Introduction
During an 11-year period, Darwin made numerous comparisons on the effects of self- and cross-pollination on 54 species in 52 genera and 30 families of herbaceous plants, all eudicots except three monocots, i.e. two grasses and a Canna species. In The effects of cross and self fertilisation in the vegetable kingdom, Darwin (Reference Darwin1876) reported on the effects of self- vs. cross-pollination on plant height and shoot mass; relative time to and productiveness of flowering; number and mass of fruits per plant; number and mass of seeds per fruit and per plant; seed size (mass); and relative time to germination. His general conclusion was that, ‘The first and most important of the conclusions which may be drawn from observations given in this volume, is that cross-fertilisation is generally beneficial, and self-fertilisation injurious’ (Darwin, Reference Darwin1876, p. 436).
However, his limited results on seed mass and germination do not offer strong support for this general conclusion. Thus, in 10 of the 16 comparisons of seed size, selfed seeds were larger than outcrossed seeds. In the other six comparisons, outcrossed seeds were larger than selfed seeds. Interestingly, ‘The lighter seeds, whether produced from crossed or self fertilised flowers, generally germinated before the heavier seeds’ (Darwin, Reference Darwin1876, p. 58). Furthermore, in 10 of 21 comparisons of relative time to germination, crossed seeds germinated faster than selfed seeds; in ten, selfed seeds germinated faster than crossed seeds; and in one there was no difference in timing. Darwin considered earlier germination to be superior to late germination.
The first extensive literature survey on the effects of inbreeding depression (ID) on seed germination was contained in a review paper by Husband and Schemske (Reference Husband and Schemske1996) on the magnitude and timing of ID in plants. Their survey included information on 79 populations: 2 families and 13 species of gymnosperms, and 23 families and 41 species of angiosperms (sensu Mabberley, Reference Mabberley2008; APG-III, 2009). All species of angiosperms in their survey were herbaceous, except one tree (Eucalyptus regnans, Myrtaceae) and one shrub (Decodon verticillatus, Lythraceae). A paper by Winn et al. (Reference Winn, Elle, Kalisz, Cheptou, Eckert, Goodwillie, Johnston, Moeller, Ree, Sargent and Vallejo-Marin2011) contains information on 59 species (49 angiosperms, 10 gymnosperms; sensu Mabberley, Reference Mabberley2008; APG-III, 2009). Forty-four of the 68 entries (populations) in this paper are included in the one by Husband and Schemske (Reference Husband and Schemske1996). D. verticillatus and E. regnans are also the only woody angiosperms on the species list of Winn et al. (Reference Winn, Elle, Kalisz, Cheptou, Eckert, Goodwillie, Johnston, Moeller, Ree, Sargent and Vallejo-Marin2011). Relative fitness values for germination are given for all entries in these two surveys. Our primary purpose was to make an updated and extensive survey of the literature on the effects of ID on seed germination. In addition, dormancy breaking and germination procedures used in studies on ID in plants are reviewed, and recommendations are made on how to improve such studies to make the results more relevant to the real world.
Inbreeding depression
Formulae and symbols used in this section follow those of many authors, including Ågren and Schemske (Reference Ågren and Schemske1993), Husband and Schemske (Reference Husband and Schemske1996) and Barringer and Geber (Reference Barringer and Geber2008). Inbreeding depression (δ) is the reduction in mean fitness of a trait, e.g. number of seeds produced, germination percentage/rate, of selfed progeny (W s) compared to that of outcrossed progeny (W o). It is typically estimated using the following equation:
Thus, when W s ≤ W o the value of δ is bound between 0 (W s = W o) and +1 (W s = 0). Inbreeding depression (ID) also applies to the reduction in mean fitness of offspring that results from crosses between close relatives (i.e. biparental ID). In which case, W s is the reduction in mean fitness of offspring due to crossing with close relatives. Some general conclusions related to ID are given in Table 1.
Table 1 A general summary of the results obtained in studies on inbreeding depression (δ) in plants

ID may be caused by increased homozygosity of deleterious recessive or partially recessive alleles (mutations) – the partial dominance hypothesis – or by ‘… increased homozygosity for alleles at loci with heterozygote advantage’– the overdominance hypothesis (Charlesworth and Willis, Reference Charlesworth and Willis2009). Thus, according to the partial dominance hypothesis, inbreeding increases the chance that two diploid individuals carrying recessive detrimental mutations will mate with each other, and only in the homozygous state will the negative effects of deleterious alleles be expressed in the offspring. In the overdominance hypothesis, heterozygosity is superior to homozygosity, i.e. individuals carrying two different copies of an allele are more fit than those carrying two identical copies of the same allele. It appears that most ID is caused by mildly deleterious alleles (partial dominance hypothesis), which may be purged by inbreeding (Lande and Schemske, Reference Lande and Schemske1985; Charlesworth and Charlesworth, Reference Charlesworth and Charlesworth1987; Charlesworth and Willis, Reference Charlesworth and Willis2009), but see comments under ‘Population size and history’ in Table 1 with regard to purging.
However, fitness of a trait is not always enhanced by outcrossing, i.e. W o is not always greater than W s. Thus, one could also get a higher mean fitness of inbred than of outbred progeny. In which case, W s >W o, and the value of δ is negative (to − ∞), when the fitness value of outcrossed progeny approaches 0. Thus, using the formula δ = 1 − (W s/W o) to calculate ID does not yield a symmetrical value around zero ( − 1 to +1), i.e. it does not give equal weight to W s > W o (to − ∞) vs. W s < W o (to +1.0).
A more meaningful way to compare the fitness of inbred and outcrossed individuals and families is to use a measure of relative performance (RP), for which the phenotypic values will be equidistant from zero, when W s > W o (to − 1) and when W s < W o (to +1). Thus, a positive value indicates that outbred plants outperformed inbred plants, and the closer the value to 1.0 the greater the ID. A negative value indicates that inbred plants outperformed outbred plants, and the more negative the value the greater the inbreeding benefit. The equation for relative performance is:
where W max is the larger of the two values, i.e. W o or W s. RP is the same as δ = 1 − (W s/W o) when W s < W o and the same as δ = (W o/W s) – 1 when W s > W o.
For most traits measured in ID studies, higher numbers represent better performance. However, in calculating rate (speed), e.g. days to 50% germination of selfed vs. outcrossed seeds, the higher value represents reduced performance. Thus, if W o has a faster rate (speed, i.e. fewer days to germinate) than W s the equation to use in calculating performance is 1 – (W o/W s), i.e. relative fitness = 1/(W s/W o) = W o/W s, whereas when W s has a faster rate the equation to use is (W s/W o) − 1.
Relation of inbreeding effect to inbreeding coefficient (F)
Theoretical aspects
The material in this section is based primarily on that in Sorensen (1969), Anderson et al. (Reference Anderson, Ascher and Widmer1992), Keller and Waller (Reference Keller and Waller2002) and Charlesworth and Willis (Reference Charlesworth and Willis2009). If deleterious mutations at different loci affecting fitness have independent (multiplicative) effects (no epistasis), then fitness is expected to decline exponentially with an increase in F (Fig. 1). This relationship is as follows.
A is a decrease in fitness due to environmental causes and genetic damage in a randomly mating population (F= 0), i.e. a decrease in fitness not attributed to inbreeding. B is the inbreeding load, i.e. an estimate of the number of lethal equivalents (a group of alleles that would be lethal if homozygous) per gamete. B describes the rate at which fitness declines with inbreeding and is equal to 0 when there is no inbreeding depression.
–B is the slope of the line (Δy/Δx) (Fig. 1).
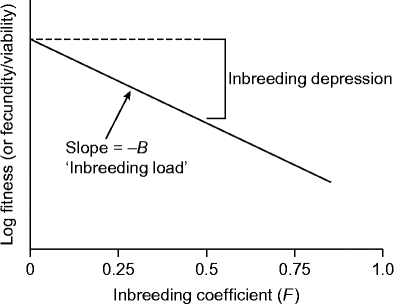
Figure 1 Theoretical decline in fitness (increase in inbreeding depression, δ) with increase in inbreeding coefficient (F). The inbreeding coefficient of progeny of randomly outbred plants (F= 0) will be 0.5 after one generation of selfing. From Keller and Waller (Reference Keller and Waller2002), with permission.
e− (A+BF) is a measure of fitness of an inbred trait, e.g. the proportion of inbred seeds that germinates. e− A is a measure of fitness of an outbred trait, e.g. the proportion of outbred seeds that germinates.
F is the coefficient of inbreeding, a mathematical expression of the level of homozygosity (% homozygosity = F × 100) at selected loci for individuals or populations submitted to inbreeding. F values range from 0.0 (no homozygosity) to 1.0 (complete homogygosity).
Thus, the equation for inbreeding depression [δ = 1 − relative fitness = 1 – (W s/W o)] can be written as 1 − (Δy/Δx) ( = 1 − e− BF ).
Now, let us look at the relationship between δ and B. The example given is for selfed progeny (F= 0.5) of randomally outbred plants (F= 0) that have undergone one generation of selfing.
B= − 2 loge R (i.e. the number of lethal equivalents per gamete).
 $$\begin{eqnarray} Number\,of\,lethal\,equivalents\,per\,zygote\,(2B)\, = - 4\hairsp log_{e}\, R \end{eqnarray}$$
$$\begin{eqnarray} Number\,of\,lethal\,equivalents\,per\,zygote\,(2B)\, = - 4\hairsp log_{e}\, R \end{eqnarray}$$
Thus the equation for calculating inbreeding depression for first-generation progeny of outbred plants species is:
For all other levels of inbreeding, the equation is:
Effect of level of inbreeding (F) on germination
Germination may or may not decline with an increase in F (Table 2). In the 35 case studies on the 25 species included in Table 2, there was a negative relationship between germinability and F in 12 and no relationship in 23. In a few cases (e.g. Reference RichardsRichards, 2000a) the relationship between germination and F was negative-linear, but in none of the cases did germination decline exponentially with increase in F (cf. Fig. 1). Apparently, this indicates that there were epistatic interactions among loci with deleterious mutations affecting germination.
Table 2 Effect of inbreeding coefficient (F) on seed germination

ID, inbreeding depression; RP, relative performance; S refers to number of generations of selfing.
Effect of population genetic diversity on germination
In contrast to what might be expected, germination may not be positively related to population genetic diversity (Table 3). For only four of the ten species listed in this table was there a positive relationship between germination and genetic diversity; for the other six species, there was no relationship between the two characters.
Table 3 Effect of population genetic diversity on seed germination
Outbreeding depression and optimum crossing distance
Crosses within (intra) and between (inter) sites or populations could lead to reduced fitness of the hybrid offspring through outbreeding depression (OD). Such a decline in fitness is caused by (1) disruption of segregation and recombination in hybrid offspring of unique co-adapted gene complexes that exist within the genomes of the parents (disruption of favourable epistatic interactions); or (2) loss (dilution with foreign genes) of adaptation in hybrid offspring to the local environment of both parents. In the first case, the decline in fitness may not occur until the F2 generation, whereas in the second case it can occur in the first generation (i.e. F1 hybrids) (Price and Waser, Reference Price and Waser1979; Templeton, Reference Templeton and Soulé1986; Reference Waser and Price1989 Reference Waser and Price1994; Parker, Reference Parker1992; Edmands, Reference Edmands2002, Reference Edmands2007). Outbreeding depression (RPo) can be calculated by the following equation (Bermingham and Brody, Reference Bermingham and Brody2011):
where Z intra is the mean performance for intrasite hybrid progeny; Z inter, mean performance of intersite hybrid progeny; and Z max = Z intra when Z intra > Z inter and Z max = Z inter when Z inter > Z intra. Positive values indicate outbreeding depression.
Since inbreeding depression can occur in offspring from crosses between close neighbours that are relatives and outbreeding depression in offspring between crosses of spatially widely separated individuals that are not closely related, it is not too surprising that an optimal crossing distance between nearby (ID) and far-off (OD) plants has been demonstrated in several studies (e.g. Price and Waser, Reference Price and Waser1979; Waser and Price, Reference Waser and Price1989; Fischer and Matthies, Reference Fischer and Matthies1998; Waser et al., Reference Waser, Price and Shaw2000). However, many studies have found no evidence of an optimal outcrossing distance in general (e.g. Newport, Reference Newport1989; Dudash, Reference Dudash1990; Richter and Weis, Reference Richter and Weis1998). An optimal outcrossing distance for seed germination was found for Gentianella germanica (Fischer and Matthies, Reference Fischer and Matthies1997) and Zostera marina (Billingham et al., Reference Billingham, Simões, Reusch and Serrão2007). For example, in Z. marina, the percentage of germination was: intermediate (71%) > near (37%) > far (29%); and for germination rate (speed): intermediate (6 d) >[near (12 d) = far (13 d)]. Germination of field-sown seeds of Ipomopsis aggregata had an optimum outcrossing distance in the 1981 cohort but not in the 1987 and 1990 cohorts (Waser et al., Reference Waser, Price and Shaw2000). For the tetraploid Digitalis purpurea, an optimal outcrossing distance was found for germination speed but not for germination percentage (Grindeland, Reference Grindeland2008). On the other hand, an optimal outcrossing distance was not found for germination/emergence in Agave schottii (Trame et al., Reference Trame, Coddington and Paige1995), Campanula americana (Galloway and Etterson, Reference Galloway and Etterson2005), Chamaecrista fasciculata (Sork and Schemske, Reference Sork and Schemske1992), Cyclamen spp. (Affre and Thompson, Reference Affre and Thompson1999), Eupatorium perfoliatum, E. resinosum (Byers, Reference Byers1998), Gentiana pneumonanthe (Oostermeijer et al., Reference Oostermeijer, Altenburg and den Nijs1995), Impatiens capensis (McCall et al., Reference McCall, Waller and Mitchell-Old1994), Lobelia cardinalis (Schlichting and Devlin, Reference Schlichting and Devlin1992), Sabatia angularis (Dudash, Reference Dudash1990), Scabiosa columbaria (van Treuren et al., Reference van Treuren, Bijlsma, Ouborg and van Delden1993), Silene acaulis (Delph, Reference Delph2004), Yucca whipplei subsp. whipplei (Richter and Weis, Reference Richter and Weis1998) and several other species.
Heterosis
Mixing of genes from different sites or populations may also result in heterosis (hybrid vigour), i.e. an increase in offspring fitness due to increased heterozygosity resulting from outcrossing individuals of inbred populations or sites (Luitjen et al., 2002; Busch, Reference Busch2006). Heterosis (H) can be calculated by the following equation (Busch, Reference Busch2006; Bermingham and Brody, Reference Bermingham and Brody2011):
where Z intra is the mean performance of progeny of intrasite (or intrapopulation) crosses and Z inter the mean performance of progeny of intersite (or interpopulation) crosses. Positive values indicate heterosis. An example of hybrid vigour for seed germination is the study by Busch (Reference Busch2006) on the cedar glade endemic Leavenworthia alabamica. Seeds from crosses within a small, geographically isolated, self-incompatible population of this species germinated to 38%, whereas those from crosses between this isolated population and other (non-isolated) populations (pollen donors) germinated to 80% (H= 1.105), i.e. substantial heterosis. In Polemonium vanbruntiae, seeds from intersite crosses germinated to 79% and those from intrasite crosses to 70% (H= 0.13) (Bermingham and Brody, 2011).
However, most within-population (WP) and between-population (BP) crosses have not resulted in heterosis for germination. For 40 other such studies, WP < BP (5), WP = BP (29) and WP >BP (6) (see references in Table 1 under ‘Within vs. between population crosses’). Five of these 40 cases were on isolated (vs. central) populations. In one of the five cases WP < BP, and in four WP = BP.
Other studies have also included in their crossing scheme near (WPnear), far (WPfar) and very far (WPvery far) distances within populations, and populations within (WR) and between (BR) regions. The results of these studies are as follows: WPnear= WPfar= WPvery far (Hymenoxys acaulis) (Moran-Palma and Snow, Reference Moran-Palma and Snow1997); WPnear > WPfar= BP (Eupatorium perfoliatum, E. resinosum) (Byers, Reference Byers1998); WR = BR (Anthericum liliago , A. ramosum) (Rosquist, Reference Rosquist2001); WPnear= WPfar (for each of two populations of Hypericum cumulicola) (Trager et al., Reference Trager, Menges, Quintana-Ascencio and Weekley2005); (WP = BR) > BP (Hypochoeris radicata) (Becker et al., Reference Becker, Reinhold and Matthies2006); (WPfar= BP) > WPnear (Stenocereus eruca) (Ricardo et al., Reference Ricardo, Corrado, Mandujano and Molina-Freaner2006); WP = BP = BR (Aster amellus) (Raabová et al., Reference Raabová, Münzbergová and Fischer2009) and Polylepis australis (Seltmann et al., Reference Seltmann, Cocucci, Renison, Cierjacks and Hensen2009).
RP of germination and lifetime fitness
The effects of inbreeding are cumulative (multiplicative) across the plant life cycle. Lifetime ID is estimated by calculating the product of relative fitness (W s/W o) of all stages of the life cycle ( = cumulative relative fitness, CRF) and then subtracting CRF from 1.
 $$\begin{eqnarray} CRF = ( W _{s1}/ W _{o1})\times ( W _{s2}/ W _{o2})\times ( W _{s3}/ W _{o3})\ldots ( W _{sx}/ W _{ox}) \end{eqnarray}$$
$$\begin{eqnarray} CRF = ( W _{s1}/ W _{o1})\times ( W _{s2}/ W _{o2})\times ( W _{s3}/ W _{o3})\ldots ( W _{sx}/ W _{ox}) \end{eqnarray}$$
Thus, as a component of multiplicative CRF, low relative fitness for germination can have a big influence on lifetime fitness of plants.
In the majority of cases, W s < W o for most (or all) life-cycle stages, and total ID will be positive but < 1.0. However, in one or more stage(s) of the life cycle, such as seed germination, survival and flowering, selfed offspring may outperform outcrossed offspring, i.e. W s > W o. In which case, one could get a CRF of >1.0 and thus a negative lifetime ID (e.g. Culley et al., Reference Culley, Weller, Sakai and Rankin1999; Kephart et al., Reference Kephart, Brown and Hall1999).
Comparison of inbreeding vs. outcrossing on seed germination
The purpose of this section of the review was to determine the proportion of cases in a large number of plant taxa in which: (1) inbred seeds germinated less well than outbred seeds (I < O); (2) inbred seeds germinated equally well as outbred seeds (I = O); and (3) inbred seeds germinated better than outbred seeds (I > O). We report 743 cases (‘case studies’) in which germination, based on percentage and/or rate (speed), of inbred seeds were (was) compared to that of outbred seeds. Cross-type (i.e. inbred vs. outbred) comparisons used in selecting inbred vs. outbred cases of seed germination are given in Table 4. The study by Kennedy and Elle (Reference Kennedy and Elle2008) will be used to illustrate what we mean by ‘case study’. This study on Collinsia parviflora included eight populations with two levels of parental plant competition (with and without), thus 16 case studies (see their Appendix S1). Without competition, selfed and outcrossed seeds germinated equally well in all eight populations, thus eight cases of I = O. With competition, selfed seeds germinated better than outcrossed seeds in two populations, thus two cases of I > O; selfed and outcrossed seeds germinated equally well in five populations, thus five more cases of I = O; and selfed seeds germinated less well than outcrossed seeds in one population, thus one case of I < O.
Table 4 Cross-type comparisons used in selecting inbred vs. outbred cases of seed germination

For the 743 case studies (Table 5), inbred seeds germinated less well than outbred seeds in 311 (41.9%); inbred and outbred seeds germinated equally well in 372 (50.1%); and inbred germinated better than outbred seeds in 60 (8.1%). A taxonomic analysis of the data for gymnosperms, angiosperms, monocots, eudicots and the three plant families with the highest number of case studies in the data set is presented in Table 6.
Table 5 A taxonomic survey of the effect of selfing vs. outcrossing on seed germination. I, inbred; O, outbred. The numbers in parentheses indicate the number of case studies for that particular I/O relationship. Nomenclature/taxonomy follows Mabberley (Reference Mabberley2008) and APG-III (2009). For the few gynodioecious species listed in this table, information is only for hermaphrodites

a Central achenes
b Peripheral achenes
c One species not otherwise on list
d None of four species otherwise on list
e Three species not otherwise on list
f Neither species otherwise on list
g Seven species not otherwise on list
h Only filled seeds used in germination tests
i All three species on list
Table 6 Partial analysis of the I/O data by taxonomic group; I, inbred; O, outbred

The proportions of I < O, I = O and I > O can vary between plant families; it was much more similar between Asteraceae and Pinaceae than it was between either of these families and Caryophyllaceae (Table 6). The I/O proportions were quite similar between gymnosperms and angiosperms, and they did not differ greatly between monocots and eudicots. Like the entire data set, this analysis found that outbred seeds germinated better than (I < O) or equally well as (I = O) inbred seeds in a high proportion of the cases, and only in a small proportion of the cases did inbred seeds germinate better than outbred ones (I > O). In six of the seven taxonomic groups analysed in Table 6, inbred seeds germinated equally as well or better than outbred seeds in >50% of the cases. The most ‘deviant’ taxonomic group in this analysis is the Caryophyllaceae, in which (I < O) >> (I = O) > (I > O). For the other taxonomic groups analysed, [(I < O) < (I = O)] >> (I > O) or [(I < O) = (I = O)] >> (I > O) (monocots only).
Relationship between mass and germination in inbred vs. outbred seeds
Here, we report the effect of inbreeding on both seed mass and germination for 216 case studies obtained from papers on the effect of ID on germination (Table 7), which also reported individual seed mass. Mean mass of outbred seeds was greater than that of inbred seeds (I < O) in 107 (49.5%) cases; in 82 (38.0%) cases, I = O; and in 27 (12.5%), I > O. For only 125 (57.9%) cases was there a direct relationship of inbreeding/outcrossing between seed size and germination, i.e. 54 cases of (I < O, germination) = (I < O, seed mass); 61 cases of (I = O, germination) = (I = O, seed mass); and 10 cases of (I > O, germination) = (I > O, seed mass). Large seeds germinated better than small ones in 54 (50.0%) of the 107 cases in which outbred seeds were larger than inbred seeds. In the other 53 (50.0%) cases, inbred and outbred seeds germinated equally well in 43 (40.2%) cases, and in 10 (9.3%) cases inbred seeds germinated better than outbred seeds. There was a direct relationship between large seed size and best germination in only 54 (25.0%) of the 216 case studies. In the 54 (67.7%) of the 80 cases in which germination of I < O, mass was I < O. This suggests that ID for germination in these 54 cases may have been mediated by seed size. However, in the other 26 (32.5%) of the 80 cases in which germination of I < O, mass of I = O (15 cases) or mass of I > O (11 cases), thus ID was not mediated by seed size. Outbred seeds were larger than inbred seeds (I < O) in 43 of the 107 cases in which germination of I = O and in 10 of the 27 cases in which germination of I > O.
Table 7 Relationship between percentage/rate of germination and mass of inbred (I) and outbred (O) seeds in 216 case studies
Magnitude of ID for seed germination
In many cases, ID per se for seed germination was not given in a paper. Thus, we calculated these values primarily from information presented by the authors in tables or graphs on percentage and/or speed of germination of selfed vs. outcrossed seeds, based on the methods described under ‘Inbreeding depression’, above. In a few cases, especially those involving germination and emergence rate, RP was incorrect and needed to be recalculated. For example, in one study RP for speed of germination is given as +0.12. However, since selfed seeds germinated in fewer days (7.923) than outcrossed seeds (9.016), i.e. selfed seeds performed better than outcrossed seeds, the RP should be − 0.12, i.e. (W s/W o) – 1, (7.923/9.016) – 1 = − 0.12, not 1 − (W s/W o) = +0.12. In another study, RP for days to emergence of selfed vs. outcrossed seedlings with and without competition was reported graphically as − 0.12 and +0.19, respectively, when in fact they were +0.12 and − 0.19, respectively. With competition, outcrossed seedlings emerged faster than selfed seedlings, thus the positive value for RP. Without competition, on the other hand, selfed seedlings emerged faster than outcrossed seedlings, thus the negative value for RP.
ID for seed germination covered most of the − 1 to +1 range possible for RP. The most extreme cases reported for germination in which W s < W o are 0.89 for Silene alba ( = S. latifolia) (Reference RichardsRichards, 2000a), 0.90 for Anacamptis morio (Smithson, Reference Smithson2006) and 1.0 for for Silene vulgaris subsp. maritima var. petraea (Pettersson, Reference Pettersson1992), and the most extreme case for W s > W o was − 0.88 for Cyclamen repandum (Affre and Thompson, Reference Affre and Thompson1999). For several cases RP ≥ 0.50, and in a few cases RP was equal to or more negative than − 0.40. On the other hand, we found many cases of RP ≤ 0.10 and a considerable number of cases in which it is less negative than − 0.10. RP values for germination in the study by Husband and Schemske (Reference Husband and Schemske1996) (calculated from their mean relative fitness values) ranged from − 0.36 to +0.40.
The magnitude of ID is relative, thus by themselves values generated for ID and RP do not necessarily tell us anything about actual performance, i.e. seed size, percentage of seeds germinating, grams of biomass accumulated, etc. For example, the level of ID will be the same (0.20) for inbred vs. outbred seeds that germinated to 8% and 10%, respectively, as it would be for inbred vs. outbred seeds that germinated to 80% and 100%, respectively.
We scored the case studies of I < O, I = O and I > O based on the results of statistical tests on germination percentage/rate (speed) by the authors of the papers and/or on RP values. It should be pointed out that in some cases P values and RP values for comparison of germination data on crossed vs. selfed seeds seem to merit a different interpretation with regard to significance. Three examples of the difficulty of making assignments to I/O categories will be given. In the study by Dudash and Fenster (Reference Dudash and Fenster2001) on Silene virginica, the average family mean for germination of selfed seeds from two populations was 39% and that of outcrossed seeds 49%. The P value for this comparison was 0.125, i.e. non-significant. Yet, the RP value calculated for these means is 0.204, i.e. average family mean for ID in the germination stage of the life cycle was 20.4%, which would seem to be biologically significant. Thus, we have scored this case as I < O. In the study by Takagawa et al. (Reference Takagawa, Washitani, Uesugi and Tsumura2006) on Nymphoides peltata, mean germination percentages for legitimate and selfed families of this heterostylous species were 96.1 and 91.0%, respectively, which was significant (P= 0.006), but the RP was only 0.05; we recorded this as I = O. In the study by Kennedy and Elle (Reference Kennedy and Elle2008) on Collinsia parviflora mentioned earlier, there was a significant difference (P < 0.05) in germination in one case of selfed vs. outcrossed seeds in which ID was 0.08. Yet, in another case of selfed vs. outcrossed seeds in the same paper the germination difference was not significant (P > 0.05), but ID was 0.11. For this study, we have made assignments based on statistical significance of the data. Thus, it is obvious that some case-by-case decisions had to be made on how to score the I/O relationship. For most assignments to I/O categories based on RP only, the following values were used: RP ≥ 0.10, I < O; − 0.10 < RP < 0.10, I = O; and RP ≤ − 0.10, I > O.
Below, we discuss the different categories about ID and seed germination. See Table 1 for additional information on topics discussed in this section.
Congeneric species
ID for germination may (e.g. Schemske, Reference Schemske1983; Latta and Ritland, Reference Latta and Ritland1994; Affre and Thompson, Reference Affre and Thompson1999) or may not (e.g. Ågren and Schemske, Reference Ågren and Schemske1993; Carr and Dudash, Reference Carr and Dudash1996; Johnston and Schoen, Reference Johnston and Schoen1996) vary considerably between congeneric species. RP was 0.02 for Begonia hirsuta and 0.05 for B. semiovata (Ågren and Schemske, Reference Ågren and Schemske1993), whereas it was − 0.44 for Diplusodon hirsutus and 0.62 for D. orbiculatus. RP for germination of four species of Cyclamen ranged from − 0.88 to 0.45 (Affre and Thompson, Reference Affre and Thompson1997).
Populations
Considerable variation in ID for germination has been found between populations of some species (e.g. Levin and Bulinska-Radomska, Reference Levin and Bulinska-Radomska1988; Latta and Ritland, Reference Latta and Ritland1994; Belaoussoff and Shore, Reference Belaoussoff and Shore1995; Ferdy et al., Reference Ferdy, Loriot, Sandmeier, Lefranc and Raquin2001; Lofflin and Kephart, Reference Lofflin and Kephart2005) but not of others (e.g. Reference WillisWillis, 1993a; Eckert and Barrett, Reference Eckert and Barrett1994; Johnston and Schoen, Reference Johnston and Schoen1996; Goodwillie and Knight, Reference Goodwillie and Knight2006). RP values in populations A, B and C of the orchid Dactylorhiza pratermissa were 0.140, 0.382 and − 0.778, respectively (Ferdy et al., Reference Ferdy, Loriot, Sandmeier, Lefranc and Raquin2001). However, the range of RP values for three populations of Linanthus (Leptosiphon) bicolor was only 0.00–0.03 (Goodwillie, Reference Goodwillie2000). In Chionographis japonica var. kurohimensis, ID for seed germination in the same population was 0.34 in 1989 and 0.05 in 1990 (Maki, Reference Maki1993). ID for germination of central populations of Clarkia concinna ranged from 0.28 to 0.32 and that of isolated populations from 0.22 to 0.27 (Groom and Preuninger, Reference Groom and Preuninger2000). ID for seed germination was − 0.01 and 0.37 for self-compatible and self-incompatible populations, respectively, of the cedar glade endemic Leavenworthia alabamica (Busch, Reference Busch2005), whereas it was 0.05 and − 0.03 for germination in selfing and outcrossing populations, respectively, of the rock-outcrop endemic Minuartia (Arenaria) uniflora (Fishman, Reference Fishman2001).
Maternal families
Maternal families of many taxa seem to exhibit a wide range of among-family variation in ID (e.g. Pettersson, Reference Pettersson1992; Husband and Schemske, Reference Husband and Schemske1995; Kephart et al., Reference Kephart, Brown and Hall1999). RP for germination of eight families of Silene douglasii var. oraria ranged from 0.00 to 0.80 (Kephart et al., Reference Kephart, Brown and Hall1999). For three maternal fig trees, on the other hand, progeny ID for germination was only 0.04 to 0.06 (Hossaert-McKey and Bronstein, Reference Hossaert-McKey and Bronstein2001). Furthermore, ID can carry over to the next generation in the form of maternal effects (Vogler et al., Reference Vogler, Filmore and Stephenson1999; Hayes et al., Reference Hayes, Winsor and Stephenson2005a). Thus, germination of progeny may be due to maternal inbreeding (δm), although F= 0 for progeny via maternal outcrossing.
Physical environment
The physical environment of parental parents may (e.g. Schemske, Reference Schemske1983) or may not (e.g. Dudash, Reference Dudash1990; Groom and Preuninger, Reference Groom and Preuninger2000) have a considerable effect on ID for germination of progeny. RP values for germination of progeny of Clarkia tembloriensis grown in a lath house and in the field were − 0.01 to 0.08 and − 0.04 to 0.03, respectively (Holtsford and Ellstrand, Reference Holtsford and Ellstrand1990). On the other hand, RPs for germination of Costus laevis grown in sun and in shade in the field and in a greenhouse were 0.09, 0.27 and − 0.09, respectively (Schemske, Reference Schemske1983).
Competition
Competition among parental plants may or may not increase ID of progeny. For germination (emergence date) of Hydrophyllum appendiculatum seeds, RP increased from − 0.19 without competition to 0.11 with competition (Wolfe, Reference Wolfe1993). However, there was little or no effect of competition on ID for germination of seeds of Collinsia parviflora (Kennedy and Elle, Reference Kennedy and Elle2008). For eight populations of this species, RP for germination with competition ranged from − 0.14 to 0.08 and that without competition from − 0.04 to 0.11.
Heterostyly and heterocarpy
For the two heterostylous species Lythrum salicaria (O'Neil, Reference O'Neil1994) and Nymphoides peltata (Takagawa et al., Reference Takagawa, Washitani, Uesugi and Tsumura2006), magnitudes of ID for seed germination {δ = 1 – (mean of selfed progeny)/(mean of [legitimate] progeny)} were relatively low. RP values for two populations of L. salicaria were 0.11 and 0.14. Mean germination percentages were 96.1% and 91.0% for legitimate and selfed families, respectively, of N. peltata, a mean RP of 0.05. There was essentially no ID for germination for either of two heterostylous populations of Amsinckia douglasiana or of A. spectabilis (Johnston and Schoen, Reference Johnston and Schoen1996).
ID for germination of central achenes (%, 0.26 and speed, 0.10) of the heterocarpic species Leontodon autumnalis was higher than it was for peripheral achenes (%, − 0.21 and speed, 0.02) (Picó and Koubek, Reference Picó and Koubek2003).
Ploidy level
In general, there does not seem to be much difference in germination of diploids and tetraploids, especially for the same cross type, i.e. selfing or outcrossing. ID for seed germination was 0.00 for two populations of the tetraploid species Amsinckia gloriosa; 0.00 and 0.012 for two populations of the diploid species A. douglasiana; and 0.00 to 0.087 for five diploid populations of A. spectabilis (Johnston and Schoen, Reference Johnston and Schoen1996). The mean ID values for seed germination of diploid and tetraploid cytotypes of Epilobium angustifolium were 0.22 and 0.11, respectively. In all five populations, selfed seeds germinated to a lower percentage than outcrossed seeds (Husband and Schemske, Reference Husband and Schemske1997). For germination percentage in four species of Clarkia: [diploid outcrossing species (ID 41) = polyploid outcrossing species (0.33)] > [diploid selfing species (0.06) = polyploid selfing species (0.05)]. There was no effect of inbreeding on days to germination: diploid outcrossing species = diploid selfing species = polyploid outcrossing species = polyploid selfing species (Barringer and Geber, Reference Barringer and Geber2008).
Herkogamy class
RP values for narrow and wide herkogamy classes of Mimulus guttatus were 0.05 and 0.06, respectively, in one population and 0.17 and 0.04, respectively, in another population (Carr et al., Reference Carr, Fenster and Dudash1997).
Endemics
ID for germination was low or non-existent for most endemics, e.g. 0.07 for Amsinckia douglasiana (Cheptou and Schoen, 2002); − 0.08 for Anchusa crispa (Quilichini et al., Reference Quilichini, Debussche and Thompson2001); − 0.03 and 0.05 for outcrossing and selfing populations, respectively, of Minuartia (Arenaria) uniflora (Fishman, Reference Fishman2001); − 0.20 for Astragalus linifolius (Karron, Reference Karron1989); − 0.04 for Brassica cretica (Rao et al., Reference Rao, Widen and Andersson2002); 0.00 for Hymenoxys herbacea (Moran-Palma and Snow, Reference Moran-Palma and Snow1997); 0.001 (%) and 0.03 (speed) for Leavenworthia crassa (Charlesworth et al., Reference Charlesworth, Lyons and Litchfield1994); 0.00 to 0.04 for Linanthus (Leptosiphon) jepsonii (Goodwillie and Knight, Reference Goodwillie and Knight2006); and − 0.02 for Sedum pusillum (Wyatt, Reference Wyatt1983). However, ID for germination is not low for all endemic species. It was 0.42 (Kephart et al., Reference Kephart, Brown and Hall1999) and 0.34 (Lofflin and Kephart, Reference Lofflin and Kephart2005) for Silene douglasii var. oraria. RP values for 19 families of the Öland (Sweden) alvar endemic Silene vulgaris subsp. maritima var. petraea ranged from − 0.27 to 1.0 (Pettersson, Reference Pettersson1992), and it was 0.37 for self-incompatible populations of the narrow Alabama cedar glade endemic Leavenworthia alabamica (Busch, Reference Busch2005).
Endemic vs. common species
Several studies on endemic species also included one or more congeners that are more geographically widespread than the narrow endemics, thus presenting an opportunity to compare ID values between these two groups. Some results are: Amsinckia douglasiana (endemic) 0.00 to 0.013 vs. A. spectabilis (widespread) − 0.087 to 0.097 (Johnston and Schoen, Reference Johnston and Schoen1996); Astragalus linifolius (endemic) − 0.20 vs. A. lonchocarpus (widespread) − 0.03 (Karron, Reference Karron1989); Leavenworthia crassa (endemic) 0.001 (%) and 0.03 (speed) vs. L. uniflora (widespread) 0.05 (%) and 0.08 (speed) (Charlesworth et al., Reference Charlesworth, Lyons and Litchfield1994); Linanthus (Leptosiphon) jepsonii (endemic) vs. L. bicolor (widespread), in which ID in all populations studied was 0.00 or nearly so (Goodwillie, Reference Goodwillie2000); Mimulus micranthus (endemic) 0.19 (within population) and 0.20 (between populations) vs. M. guttatus (widespread) 0.21 (within population) and 0.29 (between populations) (Carr and Dudash, Reference Carr and Dudash1996); and Silene douglasii var. oraria (endemic) 0.34 vs. S. douglasii var. douglasii (widespread) 0.44 (Cascades Jack Creek population) and 0.04 (Cascades Cove Creek population) (Lofflin and Kephart, Reference Lofflin and Kephart2005).
Procedures used in germinating seeds in studies on inbreeding depression
Dormancy occurs in seeds of a high proportion of the species in all major vegetation zones on Earth (Baskin and Baskin, Reference Baskin, Baskin, Smith, Dickie, Linington, Pritchard and Probert2003, 2014), and dormancy, along with temperature and light, are three of the most important factors regulating seed germination (Baskin and Baskin, Reference Baskin and Baskin2014). Further, seeds of nearly all of the families and genera containing species for which germination of inbred and outbred seeds have been compared (Table 5) have some kind of dormancy (most of them non-deep physiological) at maturity (Baskin and Baskin, Reference Baskin and Baskin2014). Thus, since germination percentage and/or rate (speed) are used as (a) measure(s) of fitness in inbred vs. outbred seeds in studies of ID in this stage of the plant life cycle, it is essential that careful attention be given to how to break dormancy and germinate the seeds.
Percentage of germination/seedling emergence in the case studies in our survey ranged from very low/low (e.g. Wolfe, Reference Wolfe1993; Mandujano et al., Reference Mandujano, Montana and Eguiarte1996; Puterbaugh, 1997; Affre and Thompson, Reference Affre and Thompson1999; Routley et al., Reference Routley, Mavraganis and Eckert1999) to high/very high (e.g. Schoen, Reference Schoen1983; Hauser and Loescheke, Reference Hauser and Loeschcke1995; Johnston and Schoen, Reference Johnston and Schoen1996; Cheptou et al., Reference Cheptou, Imbert, Lepart and Escarré2000b; Goodwillie, Reference Goodwillie2000). In many of the papers, there was no mention of giving the seeds a dormancy-breaking treatment. Except in a few cases, e.g. those of the three species of Dipterocarpaceae, the seeds cannot necessarily be considered to have been (fully) non-dormant, even though germination percentage was high in the limited range of conditions in which they were tested/sown. They may have been conditionally dormant, i.e. in a state of dormancy between ‘true dormancy’ and non-dormancy (Vegis, Reference Vegis1964; Baskin and Baskin, Reference Baskin and Baskin2004, 2014). In the paper by Seltmann et al. (Reference Seltmann, Cocucci, Renison, Cierjacks and Hensen2009) on Polylepis australis (Rosaceae), it is stated that the seeds were non-dormant. However, when the seeds were tested for germination they were 1 month old, having been stored under ambient laboratory (afterripening) conditions during this time. Furthermore, highest germination was 30%; viability of non-germinated seeds was not tested. We suggest that the seeds were physiologically dormant at maturity and that many of them were still dormant at the time they were tested, i.e. the ≥ 70% that did not germinate, assuming they were viable.
A wide variety of storage conditions used in the studies can be included under the ‘storage/afterripening’ dormancy-breaking category, e.g. seeds afterripened at room temperature and then maintained at approximately 4°C with a desiccant; seeds stored in paper bags from spring 1999 to December 1999; seeds afterripened for 1 month; seeds stored at room temperature for 6 months; seeds stored dry in laboratory at 40°C; and seeds stored for 13–14 months in screw-capped vials at 4°C. While in some cases ‘afterripening/storage’ was definitely planned as a dormancy-breaking treatment, it appears that in others the seeds were stored simply for the sake of keeping them until a later date, i.e. until they could be used in a study.
For cold-stratified seeds, the length of the cold-stratification period ranged from 5 d, which is a very short period of cold stratification and unlikely to be effective in breaking dormancy in seeds of most species, to 12 weeks, usually at 4 or 5°C. In some cases, we could not determine whether the seeds were moist-cold treated or simply dry-cold stored. In the former case, water-permeable, but not water-impermeable, seeds would have been cold stratified, whereas in the latter case neither water-permeable nor water-impermeable seeds would have been cold stratified, but water-permeable seeds might have afterripened.
Perhaps the most unnatural dormancy-breaking treatment was soaking seeds of an orchid in a calcium hypochlorite solution to chemically scarify the seed coat (Ferdy et al., Reference Ferdy, Loriot, Sandmeier, Lefranc and Raquin2001), and the most natural one was sowing seeds in the field/outdoors, where they are exposed to warm and/or cold temperatures between dispersal and germination. Other chemicals used in dormancy-breaking treatments included potassium nitrate (KNO3) and gibberellin (GA). NO3 − has been reported to break seed dormancy in nature, and GAs are natural plant growth regulators known to be intimately involved in the biochemical mechanism(s) of breakage of physiological dormancy in seeds (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Footitt et al., Reference Footitt, Douterelo-Soler, Clay and Finch-Savage2011; Graeber et al., Reference Graeber, Nakabayashi, Miatton, Leubner-Metzger and Soppe2012). Moist cold-stratification and dry-storage (afterripening) treatments simulate dry, warm and moist, cold conditions, respectively, that seeds are exposed to in nature (Baskin and Baskin, Reference Baskin and Baskin2004, 2014).
However, scarification is not a treatment that simulates a dormancy-breaking process in nature (Baskin and Baskin, Reference Baskin and Baskin2000, Reference Baskin and Baskin2014). In our survey, dormancy was broken in most of the case studies in the hardseeded (i.e. water-impermeable seed coat) families Cistaceae, Convolvulaceae, Fabaceae, Geraniaceae and Malvaceae (Baskin et al., Reference Baskin, Baskin and Li2000) by mechanical scarification. In a few of the studies, even seeds with a water-permeable seed coat were scarified or ‘pricked’. In two of these cases, the whole seed coat was removed, and only the embryo was tested for ‘germination’. Scarifying seeds with non-deep physiological dormancy allows the seed to germinate by lowering the mechanical restraint of the seed coat on embryo growth (radicle emergence) and not by creating an opening for the entrance of water (Baskin and Baskin, Reference Baskin and Baskin2004). Scarification of neither water-impermeable nor water-permeable seed coats has been demonstrated to be a way in which seed dormancy is overcome in nature.
We suggest that the scarification treatment is a good one to use to learn about viability of seeds with water-impermeable seed coats but not about dormancy, or thus seed germination, in nature, which may take up to two decades or longer to be completed by a seed population (or lot) (Baskin and Baskin, Reference Baskin and Baskin2014). In the great majority of seeds with physical dormancy, the fully developed embryo is non-dormant, and thus when the seed coat is made water permeable the radicle emerges, usually within a few days. In nature, physical dormancy is broken by high (including heat from fires) and fluctuating temperatures (including low fluctuating temperatures) (Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003; Moreira and Pausas, Reference Moreira and Pausas2012; Baskin and Baskin, Reference Baskin and Baskin2014).
A few of the papers did not include any description of germination procedures. Many of the papers reported both temperature and light conditions for germination; most such studies were done in controlled growth cabinets. In a high percentage of these studies, seeds were germinated under a single temperature regime. A high percentage of the studies in which seeds were germinated in the laboratory or greenhouse did not provide information on either temperature or light conditions. Some studies provided information on either temperature or light but not on both.
Conclusions, recommendations and remarks
-
● We recommend using the equation for RP to calculate values for traits, such as percentage germination, in studies on the effects of inbreeding vs. outbreeding in plants. When W o ≥ W s, equations for δ and RP will give the same positive value (to +1). When W o < W s, however, δ ≠ RP. That is, whereas the RP equation can give a negative value to only − 1, the equation for δ can give a negative value to − ∞. Thus, the equation for RP gives equal weight to the same phenotype trait value for the best and worst performer, whereas the equation for δ does not. Using RP in cases where W s > W o certainly would make it easier to compare the effects of selfing and outcrossing.
-
● Keep in mind that in studies of rates (speed) of a process or of an event in the plant life cycle, such as days to germinate, the higher number means lower performance. Thus, use the equation 1 − (W o/W s) when number of days for outcrossed seeds to germinate is fewer than that for inbred seeds, and the equation (W s/W o) – 1 when the number of days for inbred seeds to germinate is fewer than that for outcrossed seeds.
-
● Relative fitness values in the multiplicative fitness portion (CRF) of the equation for calculating cumulative (lifetime) inbreeding depression (1 − CRF) will be >1 when inbreeders outperform outbreeders. Thus, one may get either a positive or a negative value for events across the life cycle in selfers vs. outcrossers. If the speed of an event in the life cycle is used in this equation, when W s > W o (in terms of number of days) relative fitness should be expressed as (W o/W s) and when W o > W s as (W s/W o).
-
● ‘Optimal outcrossing distance’ for germination has been reported for only a few taxa. Most studies that have tested the effect of various distances between the seeds’ parents on germination (and also other stages in the plant life cycle) have found no evidence for it. That is, in most studies there was no evidence that seeds germinate better at some intermediate distance between the parents than at far distances from them. Neither outbreeding depression nor heterosis for germination appears to be common for crosses between populations. However, crosses between different populations can sometimes reduce the performance of hybrid offspring via outbreeding depression (OD). Thus, transfer of genes into a population via pollen, seeds or transplants could lead to reduced fitness through disruption of gene complexes or by disruption of local adaptation. Conservationists need to be aware of the possibility of these negative consequences when obtaining seeds for restoration [see Johnson et al. (Reference Johnson, Sorensen, St. Clair and Cronn2004), who discuss the concept and use of ‘tree seed zones’ in research and management of forests; but also see Broadhurst et al. (Reference Broadhurst, Lowe, Coates, Cunningham, McDonald, Vesk and Yates2008), who, while accepting the existence of local adaptation and outbreeding depression, challenge the view among restoration ecologists that local is best as a guiding principle for seed sourcing].
-
● Outbred seeds germinate better than, equal to or less well than inbred seeds. In 50.1% of the cases surveyed, inbred seeds germinated as well as outbred seeds, and in 8.1% inbred seeds germinated better than outbred seeds. Our results for 743 cases of germination of inbred vs. outbred seeds differ considerably from those of Darwin's (1876) very limited study on 21 comparisons of speed of germination of inbred vs. outbred seeds. For I < O, I = O and I > O, Darwin reported 47.6%, 4.8% and 47.6%, respectively, whereas we report 41.9%, 50.1% and 8.1%, respectively. Black (Reference Black2009) does not mention this part of Darwin's research on seeds, and although Owens and Miller (Reference Owens and Miller2009) say that Darwin recorded time from planting to seed germination, they do not give any results of his observations.
-
● Proportional relationships of I < O, I = O and I >O for germination of gymnosperms and angiosperms are quite similar; in both groups [(I < O) < (I = O)] >> (I > O).
-
● There does not seem to be a strong relationship between decrease in germination with increase in F, or between increase in germination and increase in population genetic diversity.
-
● There is a huge range of variation in the magnitude of ID for seed germination, and the level of ID may depend on species, population, maternal family, year, breeding system, degree of inbreeding, number of pollen parents, degree of seed-set autogamy, herkogamy class, physical environment or degree of competition in which seeds were produced, outcrossing distance, crosses within vs. among populations, seed morph in seed/fruit heteromorphic species, ploidy level, degree of relatedness of parents and dormancy-breaking treatment and germination conditions; and probably several other things.
-
● There is not a particularly strong relationship between seed size and germination in inbred vs. outbred seeds. In some cases of ID for seed germination, small seeds may germinate equally well or even better than large seeds; also, for seeds of equal size, W s may be greater than W o. In which cases, ID for seed germination is not mediated by large seed size. Our results of 216 case studies on size of inbred vs. outbred seeds do not agree with those of Darwin's (1876) 16 comparisons of seed size in inbred and outbred seeds. For I < O, I = O and I > O, Darwin's relative proportions are 6, 0 and 10, respectively, and ours are 107, 82 and 27, respectively. Both Black (Reference Black2009) and Owens and Miller (Reference Owens and Miller2009) note that for 10 of the 16 species Darwin (Reference Darwin1876) examined for seed mass, mass of inbred seeds was greater than that of outbred seeds.
-
● ID for seed germination for the majority of narrow endemics in our survey was low and, in general, did differ substantially from that of geographically widespread congeners.
-
● In general, more attention needs to be given to seed dormancy and germination in studies of the effects of inbreeding in plants. In particular, germinating/testing seeds at near-natural field conditions would allow one to extrapolate the results to the real world.
-
● We recommend that before beginning a study of ID on a species the investigator first become familiar with its natural history, thus following the advice of Bernhardt (Reference Bernhardt1999, p. 69): ‘If you want to find, grow, or study any living thing you must first become familiar with its season of activity.’ In the case of seed germination, the investigator needs to become familiar with the seasons of dormancy break and germination. In sum, researchers need to incorporate a stronger element of whole-seed physiology and plant life cycle phenology into their studies on ID that include seed germination.
-
● Furthermore, plants obtained from seeds whose dormancy is broken by artificial, non-natural treatments (e.g. GA) may differ in growth and morphology from those obtained from seeds whose dormancy is broken by natural means (Baskin and Baskin, Reference Baskin and Baskin1975; Fox et al., Reference Fox, Evans and Keefer1995; Evans et al., Reference Evans, Mitchell and Cabin1996). This being the case, then, it is easy to imagine that the results for ID, not only for germination but also of other stages of the plant life cycle, would not be representative of what is happening in the real world. Additionally, ‘forced’ germination may affect families (Fox et al., Reference Fox, Evans and Keefer1995; Evans et al., Reference Evans, Mitchell and Cabin1996), and perhaps even inbred and outbred progeny, differently. In the study by Evans et al. (Reference Evans, Mitchell and Cabin1996), ‘The magnitude of the GA3 effect was strongly influenced by both germination environment and maternal sibship.’
-
● Our overall impression of the thinking of many researchers who do studies on ID in plants is that at a given time it is better for a seed to germinate than not to germinate, i.e. not to remain dormant and thus delay germination until a later date. Thus, seeds that germinate to high percentages are more beneficial to the plant (via increased fitness) than are seeds that germinate to low percentages. However, undoubtedly in many cases/circumstances the plant would gain more long-term fitness by some of the seeds delaying germination than it would by all of them germinating at the same time. Importantly, delaying germination in an unpredictable environment such as deserts can be an adaptive bet-hedging strategy, i.e. increasing the geometric mean fitness of the genotype over generations (Cohen, Reference Cohen1966; Venable, Reference Venable1985; Mandák and Pyšek, Reference Mandák and Pyšek1999; Clauss and Venable, Reference Clauss and Venable2000; Simons, Reference Simons2011; Gremer and Venable, Reference Gremer and Venable2014). Considering the long term, then, at least for annual species in temporally stochastic environments, low germination (high dormancy) percentages of selfed seeds compared to those of outcrossed seeds may be more beneficial to the species.
-
● With some exceptions (e.g. Schoen, Reference Schoen1983; Norman et al., Reference Norman, Sakai, Weller and Dawson1995; Ferdy et al., Reference Ferdy, Loriot, Sandmeier, Lefranc and Raquin2001; Heenan et al., Reference Heenan, Smissen and Dawson2005; Ferrer et al., Reference Ferrer, Good-Avila, Montaña, Domínguez and Eguiarte2009), information on seed viability was not reported in the studies included in our survey. In which cases, germination percentages were based on the total number of seeds, a portion of which may have been non-viable when sown/incubated. This raises a question: should the non-viable seeds be included in the germination or in the seed development (or seed production) stage of the life cycle? We suggest that non-viability in fresh seeds be considered to have occurred during seed development, and loss of viability thereafter, e.g. during dormancy-breaking treatment, in the seed-germination stage. Further, in many cases inbred seeds are more likely to lose viability during development than are outcrossed seeds (Husband and Schemske, Reference Husband and Schemske1996). Thus, including freshly matured non-viable seeds in the seed development stage of the plant life cycle should lower the magnitude of ID for seed germination, since non-viable seeds that cannot germinate regardless of treatment are ‘replaced’ by viable seeds that can germinate either with (dormant) or without (non-dormant) dormancy-breaking treatments.
Note added in proof
A recently published paper [Carta, A., Bedini, G., Giannotti, A., Savio, L. and Peruzzi, L. (2015) Mating system modulates degree of seed dormancy in Hypericum elodes L. (Hypericaceae). Seed Science Research 25, 299–305] also calls for persons doing research on inbreeding depression in plants to pay attention not only to seed germination but also to seed dormancy. These authors found that, for germination, I>O for seeds cold-stratified for 0 and 3 weeks, whereas I = O for seeds cold-stratified for 8 weeks. They concluded that, ‘… seed germination alone is not an appropriate fitness measure for inbreeding depression estimates, unless dormancy is removed’. Otherwise, lack of germination may be related to dormancy and not to ID.
Conflicts of interest
None.










