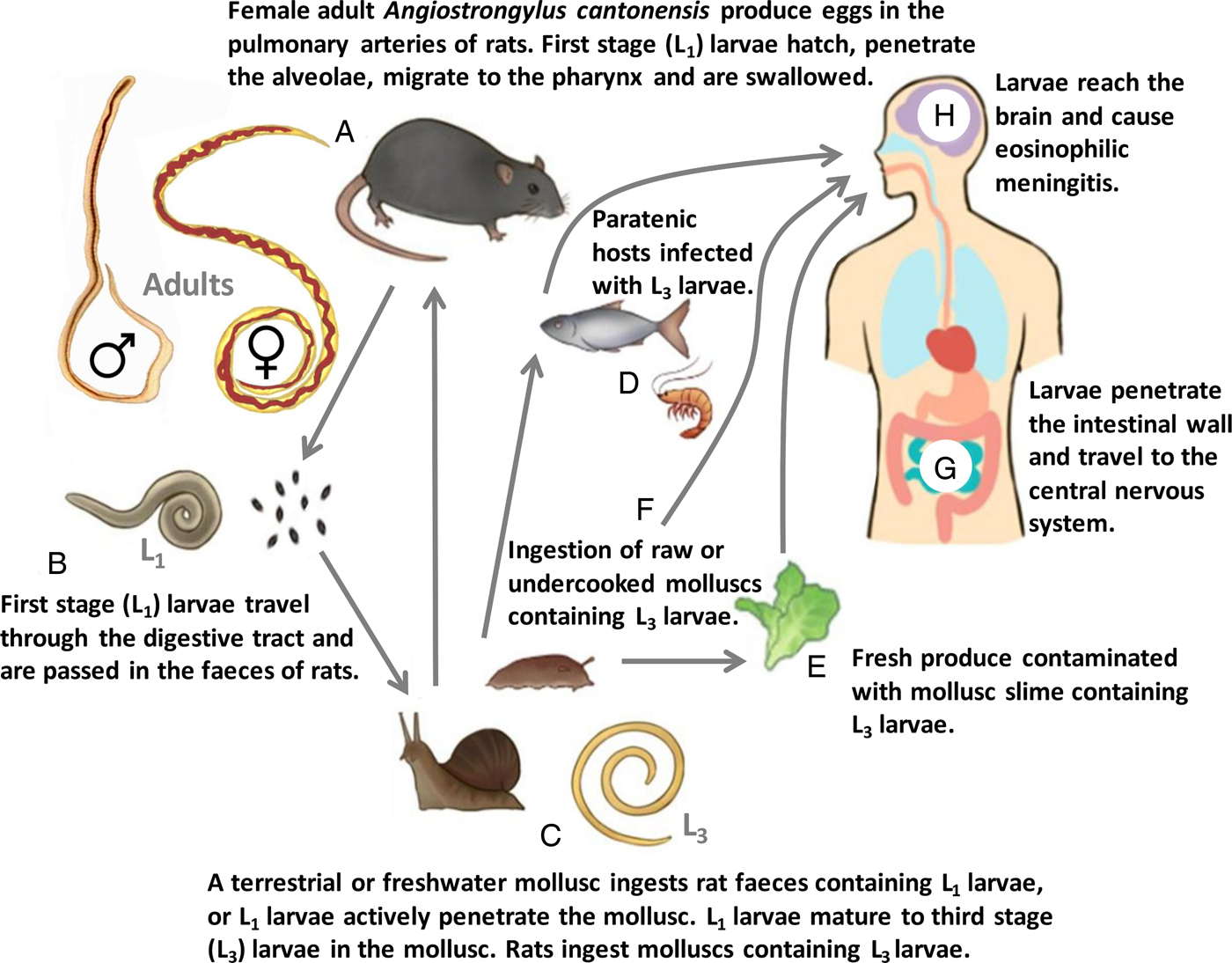
Fig. 1. Life cycle of A. cantonensis. (A) Male (♂) and female (♀) adult A. cantonensis live in the pulmonary arteries of Rattus rats, their preferred definitive host. Females lay eggs that hatch in the terminal branches of the pulmonary arteries, liberating first-stage (L1) larvae. The L1 larvae penetrate the alveolae, migrate to the pharynx and are swallowed. (B) The L1 larvae travel through the digestive tract and are passed in the rat feces. (C) A terrestrial or freshwater mollusc ingests the rat feces containing L1 larvae, or L1 larvae actively penetrate the mollusc tegument. The L1 larvae undergo two moults in the mollusc to become third-stage (L3) larvae. Infected molluscs are then ingested by a rat. The L3 larvae penetrate the rats’ intestine and migrate via the circulation to the brain where they undergo two additional moults to become young adult (L5) worms. The L5 worms leave the CNS and travel through the circulation to the pulmonary arteries where they mature to adulthood and reproduce. (D) Paratenic hosts eat molluscs infected with L3 larvae, and the larvae become quiescent in these hosts. Infected paratenic hosts remain infectious to accidental hosts such as humans. (E) Fresh produce contaminated with mollusc slime may also represent a source of human infection, though direct ingestion of raw or undercooked molluscs (F) is the most common route of human infection. (G) Once ingested, L3 larvae penetrate the intestinal wall and travel through the blood stream to the central nervous system. (H) The larvae enter the brain and in accidental hosts such as humans, eventually die. A granulomatous inflammatory reaction in the CNS is caused in response to dead worms, which manifests as eosinophilic meningitis.
Rattus rattus (the black rat) and R. norvegicus (the brown rat) are the favoured definitive hosts of A. cantonensis, though at least 17 rodent species may behave as definitive hosts, capable of passing first-stage (L1) larvae (Fig. 2) in their feces (Yong and Eamsobhana, Reference Yong and Eamsobhana2013). Simoes et al. (Reference Simoes, Maldonado Junior, Olifiers, Garcia, Bertolino and Luque2014) postulated that the movement of A. cantonensis to new regions is mediated mostly by male rats that often have greater dispersal ability than females, while females are important for maintenance of A. cantonensis on a small, local scale. Rats become infected by ingestion of an intermediate or a paratenic host, containing third-stage (L3) A. cantonensis larvae (Fig. 2). A few hours after ingestion, L3 larvae penetrate the rats’ intestinal wall and enter its blood stream where they are dispersed via the circulation. Larvae that reach the brain undergo an additional moult to become fourth-stage (L4) larvae. A fifth moult ensues in the subarachnoid space, where larvae enter the young adult (L5) stage. Young adult worms leave the central nervous system (CNS) and migrate through the circulation, and at 25 dpi, are found in the pulmonary arteries. At 35 dpi, adults have reached sexual maturity and females begin producing eggs that hatch in the terminal branches of the pulmonary arteries, liberating L1 larvae. These larvae penetrate the alveolae, migrate to the pharynx and are swallowed by the rat. The L1 larvae then travel through the digestive tract and appear in the rats’ feces approximately 42 days after the initial exposure (Thiengo et al. Reference Thiengo, Simoes Rde, Fernandez and Maldonado2013).
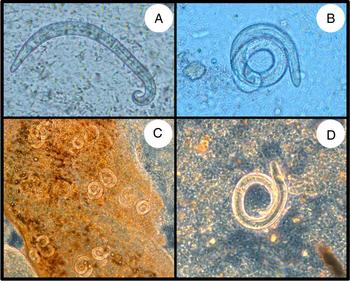
Fig. 2. Microscopic observation of L1 (A, B) and L3 (C, D) A. cantonensis larvae from rat feces and mollusc tissue, respectively. Each L1 larvae is approximately 320 µ m in length, while L3 larvae are slightly larger, at approximately 400 µ m in length. The L1 larvae were imaged under bright-field microscopy, while the L3 larvae were imaged under phase-contrast microscopy.
Definitive host competency may vary between rat species. For example, the Australian indigenous rat, Rattus fuscipes, seems a poor definitive host for A. cantonensis but is thought to be the preferred host of A. mackerrasae (Prociv and Carlisle, Reference Prociv and Carlisle2001). Similarly, the prevalence of A. cantonensis is usually much higher in R. norvegicus compared with Rattus flavipectus (Zhang et al. Reference Zhang, Chen, Gao, Geng, Huang, Liu, Wu and Zhu2008a ; Deng et al. Reference Deng, Zhang, Huang and Jones2012). This could be attributable to the biological competencies of different rat species, or simply, due to differences in the dietary habits of different rat species, though this is yet to be ascertained (Zhang et al. Reference Zhang, Chen, Gao, Geng, Huang, Liu, Wu and Zhu2008a ). Non-Rattus rats such as the bandicoot rat (Bandicota indica) and the white-toothed rat (Berylmys berdmorei), are also definitive hosts of A. cantonensis (Pipitgool et al. Reference Pipitgool, Sithithaworn, Pongmuttasaya and Hinz1997; Deng et al. Reference Deng, Zhang, Huang and Jones2012; Yong and Eamsobhana, Reference Yong and Eamsobhana2013). The finding of adult A. cantonensis in the Ryukyu Islands tree rat (Diplothrix legata) (Okano et al. Reference Okano, Haga, Mizuno, Onuma, Nakaya and Nagamine2014) also implicates this species as a definitive host, though this requires further investigation. Experimental infections in Mongolian gerbils (Meriones unguiculatus) confirmed they are highly susceptible to angiostrongyliasis yet behave as poor definitive hosts; worms developed to sexual maturity in gerbils though very few L1 larvae were shed in their feces (Wei et al. Reference Wei, Hong, Chen, Liang, Liu, Luo and Zhu2014).
Molluscs become infected by ingesting rat feces or by active penetration of L1 larvae through their tegument (Morassutti et al. Reference Morassutti, Thiengo, Fernandez, Sawanyawisuth and Graeff-Teixeira2014). First-stage larvae remain viable for several days after being excreted, though viability drops sharply after this (Yousif and Lammler, Reference Yousif and Lammler1975a ). The efficiency of mollusc infection is temperature dependent; it is greater at 26 °C compared with 24 °C (Yousif and Lammler, Reference Yousif and Lammler1975a ). Within the mollusc, L1 larvae undergo two moults to become L3 larvae; a roughly 20-day process marked by distinct morphological changes (Lv et al. Reference Lv, Zhang, Liu, Zhang, Steinmann, Zhou and Utzinger2009c ; Thiengo et al. Reference Thiengo, Simoes Rde, Fernandez and Maldonado2013; Zeng et al. Reference Zeng, Wei, Wang, Wu, Fung, Wu, Sun, Zheng, Lv and Wu2013b ). Larvae often migrate to the muscular layer of the foot given its excellent vascular supply, which provides favourable conditions for metastrongyloid larvae development (Mendonca et al. Reference Mendonca, Carvalho, Mota, Pelajo-Machado, Caputo and Lenzi1999; Giannelli et al. Reference Giannelli, Colella, Abramo, do Nascimento Ramos, Falsone, Brianti, Varcasia, Dantas-Torres, Knaus, Fox and Otranto2015). Larvae also migrate to the lung tissues (Yousif et al. Reference Yousif, Blahser and Lammler1980; Lv et al. Reference Lv, Zhang, Liu, Zhang, Steinmann, Zhou and Utzinger2009c ). Consequently, mollusc lungs are often examined microscopically in field studies, for the characteristic nodules that contain L3 larvae (Lv et al. Reference Lv, Zhang, Liu, Zhang, Steinmann, Zhou and Utzinger2009c ; Hu et al. Reference Hu, Du, Tong, Wang, Liu, Li and He2011; Qvarnstrom et al. Reference Qvarnstrom, Bishop and da Silva2013). In the aquatic snail Pila polita, ingested larvae migrate from the digestive gland to the intestine and then to the mantle, where the highest parasite burdens were recorded (Tesana et al. Reference Tesana, Srisawangwong, Sithithaworn and Laha2008). In Parmarion martensi semi-slugs (semi-slugs are molluscs with a shell that is too small to retract into), the midsection and tail had the highest larval densities; few larvae were found in the head (Jarvi et al. Reference Jarvi, Farias, Howe, Jacquier, Hollingsworth and Pitt2012). Angiostrongylus cantonensis is also pathogenic to molluscs, inducing biochemical disturbances, inflammation and reduction in reproductive capacity (Yousif et al. Reference Yousif, Blahser and Lammler1980; Tunholi-Alves et al. Reference Tunholi-Alves, Tunholi, Lustrino, Amaral, Thiengo and Pinheiro2011, Reference Tunholi-Alves, Tunholi, Pinheiro and Thiengo2012, Reference Tunholi-Alves, Tunholi, Castro, Sant'Ana, Santos-Amaral, de Oliveira, Garcia, Thiengo, Pinheiro and Maldonado2014, Reference Tunholi-Alves, Tunholi, Amaral, Mota, Maldonado Junior, Pinheiro and Garcia2015).
Intermediate hosts of A. cantonensis include species from as many as 51 mollusc families, though some species may harbour more L3 larvae than others (Table 1) (Yousif and Lammler, Reference Yousif and Lammler1975b ; Kim et al. Reference Kim, Hayes, Yeung and Cowie2014). When the aquatic snails Pomacea canaliculata and P. polita were experimentally infected with A. cantonensis, higher larval burdens were observed in P. polita (Tesana et al. Reference Tesana, Srisawangwong, Sithithaworn and Laha2008). The giant African land snail, Achatina fulica, may harbour thousands of L3 larvae, and is thought to be a major contributor to the spread of A. cantonensis globally (Alicata, Reference Alicata1965a ), though this is controversial (Civeyrel and Simberloff, Reference Civeyrel and Simberloff1996; Cowie, Reference Cowie2013a ). The effect of mollusc size on larval burden has also been considered, though correlations were only observed in some species (Yousif and Lammler, Reference Yousif and Lammler1975a ; Pipitgool et al. Reference Pipitgool, Sithithaworn, Pongmuttasaya and Hinz1997; Ibrahim, Reference Ibrahim2007; Tesana et al. Reference Tesana, Srisawangwong, Sithithaworn, Laha and Andrews2009). It is suggested that parasite burdens are more closely linked to the number of L1 larvae that the molluscs have been exposed to (Yousif and Lammler, Reference Yousif and Lammler1975a ). The prevalence of A. cantonensis in mollusc populations also varies between locations (Table 2), probably due to environmental factors such as temperature, humidity, the distribution of molluscs and the behaviours of local rats (Kim et al. Reference Kim, Hayes, Yeung and Cowie2014). Water salinity is also a contributing factor, with higher prevalences observed in aquatic molluscs living in water with lower salinity (Ibrahim, Reference Ibrahim2007).
Table 1. Larval parasite loads (infection intensities) in naturally infected intermediate and paratenic hosts of A. cantonensis

a Mean infection intensity varied between months.
b Mean infection intensity is per 25 mg of tissue.
c The mean infection intensity for L3 larvae from all worm species was 13·6. Infection of Wistar rats with these larvae resulted in a recovery rate of 48·3% adult worms and 91·7% of these (~ 6) were A. cantonensis.
d Mean infection intensity is per 50 mg of tissue.
e Mean infection intensity varied between seasons and/or locations.
f Snails from irrigation canals in five different locations (designated A–E).
g Mean infection intensity is per 1 mg of tissue. Also, the mean infection intensity varied depending on the mollusc tissue sample screened (mollusc head, mid-section, tail or slime), and the location from which molluscs were collected.
Table 2. Prevalence of A. cantonensis in definitive, intermediate and paratenic hosts

a This study compared the necropsy of rats with real-time PCR, for confirmation of an A. cantonensis infection. This table shows the higher prevalence value.
b This is a study on the seroprevalence of A. cantonensis in rats and indicates exposure to worms rather than an active, current infection.
c Sigmodon hispidus is an important definitive host of A. costaricensis (Graeff-Teixeira et al. Reference Graeff-Teixeira, de Avila-Pires, Machado Rde, Camillo-Coura and Lenzi1990), though it has not yet been described as a definitive host of A. cantonensis (Yong and Eamsobhana, Reference Yong and Eamsobhana2013).
d A meta-analysis involving 38 studies from different regions in China, over a 10-year period.
e This study compared conventional and real-time PCR for detection of A. cantonensis in mollusc tissues. This table shows the higher prevalence value.
f This study involved 164 counties from 19 provinces, predominantly along southeastern coastline of China.
g This study compared microscopic identification of worms from pepsin digested mollusc tissue to PCR, for detection of A. cantonensis in mollusc tissues. This table shows the higher prevalence value.
h The prevalence shown for this study was generated from a group of molluscs containing mixed species, in which one C. aspersum was infected only.
i This study compared microscopic identification of worms from pepsin digested mollusc tissue to a loop-mediated isothermal amplification assay, for detection of A. cantonensis. This table shows the higher prevalence value.
In China and parts of Southeast Asia, P. canaliculata, and in Thailand, Pila spp., are considered important intermediate hosts of A. cantonensis (Lv et al. Reference Lv, Zhang, Liu, Hu, Yang, Steinmann, Chen, Wang, Utzinger and Zhou2009b ; Tesana et al. Reference Tesana, Srisawangwong, Sithithaworn, Laha and Andrews2009; Eamsobhana, Reference Eamsobhana2013; Yang et al. Reference Yang, Wu and Lun2013a ). The number of known intermediate host species is increasing, with recent reports describing infections in new species from mollusc families such as the Achatinellidae, Assimineidae, Oxychilidae, and the species Theba pisana, Plutonia lamarckii, Zachrysia provisoria, Cornu aspersum and Cryptozona siamensis (Table 2) (Kim et al. Reference Kim, Hayes, Yeung and Cowie2014; Vitta et al. Reference Vitta, Polsut, Fukruksa, Yimthin, Thanwisai and Dekumyoy2016). In Australia, two terrestrial snail species, C. aspersum and Bradybaena similaris (Fig. 3) were recently confirmed as natural intermediate hosts of A. cantonensis (Table 2). Third-stage larvae of A. cantonensis also infect a multitude of paratenic hosts including freshwater prawns & shrimp, land crabs, frogs, toads, monitor lizards and planarians (Cowie, Reference Cowie2013b ; Qvarnstrom et al. Reference Qvarnstrom, Bishop and da Silva2013; Eamsobhana, Reference Eamsobhana2014). The L3 larvae remain infective to definitive and accidental hosts that eat infected paratenic hosts.

Fig. 3. Two terrestrial mollusc species that were identified as natural intermediate hosts of A. cantonensis in Australia. Cornu aspersum and Bradybaena similaris are introduced species in Australia, and are common inhabitants of gardens and parks in metropolitan areas along the eastern coast of the continent.
Humans are one of many accidental hosts that become infected via one of the three possible transmission pathways (Fig. 1D–F). In Australia, naturally acquired infections in other accidental hosts have involved dogs, horses, brushtail possums (Fig. 4), Bennett's wallabies, rufous bettongs, tamarins (in captivity), black and grey-headed flying foxes, tawny frogmouths (Fig. 4, online Supplementary materials), gang–gang cockatoos and yellow-tailed black cockatoos (McKenzie et al. Reference McKenzie, Green and Wood1978; Wright et al. Reference Wright, Kelly, Waddell and Hamilton1991; Higgins et al. Reference Higgins, Carlisle-Nowak and Mackie1997; Carlisle et al. Reference Carlisle, Prociv, Grennan, Pass, Campbell and Mudie1998; Barrett et al. Reference Barrett, Carlisle and Prociv2002; Monks et al. Reference Monks, Carlisle, Carrigan, Rose, Spratt, Gallagher and Prociv2005; Lunn et al. Reference Lunn, Lee, Smaller, MacKay, King, Hunt, Martin, Krockenberger, Spielman and Malik2012; Reece et al. Reference Reece, Perry and Spratt2013; Aghazadeh et al. Reference Aghazadeh, Jones, Aland, Reid, Traub, McCarthy and Lee2015a ; Walker et al. Reference Walker, Spielman, Malik, Graham, Ralph, Linton and Ward2015). It has been suggested that brushtail possums and tawny frogmouths are susceptible dead-end hosts native to Australia that serve as biosentinels for the presence of A. cantonensis (Ma et al. Reference Ma, Dennis, Rose, Spratt and Spielman2013; Spratt, Reference Spratt2015). In the USA, infections in several dead-end hosts have also been confirmed, including a privately owned orangutan with a history of snail consumption (Emerson et al. Reference Emerson, Walden, Peters, Farina, Fredholm, Qvarnstrom, Xayavong, Bishop, Slapcinsky, McIntosh and Wellehan2013), and a captive gibbon from a zoo in Miami (Duffy et al. Reference Duffy, Miller, Kinsella and de Lahunta2004). Burns et al. (Reference Burns, Bicknese, Qvarnstrom, DeLeon-Carnes, Drew, Gardiner and Rideout2014) described a fatal case in a captive African pygmy falcon from San Diego Zoo, Southern California, USA. Kottwitz et al. (Reference Kottwitz, Perry, Rose and Hendrix2014) reported cases of lethal angiostrongyliasis in captive Geoffroy's tamarins from a zoo in Alabama, USA.

Fig. 4. Clinical presentation of angiostrongyliasis in some Australian wildlife species, including a brushtail possum (Trichosurus vulpecula) (A) and multiple tawny frogmouths (Podargus strigoides) (B–H). These animals were described as biosentinels for A. cantonensis in the Sydney region (Ma et al. Reference Ma, Dennis, Rose, Spratt and Spielman2013). Panel (A) shows a juvenile brushtail possum that had been in care for 6 weeks. Food supplied to its large cage was gradually being left uneaten, but food offered to the possum in its nest box was eaten ravenously. Closer examination showed an inability to ambulate. Clinical examination showed hind limb and tail paralysis. Pinching the hind paws elicited no pain response but a strong and even exaggerated withdrawal reflex, typical for spinal cord damage. Panels (B–H) show tawny frogmouths displaying signs typical for spinal cord damage, prior to a diagnosis of angiostrongyliasis. The birds present with varied clinical signs, from moribund in advanced cases (B), to reduced or normal mentation (C–H). Some birds may be alert and aware but unable to fly or stand (H).
Deliberate ingestion of infected raw or undercooked molluscs is the most common route of infection for humans in Asian countries, such as Thailand, where known intermediate hosts, such as Pila spp., are regularly eaten raw as part of the local diet (Eamsobhana, Reference Eamsobhana2013). Human A. cantonensis infections have also been linked to several paratenic host species. Reports describing the ingestion of monitor lizards in Sri Lanka, Thailand & India, raw frogs in China and, freshwater shrimp, fish & crabs in the Pacific Islands, implicate ingestion of these disparate hosts as a route for acquiring Angiostrongylus infections (Hidelaratchi et al. Reference Hidelaratchi, Riffsy and Wijesekera2005; Malvy et al. Reference Malvy, Ezzedine, Receveur, Pistone, Crevon, Lemardeley and Josse2008; Tsai et al. Reference Tsai, Lai, Sy, Lee, Yen, Wann and Chen2011; Cowie, Reference Cowie2013b ; Pai et al. Reference Pai, Madi, Achappa, Mahalingam and Kendambadi2013; Eamsobhana, Reference Eamsobhana2014).
The shedding of A. cantonensis larvae in infected mollusc mucus onto fresh produce may represent another route of transmission (Fig. 1E). This pathway is relevant to those that regularly eat uncooked, inadequately washed, plant material, i.e. in salads (Barrow et al. Reference Barrow, Rose and Lindo1996; Lindo et al. Reference Lindo, Waugh, Hall, Cunningham-Myrie, Ashley, Eberhard, Sullivan, Bishop, Robinson, Holtz and Robinson2002; Slom et al. Reference Slom, Cortese, Gerber, Jones, Holtz, Lopez, Zambrano, Sufit, Sakolvaree, Chaicumpa, Herwaldt and Johnson2002; Waugh et al. Reference Waugh, Shafir, Wise, Robinson, Eberhard and Lindo2005; Yeung et al. Reference Yeung, Hayes and Cowie2013). An outbreak has also been linked to drinking raw vegetable juices (Tsai et al. Reference Tsai, Lee, Huang, Yen, Chen and Liu2004, Reference Tsai, Chen and Yen2013). Independent studies that included several mollusc species, reported A. cantonensis in mollusc mucus, though the small number of larvae shed may be negligible or insufficient to represent a major source of human infection (Qvarnstrom et al. Reference Qvarnstrom, Sullivan, Bishop, Hollingsworth and da Silva2007; Jarvi et al. Reference Jarvi, Farias, Howe, Jacquier, Hollingsworth and Pitt2012; Chan et al. Reference Chan, Barratt, Roberts, Lee, Shea, Marriott, Harkness, Malik, Jones, Aghazadeh, Ellis and Stark2015). However, mollusc slime was the predicted mode of transmission in two cases of severe Angiostrongylus eosinophilic meningitis (AEM) recently reported in young children (Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013). In these cases, a history of mollusc consumption was denied, though this is difficult to confirm in young children. Freshwater may represent another source of infection as A. cantonensis L3 larvae reportedly survive in freshwater for up to 72 h (Cheng and Alicata, Reference Cheng and Alicata1964). Studies of the feline metastrongyloid lungworms Aelurostrongylus abstrusus and Troglostrongylus brevior demonstrated that L3 larvae were also shed in mollusc mucus, and could be found in sediments from tap water containing C. aspersum that had been experimentally infected and drowned (Giannelli et al. Reference Giannelli, Colella, Abramo, do Nascimento Ramos, Falsone, Brianti, Varcasia, Dantas-Torres, Knaus, Fox and Otranto2015).
EPIDEMIOLOGY AND GLOBAL DISTRIBUTION
Angiostrongylus cantonensis is the most common cause of eosinophilic meningitis. It has been reported in over 30 countries since its identification as a human pathogen in 1945, predominantly in the tropics and sub-tropics (Beaver and Rosen, Reference Beaver and Rosen1964; Wang et al. Reference Wang, Wu, Wei, Owen and Lun2012; Eamsobhana et al. Reference Eamsobhana, Lim and Yong2013a ; Eamsobhana, Reference Eamsobhana2014) (Tables 1 and 2, Fig. 5). While more than 2877 human cases of angiostrongyliasis have been described, many have probably gone unreported because of a lack of awareness and difficulties associated with its diagnosis (Qvarnstrom et al. Reference Qvarnstrom, Sullivan, Bishop, Hollingsworth and da Silva2007). Reports of angiostrongyliasis in Europe and other regions where it was not known previously are becoming more frequent, with travel to parts of Asia, the Pacific Islands and Latin America noted as the likely route of exposure for affected individuals (Bartschi et al. Reference Bartschi, Bordmann, Blum and Rothen2004; Chancellor, Reference Chancellor2007; Leone et al. Reference Leone, De Marco, Ghirga, Nicastri, Esposito and Narciso2007; Ali et al. Reference Ali, Van den Enden, Van Gompel and Van Esbroeck2008; Malvy et al. Reference Malvy, Ezzedine, Receveur, Pistone, Crevon, Lemardeley and Josse2008; Luessi et al. Reference Luessi, Sollors, Torzewski, Muller, Siegel, Blum, Sommer, Vogt and Thomke2009; Maretic et al. Reference Maretic, Perovic, Vince, Lukas, Dekumyoy and Begovac2009; Lammers et al. Reference Lammers, Goorhuis, van de Beek, Grobusch, Bart, van Gool and van Vugt2015).
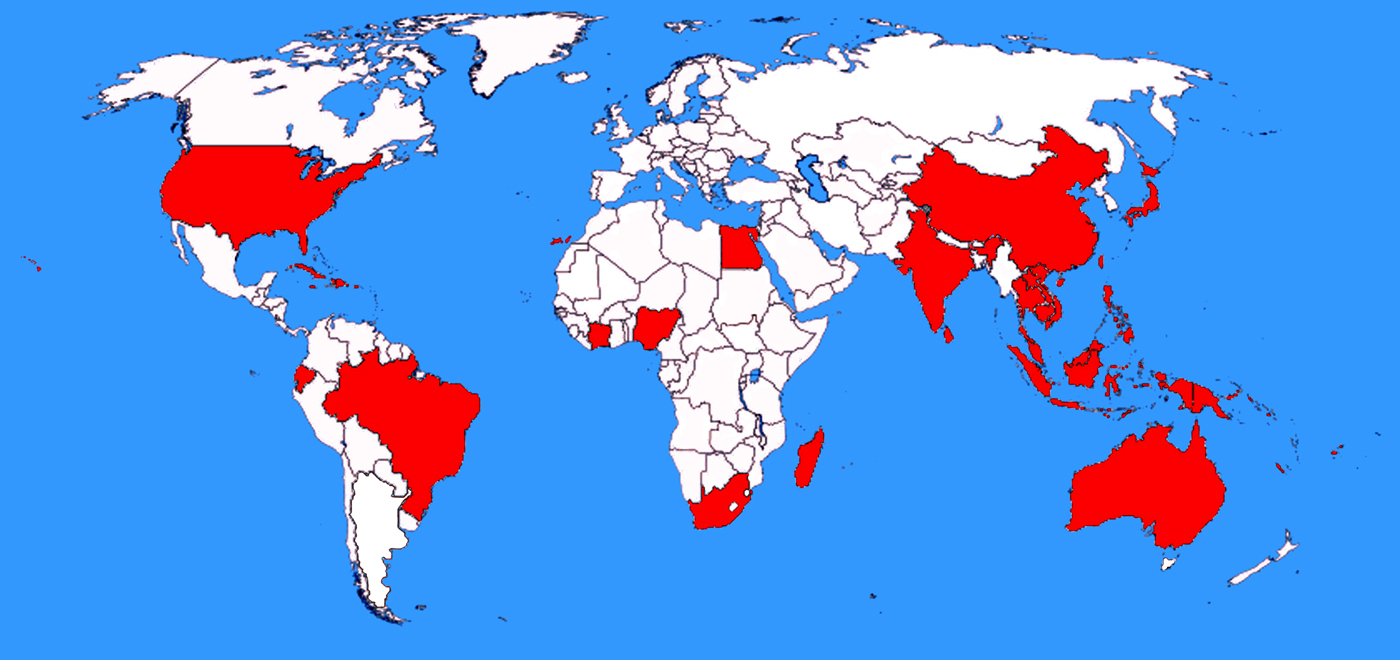
Fig. 5. Countries where A. cantonensis has been detected in naturally infected hosts. Shaded countries are those in which A. cantonensis was identified in studies screening naturally infected animals, or where humans have acquired infections. Unshaded countries include those that are yet to find evidence of A. cantonensis, or countries where studies examining potential hosts for A. cantonensis infection have not been carried out. This map does not include countries that have reported sporadic clinical cases of angiostrongyliasis that were probably acquired abroad. Mainland Australia is shaded on the map, though the island state of Tasmania is not. Native rats in Tasmania are known hosts of A. mackerrasae (Prociv et al. Reference Prociv, Spratt and Carlisle2000), though A. cantonensis has not been reported in Tasmania. Mainland Spain is also not shaded on the map, although A. cantonensis was detected in the Canary Islands, off the western coast of Africa, which are part of Spain. Currently A. cantonensis is not known to be present in mainland Spain.
While it is traditionally considered a disease of the Far East, reports of locally acquired angiostrongyliasis are becoming increasingly common in sub-tropical and temperate regions (Gutteridge et al. Reference Gutteridge, Bhaibulaya and Findlater1972; Prociv et al. Reference Prociv, Spratt and Carlisle2000; Senanayake et al. Reference Senanayake, Pryor, Walker and Konecny2003; Blair et al. Reference Blair, Orr, Delaney and Herkes2013; Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013). It may be that the range of A. cantonensis has expanded recently, possibly as a consequence of global warming or other environmental factors (York et al. Reference York, Butler and Lord2014). Hochberg et al. (Reference Hochberg, Park, Blackburn, Sejvar, Gaynor, Chung, Leniek, Herwaldt and Effler2007) attribute the spread of A. cantonensis to the sheer diversity of its intermediate hosts, and the efficient dispersion of ship-borne rats. A similar expansion in geographic range has not been reported for A. costaricensis; the causative agent of human abdominal angiostrongyliasis (Spratt, Reference Spratt2015). The first documented human A. costaricensis infection was from Costa Rica (Morera and Cespedes, Reference Morera and Cespedes1971; Morera, Reference Morera1973), and later reports confirmed its presence in Venezuela, Ecuador, Honduras, Mexico, Nicaragua, Brazil, Guatemala, Columbia, the Caribbean Islands and Southern USA (Morera et al. Reference Morera, Lazo, Urquizo and Llaguno1983; Incani et al. Reference Incani, Caleiras, Martin and Gonzalez2007; Palominos et al. Reference Palominos, Gasnier, Rodriguez, Agostini and Graeff-Teixeira2008; Spratt, Reference Spratt2015). Apparently, only one case of human A. costaricensis infection has been reported outside the Americas; an isolated case in an African man (Baird et al. Reference Baird, Neafie, Lanoie and Connor1987). The relatively restricted range of A. costaricensis is probably related to the limited range of its preferred definitive host; the hispid cotton rat (Sigmodon hispidus) (Graeff-Teixeira et al. Reference Graeff-Teixeira, de Avila-Pires, Machado Rde, Camillo-Coura and Lenzi1990), which is generally found only in southern North America and parts of Central and South America.
Most human cases of angiostrongyliasis (~1300 cases; 47% of all cases worldwide) have been reported in Thailand (Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008), where between 0·3 and 2 people per 100 000 become infected annually (Suankratay et al. Reference Suankratay, Wilde and Berger2001; Eamsobhana, Reference Eamsobhana2013). This high prevalence is attributable to the dietary habits of the local populace, involving the regular consumption of dishes like ‘koi-hoi’ that contain raw or undercooked molluscs such as Pomacea maculata, P. canaliculata and Pila spp.; common intermediate hosts of A. cantonensis (Schmutzhard et al. Reference Schmutzhard, Boongird and Vejjajiva1988; Lv et al. Reference Lv, Zhang, Chen, Wang, Fang, Chen, Jiang, Li, Du and Zhou2009a ; Eamsobhana et al. Reference Eamsobhana, Yoolek and Yong2010; Odermatt et al. Reference Odermatt, Lv and Sayasone2010; Cowie, Reference Cowie2013b ; Eamsobhana, Reference Eamsobhana2014; Kim et al. Reference Kim, Hayes, Yeung and Cowie2014). The consumption of undercooked monitor lizard livers was linked to a fatal case of AEM in Thailand (Eamsobhana, Reference Eamsobhana2014), where very high rates of monitor lizard infection have been reported; as high as 96% in one population (Eamsobhana, Reference Eamsobhana2013). Angiostrongyliasis has also been reported in the neighbouring Southeast Asian Nations of Laos (Harinasuta, Reference Harinasuta, Warren and Bowers1983), Cambodia (Brumpt et al. Reference Brumpt, Audebaud, Klein, Jolly, Mazaud and Goube1968) and Vietnam (Chau et al. Reference Chau, Thwaites, Chuong, Sinh and Farrar2003).
While the majority of AEM cases are reported from Thailand, most epidemiological data on angiostrongyliasis, including intermediate and definitive host surveys, comes from China. Several outbreaks of angiostrongyliasis have occurred in China, predominantly in provinces along the eastern coast (Lin et al. Reference Lin, Jie and Li2005; Wang et al. Reference Wang, Chen and Lun2007, Reference Wang, Qi, Diao, Zheng, Li, Ma, Ji and Yin2010; Lv et al. Reference Lv, Zhang, Steinmann and Zhou2008, Reference Lv, Zhang, Chen, Wang, Fang, Chen, Jiang, Li, Du and Zhou2009a ; Zhang et al. Reference Zhang, Chen, Gao, Geng, Huang, Liu, Wu and Zhu2008a ; Zhou et al. Reference Zhou, Barennes, Zhou, Ding, Zhu and Strobel2009; Hu et al. Reference Hu, Du, Tong, Wang, Liu, Li and He2011). Only three cases of AEM were reported in China between 1984 and 1996, and the recent increase in cases is attributable to widespread changes in human dietary patterns, where snails have become a popular food item (Chen et al. Reference Chen, Li and Lun2005; Zhou et al. Reference Zhou, Barennes, Zhou, Ding, Zhu and Strobel2009; Wang et al. Reference Wang, Qi, Diao, Zheng, Li, Ma, Ji and Yin2010). Achatina fulica and P. canaliculata are considered the most important species for A. cantonensis transmission in China, where both are introduced species, farmed as a food source (Wang et al. Reference Wang, Chen and Lun2007; Lv et al. Reference Lv, Zhang, Steinmann and Zhou2008, Reference Lv, Zhang, Liu, Hu, Yang, Steinmann, Chen, Wang, Utzinger and Zhou2009b ). Multiple Chinese outbreaks were directly linked to the consumption of raw or undercooked P. canaliculata, which is more popular as a Chinese food item than A. fulica (Wang et al. Reference Wang, Chen and Lun2007; Lv et al. Reference Lv, Zhang, Chen, Wang, Fang, Chen, Jiang, Li, Du and Zhou2009a , Reference Lv, Zhang, Liu, Hu, Yang, Steinmann, Chen, Wang, Utzinger and Zhou b ; Zhou et al. Reference Zhou, Barennes, Zhou, Ding, Zhu and Strobel2009). Consequently, P. canaliculata is aquacultured intensively for human consumption; it is often sold in local markets and served regularly in restaurants (Zhang et al. Reference Zhang, Chen, Gao, Geng, Huang, Liu, Wu and Zhu2008a ; Lv et al. Reference Lv, Zhang, Chen, Wang, Fang, Chen, Jiang, Li, Du and Zhou2009a , Reference Lv, Zhang, Liu, Hu, Yang, Steinmann, Chen, Wang, Utzinger and Zhou b ; Zhou et al. Reference Zhou, Barennes, Zhou, Ding, Zhu and Strobel2009; Wang et al. Reference Wang, Qi, Diao, Zheng, Li, Ma, Ji and Yin2010; Li et al. Reference Li, Zhang, Fang, Ouyang, Xie, Jiang, Xie, Chen and Zheng2013a ). Pomacea canaliculata is also more widespread in China than the terrestrial A. fulica, possibly because of its efficient spread via waterways during flooding (Lv et al. Reference Lv, Zhang, Liu, Hu, Yang, Steinmann, Chen, Wang, Utzinger and Zhou2009b ).
Serological surveys suggest human exposure to A. cantonensis is common in China. On Hainan Island, 92 of 459 subjects (20·04%) had antibodies to A. cantonensis (Hu et al. Reference Hu, Du, Tong, Wang, Liu, Li and He2011). In another study from Hainan Island, anti-A. cantonensis immunoglobulin G (IgG) was detected in 20·6% of 393 participants, 12·5% of whom habitually ate raw snails (Li et al. Reference Li, Hu, Tong, Liu, Li and Wang2011). In Guangdong Province, 42 of 300 people (14%) had IgG antibodies to A. cantonensis, five of whom had been recently exposed based on the detection of circulating IgM (Zhang et al. Reference Zhang, Huang, Tan, Chen and Zhan2008b ). The prevalence of A. cantonensis on Hainan Island was equal for both genders, though subjects under the age of 14 were more likely to be seropositive (Hu et al. Reference Hu, Du, Tong, Wang, Liu, Li and He2011). Chen et al. (Reference Chen, Zhang, Ai, Chen, Chen, Huang, Gao, Geng, Li and Zhu2011c ) detected circulating A. cantonensis antigen at a prevalence of 0·8% in members of the general Chinese population though differences were observed between certain groups. Males had a higher prevalence than females and those involved in the aquaculture or processing of molluscs for human consumption were more likely to have circulating antigen (Chen et al. Reference Chen, Zhang, Ai, Chen, Chen, Huang, Gao, Geng, Li and Zhu2011c ). The prevalence of A. cantonensis in molluscs and rats has also been investigated in several regions in China (Table 2).
Hundreds of AEM cases have been reported in Taiwan, many of which were linked to the handling or consumption of P. canaliculata and A. fulica (Hwang and Chen, Reference Hwang and Chen1991; Tsai et al. Reference Tsai, Lee, Huang, Yen, Chen and Liu2004, Reference Tsai, Chen and Yen2013; Wan and Weng, Reference Wan and Weng2004; Wang et al. Reference Wang, Chen and Lun2007). The aquatic snail, Bellamya chinensis, is also considered an important intermediate host of A. cantonensis in Taiwan (Lv et al. Reference Lv, Zhang, Liu, Hu, Yang, Steinmann, Chen, Wang, Utzinger and Zhou2009b ). Early Taiwanese reports of AEM described cases predominantly in children and indigenous Taiwanese (Yii et al. Reference Yii, Chen, Chen, Hsieh and Shih1975; Hwang and Chen, Reference Hwang and Chen1991), though recent reports more commonly describe infections in adults (Tseng et al. Reference Tseng, Tsai, Sy, Lee, Wann, Wang, Chen, Wu and Chen2011, Reference Tsai, Chen and Yen2013). In those earlier cases, infections peaked during the rainy season, and those affected often reported eating thoroughly cooked A. fulica prior to disease (Yii et al. Reference Yii, Chen, Chen, Hsieh and Shih1975). This suggests that A. cantonensis may have been ingested inadvertently during preparation of the snails for cooking.
Cases of AEM in Hawaii have been reported from as early as 1958 (Wallace, Reference Wallace2013). Between 2001 and 2005, 24 cases were reported though only one case was parasitologically confirmed; a case involving a young child. Most of these cases occurred in Honolulu, on the main island of Hawaii (Hochberg et al. Reference Hochberg, Park, Blackburn, Sejvar, Gaynor, Chung, Leniek, Herwaldt and Effler2007, Reference Hochberg, Blackburn, Park, Sejvar, Effler and Herwaldt2011). Deliberate consumption of raw or undercooked molluscs is rare in Hawaii and most exposures were attributed to inadvertent ingestion of molluscs or their mucus. Patients sometimes recalled finding slugs in their food or drink, and the mother of another patient recalled her infant child putting grass in her mouth (Hochberg et al. Reference Hochberg, Blackburn, Park, Sejvar, Effler and Herwaldt2011). Parmarion martensi is considered important for maintaining the endemicity of A. cantonensis in Hawaii, given its high prevalence in Hawaiian populations, and the remarkably high parasite burdens observed in some specimens (Tables 1 and 2).
In Australia, virtually all native and introduced terrestrial and freshwater molluscs can be experimentally infected with A. cantonensis (Prociv and Carlisle, Reference Prociv and Carlisle2001). Despite this, the frequency of infected molluscs in Australia is low compared to what is observed in Southeast Asia (Chan et al. Reference Chan, Barratt, Roberts, Lee, Shea, Marriott, Harkness, Malik, Jones, Aghazadeh, Ellis and Stark2015). It was suggested that the mollusc species in Australia may not be the preferred hosts of A. cantonensis (Chan et al. Reference Chan, Barratt, Roberts, Lee, Shea, Marriott, Harkness, Malik, Jones, Aghazadeh, Ellis and Stark2015). Furthermore, the climate in Sydney is temperate to sub-tropical, whereas transmission of A. cantonensis is favoured by tropical climates which experience warm temperatures, high humidity and high rainfall (Hu et al. Reference Hu, Du, Tong, Wang, Liu, Li and He2011). The minimum temperature threshold (the temperature at which larvae halt development in their mollusc hosts), is approximately 15 °C for A. cantonensis (Lv et al. Reference Lv, Zhou, Zhang, Liu, Zhu, Yin, Steinmann, Wang and Jia2006; Morley, Reference Morley2010). In Sydney, temperatures regularly fall below 15 °C in winter, probably restricting A. cantonensis transmission to the warmer months of spring and summer. In Eastern Australia the prevalence of Angiostrongylus spp. in Rattus spp. is high compared with its prevalence in molluscs (Table 2).
Human cases of AEM have been diagnosed in multiple cities along the eastern coast of Australia including Brisbane, Sydney and Melbourne (Prociv et al. Reference Prociv, Spratt and Carlisle2000). The first Australian case of human AEM was reported in 1971 in the Brisbane region (Gutteridge et al. Reference Gutteridge, Bhaibulaya and Findlater1972), and several human cases have been reported in Australia since (Prociv and Carlisle, Reference Prociv and Carlisle2001; Senanayake et al. Reference Senanayake, Pryor, Walker and Konecny2003; Blair et al. Reference Blair, Orr, Delaney and Herkes2013; Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013). Australian clinical cases mostly involved young children or infants, with or without a known history of mollusc consumption (Prociv et al. Reference Prociv, Spratt and Carlisle2000; Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013). Two cases involved young adults who knowingly ingested slugs from the Sydney area (Senanayake et al. Reference Senanayake, Pryor, Walker and Konecny2003; Blair et al. Reference Blair, Orr, Delaney and Herkes2013). The rate of human infections is proportionally lower in Australia compared with China and Thailand, probably because snails are not widely eaten in Australia. Given the rarity of human angiostrongyliasis in Australia and its mostly self-limited nature, some infections have probably gone unreported. Regardless, the recent cases of severe angiostrongyliasis and increasing range of A. cantonensis underpin the need for increased awareness of angiostrongyliasis in Australia, particularly given that the aetiological agent sometimes remains unidentified until post-mortem (Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013).
Angiostrongylus cantonensis has also been detected in Japan, parts of Southern USA, the Caribbean Islands [though it was absent in rats from Barbados (Levett et al. Reference Levett, Douglas, Waugh, Robinson and Lindo2004)], Tenerife, Brazil, Papua New Guinea, French Polynesia, Fiji, the Philippines, Indonesia, Sri Lanka, India, South Africa and Egypt (Tables 1 and 2, Fig. 5) (Alicata, Reference Alicata1965b ; Kliks and Palumbo, Reference Kliks and Palumbo1992; Uga et al. Reference Uga, Ono, Kataoka and Hasan1996; Asato et al. Reference Asato, Taira, Nakamura, Kudaka, Itokazu and Kawanaka2004; Batmanian and O'Neill, Reference Batmanian and O'Neill2004; Lindo et al. Reference Lindo, Escoffery, Reid, Codrington, Cunningham-Myrie and Eberhard2004; Abo-Madyan et al. Reference Abo-Madyan, Morsy, Motawea, El Garhy and Massoud2005; Owen, Reference Owen2005; Waugh et al. Reference Waugh, Shafir, Wise, Robinson, Eberhard and Lindo2005; Caldeira et al. Reference Caldeira, Mendonca, Goveia, Lenzi, Graeff-Teixeira, Lima, Mota, Pecora, Medeiros and Carvalho Odos2007; Chikweto et al. Reference Chikweto, Bhaiyat, Macpherson, Deallie, Pinckney, Richards and Sharma2009; Dorta-Contreras et al. Reference Dorta-Contreras, Magraner-Tarrau and Sanchez-Zulueta2009; Archer et al. Reference Archer, Appleton, Mukaratirwa and Hope2011; Constantino-Santos et al. Reference Constantino-Santos, Basiao, Wade, Santos and Fontanilla2014; Oehler et al. Reference Oehler, Ghawche, Delattre, Berberian, Levy and Valour2014; Okano et al. Reference Okano, Haga, Mizuno, Onuma, Nakaya and Nagamine2014; Lammers et al. Reference Lammers, Goorhuis, van de Beek, Grobusch, Bart, van Gool and van Vugt2015; Stockdale-Walden et al. Reference Stockdale-Walden, Slapcinsky, Qvarnstrom, McIntosh, Bishop and Rosseland2015).
PATHOPHYSIOLOGY AND CLINICAL MANIFESTATIONS
Angiostrongylus cantonensis larvae are neurotropic, preferentially infecting CNS tissue, and causing inflammation at these sites. Autopsies of AEM patients indicate that the external surfaces of the brain and the spinal cord are generally normal and gross haemorrhage is uncommon, though has been reported in severe human cases (Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008; Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013). Infiltration of lymphocytes, plasma cells and eosinophils is common in the meninges (Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008) (Fig. 6). Third-stage larvae may be observed in association with the meninges and nerve roots (Chen et al. Reference Chen, Li and Lun2005). Eosinophilic pleocytosis in the CSF and increased CSF protein are common (Schmutzhard et al. Reference Schmutzhard, Boongird and Vejjajiva1988; Dorta-Contreras et al. Reference Dorta-Contreras, Padilla-Docal, Moreira, Robles, Aroca, Alarcon and Bu-Coifiu-Fanego2011). Cellular infiltration around live worms is unusual though dead worms precipitate granuloma formation, infiltration by eosinophils and occasionally Charcot–Leyden crystals (Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008; Martins et al. Reference Martins, Tanowitz and Kazacos2015). Physical tracks and microcavities due to the burrowing movement of larvae may be observed in the brain and spinal cord (Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013; Murphy and Johnson, Reference Murphy and Johnson2013).
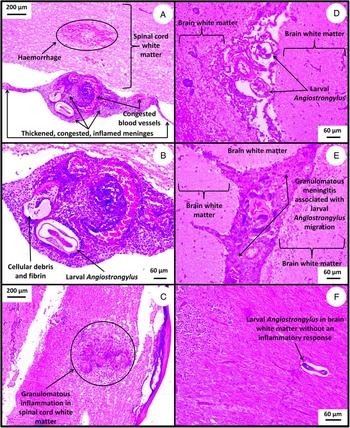
Fig. 6. Haematoxylin and eosin-stained tissue sections from Australian wildlife showing histopathological changes caused by A. cantonensis infection. (A) Spinal cord section from a sub-adult female brushtail possum that had hind limb paralysis. Focal haemorrhage of the spinal cord is apparent, along with greatly thickened, congested and inflamed meninges. (B) The same spinal cord section shown in (A), though at a higher magnification. The focal haemorrhage and thickened, congested, inflamed meninges can be seen more clearly, associated with a cross-section of a larval nematode identified as A. cantonensis in this animal. The meninges are infiltrated by plasma cells, lymphocytes, macrophages and neutrophils (marked non-suppurative meningitis). (C) Spinal cord from the same brushtail possum showing tissue damage and granulomatous inflammation in the white matter where presumably a larval Angiostrongylus travelled through. (D) Brain section from an affected tawny frogmouth showing cross-sections of larval Angiostrongylus associated with granulomatous meningitis. (E) Brain and meninges from the same tawny frogmouth as (D), showing more obvious granulomatous meningitis. (F) Brain from another affected tawny frogmouth showing a larval Angiostrongylus migrating through the white matter before an inflammatory response has occurred.
Angiostrongyliasis initiates a Th2 immune response in the CNS, characterized by the infiltration of eosinophils into the subarachnoid space, and CSF eosinophilia (Intapan et al. Reference Intapan, Kittimongkolma, Niwattayakul, Sawanyawisuth and Maleewong2008; Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009; Murphy and Johnson, Reference Murphy and Johnson2013; Martins et al. Reference Martins, Tanowitz and Kazacos2015). The role of eosinophils in the immune response to helminth infections is incompletely understood (Klion and Nutman, Reference Klion and Nutman2004). However, the classical view of eosinophils as effectors for killing worms has given way to the idea that they are probably not directly involved in killing them, but are actually important regulators of the cellular immune response (Gebreselassie et al. Reference Gebreselassie, Moorhead, Fabre, Gagliardo, Lee, Lee and Appleton2012; Gosnell and Kramer, Reference Gosnell and Kramer2013). As supported by rodent studies of AEM, microglia excrete eosinophil chemoattractants such as eotaxin and macrophage inflammatory protein in response to dead worms, to recruit eosinophils to the brain parenchyma (Chang et al. Reference Chang, Chung and Yen2004; Chang and Yen, Reference Chang and Yen2004; Intapan et al. Reference Intapan, Niwattayakul, Sawanyawisuth, Chotmongkol and Maleewong2007; Gosnell and Kramer, Reference Gosnell and Kramer2013; Zhao et al. Reference Zhao, Lv, Wang, Wei, Zhang, Li, Yang, Zeng, Wu and Wu2013; Li et al. Reference Li, Yang, Ji, Zeng, Wu, Wei, Ouyang, Liang, Zheng, Wu and Lv2014a ; Wei et al. Reference Wei, Wu, He, Zeng, Ouyang, Liu, Zheng, Lei, Wu and Lv2015). Once in the brain, eosinophils enhance this Th2 response by producing Th2 cytokines, excreting chemoattractants, and presenting antigen to CD4+ T cells (Spencer and Weller, Reference Spencer and Weller2010). Consequently, levels of Th2 cytokines such as interleukin (IL) 4, IL5, IL10 and IL13 increase in the brain and CSF (Intapan et al. Reference Intapan, Kittimongkolma, Niwattayakul, Sawanyawisuth and Maleewong2008; Yu et al. Reference Yu, Wu, Wei, Liao, Xu, Luo, Zeng, Zhao, Lv and Wu2015). Th2 cytokines may also become elevated in the periphery (Diao et al. Reference Diao, Chen, Yin, Wang, Qi and Ji2009). Angiostrongyliasis can increase CNS levels of IL33; an important mediator of eosinophil infiltration, Th2 cell differentiation and expression of IL5 and IL13 (Du et al. Reference Du, Chen, Lin and Chuang2013; Peng et al. Reference Peng, Sun, Zhang, Zhao, Wei, Zeng, Zheng and Wu2013; Chuang et al. Reference Chuang, Chen, Huang and Du2016; Saluja et al. Reference Saluja, Khan, Church and Maurer2015). IL5 and IL13 are two of several important regulators of IgE production (Deo et al. Reference Deo, Mistry, Kakade and Niphadkar2010). As a result, intrathecal IgE may be elevated in AEM (Dorta-Contreras et al. Reference Dorta-Contreras, Noris-Garcia, Escobar-Perez and Padilla Docal2005, Reference Dorta-Contreras, Padilla-Docal, Moreira, Robles, Aroca, Alarcon and Bu-Coifiu-Fanego2011; Padilla-Docal et al. Reference Padilla-Docal, Dorta-Contreras, Bu-Coifiu-Fanego, Hernandez, Barroso and Sanchez-Martinez2008). Monocyte chemotactic protein 1 is also expressed in the brain of mice in response to injury caused by migrating larvae (Yu et al. Reference Yu, Wu, Wei, Liao, Xu, Luo, Zeng, Zhao, Lv and Wu2015).
Blood brain barrier (BBB) dysfunction is a feature of AEM, associated with the activity of host matrix metalloproteinase-9 (MMP-9); a protease that degrades extracellular matrix proteins such as fibronectin and elastin (Hsu and Lai, Reference Hsu and Lai2007; Wei et al. Reference Wei, Tsai, Chiu and Lai2011). In healthy brain tissue, MMP-9 expression is low, though becomes elevated in response to certain stimuli, including brain tissue damage (Vafadari et al. Reference Vafadari, Salamian and Kaczmarek2016). In AEM, MMP-9 expression increases, possibly due to the damage inflicted by migrating worms (Tsai et al. Reference Tsai, Chung, Chen, Liu, Lee, Chen, Sy, Wann and Yen2008). Mouse studies of AEM suggest that eosinophils release MMP-9 into the subarachnoid space (Tseng et al. Reference Tseng, Tu, Lee, Chen, Chou and Lai2004), activating a proteolytic cascade that disrupts the BBB (Chen et al. Reference Chen, Liu, Lai, Hsu and Lee2006; Tsai et al. Reference Tsai, Chung, Chen, Liu, Lee, Chen, Sy, Wann and Yen2008; Chiu and Lai, Reference Chiu and Lai2013, Reference Chiu and Lai2014). In mice with AEM, MMP-9 was also observed within endothelial cells lining the vascular spaces of the brain and in leucocytes within the subarachnoid space (Lai et al. Reference Lai, Twu, Jiang, Hsu, Chen, Chiaing, Wang, Tseng, Shyu and Lee2004). Angiostrongylus cantonensis also excretes MMPs and other proteases involved in the pathogenesis of AEM (discussed in a later section). Dysfunction of the BBB in AEM may also be mediated by vascular endothelial growth factor; an inducer of vascular permeability and mediator of brain oedema (Tsai et al. Reference Tsai, Liu, Lee, Chen and Yen2007a ). Levels of pro-apoptotic proteins, plasminogen activators, and reactive oxygen species are also elevated in the CNS of mice with AEM (Hou et al. Reference Hou, Tu, Lee, Chen, Chou and Lai2004; Chen et al. Reference Chen, Liu, Lai, Hsu and Lee2006, Reference Chen, Lee, Lai, Hsu, Wang and Liu2008). Expression of the 14-3-3β protein; a marker of neuronal damage, is also elevated in CSF and serum from AEM patients (Tsai et al. Reference Tsai, Huang, Chen, Yen, Tsai, Lee and Tai2014a ).
The clinical manifestations of angiostrongyliasis occur partly as a result of increased intracranial pressure (ICP); a common clinical sign of AEM (Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009; Murphy and Johnson, Reference Murphy and Johnson2013). Increased ICP may result from vasodilation in the subarachnoid space and brain parenchyma, decreased absorption of CSF, or brain oedema (Murphy and Johnson, Reference Murphy and Johnson2013). As a consequence of high ICP, AEM patients often present with mild to severe headaches, though prolonged high ICP can eventuate in more serious neurological sequelae (Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008, Reference Wang, Qi, Diao, Zheng, Li, Ma, Ji and Yin2010; Tseng et al. Reference Tseng, Tsai, Sy, Lee, Wann, Wang, Chen, Wu and Chen2011; Murphy and Johnson, Reference Murphy and Johnson2013).
Eosinophilic meningitis is the most common manifestation of angiostrongyliasis (Wang et al. Reference Wang, Wu, Wei, Owen and Lun2012). Rarely, severe sequelae including coma, convulsion, epilepsy, amentia, hypomnesia and even death can occur (Wang et al. Reference Wang, Qi, Diao, Zheng, Li, Ma, Ji and Yin2010; Howe, Reference Howe2013; Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013). However, patients more often present with headache, neck stiffness, paraesthesia, muscle weakness, Brudzinski's sign, fever, vomiting and nausea. Symptoms such as face/limb paralysis, memory loss, confusion, dizziness, conscious disturbance, tinnitus, hyperesthesia, dystonia, urinary retention, photophobia, pneumonitis, peritonitis, abdominal pain, bowel dysfunction, orbital/retro-orbital pain and paralysis of the extra-ocular muscles, are also less common (Chau et al. Reference Chau, Thwaites, Chuong, Sinh and Farrar2003; Podwall et al. Reference Podwall, Gupta, Furuya, Sevigny and Resor2004; Furugen et al. Reference Furugen, Yamashiro, Tamayose, Naha, Miyagi, Nakasone, Uchihara, Haranaga, Azuma, Yara, Shinzato, Higa, Toma, Tateyama and Fujita2006; Jin et al. Reference Jin, Ma, Ma, He, Ji and Yin2008; Li et al. Reference Li, Xu, Gu and Chen2008; Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008, Reference Wang, Qi, Diao, Zheng, Li, Ma, Ji and Yin2010; Tseng et al. Reference Tseng, Tsai, Sy, Lee, Wann, Wang, Chen, Wu and Chen2011; Kwon et al. Reference Kwon, Ferguson, Park, Manuzak, Qvarnstrom, Morgan, Ciminera and Murphy2013). Symptoms can be protracted, taking months to disappear (Hochberg et al. Reference Hochberg, Blackburn, Park, Sejvar, Effler and Herwaldt2011). In humans and animals, neurological damage is sometimes irreversible (Chau et al. Reference Chau, Thwaites, Chuong, Sinh and Farrar2003; Batmanian and O'Neill, Reference Batmanian and O'Neill2004) (Fig. 4, online Supplementary materials). Encephalitic angiostrongyliasis is a rarer manifestation that is generally fatal (Sawanyawisuth, Reference Sawanyawisuth2008). Elderly patients who become infected with A. cantonensis and experience fever and prolonged headaches are at greater risk of developing encephalitic angiostrongyliasis (Sawanyawisuth, Reference Sawanyawisuth2008; Sawanyawisuth et al. Reference Sawanyawisuth, Takahashi, Hoshuyama, Senthong, Limpawattana, Intapan, Wilson, Tiamkao, Jitpimolmard and Chotmongkol2009).
Myelitis, sacral myeloradiculitis and inflammation of the nerve roots can occur in AEM (Hsu et al. Reference Hsu, Chuang, Chen and Huang2009; Murphy and Johnson, Reference Murphy and Johnson2013; Ueda et al. Reference Ueda, Takeuchi, Ochiai, Mabuchi and Niwa2015), though this is more often associated with gnathostomiasis; the disease caused by Gnathostoma spp. worms (Schmutzhard et al. Reference Schmutzhard, Boongird and Vejjajiva1988). Intraparenchymal cerebral haemorrhage has also been reported (Lilic and Addison, Reference Lilic and Addison2013). Presumably, disease severity and incubation period vary depending on the number of larvae consumed (Murphy and Johnson, Reference Murphy and Johnson2013). Incubation periods range from as little as 1 day to several months, with a median of 11 days following ingestion of L3 larvae (Zhou et al. Reference Zhou, Barennes, Zhou, Ding, Zhu and Strobel2009; Tseng et al. Reference Tseng, Tsai, Sy, Lee, Wann, Wang, Chen, Wu and Chen2011; Wang et al. Reference Wang, Wu, Wei, Owen and Lun2012; Murphy and Johnson, Reference Murphy and Johnson2013; Sawanyawisuth et al. Reference Sawanyawisuth, Chindaprasirt, Senthong, Limpawattana, Auvichayapat, Tassniyom, Chotmongkol, Maleewong and Intapan2013). As human angiostrongyliasis is often self-limiting, mortality rates are usually low (Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008; Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009).
While there is little supporting evidence, children seem especially predisposed to angiostrongyliasis, as evidenced by their disproportionate representation in some outbreaks (Yii, Reference Yii1976), and the particularly severe cases reported in infants and children (Prociv et al. Reference Prociv, Spratt and Carlisle2000; Li et al. Reference Li, He, Wang, Liang, Li, Men and Zhan2001; Lindo et al. Reference Lindo, Escoffery, Reid, Codrington, Cunningham-Myrie and Eberhard2004; Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013; Murphy and Johnson, Reference Murphy and Johnson2013; Evans-Gilbert et al. Reference Evans-Gilbert, Lindo, Henry, Brown and Christie2014). The reason for this remains unclear. However, in cases such as those reported from Taiwan, infected children were thought to have been playing with live snails and possibly eating them (Yii et al. Reference Yii, Chen, Chen, Hsieh and Shih1975; Yii, Reference Yii1976), which is something adults are unlikely to do. This may expose children to greater numbers of larvae, resulting in severer manifestations. Wang et al. (Reference Wang, Lai, Zhu, Chen and Lun2008) and Sawanyawisuth et al. (Reference Sawanyawisuth, Chindaprasirt, Senthong, Limpawattana, Auvichayapat, Tassniyom, Chotmongkol, Maleewong and Intapan2013) also note that the clinical manifestations of AEM differ between adults and children; somnolence, fever, constipation, abdominal pain, vomiting, nausea, hepatomegaly, neck stiffness and cranial nerve palsies were more common in children.
In approximately 1·1% of cases A. cantonensis causes ocular disease, which may or may not be concurrent with AEM (Patikulsila et al. Reference Patikulsila, Ittipunkul and Theerakittikul2003; Sawanyawisuth et al. Reference Sawanyawisuth, Kitthaweesin, Limpawattana, Intapan, Tiamkao, Jitpimolmard and Chotmongkol2007; Baheti et al. Reference Baheti, Sreedharan, Krishnamoorthy, Nair and Radhakrishnan2008; Chi et al. Reference Chi, Kim, Haug, Vagefi and Kersten2014). Patients with ocular angiostrongyliasis may present with blepharospasm, diplopia, strabismus, blurred vision, loss of colour vision or complete vision loss (Liu et al. Reference Liu, Chung, Chen and Cho2006; Wang et al. Reference Wang, Wang and Jou2006b ; Sawanyawisuth et al. Reference Sawanyawisuth, Kitthaweesin, Limpawattana, Intapan, Tiamkao, Jitpimolmard and Chotmongkol2007; Sinawat et al. Reference Sinawat, Sanguansak, Angkawinijwong, Ratanapakorn, Intapan and Yospaiboon2008; Qi et al. Reference Qi, Diao and Yin2009). The route taken by A. cantonensis to enter the eye is unknown, though worms may travel from the brain via the optic nerve, through the circulation via the retinal artery, or enter the eye directly from the environment (Martins et al. Reference Martins, Tanowitz and Kazacos2015). Worms may be found in the anterior chamber, the vitreous cavity or the subretinal space (Kumar et al. Reference Kumar, Kyprianou and Keenan2005; Malhotra et al. Reference Malhotra, Mehta, Arora, Chauhan, Ray and Jain2006; Sawanyawisuth et al. Reference Sawanyawisuth, Kitthaweesin, Limpawattana, Intapan, Tiamkao, Jitpimolmard and Chotmongkol2007; Crane et al. Reference Crane, Weiss and Galor2013; Sinawat and Yospaiboon, Reference Sinawat and Yospaiboon2013; Galor and Eberhard, Reference Galor and Eberhard2014). Clinical signs of ocular angiostrongyliasis are diverse and may include uveitis, macular oedema, retinal oedema, necrotic retinitis, panophthalmitis, papilledema, optic neuritis, optic nerve compression, orbital inflammation, increased intraocular pressure, retinal oedema, macular oedema and a pale optic disc (Kumar et al. Reference Kumar, Kyprianou and Keenan2005; Liu et al. Reference Liu, Chung, Chen and Cho2006; Wang et al. Reference Wang, Wang and Jou2006b ; Sawanyawisuth and Kitthaweesin, Reference Sawanyawisuth and Kitthaweesin2008; Sinawat et al. Reference Sinawat, Sanguansak, Angkawinijwong, Ratanapakorn, Intapan and Yospaiboon2008; Qi et al. Reference Qi, Diao and Yin2009; Feng et al. Reference Feng, Nawa, Sawanyavisuth, Lv and Wu2013; Sinawat and Yospaiboon, Reference Sinawat and Yospaiboon2013; Chi et al. Reference Chi, Kim, Haug, Vagefi and Kersten2014). Altered epithelial pigment and subretinal tracks may also be observed (Sinawat et al. Reference Sinawat, Sanguansak, Angkawinijwong, Ratanapakorn, Intapan and Yospaiboon2008).
DIAGNOSIS
Diagnosis of AEM is often overlooked, particularly in regions previously considered non-endemic. The lack of standardization in diagnostic procedures for angiostrongyliasis often results in a presumptive diagnosis; based on patient history and suggestive clinical findings (i.e. eosinophilic meningitis) (Murphy and Johnson, Reference Murphy and Johnson2013). A history of residence or travel to endemic regions and/or a history of raw mollusc consumption are integral in establishing the diagnosis (Tsai et al. Reference Tsai, Liu, Kunin, Lai, Lee, Chen, Wann, Lin, Huang, Ger, Lin and Yen2003; Cowie, Reference Cowie2013b ). A history of eating unwashed fresh produce, such as lettuce, is also informative (Lindo et al. Reference Lindo, Waugh, Hall, Cunningham-Myrie, Ashley, Eberhard, Sullivan, Bishop, Robinson, Holtz and Robinson2002; Waugh et al. Reference Waugh, Shafir, Wise, Robinson, Eberhard and Lindo2005). An accurate patient history may differentiate AEM from neural gnathostomiasis, which can also manifest as eosinophilic meningitis. While AEM patients often have a history of eating raw molluscs, gnathostomiasis patients usually recall eating undercooked poultry or fish (Senthong et al. Reference Senthong, Chindaprasirt and Sawanyawisuth2013).
Microscopic detection of L3 larvae (Fig. 2) from a patient's CSF or eye provides a definitive diagnosis. However, the sensitivity of CSF microscopy depends on sample volume, which is generally limited, often leading to poor sensitivity and false negative results (Prociv et al. Reference Prociv, Spratt and Carlisle2000; Chen et al. Reference Chen, Li and Lun2005; Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009). Molecular and immunological tests offer greater sensitivity and several of these have been described for angiostrongyliasis (discussed in later sections). Peripheral blood and CSF eosinophil counts, and CT or MRI imaging can aid the diagnosis (Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009). In ocular angiostrongyliasis, worms may be observed by slit lamp examination of the eye (Malhotra et al. Reference Malhotra, Mehta, Arora, Chauhan, Ray and Jain2006; Mattis et al. Reference Mattis, Mowatt, Lue, Lindo and Vaughan2009).
During a helminth infection, the proportion of eosinophils may reach 7–36% of the total white blood cell count in peripheral blood (normal range is 0·5–5%) and 10% or more (100–1000 eosinophils per μL) of the white cell count in CSF (normal range is <10 eosinophils/μL−1) (Schulte et al. Reference Schulte, Krebs, Jelinek, Nothdurft, von Sonnenburg and Loscher2002; Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008; Sawanyawisuth and Chotmongkol, Reference Sawanyawisuth and Chotmongkol2013). Eosinophils can be difficult to differentiate from neutrophils using some staining techniques such as the toluidine blue wet film. In aseptic meningitis, particularly associated with peripheral eosinophilia, more specific Romanowsky-based stains such as the May–Grünwald–Giemsa stain or Wright stain should be performed (Senanayake et al. Reference Senanayake, Pryor, Walker and Konecny2003; Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009). The Diff-Quik stain may also be useful, having been used to confirm eosinophilia in cases of canine angiostrongyliasis (Lunn et al. Reference Lunn, Lee, Smaller, MacKay, King, Hunt, Martin, Krockenberger, Spielman and Malik2012). While A. cantonensis is the leading cause of eosinophilic meningitis, other aetiological agents must be considered in the differential diagnosis (Table 3).
Table 3. Causative agents to be considered in the differential diagnosis of eosinophilic meningitis
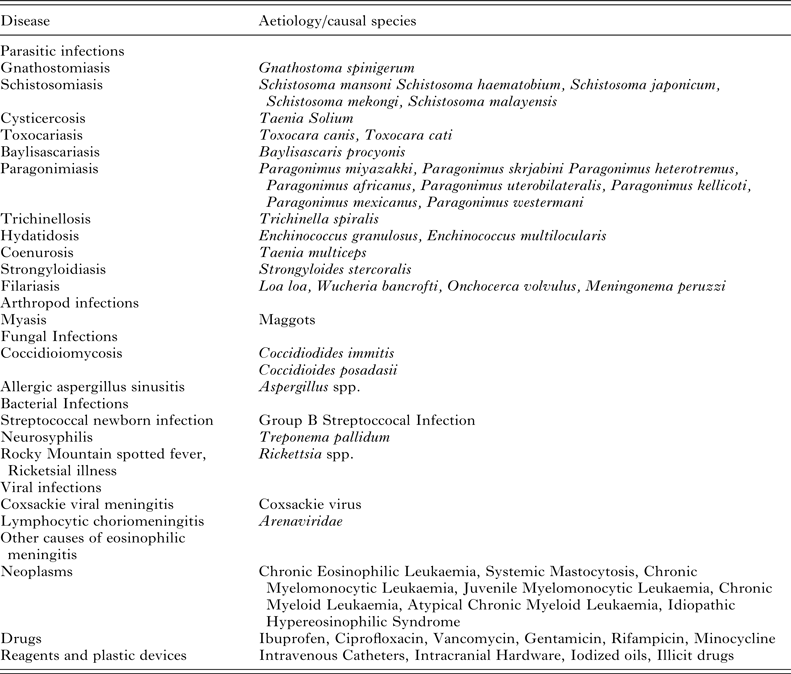
Note: This table was prepared using information presented by Graeff-Teixeira et al. (Reference Graeff-Teixeira, da Silva and Yoshimura2009) and Diaz (Reference Diaz2009).
Brain MRI or CT scans may reveal some abnormalities in AEM patients, though lesions resulting from gnathostomiasis are often distinct from those seen in AEM (Senthong et al. Reference Senthong, Chindaprasirt and Sawanyawisuth2013). In gnathostomiasis with CNS involvement, abnormal CT or MRI findings are common (Kanpittaya et al. Reference Kanpittaya, Sawanyawisuth, Intapan, Khotsri, Chotmongkol and Maleewong2012). Conversely, AEM patients may have normal CT or MRI findings though non-specific cerebral oedema, focal oedematous changes, nodular enhancing lesions, meningeal/leptomeningeal enhancement and mild ventricular dilatation may be apparent (Tsai et al. Reference Tsai, Liu, Kunin, Lai, Lee, Chen, Wann, Lin, Huang, Ger, Lin and Yen2003, Reference Tsai, Tseng, Yen, Chen, Sy, Lee, Wann and Chen2012; Jin et al. Reference Jin, Ma, Ma, He, Ji and Yin2008; Wang et al. Reference Wang, Qi, Diao, Zheng, Li, Ma, Ji and Yin2010; Tseng et al. Reference Tseng, Tsai, Sy, Lee, Wann, Wang, Chen, Wu and Chen2011; Kanpittaya et al. Reference Kanpittaya, Sawanyawisuth, Intapan, Khotsri, Chotmongkol and Maleewong2012; Nalini et al. Reference Nalini, Ramakrishna, Dekumoy, Kumar, Pakdee, Saini and Hegde2013). Intracerebral haemorrhage and myelitis are more suggestive of gnathostomiasis (Kanpittaya et al. Reference Kanpittaya, Sawanyawisuth, Intapan, Khotsri, Chotmongkol and Maleewong2012). Lesions may also be present on the spinal cord of AEM patients (Diao et al. Reference Diao, Jin and Yin2010). Brain abnormalities may become apparent in MRI scans as early as 3 weeks after the onset of AEM symptoms (Jin et al. Reference Jin, Ma, Liang, Ji and Gan2005).
Immunodiagnostic assays
Immunodiagnostic tests for angiostrongyliasis using purified antigens or monoclonal antibodies have been available for decades. The earliest enzyme-linked immunosorbant assays (ELISAs) were developed using crude or partially purified adult A. cantonensis antigens (Chuan-Min and Eng-Rin, Reference Chuan-Min and Eng-Rin1991). Two immunodominant A. cantonensis antigens were identified; a 29 and a 31 kDa antigen, and most immunoassays described target these (Wilkins et al. Reference Wilkins, Qvarnstrom, Whelen, Saucier, da Silva and Eamsobhana2013) (Table 4). Immunoblots targeting the 29 kDa antigen cross-reacted with sera from patients infected with other tissue-invading helminths, while tests targeting the 31 kDa antigen exhibited greater specificity (Wilkins et al. Reference Wilkins, Qvarnstrom, Whelen, Saucier, da Silva and Eamsobhana2013).
Table 4. Assays recently evaluated for the specific detection of A. cantonensis
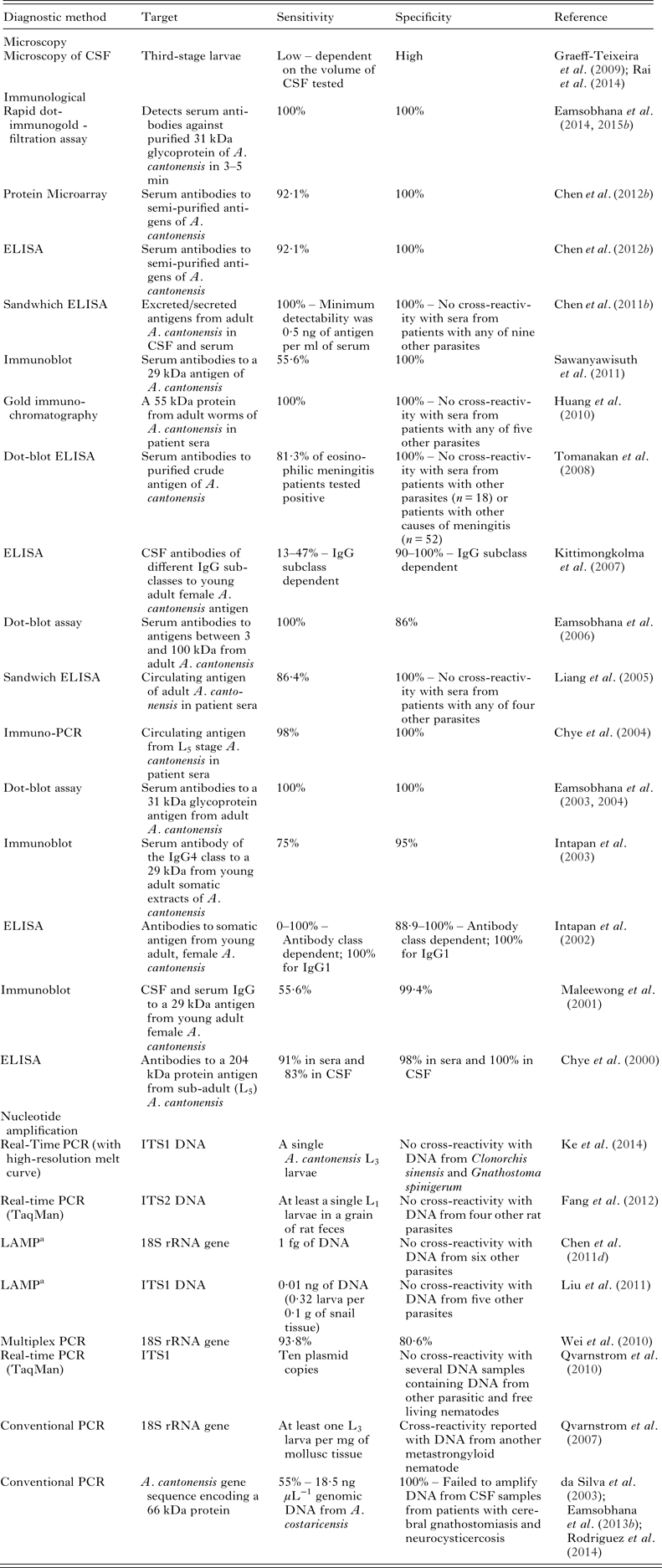
Note: This table only lists assays described in the literature during or after the year 2000. For information on earlier assays see the reviews by Graeff-Teixeira et al. (Reference Graeff-Teixeira, da Silva and Yoshimura2009), Eamsobhana and Yong (Reference Eamsobhana and Yong2009) and Wilkins et al. (Reference Wilkins, Qvarnstrom, Whelen, Saucier, da Silva and Eamsobhana2013).
a LAMP, Loop-mediated isothermal amplification.
As most cases of AEM originate in Thailand, routine serological testing has been implemented there in several regional hospital laboratories (Eamsobhana and Yong, Reference Eamsobhana and Yong2009). These laboratories utilize an in-house dot-blot assay based on the 31 kDa antigen, purified from A. cantonensis worms (Eamsobhana et al. Reference Eamsobhana, Yoolek and Kreethapon2003, Reference Eamsobhana, Ongrotchanakun, Yoolek, Punthuprapasa, Monkong and Dekumyoy2006; Eamsobhana, Reference Eamsobhana2013; Wilkins et al. Reference Wilkins, Qvarnstrom, Whelen, Saucier, da Silva and Eamsobhana2013). This 31 kDa antigen consists of up to four distinct glycoproteins that react to sera from angiostrongyliasis patients (Morassutti et al. Reference Morassutti, Levert, Perelygin, da Silva, Wilkins and Graeff-Teixeira2012a ). As purification of these glycoproteins from worms is laborious, attempts were made to generate recombinants of them for immunodiagnostic purposes (Morassutti et al. Reference Morassutti, Perelygin, Levert, Lin, Lee, da Silva, Wilkins and Graeff-Teixeira2013a ). This endeavour has been met with limited success so far, which may be attributable to the choice of expression system (Morassutti et al. Reference Morassutti, Perelygin, Levert, Lin, Lee, da Silva, Wilkins and Graeff-Teixeira2013a ). Eamsobhana et al. (Reference Eamsobhana, Prasartvit, Gan and Yong2015b ) recently described a rapid dot-immunogold filtration assay for detecting serum antibodies reactive to the 31 kDa antigen. This assay boasts 100% sensitivity and specificity and produces a result in 3–5 min, compared to the 3 h required for an immunoblot test result (Eamsobhana et al. Reference Eamsobhana, Prasartvit, Gan and Yong2015b ).
Currently, Shenzhen Combined Biotech Co. Ltd, China, provides the only commercial immunoassays for detecting anti-A. cantonensis antibodies, though these have not been implemented for routine diagnostic use based on current literature. The company produces three ELISA tests; one for detecting human IgG, another for detecting human IgM, and a third for detecting rat IgG (Hu et al. Reference Hu, Du, Tong, Wang, Liu, Li and He2011).
Molecular assays
Several nucleic acid amplification assays have been described for the detection of A. cantonensis DNA, most targeting the ribosomal RNA genes (rDNA) (Table 4). Given the limited genetic variation in the rDNA between the various Angiostrongylidae, new tests that differentiate them with improved resolution must focus on different markers (Chan et al. Reference Chan, Barratt, Roberts, Lee, Shea, Marriott, Harkness, Malik, Jones, Aghazadeh, Ellis and Stark2015). The development of molecular tests that differentiate closely related Angiostrongylidae was initially hampered by the limited availability of sequence data for them. A technique for distinguishing certain Angiostrongylus spp. was developed several years ago; a PCR restriction fragment length polymorphism technique targeting the mitochondrial cytochrome c oxidase subunit I (COI) gene, and the internal transcribed spacer 2 (ITS2) DNA (Caldeira et al. Reference Caldeira, Carvalho, Mendonca, Graeff-Teixeira, Silva, Ben, Maurer, Lima and Lenzi2003). It is unknown however, whether this technique can differentiate between the closely related A. cantonensis and A. mackerrasae. With the current availability of several Angiostrongylus spp. mitochondrial genomes (discussed in a later section), the development of improved, species-specific assays may now be achievable. Molecular detection, though currently not used routinely, holds potential for the future diagnosis of angiostrongyliasis, enabling specific detection of A. cantonensis DNA in patient CSF (Wilkins et al. Reference Wilkins, Qvarnstrom, Whelen, Saucier, da Silva and Eamsobhana2013; Qvarnstrom et al. Reference Qvarnstrom, Xayavong, da Silva, Park, Whelen, Calimlim, Sciulli, Honda, Higa, Kitsutani, Chea, Heng, Johnson, Graeff-Teixeira, Fox and da Silva2016).
One conventional PCR assay amplifying a 1134 bp fragment of the 18S rDNA was used to detect A. cantonensis DNA in molluscs from Hawaii, in response to an angiostrongyliasis outbreak (Qvarnstrom et al. Reference Qvarnstrom, Sullivan, Bishop, Hollingsworth and da Silva2007). Sequencing of PCR products obtained in that study confirmed three false positives resulting from cross-reactivity with other nematode species; a consequence of the highly conserved nature of 18S rDNA (Qvarnstrom et al. Reference Qvarnstrom, Sullivan, Bishop, Hollingsworth and da Silva2007). Another PCR assay targeting a 66 kDa protein from A. cantonensis/A. costaricensis is also available. This assay detected A. costaricensis DNA in patient sera and in paraffin-embedded tissues (da Silva et al. Reference da Silva, Graeff-Teixeira and Zaha2003; Rodriguez et al. Reference Rodriguez, da Silva, Muller, Alves, Graeff-Teixeira and Fornari2014). It also detected A. cantonensis DNA in patient CSF, though with comparatively low sensitivity (Table 4) (Eamsobhana et al. Reference Eamsobhana, Wanachiwanawin, Dechkum, Parsartvit and Yong2013b ).
A TaqMan qPCR assay was developed for detecting A. cantonensis DNA in molluscs collected in the field in Hawaii (Qvarnstrom et al. Reference Qvarnstrom, da Silva, Teem, Hollingsworth, Bishop, Graeff-Teixeira and da Silva2010). This assay amplifies the ITS1 DNA region, which is less conserved than the 18S rDNA among helminth species (Qvarnstrom et al. Reference Qvarnstrom, da Silva, Teem, Hollingsworth, Bishop, Graeff-Teixeira and da Silva2010). The assay has also been trialed on human CSF for diagnosing AEM, with promising results (Thyssen et al. Reference Thyssen, Mitchell, Qvarnstrom, Rao, Benke and Glode2013; Qvarnstrom et al. Reference Qvarnstrom, Xayavong, da Silva, Park, Whelen, Calimlim, Sciulli, Honda, Higa, Kitsutani, Chea, Heng, Johnson, Graeff-Teixeira, Fox and da Silva2016). However, some patients returned intermittently positive and negative results, indicating that the amount of A. cantonensis DNA in CSF changes over the course of infection (Qvarnstrom et al. Reference Qvarnstrom, Xayavong, da Silva, Park, Whelen, Calimlim, Sciulli, Honda, Higa, Kitsutani, Chea, Heng, Johnson, Graeff-Teixeira, Fox and da Silva2016). It has also been used to screen molluscs in field studies from various other locations (Tables 1 and 2), and to detect A. cantonensis DNA in the blood and peripheral tissues of experimentally infected, wild-type R. rattus from Hawaii (Jarvi et al. Reference Jarvi, Pitt, Farias, Shiels, Severino, Howe, Jacquier, Shiels, Amano, Luiz, Maher, Allison, Holtquist and Scheibelhut2015). However, this assay was recently found to cross-react with DNA from A. mackerrasae (Chan et al. Reference Chan, Barratt, Roberts, Lee, Shea, Marriott, Harkness, Malik, Jones, Aghazadeh, Ellis and Stark2015). Consequently, a more specific test is required to confirm the prevalence of A. cantonensis in Australia, where A. mackerrasae is endemic (Mackie et al. Reference Mackie, Lacasse and Spratt2013). Angiostrongylus mackerrasae causes disease in animals though its zoonotic potential remains unknown (Spratt, Reference Spratt2015). Some human cases of angiostrongyliasis may have been attributable to A. mackerrasae in Australia, though the absence of an assay that differentiates it from A. cantonensis makes this possibility difficult to explore. Mitochondrial genes such as the COI gene and NADH dehydrogenase subunit 6 gene represent possible targets for differentiating closely related Angiostrongylidae as they possess greater variability between populations compared with the chromosomal rDNA sequences (Gasser et al. Reference Gasser, Jabbar, Mohandas, Schnyder, Deplazes, Littlewood and Jex2012; Lv et al. Reference Lv, Zhang, Zhang, Liu, Liu, Hu, Wei, Steinmann, Graeff-Teixeira, Zhou and Utzinger2012, Reference Lv, Zhang, Guo, Liu, Zhou, Jiang and Gu2014; Tokiwa et al. Reference Tokiwa, Harunari, Tanikawa, Komatsu, Koizumi, Tung, Suzuki, Kadosaka, Takada, Kumagai, Akao and Ohta2012; Aghazadeh et al. Reference Aghazadeh, Traub, Mohandas, Aland, Reid, McCarthy and Jones2015c ; Yong et al. Reference Yong, Song, Eamsobhana, Goh and Lim2015c ).
Two loop-mediated isothermal amplification (LAMP) assays have been developed for A. cantonensis, one targeting the ITS1 DNA (Liu et al. Reference Liu, Song, Zhang, Chen, Xu, Ai, Chen, Zhan, Liang, Yuan, Lin and Zhu2011) and the other targeting the 18S rDNA (Chen et al. Reference Chen, Tong, Zhang, Lou, Kong, Lv, Zhuo, Wen and Lu2011d ). These assays were up to ten times more sensitive than conventional PCR (Chen et al. Reference Chen, Tong, Zhang, Lou, Kong, Lv, Zhuo, Wen and Lu2011d ; Liu et al. Reference Liu, Song, Zhang, Chen, Xu, Ai, Chen, Zhan, Liang, Yuan, Lin and Zhu2011) and have the added benefit of being performed at a single temperature (~65 °C), on a simple heat block or in a warm water bath.
MOLECULAR BIOLOGY
Several wide-scale genomic, transcriptomic and proteome studies have contributed to our current understanding of Angiostrongylus spp. molecular biology. These studies will potentially underpin the development of improved diagnostics, therapeutics and novel molecular tools for differentiating species and strains within the Angiostrongylidae. As more data becomes available, it is becoming increasingly apparent that A. cantonensis genetically diverse, probably attributable to its sexual reproductive cycle. Sex determination occurs via an XX/XO system, whereby males receive a single X-chromosome and females receive two, resulting in a diploid chromosome number of 11 and 12, for males and females, respectively (Eamsobhana et al. Reference Eamsobhana, Yoolek and Yong2009). Genetically distinct strains of A. cantonensis can possess significant differences in infectivity, fecundity and virulence (Lee et al. Reference Lee, Chung, Wang, Lin, Wang, Tu, Wu and Yen2014).
Certain Angiostrongylus spp. mitochondrial genes are recognized as a source of genetic diversity. Nine unique COI gene haplotypes were identified in a study of Brazilian A. cantonensis isolates (Monte et al. Reference Monte, Simoes, Oliveira, Novaes, Thiengo, Silva, Estrela and Maldonado2012). Wang and Lv (Reference Wang and Lv2014) identified 39 COI haplotypes in isolates from China. In studies of Thai isolates, as many as 15 mitochondrial cytochrome b gene haplotypes were identified (Dusitsittipon et al. Reference Dusitsittipon, Thaenkham, Watthanakulpanich, Adisakwattana and Komalamisra2015; Yong et al. Reference Yong, Eamsobhana, Song, Prasartvit and Lim2015b ). By comparison, only six 18S rDNA haplotypes are known (Eamsobhana et al. Reference Eamsobhana, Lim and Yong2013a , Reference Eamsobhana, Lim and Yong2015a ).
Nuclear genomes
The A. cantonensis nuclear genome is large compared with other nematodes (Zarowiecki and Berriman, Reference Zarowiecki and Berriman2015). Two A. cantonensis nuclear genomes have been sequenced; one (~253 Mb) from a Taiwanese isolate (Morassutti et al. Reference Morassutti, Perelygin, Carvalho Marcos, Lemos, Pinto, Frace, Wilkins, Graeff-Teixeira and Da Silva2013b ) (GenBank accession: PRJEB493) and the other (~260 Mb) from a Thai isolate (Yong et al. Reference Yong, Eamsobhana, Lim, Razali, Aziz, Rosli, Poole-Johnson and Anwar2015a ) (GenBank accession: PRJNA260338). Morassutti et al. (Reference Morassutti, Perelygin, Carvalho Marcos, Lemos, Pinto, Frace, Wilkins, Graeff-Teixeira and Da Silva2013b ) detected 28 080 putative open reading frames, 3370 of which possessed homology to protein sequences in public databases. Yong et al. (Reference Yong, Eamsobhana, Lim, Razali, Aziz, Rosli, Poole-Johnson and Anwar2015a ) detected 17 482 genes greater than 300 base pairs, 7737 of which were predicted to encode proteins. Of the proteins with known function, most were kinases and transferases. Genes encoding cellular components, including components of the cytoskeleton, were the most abundant (Yong et al. Reference Yong, Eamsobhana, Lim, Razali, Aziz, Rosli, Poole-Johnson and Anwar2015a ). While most genes obtained matches Caenorhabditis spp. sequences, A. cantonensis shares a closer phylogenetic relationship to other bursate nemotodes such as Haemonchus contortus and Necator americanus (Morassutti et al. Reference Morassutti, Perelygin, Carvalho Marcos, Lemos, Pinto, Frace, Wilkins, Graeff-Teixeira and Da Silva2013b ; Yong et al. Reference Yong, Eamsobhana, Lim, Razali, Aziz, Rosli, Poole-Johnson and Anwar2015a ).
Morassutti et al. (Reference Morassutti, Perelygin, Carvalho Marcos, Lemos, Pinto, Frace, Wilkins, Graeff-Teixeira and Da Silva2013b ) identified genes encoding immunogenic proteins using previously published mass spectrometry data to interrogate their genome. A cDNA walking strategy was used to obtain the complete coding sequence for these genes, with the intention of generating recombinants that might facilitate diagnostic development. Of the immunogenic proteins of interest, six galectins were identified as candidates for expression (Morassutti et al. Reference Morassutti, Levert, Pinto, da Silva, Wilkins and Graeff-Teixeira2012b ). Yong et al. (Reference Yong, Eamsobhana, Lim, Razali, Aziz, Rosli, Poole-Johnson and Anwar2015a ) noted nine galectins amongst 34 excreted-secreted proteins identified in their A. cantonensis genome.
Wolbachia spp. are endosymbionts of some arthropods and certain filarial nematodes including Brugia, Wuchereria and Onchocerca spp. (Ferri et al. Reference Ferri, Bain, Barbuto, Martin, Lo, Uni, Landmann, Baccei, Guerrero, de Souza Lima, Bandi, Wanji, Diagne and Casiraghi2011; Slatko et al. Reference Slatko, Luck, Dobson and Foster2014). In Brugia malayi the Wolbachia endosymbiont is essential for survival and fertility (Foster et al. Reference Foster, Ganatra, Kamal, Ware, Makarova, Ivanova, Bhattacharyya, Kapatral, Kumar, Posfai, Vincze, Ingram, Moran, Lapidus, Omelchenko, Kyrpides, Ghedin, Wang, Goltsman, Joukov, Ostrovskaya, Tsukerman, Mazur, Comb, Koonin and Slatko2005). Tsai et al. (Reference Tsai, Huang, Wang, Yu, Wu and Chen2007b ) provided PCR evidence for a Wolbachia endosymbiont in A. cantonensis which was unusual, given that Wolbachia had never been reported in a non-filarial nematode (Foster et al. Reference Foster, Kumar, Ford, Johnston, Ben, Graeff-Teixeira and Taylor2008). To confirm this, Foster et al. (Reference Foster, Kumar, Ford, Johnston, Ben, Graeff-Teixeira and Taylor2008) used PCR and immunohistochemistry to examine A. cantonensis and A. costaricensis but found no evidence of Wolbachia. In support of this, Morassutti et al. (Reference Morassutti, Perelygin, Carvalho Marcos, Lemos, Pinto, Frace, Wilkins, Graeff-Teixeira and Da Silva2013b ) and Yong et al. (Reference Yong, Eamsobhana, Lim, Razali, Aziz, Rosli, Poole-Johnson and Anwar2015a ) report no evidence of Wolbachia in their genome sequences. While Foster et al. (Reference Foster, Kumar, Ford, Johnston, Ben, Graeff-Teixeira and Taylor2008) suggest that the findings of Tsai et al. (Reference Tsai, Huang, Wang, Yu, Wu and Chen2007b ) may be the result of contamination, it should be noted that not all specimens within a filarial nematode species harbour Wolbachia even if that species is known to possess a Wolbachia endosymbiont (Ferri et al. Reference Ferri, Bain, Barbuto, Martin, Lo, Uni, Landmann, Baccei, Guerrero, de Souza Lima, Bandi, Wanji, Diagne and Casiraghi2011).
Mitochondrial genomes
The mitochondrial (mt) genomes of A. cantonensis, A. mackerrasae, A. costaricensis and A. vasorum are similar in size and nucleotide composition. Each circular mt genome encodes 36 genes; 12 proteins, 22 transfer RNAs and two rRNAs (Gasser et al. Reference Gasser, Jabbar, Mohandas, Schnyder, Deplazes, Littlewood and Jex2012; Lv et al. Reference Lv, Zhang, Zhang, Liu, Liu, Hu, Wei, Steinmann, Graeff-Teixeira, Zhou and Utzinger2012, Reference Lv, Zhang, Guo, Liu, Zhou, Jiang and Gu2014; Aghazadeh et al. Reference Aghazadeh, Traub, Mohandas, Aland, Reid, McCarthy and Jones2015c ; Yong et al. Reference Yong, Song, Eamsobhana, Goh and Lim2015c ). The first mt genomes of A. cantonensis and A. costaricensis to be sequenced were 13 497 and 13 585 bp in length, respectively, and were 81·6% similar at the nucleotide level (Lv et al. Reference Lv, Zhang, Zhang, Liu, Liu, Hu, Wei, Steinmann, Graeff-Teixeira, Zhou and Utzinger2012). The mt genome of the canine pathogen, A. vasorum is 13 422 bp (Gasser et al. Reference Gasser, Jabbar, Mohandas, Schnyder, Deplazes, Littlewood and Jex2012), and that of A. mackerrasae is 13 640 bp (Aghazadeh et al. Reference Aghazadeh, Traub, Mohandas, Aland, Reid, McCarthy and Jones2015c ). Pairwise comparisons of translated mt protein coding genes from A. mackerrasae to those of A. cantonensis revealed only a 2·4% difference, indicating that these species diverged very recently (Aghazadeh et al. Reference Aghazadeh, Traub, Mohandas, Aland, Reid, McCarthy and Jones2015c ). This is in contrast to a 16·8% difference observed between A. mackerrasae and A. costaricensis (Aghazadeh et al. Reference Aghazadeh, Traub, Mohandas, Aland, Reid, McCarthy and Jones2015c ).
Lv et al. (Reference Lv, Zhang, Zhang, Liu, Liu, Hu, Wei, Steinmann, Graeff-Teixeira, Zhou and Utzinger2014) sequenced mt genomes from seven Chinese isolates of A. cantonensis which revealed five unique types. These Chinese mt genomes were compared with that of a Taiwanese and Thai isolate (Yong et al. Reference Yong, Song, Eamsobhana, Goh and Lim2015c ). The Taiwanese and Chinese isolates possess smaller mt genomes than the Thai isolate (Yong et al. Reference Yong, Song, Eamsobhana, Goh and Lim2015c ), which also possessed a genome that was more distantly related compared with the Chinese/Taiwanese genomes. Eighteen tRNAs encoded on the Thai mt genome lacked a TΨC-arm (Yong et al. Reference Yong, Song, Eamsobhana, Goh and Lim2015c ); a tRNA structure that interacts with elongation factor Tu during protein synthesis (Watanabe et al. Reference Watanabe, Suematsu and Ohtsuki2014). While the absence of a TΨC-arm is not uncommon in tRNAs encoded on nematode mt genomes (Watanabe et al. Reference Watanabe, Suematsu and Ohtsuki2014), differences were observed between isolates; 17 and 16 tRNAs lacked the TΨC-arm in the Chinese and Taiwanese mt genomes, respectively (Yong et al. Reference Yong, Song, Eamsobhana, Goh and Lim2015c ). A second 13 652 bp A. costaricensis mt genome was sequenced from a Costa Rican isolate (Yong et al. Reference Yong, Song, Eamsobhana, Goh, Lim, Chow, Chan and Abrahams-Sandi2015d ) and compared to the original A. costaricensis mt genome, derived from a Brazilian parasite (Lv et al. Reference Lv, Zhang, Zhang, Liu, Liu, Hu, Wei, Steinmann, Graeff-Teixeira, Zhou and Utzinger2012). Based on phylogenetic analyses and genetic distances calculated for the mt genes, it was proposed that these parasites should be considered distinct members of a species complex, or sibling species (Yong et al. Reference Yong, Song, Eamsobhana, Goh, Lim, Chow, Chan and Abrahams-Sandi2015d ).
Generally, comparisons of mt genes demonstrate greater resolution for differentiating species/strains within the Angiostrongylidae, compared with the nuclear rDNA. These differences could be exploited to develop molecular tools that differentiate Angiostrongylus spp., and may facilitate the identification of new, cryptic species (Gasser et al. Reference Gasser, Jabbar, Mohandas, Schnyder, Deplazes, Littlewood and Jex2012; Yong et al. Reference Yong, Song, Eamsobhana, Goh, Lim, Chow, Chan and Abrahams-Sandi2015d ).
Transcriptomes, proteomes and virulence factors
The transition between larval and adult stages of A. cantonensis is a complex process requiring stringent regulation of gene expression. Several studies have investigated differences in gene expression at the transcript and protein level between stages, to identify mechanisms associated with stage transition and function in Angiostrongylus spp. While some genes are expressed constitutively across all stages, some are stage specific. Chang et al. (Reference Chang, Tang, Yen, Chow and Wang2014a ) found that transcripts exclusive to L5 worms were related to growth, development, sexual differentiation and reproduction, while L3-specific transcripts were mostly related to metabolism. The classes of proteases expressed between these two stages also differed (Chang et al. Reference Chang, Tang, Yen, Chow and Wang2014a ). Huang et al. (Reference Huang, Yao, Song, Li, Hua, Li, Pan and Xia2013) observed differences in galectin abundance in the soluble proteomes of male L5 & male adult worms, and female adult & male adult worms. Stage- and sex-dependent differences in expression of aspartic protease and ornithine decarboxylase antizyme were also noted (Hwang et al. Reference Hwang, Chang and Wang2010; Chen et al. Reference Chen, Liu, Yang, Wu, Zhang, He and Zhan2013), as were differences in antioxidant capacity (Song et al. Reference Song, Huang, Tan, Zhang, Hu and Pan2012; Huang et al. Reference Huang, Yao, Song, Li, Hua, Li, Pan and Xia2013).
Transcriptomes sequenced from all worm stages suggest that proteases are fundamental to Angiostrongylus spp. (Ansell et al. Reference Ansell, Schnyder, Deplazes, Korhonen, Young, Hall, Mangiola, Boag, Hofmann, Sternberg, Jex and Gasser2013; Wang et al. Reference Wang, Chen, Chang, Chung, Gan, Cheng and Tang2013b ). Proteases are important virulence factors in other pathogenic helminths, essential for tissue penetration and nutrient acquisition (Stack et al. Reference Stack, Dalton and Robinson2011; McVeigh et al. Reference McVeigh, Maule, Dalton and Robinson2012; Morassutti and Graeff-Teixeira, Reference Morassutti and Graeff-Teixeira2012). Chang et al. (Reference Chang, Tang, Yen, Chow and Wang2014a ) identified transcripts for proteases of various types in the L3 and L5 stages; most notably metalloproteases, aspartic proteases & cysteine proteases in the L3 stage and cysteine, aspartic & serine proteases in the L5 stage. Serine proteases of A. cantonensis react to sera from infected patients (Morassutti et al. Reference Morassutti, Levert, Pinto, da Silva, Wilkins and Graeff-Teixeira2012b ), and a recombinant asparaginyl endopeptidase was recognized by serum from infected BALB/c and ICR strain mice and, by serum from human angiostrongyliasis patients (Chang et al. Reference Chang, Chen and Wang2014b ). This supports their role at the host–parasite interface.
High expression of cathepsin B-like proteases 1 and 2 (AC-cathB-1, AC-cathB-2) and haemoglobin-type cysteine protease (AC-haem) occurs in L3 larvae (Ni et al. Reference Ni, Wang, Zhang, Yu, Fang and Luo2012; Yu et al. Reference Yu, Liao, Chen, Xu, Zeng, Lv, Sun, Zhen and Wu2014a ). As with other helminths, cathepsin B- and D-like proteases of A. cantonensis degrade host proteins, including haemoglobin and IgG (Brindley et al. Reference Brindley, Kalinna, Wong, Bogitsh, King, Smyth, Verity, Abbenante, Brinkworth, Fairlie, Smythe, Milburn, Bielefeldt-Ohmann, Zheng and McManus2001; Baig et al. Reference Baig, Damian and Peterson2002; Beckham et al. Reference Beckham, Law, Smooker, Quinsey, Caffrey, McKerrow, Pike and Spithill2006; Jilkova et al. Reference Jilkova, Rezacova, Lepsik, Horn, Vachova, Fanfrlik, Brynda, McKerrow, Caffrey and Mares2011; Cheng et al. Reference Cheng, Yang, Li, He, Qu, He, Wu and Zhan2012). Expression of AC-cathB-2 is higher in L3 larvae than in L1 larvae and adults (Ni et al. Reference Ni, Wang, Zhang, Yu, Fang and Luo2012), while AC-cathB expression is greater in L4 and L5 worms (Han et al. Reference Han, Li, Li, Sun, Zhu, Ling, Zheng, Wu and Lv2011). High expression of AC-cathBs occurs in the oesophagus of L3 larvae, supporting their role in host gut penetration (Yu et al. Reference Yu, Wang, Zhang, Fang and Luo2014a ; Long et al. Reference Long, Cao, Yu, Tukayo, Feng, Wang and Luo2015). In L4 worms, AC-cathB-1 is excreted/secreted and localizes to the intestine (Cheng et al. Reference Cheng, Yang, Li, He, Qu, He, Wu and Zhan2012). These studies indicate that in L3 larvae, AC-cathBs are probably important for host gut penetration, and in L4 and L5 worms, are involved in host tissue destruction during migration.
Metalloprotease I, AC-cathB-1 and AC-cathB-2 were amongst the most abundant transcripts in pepsin-HCl activated L3 larvae (Chang et al. Reference Chang, Tang and Wang2011). Excreted/secreted products of L1 and L3 larvae possess demonstrable proteolytic activity (Lai et al. Reference Lai, Jiang, Chen and Lee2005b ; Lee and Yen, Reference Lee and Yen2005; Rebello et al. Reference Rebello, Siqueira, Ribeiro, Valente, Mota, Perales, Neves-Ferreira and Lenzi2012). Furthermore, serine protease and metalloprotease inhibitors reduced penetration of L3 larvae through the intestinal wall of mice (Lee and Yen, Reference Lee and Yen2005). Metalloprotease activity is present in L1, L3, L5 and adult worms (Lai et al. Reference Lai, Jiang, Chen and Lee2005b ), though metalloprotease transcripts were not detected in L4 worms, an absence that is not understood (He et al. Reference He, Cheng, Yang, Meng, He, Zheng, Li, Guo, Pan and Zhan2009). Metalloproteases of A. cantonensis also degrade gelatin and host metalloprotease (Lai et al. Reference Lai, Jiang, Chen and Lee2005b ; Adisakwattana et al. Reference Adisakwattana, Nuamtanong, Yenchitsomanus, Komalamisra and Meesuk2012). In adult A. vasorum 4·5% of all functionally annotated transcripts classified as proteases by homology, including cysteine proteinase 3, aspartyl protease and cathBs (Ansell et al. Reference Ansell, Schnyder, Deplazes, Korhonen, Young, Hall, Mangiola, Boag, Hofmann, Sternberg, Jex and Gasser2013). In adult worms, these proteases probably play a role in the digestion of haemoglobin and other host proteins (Cheng et al. Reference Cheng, Yang, Li, He, Qu, He, Wu and Zhan2012; Ansell et al. Reference Ansell, Schnyder, Deplazes, Korhonen, Young, Hall, Mangiola, Boag, Hofmann, Sternberg, Jex and Gasser2013). A role for AC-cathB-2 in reproduction is also proposed, given its localization to the vas deferens, uterus and oviduct of adult worms (Yu et al. Reference Yu, Wang, Zhang, Fang and Luo2014a ).
Helminth-derived molecules that regulate host immune responses are considered important virulence factors; inducing an immune state that tolerates the parasites’ presence. Extracts from Angiostrongylus spp. reduced inflammation in a rodent model of asthma (Pitrez et al. Reference Pitrez, Gualdi, Barbosa, Sudbrack, Ponzi, Cao, Silva, Machado, Jones, Stein and Graeff-Teixeira2015). Similarly, asthmatic rats treated with A. cantonensis cystatin experienced reduced airway inflammation (Ji et al. Reference Ji, Hu, Yang, Wei, Zhu, Liu, Feng, Yang, Okanurak, Li, Zeng, Zheng, Wu and Lv2015). Transcripts for immunomodulatory proteins were among the most abundant in adult A. vasorum (Ansell et al. Reference Ansell, Schnyder, Deplazes, Korhonen, Young, Hall, Mangiola, Boag, Hofmann, Sternberg, Jex and Gasser2013). Homologues of Ancylostoma secreted protein (activation-associated secreted proteins or ASPs) were noted in A. vasorum. Helminth ASPs modulate host leucocyte activity and recruit neutrophils by neutrophil receptor binding (Ansell et al. Reference Ansell, Schnyder, Deplazes, Korhonen, Young, Hall, Mangiola, Boag, Hofmann, Sternberg, Jex and Gasser2013). Additionally, ASP was identified in a cDNA library of L4 A. cantonensis larvae and a recombinant version of this protein was recognized by sera from infected mice but not from infected rats (Yang et al. Reference Yang, Li, He, Cheng, Liu, Zhang, Chen, Wu, He, Zheng, Wu, Wu and Zhan2013b ). The ASP gene was highly transcribed in brain stage L4 larvae, and immunization of mice with recombinant ASP before infection reduced neutrophil infiltration in mouse brains, implicating ASPs as being directly involved in the pathogenesis of AEM (Yang et al. Reference Yang, Li, He, Cheng, Liu, Zhang, Chen, Wu, He, Zheng, Wu, Wu and Zhan2013b ). Ansell et al. (Reference Ansell, Schnyder, Deplazes, Korhonen, Young, Hall, Mangiola, Boag, Hofmann, Sternberg, Jex and Gasser2013) also identified homologues of high mobility group box protein in adult A. vasorum. Homologues of this protein from filarial nematodes possess an immunomodulatory role, triggering secretion of pro-inflammatory cytokines such as IL-6 and tumour necrosis factor (TNF)-α by mouse peritoneal macrophages (Thirugnanam et al. Reference Thirugnanam, Munirathinam, Veerapathran, Dakshinamoorthy, Reddy and Ramaswamy2012).
Constituents of the cytoskeleton, galectins and heat-shock proteins are among the most abundant proteins expressed by A. cantonensis, based on proteomic studies (Leon et al. Reference Leon, Neves-Ferreira, Valente, Mota, Lenzi and Perales2007; Song et al. Reference Song, Huang, Tan, Zhang, Hu and Pan2012; Huang et al. Reference Huang, Yao, Song, Li, Hua, Li, Pan and Xia2013; Chen et al. Reference Chen, Cheng, Yen, Tang and Wang2014a ). Galectins participate in a diverse range of processes including cell adhesion and host immune modulation (Morassutti and Graeff-Teixeira, Reference Morassutti and Graeff-Teixeira2012; Huang et al. Reference Huang, Yao, Song, Li, Hua, Li, Pan and Xia2013). Galectins also react with serum from angiostrongyliasis patients (Morassutti et al. Reference Morassutti, Levert, Pinto, da Silva, Wilkins and Graeff-Teixeira2012b ). Stage-specific expression of galectin 1 has been noted; its expression is elevated 11-fold in male L5 worms compared with male adults (Huang et al. Reference Huang, Yao, Song, Li, Hua, Li, Pan and Xia2013). Expression of some galectins is also higher in female adult worms compared with males and vice versa (Song et al. Reference Song, Huang, Tan, Zhang, Hu and Pan2012; Huang et al. Reference Huang, Yao, Song, Li, Hua, Li, Pan and Xia2013), though the significance of this is unknown.
Nitric oxide is produced by the host immune system in response to infections with helminths and other pathogens, to subject them to conditions of oxidative stress (Muro and Perez-Arellano, Reference Muro and Perez-Arellano2010). Consequently, helminths have evolved defences to counter this. Expression of A. cantonensis galectin 10 increased following exposure to H2O2, suggesting its involvement in the prevention of oxidative stress (Liu et al. Reference Liu, He, Lv, Wei, Zeng, Liang, Zheng, Yu, Sun and Wu2013). Glutathione-S-transferase (GST) is also involved in helminth defences against oxidative stress, by detoxifying harmful compounds produced by oxygen free radicals (Vibanco-Perez and Landa-Piedra, Reference Vibanco-Perez and Landa-Piedra1998; Kampkotter et al. Reference Kampkotter, Volkmann, de Castro, Leiers, Klotz, Johnson, Link and Henkle-Duhrsen2003). Consequently, GST has long been considered a potential target for anthelmintic chemotherapy (Vibanco-Perez and Landa-Piedra, Reference Vibanco-Perez and Landa-Piedra1998). GST family proteins are highly expressed in adult female A. costaricensis (Leon et al. Reference Leon, Neves-Ferreira, Valente, Mota, Lenzi and Perales2007). Antioxidants such as peroxiredoxin and thioredoxin peroxidase are also highly expressed, and represent potential chemotherapeutic targets (Leon et al. Reference Leon, Neves-Ferreira, Valente, Mota, Lenzi and Perales2007; Huang et al. Reference Huang, Yao, Song, Li, Hua, Li, Pan and Xia2013). Female adult A. cantonensis possess increased antioxidant capacity compared with female L5 worms (Huang et al. Reference Huang, Yao, Song, Li, Hua, Li, Pan and Xia2013), suggesting that female worms increase defences against oxidative stress as they mature, and possibly in preparation for embryogenesis.
MicroRNAs
MicroRNAs (miRNA) are small non-coding RNAs (~21–23 nt) that regulate a wide range of biological processes in parasitic helminths, including development, metabolism and cell differentiation/proliferation (Chen et al. Reference Chen, Ai, Xu, Zhang, Chen, Zhang, Guo, Cai, Tian, Zhang, Zhu and Chen2011a ; Britton et al. Reference Britton, Winter, Gillan and Devaney2014). Consequently, miRNAs have been investigated for their role in helminth biology and as potential diagnostic biomarkers of helminth infection (Britton et al. Reference Britton, Winter, Gillan and Devaney2014).
Chen et al. (Reference Chen, Ai, Xu, Zhang, Chen, Zhang, Guo, Cai, Tian, Zhang, Zhu and Chen2011a ) detected 1 072 876 miRNA species in adult A. cantonensis; 122 051 were common to both sexes, 548 720 were female specific and 402 105 were male specific. The miRNA profiles differed between young adult and mature adult worms, though certain miRNA species, such as mirR-1, mirR-71 and miR-44, were expressed highly in both stages (Chang et al. Reference Chang, Tang, Lai, Kuo and Wang2013). Mir-71 family members are the most abundant miRNA species in young adult and mature adult worms (Chen et al. Reference Chen, Ai, Xu, Zhang, Chen, Zhang, Guo, Cai, Tian, Zhang, Zhu and Chen2011a ; Chang et al. Reference Chang, Tang, Lai, Kuo and Wang2013). Mir-71 is thought to promote longevity and stress resistance and along with aca-miR-1–1, may also affect sexual differentiation and development (Chang et al. Reference Chang, Tang, Lai, Kuo and Wang2013). In L3 larvae, mir-200c was the most abundant miRNA identified, and its expression was 352-fold higher in L3 larvae compared with L4 larvae (Li et al. Reference Li, Chen, Zen, Liang, Wei, Lv, Sun and Wu2014b ). Li et al. (Reference Li, Chen, Zen, Liang, Wei, Lv, Sun and Wu2014b ) noted higher expression of miR-124 in L4 worms, and implicate it as an immunomodulator. Mouse microglial cells transfected with miR-124 mimics significantly down-regulated transcription of IL-6, IL-1β and TNF-α (Li et al. Reference Li, Chen, Zen, Liang, Wei, Lv, Sun and Wu2014b ).
The utility of miRNAs as biomarkers for A. cantonensis infection is also under investigation. Expression of miR-132-3p in mouse brains, and of aca-miR-146a/aca-miR-146a-5p in rodent sera and brains, is elevated in infected animals compared with uninfected controls (Chen et al. Reference Chen, Li, Maleewong, Maleewong, Liang, Zeng, Zheng, Wu and Sun2014b ; Li et al. Reference Li, Chen, Zen, Liang, Wei, Lv, Sun and Wu2014b ; Yu et al. Reference Yu, Liao, Chen, Xu, Zeng, Lv, Sun, Zhen and Wu2014b ). Consequently, aca-miR-146a and aca-miR-146a-5p could serve as useful biomarkers for early-stage A. cantonensis infection.
TREATMENT
Treatment of angiostrongyliasis involves reducing inflammation, reducing ICP, eradicating the worms and reducing pain. Mild cases often resolve spontaneously without therapy, though rarely, severe cases can lead to permanent neurologic damage or, death in 2–3% of cases (Eamsobhana and Yong, Reference Eamsobhana and Yong2009; Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013). Repeated lumbar puncture is performed to reduce ICP, thereby relieving severe headaches and other neurological derangements (Jitpimolmard et al. Reference Jitpimolmard, Sawanyawisuth, Morakote, Vejjajiva, Puntumetakul, Sanchaisuriya, Tassaneeyakul, Tassaneeyakul and Korwanich2007; Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009; Diao et al. Reference Diao, Wang, Qi, Li, Zheng and Yin2011; Murphy and Johnson, Reference Murphy and Johnson2013). Analgesics such as ibuprofen can also be administered to relieve headache and fever (Wang et al. Reference Wang, Qi, Diao, Zheng, Li, Ma, Ji and Yin2010). Administration of intravenous mannitol or glycerol/fructose can reduce ICP in AEM patients (Li et al. Reference Li, Xu, Gu and Chen2008; Wang et al. Reference Wang, Qi, Diao, Zheng, Li, Ma, Ji and Yin2010). Gamma-globulin has also been administered to AEM patients to support immunity (Li et al. Reference Li, Xu, Gu and Chen2008).
Ocular angiostrongyliasis though non-fatal, can lead to permanent visual impairment if untreated (Sawanyawisuth et al. Reference Sawanyawisuth, Kitthaweesin, Limpawattana, Intapan, Tiamkao, Jitpimolmard and Chotmongkol2007; Feng et al. Reference Feng, Nawa, Sawanyavisuth, Lv and Wu2013). Surgical removal of larvae from the eye is often performed, along with administration of topical or oral corticosteroids to reduce inflammation (Patikulsila et al. Reference Patikulsila, Ittipunkul and Theerakittikul2003; Kumar et al. Reference Kumar, Kyprianou and Keenan2005; Sawanyawisuth et al. Reference Sawanyawisuth, Kitthaweesin, Limpawattana, Intapan, Tiamkao, Jitpimolmard and Chotmongkol2007; Feng et al. Reference Feng, Nawa, Sawanyavisuth, Lv and Wu2013). Laser killing of larvae in the eye can be performed, and is considered preferable to surgery, provided the worms have caused minimal damage at the time of diagnosis (Feng et al. Reference Feng, Nawa, Sawanyavisuth, Lv and Wu2013). However, some suggest there is little evidence that laser interventions or surgical removal of larvae improve clinical outcomes (Kumar et al. Reference Kumar, Kyprianou and Keenan2005; Sawanyawisuth et al. Reference Sawanyawisuth, Kitthaweesin, Limpawattana, Intapan, Tiamkao, Jitpimolmard and Chotmongkol2007). Improvements have been noted upon administration of anthelmintics (Wang et al. Reference Wang, Wang and Jou2006b ), though some investigators advise against this given the risk that dead worms may activate serious intraocular inflammation (Feng et al. Reference Feng, Nawa, Sawanyavisuth, Lv and Wu2013).
Anthelmintics such as albendazole, mebendazole, flubendazole and ivermectin have been administered in many AEM cases, though their usefulness in AEM management is controversial (Murphy and Johnson, Reference Murphy and Johnson2013). Some studies suggest that early administration of anthelmintics in AEM may exacerbate symptoms and hasten disease progression (Slom et al. Reference Slom, Cortese, Gerber, Jones, Holtz, Lopez, Zambrano, Sufit, Sakolvaree, Chaicumpa, Herwaldt and Johnson2002; Hidelaratchi et al. Reference Hidelaratchi, Riffsy and Wijesekera2005; Liu et al. Reference Liu, Chung, Chen and Cho2006; Wang et al. Reference Wang, Jung, Chen, Wong, Wan and Wan2006a ). Transaminitis has also been reported following albendazole treatment in an AEM case (Morton et al. Reference Morton, Britton, Palasanthiran, Bye, Sugo, Kesson, Ardern-Holmes and Snelling2013). However, a double blind, placebo controlled trial confirmed a reduction in headache duration in AEM patients, with no adverse effects, though with borderline efficacy, using albendazole (Jitpimolmard et al. Reference Jitpimolmard, Sawanyawisuth, Morakote, Vejjajiva, Puntumetakul, Sanchaisuriya, Tassaneeyakul, Tassaneeyakul and Korwanich2007; Murphy and Johnson, Reference Murphy and Johnson2013). Albendazole alone or in combination with corticosteroids, was efficacious, and caused no adverse reactions in AEM mouse models (Lan et al. Reference Lan, Wang, Lai, Chen, Lee, Hsu and Lee2004; Tu and Lai, Reference Tu and Lai2006). Similarly, administration of albendazole and prednisolone to a group of 53 Thai patients with eosinophilic meningitis caused no serious adverse reactions (Chotmongkol et al. Reference Chotmongkol, Kittimongkolma, Niwattayakul, Intapan and Thavornpitak2009).
Corticosteroids such as dexamethasone and prednisolone form the basis of AEM treatment, by reducing granulomatous inflammation and headache intensity (Sawanyawisuth et al. Reference Sawanyawisuth, Limpawattana, Busaracome, Ninpaitoon, Chotmongkol, Intapan and Tanawirattananit2004; Sawanyawisuth, Reference Sawanyawisuth2008; Thanaviratananich and Ngamjarus, Reference Thanaviratananich and Ngamjarus2015). The efficacy of corticosteroid & anthelmintic combination therapy for alleviating AEM symptoms is supported by several independent reports (Wan and Weng, Reference Wan and Weng2004; Chotmongkol et al. Reference Chotmongkol, Sawadpanitch, Sawanyawisuth, Louhawilai and Limpawattana2006; Leone et al. Reference Leone, De Marco, Ghirga, Nicastri, Esposito and Narciso2007; Diao et al. Reference Diao, Chen, Yin, Wang, Qi and Ji2009; Lv et al. Reference Lv, Zhang, Chen, Wang, Fang, Chen, Jiang, Li, Du and Zhou2009a ; Zhou et al. Reference Zhou, Barennes, Zhou, Ding, Zhu and Strobel2009; Ueda et al. Reference Ueda, Takeuchi, Ochiai, Mabuchi and Niwa2015). Furthermore, experiments using peripheral blood mononuclear cells extracted from AEM patients indicate that dexamethasone & albendazole combination therapy dampens the Th2 cytokine response in favour of a Th1 response (Diao et al. Reference Diao, Chen, Yin, Wang, Qi and Ji2009). Generally, corticosteroids are administered in doses ranging from 3 mg day−1 to 20 mg four times daily, for 1–2 weeks (Chotmongkol et al. Reference Chotmongkol, Sawanyawisuth and Thavornpitak2000, Reference Chotmongkol, Sawadpanitch, Sawanyawisuth, Louhawilai and Limpawattana2006; Li et al. Reference Li, Xu, Gu and Chen2008; Zhou et al. Reference Zhou, Barennes, Zhou, Ding, Zhu and Strobel2009; Wang et al. Reference Wang, Qi, Diao, Zheng, Li, Ma, Ji and Yin2010; Murphy and Johnson, Reference Murphy and Johnson2013). Albendazole or mebendazole are administered at doses of 10–20 mg kg−1 day−1 for 1–2 weeks (Chotmongkol et al. Reference Chotmongkol, Sawadpanitch, Sawanyawisuth, Louhawilai and Limpawattana2006; Zhou et al. Reference Zhou, Barennes, Zhou, Ding, Zhu and Strobel2009; Wang et al. Reference Wang, Qi, Diao, Zheng, Li, Ma, Ji and Yin2010). However, no randomized, double-blind, placebo-controlled trials have been performed to assess the efficacy of these regimens, and some investigators report little or no benefit following their administration (Ali et al. Reference Ali, Van den Enden, Van Gompel and Van Esbroeck2008; Hochberg et al. Reference Hochberg, Blackburn, Park, Sejvar, Effler and Herwaldt2011; Tseng et al. Reference Tseng, Tsai, Sy, Lee, Wann, Wang, Chen, Wu and Chen2011).
Chotmongkol et al. (Reference Chotmongkol, Kittimongkolma, Niwattayakul, Intapan and Thavornpitak2009) found no statistical difference between two study groups of more than 50 eosinophilic meningitis patients, treated with either albendazole and prednisolone combination therapy or with prednisolone alone. Furthermore, in rodent models of AEM, dexamethasone treatment alone reduced brain cell apoptosis, BBB permeability and expression of the 14-3-3β protein in CSF (Tsai et al. Reference Tsai, Lee, Yen, Wann, Lee, Chen and Tai2014b , Reference Tsai, Lee, Yen, Wann, Lee and Chen2015), casting doubt on the therapeutic benefit of administering anthelmintic drugs for the treatment of AEM.
Alternative therapies are also under investigation in rodents. Tribendimidine, a broad spectrum anthelmintic developed in China in the 1980s (Robertson et al. Reference Robertson, Puttachary, Buxton and Martin2015), killed A. cantonensis in rodents, possibly by damaging its tegument (Wang et al. Reference Wang, Wei, Zeng, Liang, Wu, Li, Zheng, He and Wu2013a ; Zeng et al. Reference Zeng, Wang, Wei, Wu, Fung, Wu, Sun, Zheng, Lv and Wu2013a ; Feng et al. Reference Feng, Zeng, Li, Wang, Chen, Ou-Yang, Sun, Feng and Wu2014). Treatment of mice with IL-12 and mebendazole reduces AEM severity and causes a shift towards a Th1 response (Du et al. Reference Du, Liao, Fan and Su2003). Lai et al. (Reference Lai, Jiang, Chen, Hsu, Shyu and Lee2005a ) used albendazole in combination with GM6001 (a matrix MMP-9 inhibitor) to reduce AEM severity in mice. In combination with albendazole, certain Chinese herbs and their extracts exhibited some beneficial effects in mouse models of Angiostrongylus optic neuritis and AEM (Lai, Reference Lai2006; Feng et al. Reference Feng, Feng, Liu, Li, Wang, Wu and Lv2015). Ginger extracts possess some activity against A. cantonensis (Lin et al. Reference Lin, Chen, Chung and Yen2010). Curcumin (a compound found in turmeric) was not efficacious in AEM mouse models when used alone, though in combination with albendazole, reduced CSF eosinophilia to a greater extent than albendazole mono-therapy (Shyu et al. Reference Shyu, Chang, Hsu, Lin, Teng and Lee2012). Similarly, the combination of diammonium glycyrrhizinate (a liquorice root extract) and albendazole was more effective in a mouse model of AEM than albendazole/dexamethasone co-therapy (Li et al. Reference Li, Tang, Chen, Fu, Wang, Li, Wei, Li and Dong2013b ). The marine fungal extract m2-9 had synergistic effects when used in combination with albendazole for treatment of murine AEM (Li et al. Reference Li, Sun, Li, Song, Lin, Zeng, He, Wei, Yang, Zheng, Lv and Wu2012). Treatment with albendazole and thalidomide was time-dependent; early treatment produced better outcomes in murine AEM (Chen and Lai, Reference Chen and Lai2007).
To summarize, lumbar puncture is effective at reducing ICP in AEM, relieving patients of the associated headaches (Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009; Murphy and Johnson, Reference Murphy and Johnson2013). Administration of corticosteroids to reduce inflammation in AEM is also warranted based on multiple reports (Chotmongkol et al. Reference Chotmongkol, Kittimongkolma, Niwattayakul, Intapan and Thavornpitak2009; Diao et al. Reference Diao, Chen, Yin, Wang, Qi and Ji2009; Murphy and Johnson, Reference Murphy and Johnson2013). Conversely, the value of administering anthelmintics is uncertain (Jitpimolmard et al. Reference Jitpimolmard, Sawanyawisuth, Morakote, Vejjajiva, Puntumetakul, Sanchaisuriya, Tassaneeyakul, Tassaneeyakul and Korwanich2007; Chotmongkol et al. Reference Chotmongkol, Kittimongkolma, Niwattayakul, Intapan and Thavornpitak2009; Murphy and Johnson, Reference Murphy and Johnson2013). As reports of severe adverse events resulting from albendazole use are rare, it should generally be considered safe for AEM treatment, particularly in combination with corticosteroids (Murphy and Johnson, Reference Murphy and Johnson2013). However, given the borderline efficacy of albendazole monotherapy (Jitpimolmard et al. Reference Jitpimolmard, Sawanyawisuth, Morakote, Vejjajiva, Puntumetakul, Sanchaisuriya, Tassaneeyakul, Tassaneeyakul and Korwanich2007), and the lack of any difference in clinical outcome when comparing corticosteroid monotherapy and corticosteroid/albendazole co-therapy (Chotmongkol et al. Reference Chotmongkol, Kittimongkolma, Niwattayakul, Intapan and Thavornpitak2009), the value of administering anthelmintics for AEM treatment is questionable. Given the inconsistencies, these studies represent a useful guide for the treatment of angiostrongyliasis at best. Clearly, further research, including randomized, double-blind, placebo-controlled trials, is required to establish an improved consensus for the treatment of this disease.
PREVENTION AND CONTROL
Given its wide distribution and zoonotic nature, it is unlikely that A. cantonensis can be completely eradicated. However, disrupting its transmission to humans through the control of its preferred hosts is plausible. Molluscicides and other methods to control snail and slug numbers have been implemented in Hawaii and China (Hata et al. Reference Hata, Hara and Hu1997; Hollingsworth et al. Reference Hollingsworth, Howe and Jarvi2013; Yang et al. Reference Yang, Wu and Lun2013a ). In Jamaica, molluscicides are routinely applied to growing produce but this practice was deemed superficial as molluscs remain in the surrounding vegetation and return presumably when the molluscicides lose their potency (Waugh et al. Reference Waugh, Shafir, Wise, Robinson, Eberhard and Lindo2005). In China, the collection of P. canaliculata egg masses and the use of commercial molluscicides have proved effective, though their use is limited by their laborious nature and potential environmental side effects (Yang et al. Reference Yang, Wu and Lun2013a ). Biological controls, including the introduction of ducks and Mylopharyngodon piceus (black carp) to rice paddy fields and ponds drastically reduced snail numbers (Yang et al. Reference Yang, Wu and Lun2013a ). Hollingsworth et al. (Reference Hollingsworth, Howe and Jarvi2013) suggests drowning of terrestrial slugs or snails for several days in a covered bucket filled with soapy water or a 15% solution of salt water. The salt water treatment is not only lethal to terrestrial molluscs, but is also expected to kill any A. cantonensis larvae that may be present (Hollingsworth et al. Reference Hollingsworth, Howe and Jarvi2013).
Standardized food treatment measures may be effective in some areas, though would be ineffective in locations where food is often home grown or collected from the local environment. Thorough washing of vegetables prior to consumption is a simple measure to reduce A. cantonensis transmission (Cowie, Reference Cowie2013b ). Washing experiments carried out on grown produce by Yeung et al. (Reference Yeung, Hayes and Cowie2013) showed no difference between simple water washes and washing with other domestic chemicals such as bleach, acetic acid and sodium chloride. While some slug slime residue remained after washes, washing was still considered the most appropriate measure for reducing the transmission of larvae in produce (Yeung et al. Reference Yeung, Hayes and Cowie2013). As evidenced by Wang et al. (Reference Wang, Chung, Lin, Lee, Lin and Yen2011), thorough roasting of molluscs for more than 20 min is required to ensure that all A. cantonensis larvae are destroyed in mollusc tissue.
The major difficulty in controlling the spread of angiostrongyliasis lies in its link with diverse social, economic, cultural and environmental factors. In some endemic areas, awareness of A. cantonensis is low in the general community (Li et al. Reference Li, Hu, Tong, Liu, Li and Wang2011). Consequently, educating the public to the dangers of raw mollusc consumption in these regions may be an effective starting measure. Educating laypeople on strategies for controlling the spread of A. cantonensis would be helpful, particularly in regions where food is home grown or collected locally. However, public education programmes must be executed with caution, as the public concern generated could be excessive given the rarity of angiostrongyliasis. Despite this, public education campaigns have had favourable results in China (Lv et al. Reference Lv, Zhang, Steinmann and Zhou2008). Finally, programmes that raise awareness amongst public health workers and physicians might enable them to provide better advice to patients planning overseas travel, and facilitate a more rapid diagnosis with improved clinical outcomes when future cases of angiostrongyliasis are encountered.
Concluding remarks
While angiostrongyliasis is generally considered rare, its global presence is steadily increasing with both clinical cases and epidemiological surveys confirming A. cantonensis in regions where it was previously considered absent. AEM is often overlooked at the outset, particularly in regions where angiostrongyliasis is rarely observed. There is a lack of standardization in the procedures for diagnosing angiostrongyliasis. Serological tests are commercially available, though are not widely used. Other diagnostic tools are under development. There is no consensus on the appropriate treatment for angiostrongyliasis and some treatments are controversial. A combination of anthelmintics and corticosteroids seems effective in most cases, though there have been no randomized, double-blind, placebo-controlled trials to support any treatment regimen described for angiostrongyliasis. Blocking transmission is the most appropriate method of reducing infections. Implementing simple wash protocols for vegetables, public education on the dangers of raw mollusc consumption and implementation of mollusc and rat control measures may be useful. In developed nations, the prevalence of angiostrongyliasis in companion animals and wildlife is usually greater than in humans. Thus, animals play a role in alerting public health authorities of the risk to humans in locations where animals are affected by angiostrongyliasis. Considering the potentially lethal nature of angiostrongyliasis and the recent reports of its increasing geographical range, it is important that this disease is given due consideration in the differential diagnosis of eosinophilic meningitis, even in areas where A. cantonensis is rarely reported. This is essential to improve patient prognosis and reduce human and companion animal suffering.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0031182016000652.
ACKNOWLEDGEMENTS
We acknowledge the University of Technology Sydney and St. Vincent's Hospital, Sydney in support of Ph.D. student, Douglas Chan (scholarship and infrastructure, respectively).













