Introduction
Agents of disease (parasites and pathogens) are important and ubiquitous biotic factors, playing an important role in natural populations by regulating host population dynamics, maintaining host genetic diversity and modulating other trophic interactions (Jokela et al. Reference Jokela, Dybdahl and Lively2009; Hall et al. Reference Hall, Becker, Duffy and Caceres2011; Agha et al. Reference Agha, Saebelfeld, Manthey, Rohrlack and Wolinska2016). Parasites can affect their host directly – for example through reduced fecundity, increased mortality and/or altered behaviour (Hall et al. Reference Hall, Becker, Duffy and Caceres2011; Kekäläinen et al. Reference Kekäläinen, Lai, Vainikka, Sirkka and Kortet2014) – and indirectly – by inducing changes in the host's response to other stress factors, such as contaminants (Kelly et al. Reference Kelly, Poulin, Tompkins and Townsend2010; Buser et al. Reference Buser, Jansen, Pauwels, De Meester and Spaak2012a; Hanlon et al. Reference Hanlon, Lynch, Kerby and Parris2015). Research on the interactive effects of parasitism and contamination (reviewed by Marcogliese and Pietrock, Reference Marcogliese and Pietrock2011) has produced contrasting evidence, with the outcome of the interaction being favourable either to the host (Blanar et al. Reference Blanar, MacLatchy, Kieffer and Munkittrick2010; Hanlon et al. Reference Hanlon, Kerby and Parris2012) or to the parasite (Johnson et al. Reference Johnson, Chase, Dosch, Hartson, Gross, Larson, Sutherland and Carpenter2007; Coors et al. Reference Coors, Decaestecker, Jansen and De Meester2008). A previous experimental study by our team (Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2017) demonstrated that simultaneous exposure of Daphnia to a microparasitic yeast (Metschnikowia bicuspidata) and fungicides resulted in independent effects from contaminant and parasite (for copper sulphate), whereas contaminant-driven parasite suppression was found for another fungicide, tebuconazole. Such contrasting results emphasize the need for more research on this subject.
Increasing temperature is expected to change contaminant properties (Noyes et al. Reference Noyes, McElwee, Miller, Clark, Van Tiem, Walcott, Erwin and Levin2009; degradation rates, environmental mobility, bioavailability; Delcour et al. Reference Delcour, Spanoghe and Uyttendaele2014) as well as the susceptibility of organisms to contaminants (Seeland et al. Reference Seeland, Oehlmann and Müller2012; Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2016). Simultaneously, temperature plays a pivotal role in the outcome of host–parasite relationships (Wolinska and King, Reference Wolinska and King2009; Schoebel et al. Reference Schoebel, Tellenbach, Spaak and Wolinska2011; Gallana et al. Reference Gallana, Ryser-Degiorgis, Wahli and Segner2013), by influencing parasite infectivity and spore production (Mitchell et al. Reference Mitchell, Rogers, Little and Read2005; Vale et al. Reference Vale, Stjernman and Little2008), and by modulating the host's susceptibility to infection (Garbutt et al. Reference Garbutt, Scholefield, Vale and Little2014). Based on this rationale, research on interaction scenarios between parasitism and contamination should consider temperature as an important modulator of both phenomena. This is particularly important in the light of climate change predictions (namely warming), as temperature effects on pollution × disease interactions are still unresolved (Marcogliese, Reference Marcogliese2008; Noyes et al. Reference Noyes, McElwee, Miller, Clark, Van Tiem, Walcott, Erwin and Levin2009; Delcour et al. Reference Delcour, Spanoghe and Uyttendaele2014).
The crustacean zooplankter Daphnia sp. plays a central role in aquatic food webs by consuming primary producers and serving as food for predators, and is also frequently parasitized by numerous microorganisms (Lass and Ebert, Reference Lass and Ebert2006; leading to epidemics in the field; Duffy and Sivars-Becker, Reference Duffy and Sivars-Becker2007; Duncan and Little, Reference Duncan and Little2007). These parasites play a pivotal role in Daphnia population dynamics (Hall et al. Reference Hall, Becker, Duffy and Caceres2011), diversity (Duncan and Little, Reference Duncan and Little2007; Wolinska and Spaak, Reference Wolinska and Spaak2009) and community structure (Hall et al. Reference Hall, Becker, Simonis, Duffy, Tessier and Caceres2009; Buser et al. Reference Buser, Spaak and Wolinska2012b). The microparasitic yeast M. bicuspidata grows inside the host's body cavity and produces needle-like ascospores that are horizontally transmitted (Ebert, Reference Ebert2005). This virulent parasite reduces host fecundity in the late stage of the infection and kills its host within 2–3 weeks (Ebert, Reference Ebert2005; Hall et al. Reference Hall, Tessier, Duffy, Huebner and Cáceres2006; Duffy and Hall, Reference Duffy and Hall2008). Lake epidemics of M. bicuspidata can reach a prevalence up to 40% (Caceres et al. Reference Caceres, Hall, Duffy, Tessier, Helmle and MacIntyre2006; Duffy et al. Reference Duffy, Hall, Caceres, Ives, Cáceres, Ives and Caceres2009; Hall et al. Reference Hall, Becker, Duffy and Caceres2011; Wolinska et al. Reference Wolinska, Seda, Koerner, Smilauer and Petrusek2011), and they are important in the ecological dynamics of Daphnia natural populations (Duffy and Sivars-Becker, Reference Duffy and Sivars-Becker2007; Duffy et al. Reference Duffy, Hall, Caceres, Ives, Cáceres, Ives and Caceres2009, Reference Duffy, Housley, Penczykowski, Caceres and Hall2011). This host–parasite model allows experimental manipulation of spore load and parasite transmission (Lohr et al. Reference Lohr, Yin and Wolinska2010; Yin et al. Reference Yin, Laforsch, Lohr and Wolinska2011), as well as the discrimination of the interactive effects of parasite and other stressors (Duffy et al. Reference Duffy, Housley, Penczykowski, Caceres and Hall2011; Civitello et al. Reference Civitello, Forys, Johnson and Hall2012; Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2017).
The aim of this study was to assess the interactive effects of fungicide contamination and disease, considering the influence of temperature. Copper sulphate and tebuconazole were selected based on their contrasting effects on the host–parasite system described (i.e. Daphnia infected with M. bicuspidata, Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2017). We hypothesize that temperature will affect the outcome of the contaminant × disease interaction in the case of tebuconazole (which causes a suppression of infection), but this may be less important in the case of copper sulphate because of the lack of interaction between parasite and toxicant effects (Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2017).
Materials and methods
Host–parasite system: origin and maintenance
Two host genotypes were tested, belonging to the Daphnia longispina species complex (D. galeata × longispina clone AMM_12 and D. galeata clone AMM_47, from now on designated as Clone 12 and Clone 47, respectively). The studied parasite was a single isolate of the endoparasitic yeast M. bicuspidata. Host genotypes and parasite strain were isolated from Lake Ammersee (Germany) and have been used in other studies (Yin et al. Reference Yin, Laforsch, Lohr and Wolinska2011; Buser et al. Reference Buser, Spaak and Wolinska2012b; Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2017).
Daphnia genotypes (Clone 12 and Clone 47) were reared as parthenogenetic lineages in moderately hard reconstituted water (123 mg L−1 MgSO4·7H2O, 96 mg L−1 NaHCO3, 60 mg L−1 CaSO4·2H2O, 4 mg L−1 KCl) supplemented with a standard organic additive (algal extract) and vitamins (Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2016, Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2017). Cultures were maintained with a 16hL:8hD photoperiod and the medium was renewed three times a week. Along with medium renewal, daphnids were fed with a Raphidocelis subcapitata suspension (1·5 × 105 cells mL−1). Daphnia were reared in a controlled temperature room (at 20 ± 1 °C) and in a water bath equipped with a water chiller (17 ± 1 °C). The temperature was regularly checked with a temperature probe. Before the experiment, Daphnia were acclimated for at least five generations at the two tested temperatures, in order to remove potentially confounding effects of transient thermal stress (Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2016).
The parasite strain was maintained in a Daphnia magna clone, reared under the conditions described above, at 20 °C. Uninfected Daphnia juveniles were added to infected cultures every other week, guaranteeing a continuous supply of hosts and production of spores (Hesse et al. Reference Hesse, Engelbrecht, Laforsch and Wolinska2012; Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2017).
Test chemicals
Tebuconazole and copper sulphate concentrations were defined according to Cuco et al. (Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2016), which estimated reproductive ECx values that were then used to define a low dose (roughly corresponding to EC10 for both contaminants) and a high dose (corresponding to EC50 for tebuconazole and EC20 for copper). A lower ECx value was used for copper because it causes substantial mortality along with reproductive impairment that could result in a highly unbalanced design (loss of experimental units due to high mortality). Concentrated stock solutions were prepared with ethanol (for dissolving tebuconazole) or distilled water (copper sulphate) and then diluted in reconstituted water (see Host–parasite system: origin and maintenance). Final test solutions were: 0·00, 100 and 250 µg L−1 for tebuconazole, and 0·00, 30·0 and 40·0 µg L−1 for copper sulphate (expressed as free Cu2+ ion). For tebuconazole exposure, ethanol was also added in the negative control (0·00 µg L−1) at an equal volume to the one used in the tebuconazole concentrations (0·1 mL L−1).
Analytical quantification of tested substances previously confirmed the validity of nominal concentrations within the range of 25·0–43·7 µg L−1 (for copper) and 100–240 µg L−1 (for tebuconazole), although measured concentrations slightly overestimated nominal concentrations: 6–16% for copper and 1–10% for tebuconazole. Detailed analyses of measured copper and tebuconazole concentrations are presented in Supplementary Table S1. These analyses were performed by an independent analytical laboratory (certified according to ISO/IEC 17025:2005), which analysed random samples by ICP-MS (for copper, according to ISO 17294-2:2003) and LC-MS/MS (for tebuconazole, according to an internal method adapted from ISO 11369:1997). We assumed that good correspondence between nominal and measured concentrations was valid for the experiments shown here (the same procedures were used and the range of concentrations is comparable).
Experimental design
Life history assays were initiated with neonates (<24 h old, born between the 3rd and the 5th broods) from the two Daphnia genotypes reared at each acclimation temperature (17 °C and 20 °C). Daphnia were individually exposed to a range of contaminant concentrations (see Test chemicals), at the two selected temperatures (17 °C and 20 °C), in the presence (‘parasite’) or absence (‘no-parasite’) of the parasite M. bicuspidata, in a fully crossed design. These temperatures were chosen based on previous work (Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2016), which showed substantial differences in the life history of these Daphnia clones at 17 °C when compared with warmer conditions (20 °C and 23 °C). For logistical reasons, experiments with tebuconazole and copper were carried out separately. Therefore, the experimental design for each contaminant consisted of 2 Daphnia clones × 3 contaminant concentrations (see Test chemicals) × 2 parasite treatments × 2 exposure temperatures × 13 replicates = 312 experimental units. Because infection is not always 100% successful, the parasite treatment was initiated with 20 replicates, and individual Daphnia that did not become infected were then excluded. In the case of tebuconazole, only 13 replicates were set up in the parasite treatment, as we anticipated a suppression of infection (Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2017). Each replicate corresponded to one Daphnia individually placed in a glass vessel containing 25 mL of the respective test solution, prepared by adding the proper amount of contaminant stock solution to the culture medium (see Test chemicals). Assays were carried out under the same conditions as described for maintenance, except that Daphnia were fed daily with an algal ration of 1·5 × 105 cells mL−1 and test medium was renewed every 3 days. In the parasite treatment, individual Daphnia were infected on day 5 with an M. bicuspidata spore suspension, following the methodology described below (see Infection procedure). Test procedures followed OECD (Reference OECD2012) recommendations.
Infection procedure
At experimental day 5 (first day of infection), test solution volume from all experimental units was reduced from 25 to 10 mL and Daphnia were not fed; this ensured higher spore encounter and filtration rates (Yin et al. Reference Yin, Laforsch, Lohr and Wolinska2011). Infected D. magna from the host-parasite culture were crushed in distilled water to obtain a homogenized spore suspension, and spore concentration was determined using a Neubauer improved counting chamber. A placebo suspension was also obtained by crushing the same number of uninfected D. magna in distilled water. Experimental units of the parasite treatment were inoculated with an M. bicuspidata spore suspension aliquot (700 spores mL−1) while experimental units of the no-parasite treatment were given an equivalent placebo aliquot. The use of a placebo was important to rule out potential confounding effects of bacteria or alarm cue effects from the Daphnia homogenates. On day 6, test solution volume was increased to 15 mL and, on day 7, to 25 mL. Daily food ration (1·5 × 105 cells mL−1) was restored on day 6. Between day 5 and day 7, vessels were slightly shaken once per day, to promote spore resuspension and subsequent filtration by the host. From day 8 onwards, initial conditions (25 mL of test solution with renewal every 3 days) were restored, as described above.
Data collection
Survival and reproduction data were recorded daily for each Daphnia. Reproductive parameters included fecundity (cumulative number of living offspring released per surviving female) and reproductive output (total number of living offspring released per initial female, including females, which died during the course of the experiment). Fecundity was estimated at day 15 before females started dying due to parasite action. For the calculation of reproductive output (measured at day 21), we excluded replicates in which Daphnia died before day 4, as this could be the result of accidental or natural early-age mortality. The experiment ended on day 21 for copper, later for tebuconazole (see below). At the time of death or at the end of the assay, daphnids were visually inspected for signs of infection (presence of spores inside the host's body cavity) under an Olympus CKX41 inverted microscope, and prevalence of infection was calculated per treatment. For the calculation of all parameters, we excluded experimental units where Daphnia died before the infection could be confirmed. Survival and reproduction data were used to determine the per capita intrinsic rate of population increase (r) from the Euler–Lotka equation:
 $$1 = \mathop \sum \limits_{x = 0}^n e^{ - rx}l_xm_x,$$
$$1 = \mathop \sum \limits_{x = 0}^n e^{ - rx}l_xm_x,$$where r is the rate of population increase (day−1), x is the age class in days, l x is the probability of surviving to age x, and m x is the fecundity at age x (Meyer et al. Reference Meyer, Ingersoll, McDonald and Boyce1986; McCallum, Reference McCallum2000). Replicate pseudo values for r were generated using the jack-knifing technique described by Meyer et al. (Reference Meyer, Ingersoll, McDonald and Boyce1986), thus enabling the use of statistical tests for analyzing differences in the rate of increase between experimental treatments.
Previous data (Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2017) showed that tebuconazole suppresses M. bicuspidata infection, unlike copper. Therefore, with the purpose of assessing if parasite infection is delayed or definitely suppressed by this fungicide, we continued to monitor Daphnia exposed to tebuconazole (and respective negative controls) throughout their lifespan (i.e. beyond the 21-d period). During this period, we monitored survival and the presence of infection.
Statistical analysis
Three-way analyses of variance (ANOVA) were performed separately for each experiment (copper and tebuconazole) to assess the effects of temperature, parasite challenge and contaminant concentration (as fixed factors) on Daphnia life history parameters. Because we expected differences in the sensitivity of the tested clones to the stress factors (Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2016), analyses were conducted separately per clone, with a necessary adjustment in the significance level (α = 0·025). Plots of residuals were analysed to assess departures from normality and homogeneity of variances, which were found on a few occasions; however, we assumed ANOVA to be sufficiently robust to deal with such deviations. ANOVAs were carried out with the General Linear Model procedure in Minitab 16 (Minitab Inc., USA).
Results
Infection data
The infection procedure was successful in both experiments and for both clones; 55–100% of exposed Daphnia became infected. No infections were detected in the no-parasite treatment. Almost all infected hosts died within the 21-d period, confirming that M. bicuspidata considerably reduces Daphnia life span. Specifically, longevity was reduced from 6 to 2 weeks at 20 °C and from 9 to 3 weeks at 17 °C.
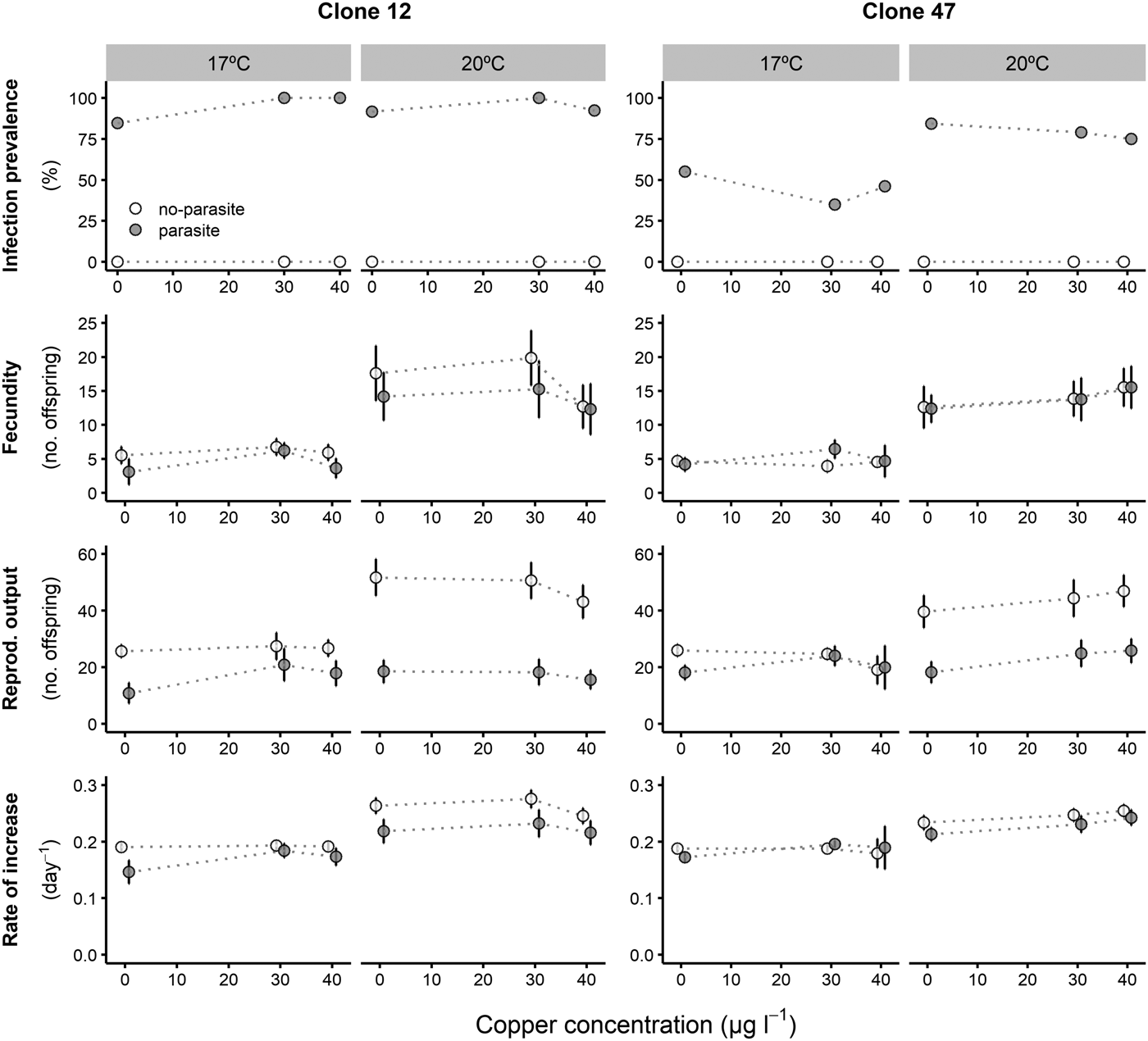
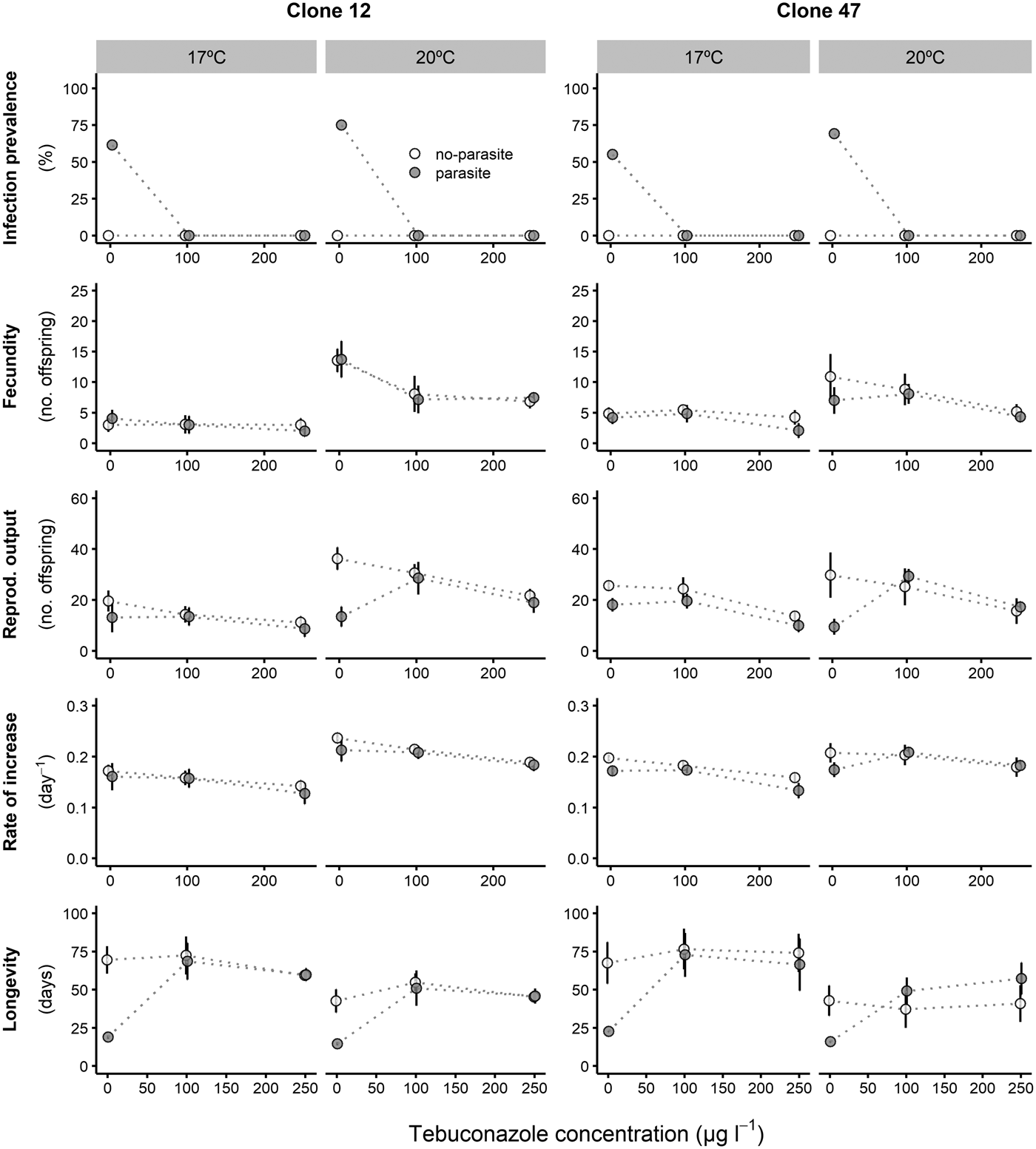
Prevalence of infection was fairly consistent for both Daphnia clones across all copper concentrations, despite an overall lower proportion of infection for Clone 47 at 17 °C (Fig. 1). By contrast, infection was suppressed in the presence of tebuconazole (only control animals became infected), which was consistent for both clones and under both temperatures (Fig. 2).
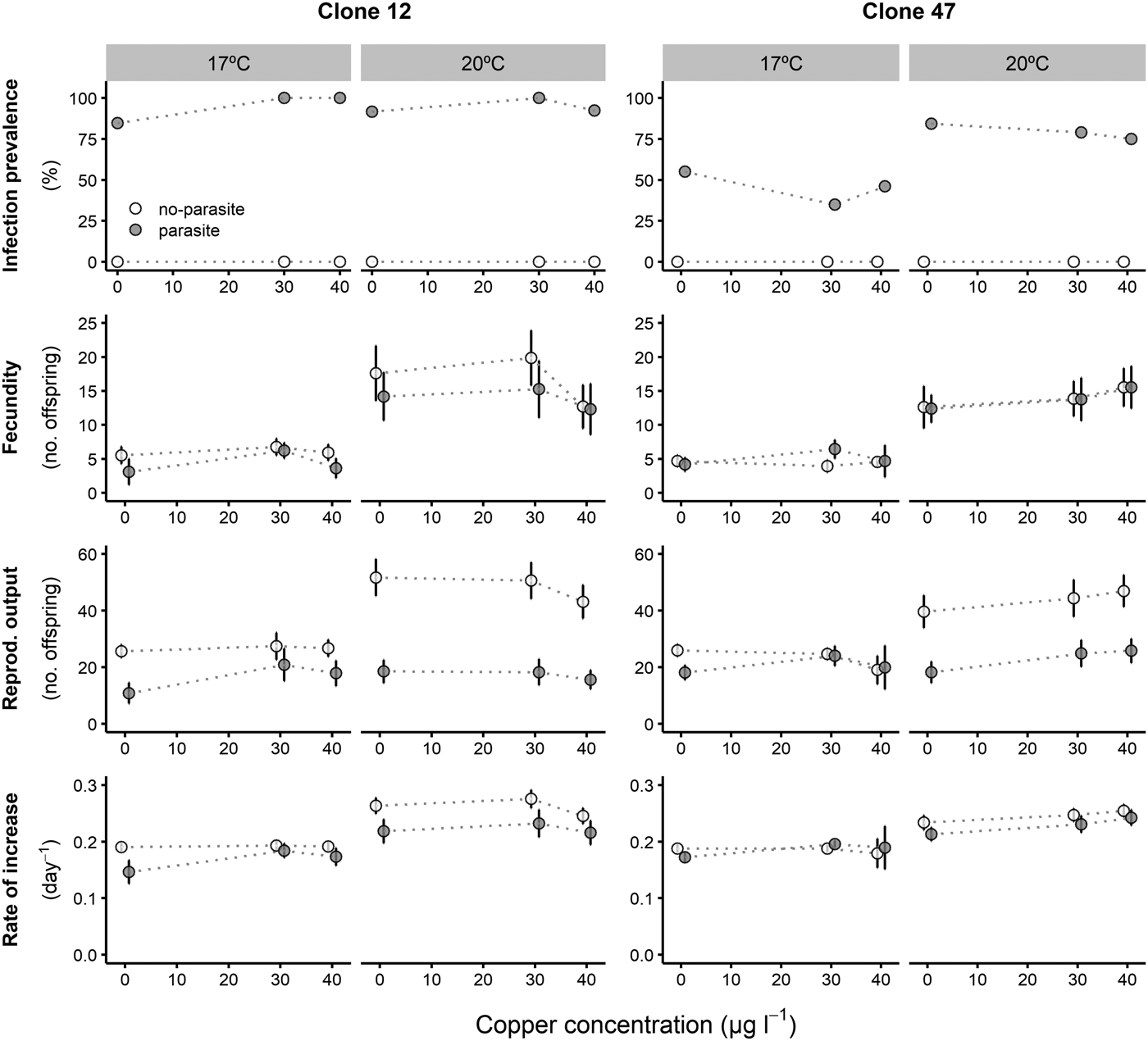
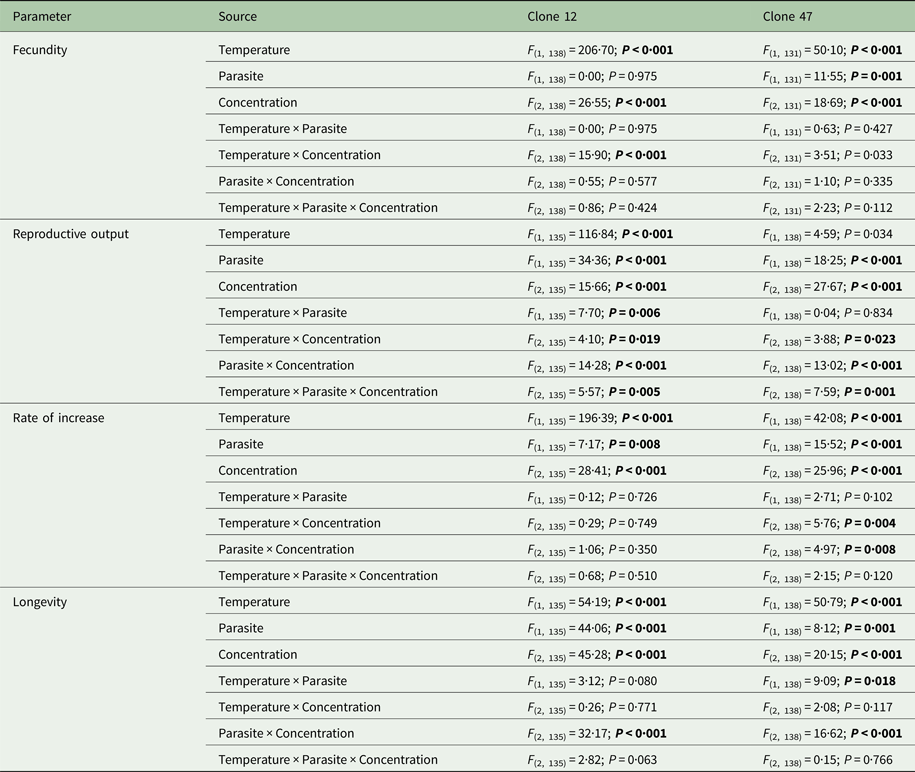
Fig. 1. Life history parameters (mean and respective 95% confidence intervals, except for infection) of two Daphnia genotypes (Clone 12 and 47) when exposed to increasing copper concentrations in the presence (‘parasite’) or absence (‘no-parasite’) of spores from the microparasitic yeast M. bicuspidata, at two temperatures (17 °C and 20 °C). For the calculation of the reproductive and demographic endpoints in the parasite treatment, only infected hosts were considered (see Methods).
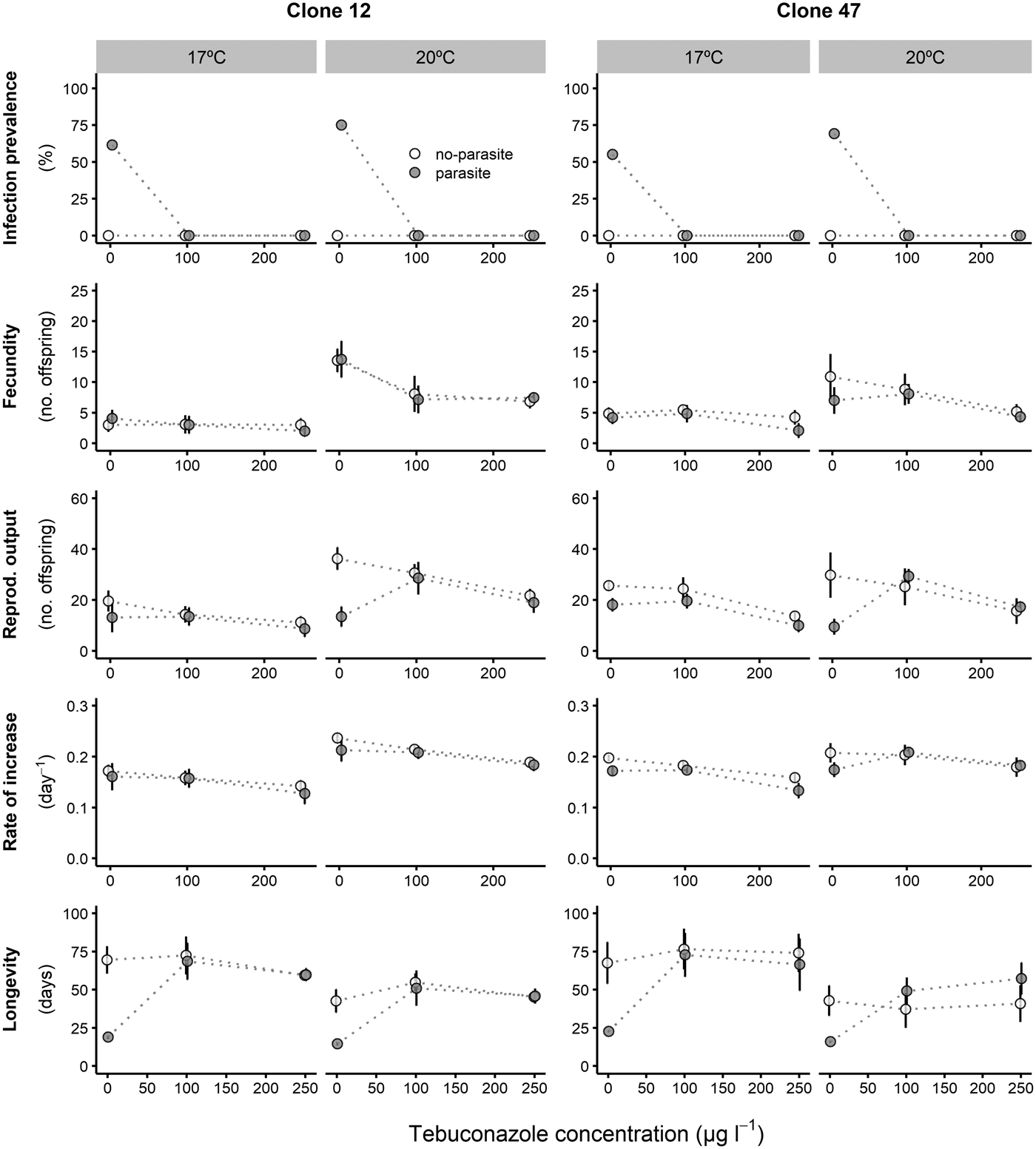
Fig. 2. Life history parameters (mean and respective 95% confidence intervals, except for infection) of two Daphnia genotypes (Clone 12 and 47) when exposed to increasing tebuconazole concentrations in the presence (‘parasite’) or absence (‘no-parasite’) of spores from the microparasitic yeast M. bicuspidata, at two temperatures (17 °C and 20 °C). Because of the antiparasitic nature of tebuconazole, no infected hosts were present in the parasite treatment, except for the negative control; in the latter case, only infected hosts were considered for the calculation of the reproductive and demographic endpoints (see Methods).
Temperature × parasite × copper interaction
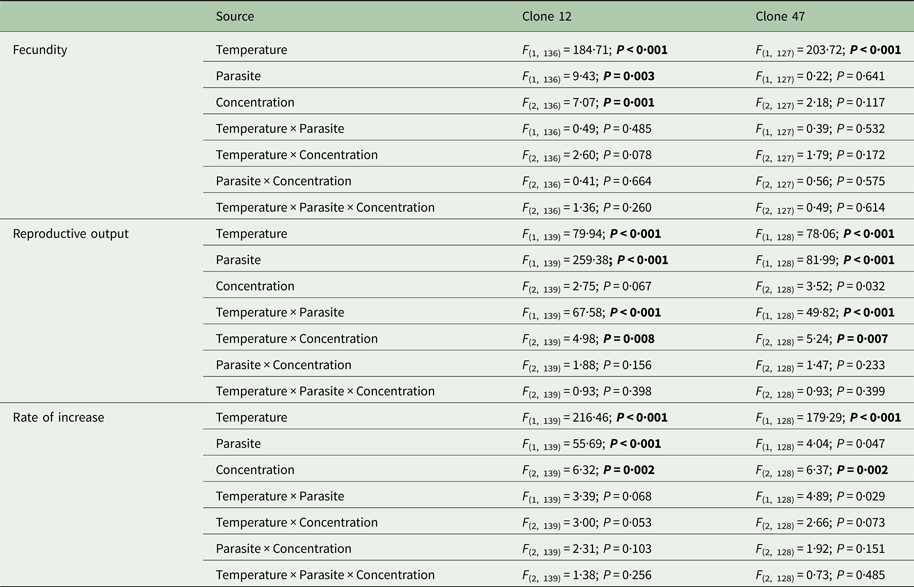
The tested copper concentrations did not elicit a pronounced effect on Daphnia reproductive parameters, although statistically significant effects (including a slight stimulation of reproduction) were observed under some circumstances (Table 1). These subtle effects were sometimes temperature-dependent (significant temperature × concentration interaction – Table 1). Temperature and the parasite caused major effects on Daphnia reproductive performance. The parasite-induced reduction in reproductive output was stronger at 20 °C (significant temperature × parasite interaction – Table 1 and Fig. 1). However, fecundity (calculated prior to the death of the hosts) was only reduced by the parasite in Clone 12, independently of temperature. The same was true for the rate of increase (Table 1 and Fig. 1). This means that the reproductive underperformance of infected Daphnia was mostly due to parasite-induced mortality rather than reproductive impairment, especially in Clone 47 (no differences in fecundity between parasite and no-parasite treatments). No significant interactions were detected for parasite × concentration or temperature × parasite × concentration (Table 1), demonstrating independent effects of parasite and contaminant action for both temperatures.
Table 1. Three-way ANOVA summary table of the effects of temperature, parasite challenge, concentration and their interactions on different life history parameters of two Daphnia genotypes (Clone 12 and 47) exposed to copper

Analyses were conducted separately per Daphnia clone. Significant effects are highlighted in bold (P ⩽ 0·025).
Temperature × parasite × tebuconazole interaction
Tebuconazole significantly decreased the reproductive performance of both Daphnia clones (Fig. 2 and Table 2). In some cases, these effects were temperature-dependent (significant temperature × concentration interactions, Table 2). Lower temperature significantly decreased the measured life history parameters of Daphnia, and exposure to the parasite also compromised host fecundity (only in clone 47), reproductive output and per capita rate of increase (both clones, Fig. 2 and Table 2). However, because infection only occurred in the absence of tebuconazole (see above), parasite effects were generally dependent on tebuconazole concentration (see significant interactions between parasite × concentration or temperature × parasite × concentration in Table 2). For example, the parasite caused a significant reduction in reproductive output and rate of increase, but only without tebuconazole (which suppressed infection), this effect is more pronounced at 20 °C (Fig. 2). Thus, unlike for copper, there is interdependence between tebuconazole contamination and parasitism, which is further modulated by temperature (Fig. 3).
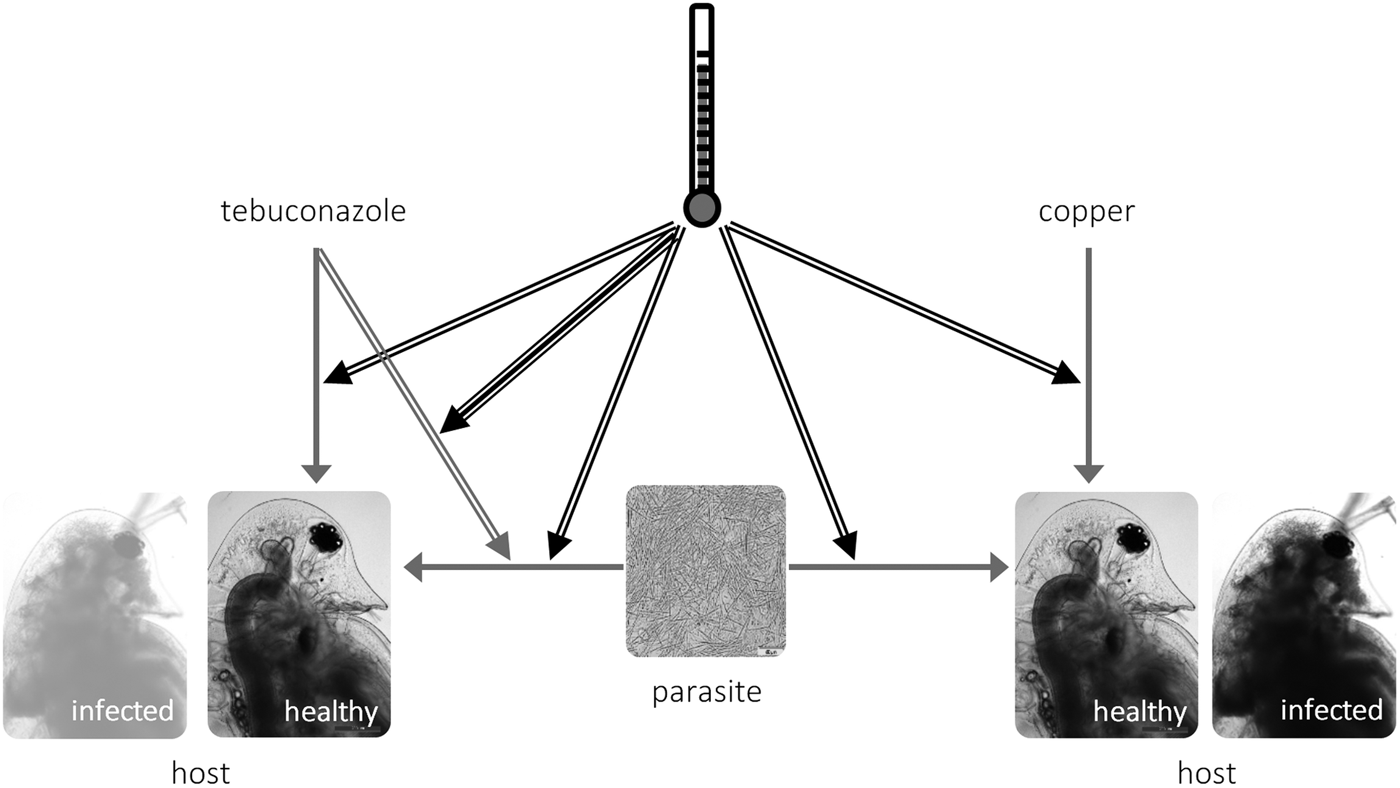
Fig. 3. Schematic representation of the interactions between temperature (black arrows), toxicant (tebuconazole, left; copper, right) and parasite (M. bicuspidata) in Daphnia life history. Simple arrows represent direct effects of a stressor (e.g. tebuconazole decreases reproduction in Daphnia), double arrows represent two-way interactions between stressors (e.g. higher temperature increases the virulence of the parasite); and the triple arrow depicts the three-way interaction between temperature × tebuconazole × parasite (see text).
Table 2. Three-way ANOVA summary table of the effects of temperature, parasite challenge, concentration and their interactions on different life history parameters of two Daphnia genotypes (Clone 12 and 47) exposed to tebuconazole
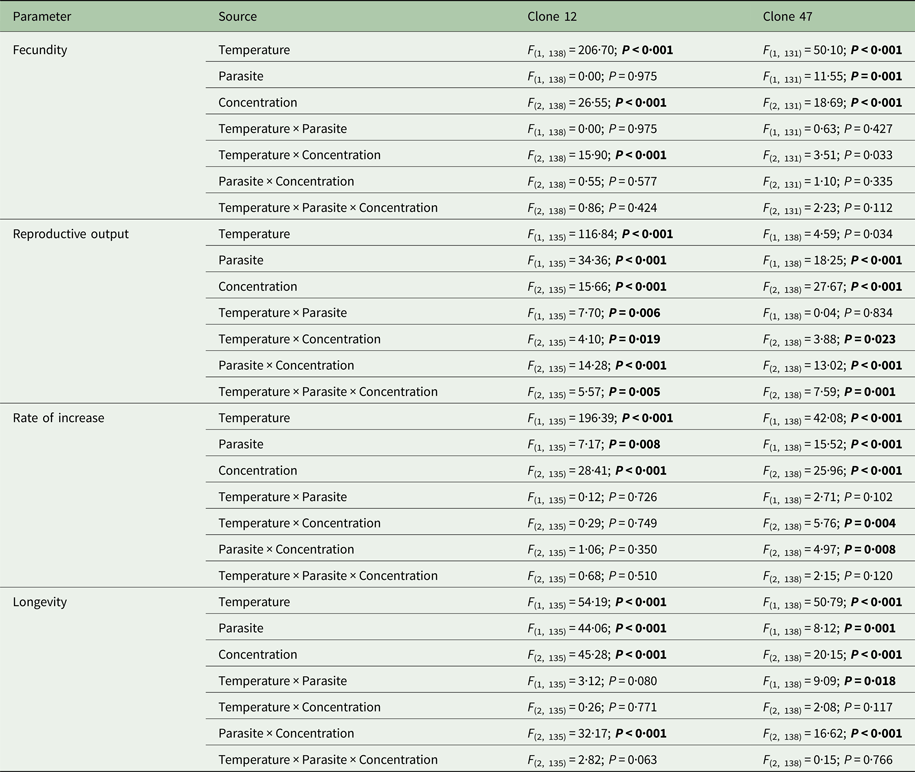
Analyses were conducted separately for each Daphnia clone. Significant effects are highlighted in bold (P ⩽ 0·025).
The extended observations (>21 days) confirmed that infection was definitely suppressed in the presence of tebuconazole, at all tested concentrations. Consequently, longevity was significantly reduced by the parasite, but only in the absence of tebuconazole (see significant parasite × concentration interaction: Table 2, Fig. 2). This pattern was similar for both clones, irrespective of exposure temperature.
Discussion
We explored how temperature modifies the outcome of the interaction between pollution and parasitism. We tested environmentally relevant scenarios, both in terms of applied pollutant concentrations (Kahle et al. Reference Kahle, Buerge, Hauser, Müller and Poiger2008; Bereswill et al. Reference Bereswill, Golla, Streloke and Schulz2012) and experienced temperatures. Confirming our expectations, elevated temperature exacerbated overall parasite effects and their interaction with tebuconazole (but not with copper sulphate) – see the conceptual model in Fig. 3. These results highlight the context-dependent nature of interactions between stress and parasitism, which might bring into question the pollution × disease debate, as pointed out by Hall et al. (Reference Hall, Vettiger and Ebert2013).
The most striking influence of temperature was the increased effect of the parasite on the host reproductive output, indicating that M. bicuspidata is more virulent at higher temperatures. At 17 °C, Daphnia still produced several broods before dying from infection, in contrast to females at 20 °C. For one of the two tested clones, higher infection was observed at 20 °C (clone 47 in the copper experiment). This may have resulted from increased filtration rates at 20 °C (Burns, Reference Burns1969), leading to increased consumption of parasite ascospores (Vale et al. Reference Vale, Stjernman and Little2008). Increased infection at higher temperatures has been observed in other host–microparasite systems (Vale et al. Reference Vale, Stjernman and Little2008; Dietrich et al. Reference Dietrich, Van Gaest, Strickland and Arkoosh2014; Frenken et al. Reference Frenken, Velthuis, de Senerpont Domis, Stephan, Aben, Kosten, van Donk and Van de Waal2016). Thus, climate change scenarios involving rising temperatures might exacerbate the negative effects of parasites in some systems (Marcogliese, Reference Marcogliese2008). However, the relationship between temperature and disease may not always be straightforward, because it depends on host physiology and interactions with other factors, such as predation (Hall et al. Reference Hall, Tessier, Duffy, Huebner and Cáceres2006). Furthermore, the degree of temperature variability has also been shown to be important in host–parasite interactions (Dallas and Drake, Reference Dallas and Drake2016).
Tested copper concentrations were mostly non-toxic to Daphnia, but they also did not alter parasite effects on the host, confirming the independent effect of copper and parasitism, as observed by Cuco et al. (Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2017). Although temperature interacted with copper concentration and parasite challenge (see the previous paragraph), it did not interfere with the inexistent copper × disease interaction. With respect to tebuconazole, tested concentrations caused a significant reduction in the reproduction of Daphnia, but the most notable effect of the toxicant was the suppression of infection. This confirms our previous results (Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2017) carried out in a distinct set of experiments. Unlike for copper, temperature clearly interfered in the interaction between tebuconazole and the parasite. The largest difference between infected and non-infected Daphnia was observed in the reproductive output in the control treatment (no tebuconazole) at 20 °C when compared with the controls at 17 °C. The temperature-dependent effect via parasite action was no longer perceptible in the presence of ecologically-relevant tebuconazole concentrations.
Few studies have addressed interactions between temperature, pollution and disease. For example, Dietrich et al. (Reference Dietrich, Van Gaest, Strickland and Arkoosh2014) demonstrated increased susceptibility of chinook salmon to the microparasite Aeromonas salmonicida when exposed to a common organophosphate insecticide (malathion) at elevated temperatures. In that study, however, the applied elevated temperature (19 °C) was above the fish optimal temperature (11 °C), which probably resulted in thermal stress. This was not the case for our Daphnia clones (Cuco et al. Reference Cuco, Abrantes, Gonçalves, Wolinska and Castro2016), and prior acclimation to each exposure temperature allowed potential effects of transient thermal shock or maladjustment to be discarded. Interactions between temperature, pollutants and biotic factors other than parasites have also been demonstrated. For example, Scherer et al. (Reference Scherer, Seeland, Oehlmann and Müller2013) found that suboptimal temperature influences the toxicity of the fungicide pyrimethanil in Daphnia, but this effect is masked by the presence of predator chemical cues. Despite the numerous appeals to consider such multi stressor scenarios (Heugens et al. Reference Heugens, Hendriks, Dekker, van Straalen and Admiraal2001; Noyes et al. Reference Noyes, McElwee, Miller, Clark, Van Tiem, Walcott, Erwin and Levin2009; Holmstrup et al. Reference Holmstrup, Bindesbøl, Oostingh, Duschl, Scheil, Köhler, Loureiro, Soares, Ferreira, Kienle, Gerhardt, Laskowski, Kramarz, Bayley, Svendsen and Spurgeon2010; Fischer et al. Reference Fischer, Pomati and Eggen2013), a consolidated body of knowledge on pollutant effects in environmentally-realistic scenarios is still missing.
According to IPCC (2014) predictions for climate change, temperatures are expected to rise ca. 4 °C by the end of the 21st century. In the tested scenarios, an increase of 3 °C was enough to intensify the effects of the parasite and its interaction with contaminant challenge (in the case of tebuconazole). This result is relevant not only because of the projected increase in mean temperatures but also in the face of projected increases in maximum temperatures, number of hot days and heat waves (IPCC, 2014), which may lead to transient periods of higher temperatures that could coincide with parasite epidemics. Thus, this is a clear indication that such an increase in temperature could lead to aggravated effects of parasitism and its interaction with anti-parasitic agents (such as tebuconazole) in natural populations.
Our extended observations (>21 days) confirmed that tebuconazole completely suppressed M. biscuspidata infection, which may have important ecological consequences. It is still not clear which stages of development (spore germination, growth, sporulation) are affected by tebuconazole exposure. Azole fungicides are considered fungistatic agents, preventing growth and sporulation of fungi (Kahle et al. Reference Kahle, Buerge, Hauser, Müller and Poiger2008), including yeasts (Manavathu et al. Reference Manavathu, Cutright and Chandrasekar1998; Álvarez-Pérez et al. Reference Álvarez-Pérez, de Vega, Pozo, Lenaerts, Van Assche, Herrera, Jacquemyn and Lievens2015). The anti-parasitic effect of tebuconazole here observed may compromise the extension and importance of parasite epidemics in the field. Such an event could affect host–parasite coevolution and endanger the ecological role of the parasite (Duffy and Sivars-Becker, Reference Duffy and Sivars-Becker2007; Hall et al. Reference Hall, Becker, Duffy and Caceres2011). The widespread use of azole fungicides (as agrochemicals, but also as pharmaceuticals) and their environmental persistence in aquatic systems (Kahle et al. Reference Kahle, Buerge, Hauser, Müller and Poiger2008) could ultimately result in a weakening of the reciprocal selection of hosts and azole-sensitive microparasites (such as M. bicuspidata). Indeed, fungi are ubiquitous in aquatic systems (Marcogliese, Reference Marcogliese2008; Dighton, Reference Dighton2016; Grossart et al. Reference Grossart, Wurzbacher, James and Kagami2016), being common parasites to a variety of organisms, such as cyanobacteria (Gleason et al. Reference Gleason, Jephcott, Küpper, Gerphagnon, Sime-Ngando, Karpov, Guillou and van Ogtrop2015; Agha et al. Reference Agha, Saebelfeld, Manthey, Rohrlack and Wolinska2016), protists (Gleason et al. Reference Gleason, Jephcott, Küpper, Gerphagnon, Sime-Ngando, Karpov, Guillou and van Ogtrop2015; Jephcott et al. Reference Jephcott, Alves-de-Souza, Gleason, van Ogtrop, Sime-Ngando, Karpov and Guillou2016), plants (Dean et al. Reference Dean, Van Kan, Pretorius, Hammond-Kosack, Di Pietro, Spanu, Rudd, Dickman, Kahmann, Ellis and Foster2012), invertebrates (Hall et al. Reference Hall, Becker, Duffy and Caceres2011), fish (Gozlan et al. Reference Gozlan, Marshall, Lilje, Jessop, Gleason and Andreou2014) and amphibians (Hanlon et al. Reference Hanlon, Kerby and Parris2012, Reference Hanlon, Lynch, Kerby and Parris2015). Besides playing important roles in energy transfer and in the regulation of host diversity, they also act as biological controllers of economically- and ecologically-important aquatic hosts, such as toxin-producing dinoflagellates or cyanobacteria (Gleason et al. Reference Gleason, Jephcott, Küpper, Gerphagnon, Sime-Ngando, Karpov, Guillou and van Ogtrop2015; Jephcott et al. Reference Jephcott, Alves-de-Souza, Gleason, van Ogtrop, Sime-Ngando, Karpov and Guillou2016). Given the common occurrence and importance of fungi at the ecosystem level (Dighton, Reference Dighton2016), studies such as this one are necessary to understand the potential effects of climate change and aquatic pollution on fungi-mediated processes (e.g. disease) and their consequences on ecosystem functioning.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017002062
Acknowledgments
Authors thank Mark Phillipo for linguistic improvement.
Financial Support
This work was supported by European Funds (through COMPETE2020 and PT2020) and by National Funds (through the FCT – Portuguese Science Foundation) within the scope of the strategic programmes UID/AMB/50017/2013 (POCI-01-0145-FEDER-007638) and UID/BIA/04050/2013 (POCI-01-0145-FEDER-007569), as well as project VITAQUA (PTDC/AAC-AMB/112438/2009). Individual support was also granted to APC (Ph.D. grant SFRH/BD/81661/2011), BBC (post-doc fellowship SFRH/BPD/109753/2015) and NA (researcher contract IF/01198/2014). The funding institutions had no role or involvement in the conception, collection, analysis and interpretation of the data.